Abstract
Rat C-CAM is a ubiquitous, transmembrane and carcino-embryonic antigen related cell adhesion molecule. The human counterpart is known as biliary glycoprotein (BGP) or CD66a. It is involved in different cellular functions ranging from intercellular adhesion, microbial receptor activity, signaling and tumor suppression. In the present study N-glyco-sylation of C-CAM immunopurified from rat liver was analyzed in detail. The primary sequence of rat C-CAM contains 15 potential N-glycosylation sites. The N-glycans were enzymatically released from glycopeptides, fluorescently labeled with 2-aminobenzamide, and separated by two-dimensional HPLC. Oligosaccharide structures were characterized by enzymatic sequencing and MALDI-TOF-MS. Mainly bi- and triantennary complex structures were identified. The presence of type I and type II chains in the antennae of these glycans results in heterogeneous glycosylation of C-CAM. Sialylation of the sugars was found to be unusual; bi- and triantennary glycans contained three and four sialic acid residues, respectively, and this linkage seemed to be restricted to the type I chain in the antennae. Approximately 20% of the detected sugars contain these unusual numbers of sialic acids. C-CAM is the first transmembrane protein found to be over-sialylated.
Keywords: glycoprotein, N-glycan structure, sialylation, carcinoembryonic antigen, C-CAM
Introduction
Rat cell-cell adhesion molecule C-CAM is a highly glycosylated, transmembrane protein with 4 Ig-like loop domains and a Mr of 110 k with highest sequence similarity to the carcinoembryonic-antigen (CEA)-subfamily (for review, see Öbrink, 1997). C-CAM has been independently described by several groups using different designations, e.g., cell-CAM 105, gp110, or Ecto-ATPase/C-CAM (Becker et al., 1985; Odin et al., 1986; Lin and Guidotti, 1989). The human counterpart is known as biliary glycoprotein (BGP) or CD66a (Svenberg et al., 1979; Hinoda et al., 1988).
C-CAM is regarded as a multifunctional molecule. Like other proteins of the Ig-superfamily, it mediates homophilic intercellular adhesion via its extracellular region, as shown in isolated hepatocytes (Odin et al., 1986; Becker et al., 1989; Becker et al., 1993;) and transfected COS 7-, CHO- or insect cells (Lin et al., 1991; Lucka et al., 1995; Olsson et al., 1995). In this process the first N-terminal Ig-like loop structure is the major adhesive component (Cheung et al., 1993). Within this region three potential glycosylation sites exist. The intracellular part of C-CAM interacts with proteins involved in the signal transduction pathway, such as pp60cscr, the insulin receptor kinase, the protein kinase C, and the protein tyrosine phosphatase SHP-1 (Brummer et al., 1995; Najjar et al., 1995; Beauchemin et al., 1997; Edlund et al., 1998). Furthermore, interaction of both isoforms with calmodulin has been reported (Blikstad et al., 1992; Edlund and Öbrink, 1993; Edlund et al., 1996). Most important, tumor suppression activity of C-CAM has been demonstrated in carcinoma cells from rat prostate, bladder, and colon (Hsieh et al., 1995; Kleinerman et al., 1996; Turbide et al., 1997). In addition, C-CAM has been shown to bind bacteria and viruses (Leusch et al., 1991; Williams et al., 1991; Dveksler et al., 1993; Virji et al., 1996; Gray-Owen et al., 1997), and its involvement in the binding and transport of bile salts from hepatocytes into the bile has been suggested (Müller et al., 1991; Sippel et al., 1993).
The human C-CAM protein from neutrophilic granulocytes has been implicated as a receptor for the lectin, galectin 3 (Ohannesian et al., 1995), and has been characterized as one of the major protein carriers of Lewis x and sialyl Lewis x structures (Stocks and Kerr, 1993) which are known to function as selectin ligands. The primary sequence of rat C-CAM contains 15 potential N-glycosylation consensus sites (Lin and Guidotti, 1989), but so far no detailed structural characterization of N-linked rat C-CAM glycan chains has been reported. Digestion with oligosaccharide-specific enzymes showed that rat liver C-CAM exists as numerous glycoforms. Desialylation reduced the Mr from 110 k to ∼90 k, while PNGaseF-treatment shifted the Mr to ∼50 k. EndoH had no effect, indicating the absence of high-mannose type oligosaccharides (Becker et al., 1993). Characterization using lectin-agarose affinity chromatography indicated that the majority of glycans were of the complex-type (Bierhuizen et al., 1989).
Although the protein structure of C-CAM is known, and detailed functional transfection studies have been performed, the glycosylation status of the protein remains to be clarified. The present study describes the complete oligosaccharide structures of rat C-CAM, as analyzed by different HPLC separations in combination with MALDI-MS and enzymatic sequencing of N-glycans.
Fig. 1.
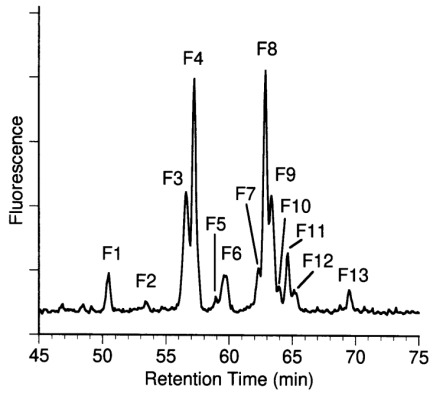
Aminophase HPLC of desialylated N-glycans from C-CAM. N-glycans were released from C-CAM by PNGase F and subsequently desialylated. Neutral oligosaccharides were separated using aminophase HPLC as described in Materials and methods. Oligosaccharide fractions F1 to F13 were further separated by RP-HPLC and characterized using mass spectrometry and carbohydrate sequencing.
Results
Monosaccharide composition
C-CAM was isolated from rat liver and purified using immunoaffinity chromatography. Purity of the resulting protein fractions was monitored by SDS-PAGE. A single protein band with an apparent mass of about of 110 kDa corresponding to the mass of C-CAM was observed. Carbohydrate composition analysis revealed the presence of N-acetylglucosamine, galactose and mannose in a molar ratio of 4.7∶2.5∶3.0 (relative to mannose = 3.0). Fucose was detected in traces only. N-Acetylgalactosamine, a typical constituent of mucin type O-glycans, was not found. The content of sialic acid is 2.2 relative to mannose (3.0), calculated from the results of Table III.
Separation of desialylated 2-AB-labeled glycans
N-Linked glycans were enzymatically released from tryptic peptides by PNGase F digestion, then fluorescently labeled with 2-AB. Labeled N-glycans were incubated with neuraminidase from Arthrobacter ureafaciens. Anion-exchange chromatography yielded only one signal corresponding to neutral glycans (data not shown). Therefore the acidic property of the sugars could be ascribed to their sialic acid residues. Desialylated N-glycans were separated with various chromatographic techniques. An aliquot of the desialylated sugars was subjected to P4 gel permeation chromatography, which separates sugars according to their hydrodynamic volume (data not shown). Due to the relatively low resolution of this chromatographic system, no separation of sugars differing in the linkage of the terminal galactose residues was achieved. The two main fractions eluted at positions corresponding to 11.1 and 14 glucose units, which is consistent with the presence of bi-and triantennary glycans. The corresponding fucosylated sugars were eluted at positions corresponding to approximately 12.1 and 15 glucose units. Four sugar fractions containing bi- and triantennary glycans with or without fucose were analyzed by enzymatic sequencing. Two dimensional HPLC was applied as a high resolution chromatographic system to obtain individual glycans. Separation of desialylated 2-AB-labeled glycans using NH2-HPLC yielded 13 fractions designated F1-F13 (Figure 1).
Table I. Mass spectrometric data of desialylated N-glycans released from C-CAM.
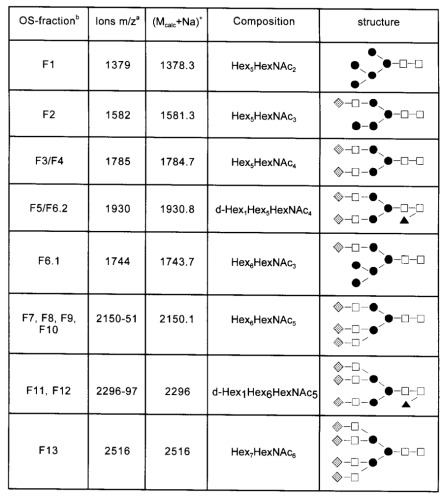
Mass spectrometric analysis of the desialylated oligosaccharides from C-CAM was performed by MALDI-TOF-MS. Detected ions could be interpreted as Na-adducts (M+Na)+ of the glycans. Mcalc refers to the calculated average molecular mass (in daltons) summed from the likely carbohydrate composition plus the 2-AB-label. Open squares, N-acetyl-D-glucosamine; solid circles, D-mannose; shaded diamonds, D-galactose; solid triangles, L-fucose.
aValues are rounded.
bOS-fraction, Oligosaccharide fraction numbers correspond to peak numbers in Figure 1.
Mass determination
Fraction F1-F13 were rechromatographed by RP-HPLC to obtain homogeneous sugar fractions. RP-HPLC-subfractions were analyzed if they constituted more than 1% of the total desialylated N-glycans. For the structural characterization of individual glycans, the oligosaccharide fractions isolated by NH2- and RP-HPLC were analyzed using mass spectrometry and carbohydrate sequencing with exoglycosidases. Oligosaccharide-fractions obtained by RP-HPLC were subjected to mass determination by MALDI-TOF-MS (Table I). Pseudomolecular ions corresponding to a high mannose structure and hybrid structures were detected for fractions F1, F2, and F6.1. Mass spectrometric data of fractions F3, F4, and F7–F10 were consistent with biantennary and triantennary structures, respectively. Data recorded for F5, F6.1 and F11, F12 revealed the presence of fucosylated biantennary and fucosylated triantennary sugar chains. Ions of tetraantennary glycans were detected for fraction F13.
Carbohydrate sequencing
Four sugar fractions obtained by P4 gel permeation chromatography were analyzed using the reagent array analysis method. The results revealed that C-CAM carried bi- and triantennary sugars with antennae consisting of GlcNAcβ1-3/4Gal, and that fucose is bound to the core region of the glycans. Only 2,2,4 but no 2,2,6 linked triantennary sugars were detected. To specify the linkage of terminally bound galactose residues as well as the linkage of the subterminal N-acetylglucosamine to the trimannosyl core, glycan fractions obtained by RP-HPLC were digested with a mixture of β1-4-galactosidase and β-N-acetylhexosaminidase. The reaction products were analyzed by MALDI-TOF-MS and for some sugars by P4 gel permeation chromatography. Treatment of fraction F3 yielded two sugars with m/z values of 1786 (biantennary glycan) and 1421 (biantennary glycan minus a lactosamine unit). Thus two isomeric biantennary glycans eluted in fraction F3 carrying one or two units of GlcNAcβ1-3Gal (type I chain) in the antennae (Table III, F3.1 and F3.2). The main compound in fraction F4 was found to be a biantennary sugar with two antennae consisting of two GlcNAcβ1-4Gal (type II chain), but an isomer with one type I chain antenna was also detected. P4 gel permeation chromatography was used to determine the ratio of these fragments in fractions F3 and F4. The linkage of the type I and II chain antennae to the 3- or 6-linked mannose in the core of the oligosaccharide was not examined. Two fucosylated biantennary structures carrying one or two type II chains were found for sugars eluted in F5 and F6.2. Mass spectrometry of the digested fractions F7, F8, F9, and F10 resulted in ions which could be interpreted as isomers of triantennary sugars. A signal at m/z 1786 detected for fraction F7 revealed a loss of one lactosamine unit consistent with a 2,2,4 branched-triantennary sugar with two type I chains in the antennae. The main fraction, F8, yielded a signal at m/z 1421; this indicates the loss of two lactosamine units from a triantennary glycan carrying one GlcNAcβ1-3Gal antenna which is 1–4-linked to mannose. Two digestion products were detected for fraction F9. The signal of m/z 1421 (F9.1) was again consistent with a fragment generated from a digested 2,2,4 branched-triantennary sugar with two type II sugar chains. A second isomer carrying three type II chains was deduced from the m/z value of 1259 recorded for fraction 9.2. The ratio of these fragments was determined by P4 gel permeation chromatography. Mass spectrometric data of exoglycosidase treated glycans F7, F8, and F9.1 suggested that one antennae of the type I chain is 1–4-linked to mannose.
In contrast, the m/z value of 1623 detected for the digested sugar fraction F10 indicated the presence of a triantennary glycan with a single type I chain 1–2-linked to mannose. Treatment of fractions F11, F12.1, and F12.2 with the enzyme mixture resulted in ions at m/z 1565 (F11), 1931 (12.1), and 1405 (F12.2). These signals could be interpreted as digestion products of fucosylated 2,2,4-branched triantennary sugars carrying one type I chain linked to the 4 arm (F11), and a corresponding glycan containing a second type I chain linked to the 2 position of mannose (F12.1). An isomer of F11 carrying three GlcNAcβ1-4Gal units was detected in fraction F12.2. Mass spectrometric data for digested glycans of fraction F13 revealed the presence of tetraantennary structures. These results are compiled in Table III.
Fig. 2.
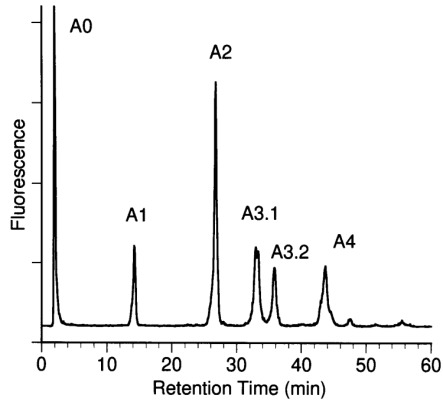
Separation of N-linked oligosaccharides from C-CAM by anion exchange chromatography. N-glycans were fractionated according their number of negative charges using anion-exchange chromatography on MonoQ as described in Materials and methods. Fractions of neutral (A0), mono- (α1), bi- (α2), tri- (A3.1, A3.2), and tetrasialylated (A4) oligosaccharides were desialylated and further characterized using aminophase HPLC.
Analysis of sialylated N-glycans
2-AB-labeled N-glycans were separated using anion-exchange chromatography and NH2-HPLC. Fractionated N-glycans were identified after desialylation by their retention times and mass spectrometric data.
Sialylated, 2-AB-labeled oligosaccharides were separated according to their number of negative charges using anion-exchange chromatography. A neutral fraction A0 and five acidic fractions α1, α2 A3.1, A3.2, and A4, containing 1, 2, 3, or 4 acidic residues, were obtained (Figure 2). These represented 3 (A0), 11 (α1), 38 (α2), 20 (A3.1), 11 (A3.2), and 17% (A4) of the total N-glycans. N-Glycans carrying three negative charges eluted in the two fractions A3.1 and A3.2. Since fraction AO coeluted with the injection peak, the ratio of sugars was quantified after rechromatography using NH2-HPLC. For further characterization the oligosaccharide-fractions α1–A4 were desialylated and rechromatographed by NH2-HPLC. Comparison of the glycan pattern of the total oligosaccharide moiety with the glycan pattern of the desialylated sugars of fractions A0, α1, α2, A3.1, A3.2, and A4 was used for structure assignment of the acidic fractions (Figure 3). The identification of the chromatographic pattern was supported by mass spectrometric data for each desialylated glycan fraction (data not shown). Analysis of the total glycan population after desialylation revealed the presence of two isomers in several of the fractions, including the main fractions F3, F4, and F9. The ratio of sugar isomers within fractions α2, A3.1, A3.2, and A4 was evaluated after enzymatic digestion with a mixture of exoglycosidases as described above. Reaction products were determined by mass spectrometry and by P4 gel permeation chromatography.
Fig. 3.
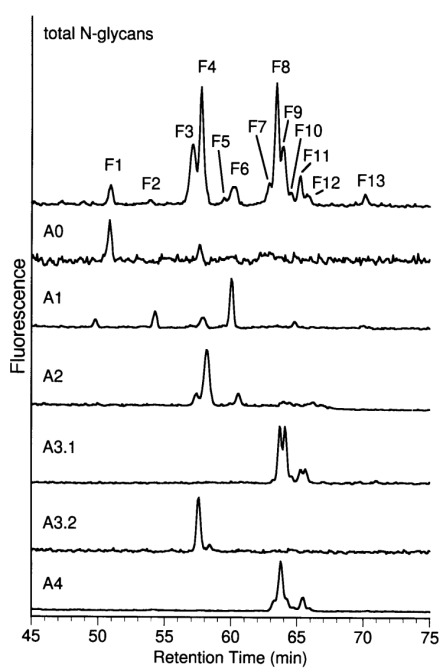
Aminophase HPLC of desialylated MonoQ subfractions. Fraction A0 and desialylated fractions α1, α2, A3.1, A3.2, and A4 of MonoQ-FPLC were rechromatographed using aminophase HPLC. Oligosaccharides of subfractions were identified by comparison of retention times, by determination of the total oligosaccharide moiety and by MALDI-TOF MS (not shown). Distribution of sialylation variants of glycans F1–F13 in fractions A0–A4 was quantified by determination of peak areas and is summarized in Table III.
The main component in the small neutral fraction A0 was a high mannose structure (F1). A hybrid glycan eluting in fraction F6 was the dominant sugar of the monosialylated oligosaccharides (α1). Biantennary glycans with or without fucose were detected in fraction α2 (Figure 3). Enzymatic digestion of the total fraction α2 and quantification of the glycan fragments showed that the ratio of the isomeric biantennary sugars carrying one/two type II chains in the antennae was approximately 1∶3. Fraction A3.1 consisted exclusively of triantennary glycans. The main components were glycans F8 and F9.2 in a ratio of approximately 2∶1. Biantennary glycans carrying three sialic acid residues were identified in fraction A3.2. Two isomers carrying one or two Galβ1-3 GlcNAc units in the antennae were found in a ratio of approximately 2∶1. Biantennary glycans with two Galβ1-4 GlcNAc units in the antennae were not found in this fraction. Glycans of fraction A4 were of the triantennary type. The main glycan structure was F8. Secondary components were identified as glycans F7, F9.1 and their corresponding fucosylated sugars F11 and F12.1. The results are summarized in Table III.
Fig. 4.
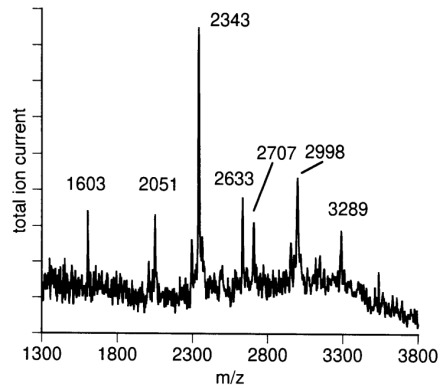
MALDI-TOF mass spectrometry of sialylated N-glycans from C-CAM. Sialylated oligosaccharides were measured in negative ion mode (see Materials and methods). Relative masses of 2051, 2343, 2633, 2707, 2998, and 3289 correspond to theoretical [M-H]−-ions of 2-AB labeled mono-, bi-, and trisialylated biantennary and bi-, tri-, and tetrasialylated triantennary complex N-glycans.
To investigate the unusually high sialylation of the bi- and triantennary glycans eluting in fraction A3.2 and A4, the mixture of the sialylated glycans were subjected to mass spectrometry (Figure 4). The mass spectrum revealed the presence of biantennary glycans carrying one, two, or three N-acetylneura-minic acid (NANA) residues and triantennary glycans containing two, three, or four N-acetylneuraminic acid residues (Table II).
The nature of the sialic acid linkage was investigated by digestion of sialylated oligosaccharide fraction with sialidase from Arthrobacter ureafaciens and Newcastle disease virus followed by anion-exchange chromatography. Sialidase from Arthrobacter ureafaciens shows broad specificity, cleaving terminal α2,3, α2,6, or α2,8 sialic acid (Uchida et al., 1979). In contrast, sialidase from Newcastle disease virus is highly α2,3 linkage specific and does not cleave α2,6-bound sialic acid (Drzeniek, 1973; Paulson et al., 1982). Figure 5 shows separation of N-glycans before (A) and after digestion with sialidases from Newcastle disease virus (B) and Arthrobacter ureafaciens (C), respectively. Digestion with Arthrobacter ureafaciens sialidase results in complete cleavage of sialic acids, whereas sialic acids are split off only partially using α2,3-specific sialidase from Newcastle disease virus. Fractions A3/2 and A4—exclusively consisting of trisialylated, biantennary and tetrasialylated, trian-tennary N-glycans, respectively—completely disappeared, indicating at least one α2,3-bound sialic acid per oversialylated oligosaccharide.
However, α2,3-linked sialic acid could not be detected by lectin binding with SNA and MAA to immobilized C-CAM. SNA binds to α2,6-linked, whereas MAA binds to α2,3-linked sialic acid. Strong binding of SNA but no binding of MAA was observed (data not shown).
Discussion
The combination of two dimensional HPLC, MALDI-TOF-MS, and carbohydrate sequencing yielded detailed analysis of all N-glycans present on C-CAM. The absence of N-acetyl-galacto-samine, as shown by monosaccharide composition analysis, suggests that C-CAM is exclusively N-glycosylated. The main structures are complex, bi- and triantennary N-glycans. Antennae consist of both Galβ1,3GlcNAc1 type I- and Galβ1,4GlcNAc1 type II-chains. Fucose was found to be exclusively α1,6-linked at the proximal GlcNAc residue of the N,N′-diacetyl-chitobiose moiety. Twenty-eight percent of C-CAM N-glycans possess an unusually high number of sialic acids, i.e., they contain more sialic acid residues than antennae. This phenomenon is known as “oversialylation.”
Table II. Mass spectrometric data for sialylated N-glycans released from C-CAM.
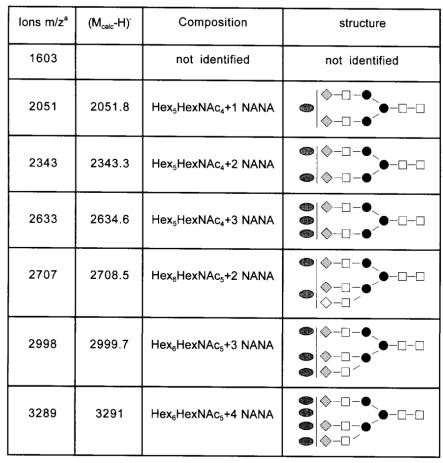
Mass spectrometric data of the sialylated oligosaccharides from C-CAM were obtained by MALDI-TOF-MS. Detected ions could be interpreted as (M-H)- of the glycans. Mcalc refers to the calculated average molecular mass (in daltons) summed from the likely carbohydrate composition plus the 2-AB-label. Open squares, N-acetyl-D-glucosamine; solid circles, D-mannose; shaded diamonds, D-galactose; shaded ovals, N-acetylneuraminic acid.
aValues are rounded.
In a former study Bierhuizen et al. (1989) characterized the glycosylation of C-CAM from rat liver using lectin-agarose affinity chromatography of desialylated, 14C-labeled glycopeptides. Lectin-binding indicated that the majority of glycans were of the complex-type, which agrees with our results. However, 16% of C-CAM N-glycans were thought to consist of oligoman-nose or incomplete complex, biantennary structures and more than half of the biantennary glycopeptides were found to be incompletely β-galactosylated. As we did not find any undergalactosylated N-glycans, occurrence of incomplete structures may be explained by degradation due to preparation of glycopeptides by extensive pronase digestion, desialylation by acid hydrolysis and 14C-labeling. Moreover, in contrast to our analysis, Bierhuizen et al. detected α(1–3)-linked fucose bound to GlcNAc of Galβ1,4GlcNAc1- of the outer chain. Detection of α(1–3)-linked fucose and a high portion of oligomannose structures may reflect the limitations of using lectin-binding studies for structural analysis of oligosaccharides.
In addition to the detailed analysis of desialylated N-glycans, we present the sialylation variants of these structures. Differences in the extent of sialylation of 19 main structures result in 39 N-glycan structures, of which 36 N-glycans contain one or more sialic acids. The most important result is that 28% of total N-glycans contain more sialic acid residues than antennae. About 38% of all biantennary structures are trisialylated, while 38% of all triantennary glycans appear to be tetrasialylated. The high content of sialic acids is responsible for the very acidic isoelectric point of 2.5–3.5 of C-CAM, as shown by the shift of its pI to 5.0–6.0 after desialylation (Becker et al., 1993).
Oversialylation of N-glycans has been described for factor IX, X, cold insoluble globulin, and fetuin from bovine blood (Takasaki et al., 1979; Mizuochi et al., 1980, 1983; Green et al., 1988) and mouse monoclonal IgG (Krotkiewski et al., 1989, 1990). In rat, oversialylation has been shown in plasma α1-acid glycoprotein, serotransferrin, and T-kininogen (Yoshima et al., 1981; Spik et al., 1991; Baussant et al., 1992). In all cases, oversialylation was due exclusively to the presence of α2,6-linked sialic acid bound to GlcNAc in NeuAc-α2,3Galβ1-3GlcNAc-antennae (type I-chains). This corresponds to our finding, that oversialylated bi-and triantennary N-glycans contained at least one type I-chain and at least one α2,3-bound sialic acid as shown by digestion with specific sialidases. Although occurrence of both α2,6- and α2,3-sialylation has been clearly demonstrated, in lectin binding experiments only α2,6-bound N-acetylneuraminic acid could be detected in C-CAM using SNA and MAA, respectively. This may be explained by inhibition of MAA-binding to disialylated type I chain, as disialylated NeuAcα2,4Galβ1,3(NeuAcα2,6)GlcNAc-, which has been found in cold-insoluble globulin from bovine plasma was also not detected by MAA-binding (Takasaki et al., 1979).
In rat hepatocytes oversialylation of biantennary N-glycan has been described only on the α1,3-bound NeuAcα2,3Galβ1,3Glc-NAcβ1,2Man-antennae of the biantennary N-glycan (Goulut-Chassaing and Bourrillon, 1995). Spik et al. (1991) and Baussant et al. (1992) also detected oversialylation in biantennary N-glycans of rat plasma glycoproteins in type I chains attached to the α1,3-linked mannose. This corresponds to our findings, as oligosaccharide F3.1 was found to be maximally trisialylated but not tetrasialylated, although it contains two type I chains. In contrast, Cumming et al. (1989) detected the NeuAc-α2,3Galβ1,3(NeuAcα2,6)GlcNAcβ1-chain in many structures of bovine fetuin, including the Manα1,6-antennae, but with marked preference for the Manα1,3-bound chain. Krotkiewski et al. (1990) found oversialylation of triantennary N-glycan exclusively in type I-chain linked to the C4-position of the 2,4-disubstituted mannose in a mouse monoclonal antibody. These findings agree with our results, as all the oversialylated triantennary structures we found (F7, F8, F9.1, F11, and F12.1) exhibit a type I chain linked to the C4-position of the 2,4-disubstituted mannose (Galβ1,3GlcNAcβ1,4Manα1,3-chain). Triantennary structures with type II chains bound at β1,4-position to mannose were not oversialylated, even when they possessed type I chains in β1,2-linkage to mannose (F9.2, F10).
Characterization of rat liver sialyltransferases using purified enzymes (Weinstein et al., 1982a,b) and biosynthesis of oligosaccharides using intact Golgi preparations from rat liver (Hayes and Varki, 1993a,b) showed distinct specificity of sialyltransferases towards type I and type II chains: while the α2,6-sialyltransferase sialylates only type II antennae, the α2,3-sialyltransferase can sialylate galactose in type I and type II chains as well. The substrate specificity of α2,6-sialyltransferase for type II oligosac-terminal β-Gal and the 2-amido group of the neighboring GlcNAc charides is explained by the requirement for the 6-hydroxyl of the for the transfer of Neu5Ac (Wlasichuk et al., 1993). In contrast, the α2,3-sialyltransferase accepts type I and type II chains as substrates, also when the 2- and 6- positions of the GlcNAc are modified by different substituents. Paulson et al. (1984) identified an N-acetylglucosaminide (α2–6)-sialyltransferase in Golgi apparatus from rat liver. This sialyltransferase had a marked preference for the sequence NeuAcα2,3Galβ1,3GlcNAcβ1- as an acceptor substrate. Sialylation of the type I antennae Galβ1,3GlcNAc1- was also demonstrated in vitro. However, this sialyltransferase has not been characterized or cloned to date.
Table III. Proposed structures for N-linked oligosaccharides of C-CAM from rat liver.
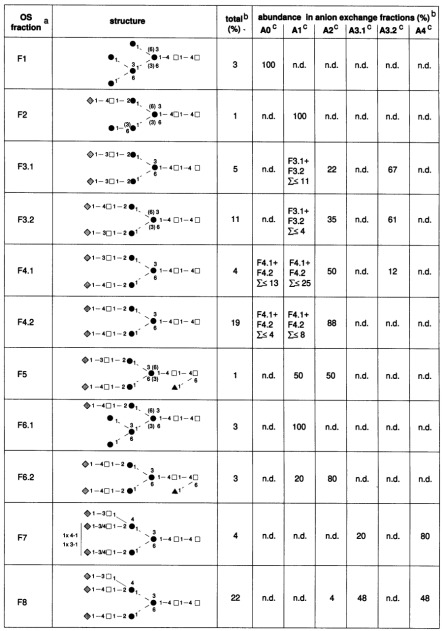
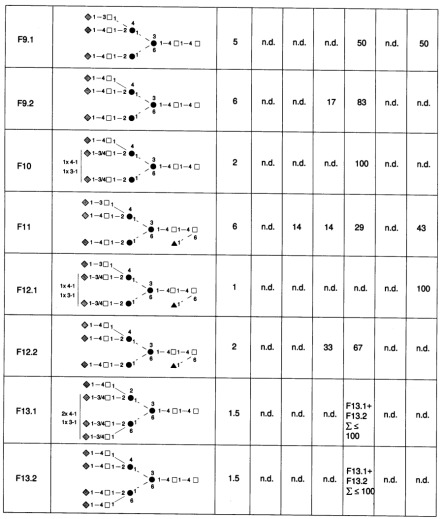
Relative abundance was estimated from peak ratios from the aminophase HPLC of desialylated fractions. Open squares, N-acetyl-D-glucosamine; solid circles, D-mannose; shaded diamonds, D-galactose; solid triangles, L-fucose; solid line, â-glycosidic; broken line, á-glycosidic.
aOS-fraction, Oligosaccharide fraction numbers correspond to the peak numbers in Figure 1.
bValues are rounded.
cA, the number of sialic acid residues (A0, neutral; A1, monosialo; A2, bisialo; A3, trisialo; A4, tetrasialo glycans). n.d., Not detected.
Comparison of carbohydrate moieties of rat and human plasma α1-acid glycoprotein (Yoshima et al., 1981) showed that all sialic acids of human α1-acid glycoprotein occurred as NeuAcα2,3Gal-or NeuAcα2,6Gal-. NeuAc in α2,6-position linked to Galβ1,3GlcNAc-chains has only been reported for rat α1-acid glycoprotein. As oversialylation has been shown to occur in rat, mouse, and bovine glycoproteins, it might be regarded as a general glycosylation feature of mammalian cells. Interestingly, oversialylation has not been reported for human glycoproteins so far.
Fig. 5.
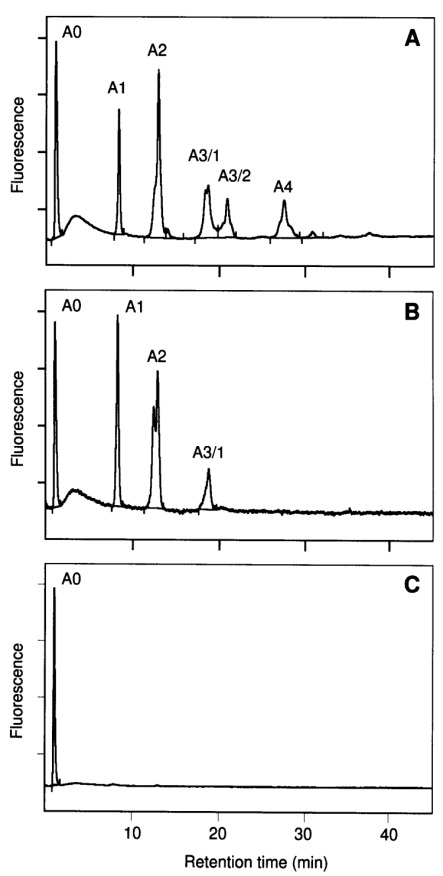
Separation of N-linked oligosaccharides from C-CAM by anion exchange chromatography before (A) or after digestion with sialidases from Newcastle disease virus (B) and Arthrobacter ureafaciens (C), respectively. N-glycans were fractionated according their number of negative charges using anion-exchange chromatography on MonoQ as described in Materials and methods. A0, Neutral; α1, mono-; α2, bi-; A3.1 and A3.2, tri- and A4 tetrasialylated structures.
Recently, Lucka et al. (1998) detected high concentrations of C-CAM in rat serum. High expression of type I chains may prevent unwanted crosslinking of soluble C-CAM with the asialoglycoprotein receptor, since oligosaccharides containing β1,3-linked Gal interact poorly with the asialoglycoprotein receptor, compared with those containing β1,4-linked Gal (Townsend et al., 1986). It remains to be shown whether glycosylation of soluble C-CAM corresponds to glycosylation of membrane bound C-CAM. However, the shielding of potential protease cleavage sites by the high degree of glycosylation and the high sialic acid content may be one reason for the long half life of C-CAM (∼50 h) in rat liver (Becker, 1989).
In summary, our study shows, for the first time, the presence of oversialylation in a membrane-bound glycoprotein. In view of the important functions of C-CAM, a separate study should be conducted to determine whether oversialylation is also detectable in human tissue.
Materials and methods
Materials
Unless otherwise stated, all chemicals and reagents were obtained from Merck, Serva or Sigma, Germany. Trypsin was purchased from Serva and trifluoroacetic acid was obtained from Sigma. Glycosidases were purchased from Boehringer Mannheim, Germany. Signal™ Labeling Kit, RAAM 2000 Neutral Sequencing Kit and sialidases were obtained from Oxford GlycoSciences Ltd., UK. Wistar rats were inbred German stocks originally derived from Charles River Wiga.
Purification of C-CAM
Liver membrane fractions of adult male Wistar rats (160 – 180 g body weight) were prepared after homogenization by differential centrifugation as described (Becker et al., 1986). Membranes were resuspended at a concentration of 5 mg/ml in PBS/1% Triton X-100 and completely solubilized for 2 h at 4°C. The supernatant after centrifugation at 100,000 g contained the extracted membrane preparation. C-CAM was isolated from rat liver membrane fractions by immunoaffinity chromatography. Immunoaffinity columns were prepared by coupling, as bridge-antibodies, 10 mg rabbit anti-mouse IgG to 1ml protein A-Sepharose (PAS) (Pharmacia, Uppsala, Sweden) followed by 10 mg purified monoclonal antibody Be 9.2 (Becker et al., 1986). The resulting PAS-immunocomplex was covalently crosslinked as described by Schneider et al. (1982). Immunoaffinity chromatography was done overnight at 4°C. After extended stringent washing with buffer containing high concentrations of salt and detergent, bound C-CAM was eluted with 50 mM diethylamine, pH 11.5. Quality was monitored by SDS-PAGE, silver staining and Western blotting according to standard procedures.
Release and fluorescence-labeling of oligosaccharide chains; carbohydrate composition analysis
C-CAM preparations were digested with trypsin. For release of N-glycans, tryptic peptides from 500 µg cell-CAM were digested with 5 mU PNGase F from Flavobacterium meningosepticum in 250 µl of a buffer containing 0.5% (w/v) NH4HCO3, pH 8.0, for 18 h at 37°C. Released N-linked glycans were fluorescently labeled by reductive amination with 2-aminobenzamide (2-AB) using a Signal™ Labeling Kit according the manufacturers protocol (Oxford GlycoSciences Ltd., UK). Monosaccharide analysis was performed by hydrolysis of the oligosaccharides with 2 M trifluoroacetic acid for 3.5 h at 100°C. Acetylated monosaccharides were analyzed by gas-chromatography as described previously (Geilen et al., 1992).
Separation of neutral N-glycans
Neutral 2-AB-labeled N-glycans were separated by aminophase (NH2)-HPLC using an APS 2-Hypersil column (4 × 250 mm, Knauer, Berlin, Germany) at room temperature with a flow rate of 1.5 ml/min. Glycans were eluted by a two step gradient, consisting of solvent A (acetonitrile) and solvent B (15 mM sodium acetate, pH 5.2) starting from 0% solvent B to 20% B in 10 min, and to 50% B within 100 min.
RP-HPLC, neutral-pH anion-exchange chromatography, and P4 gel permeation
Rechromatography of the neutral 2-AB-labeled N-glycans was performed with an ODS-Hypersil column (4 × 250 mm, Knauer, Berlin, Germany) at room temperature with a flow rate of 1 ml/min. After injection of the sample, the column was eluted with 7 ml H2O, followed by a linear gradient up to 25% acetonitrile within 75 min. Negatively charged sugars were separated on a Mono Q anion-exchange column (HR 5/5; Pharmacia). Oligosaccharides were eluted at a flow rate of 1 ml/min by a linear gradient formed from solvent A (H2O) and solvent B (0.6 M sodium acetate, pH 7.0); 5 min after injection, the gradient was increased from 0% solvent B to 35% B in 65 min. P4 gel permeation chromatography of 2-AB-labeled N-glycans was performed using a RAAM 2000 GlycoSequencer according to the manufacturers manual (Oxford GlycoSciences Ltd., UK).
Mass spectrometry
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) was carried out on a Bruker Biflex instrument (Bruker, Bremen, Germany) as described previously (Gohlke et al., 1996, 1997; Stehling et al., 1998).
Desialylation
Sialylated oligosaccharides were incubated with 0.1 U neuraminidase from Arthrobacter ureafaciens or with 10 mU sialidase from Newcastle disease virus (Oxford GlycoSciences Ltd., UK) each in 50 µl of a 0.05 M sodium acetate buffer, pH 5.5, overnight at 37°C.
Carbohydrate sequence analysis
Enzymatic sequencing of the main structures was performed using the reagent array analysis method (RAAM) (Edge et al., 1992) as described previously (Gohlke et al., 1997). Analysis was carried out using the RAAM 2000 Neutral Sequencing Kit and the RAAM 2000 GlycoSequencer (all from Oxford GlycoSciences Ltd.) according the protocol of the manufacturer. Linkage variants of terminally bound galactose and subterminal N-acetyl-glucosamine residues were studied by incubation of sugar fractions with a mixture of β-N-acetylhexosaminidase from Streptococcus pneumoniae and β-galactosidase from Streptococcus pneumoniae, both at a concentration of 80 mU/ml at pH 5.5 overnight (37°C). Digestion products were analyzed with MALDI-TOF-MS or P4 gel permeation chromatography.
Acknowledgments
We gratefully acknowledge the excellent technical assistance of Sabine Grams and Detlev Grunow. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 366), the Sonnenfeld-Stiftung, and the Fonds der Chemischen Industrie.
Glossary
Abbreviations
- 2-AB
2-aminobenzamide
- AP
polyclonal sheep anti-digoxygenin Fab fragments conjugated with alkaline phosphatase
- C-CAM
cell-cell adhesion molecule
- CEA
carcinoembryonic antigen
- DHB
2,5-dihydroxybenzoic acid
- MALDI-TOF MS
matrix-assisted laser desorption ionization-time of flight mass spectrometry
- NBT
4-nitroblue tetrazolium chloride
- RAAM
reagent array analysis method
- X-phosphate
5-bromo-4-chloro-3-indolyl-phosphate.
References
- Baussant T., Alonso C., Wieruszeski J.M., Strecker G., Montreuil J., Alhenc-Gelas F., Michalski J.C. Comparative study of asparagine-linked glycans of plasma T-kininogen in normal rats and during acute inflammation. Biochem. J. 1992;283:531–535. doi: 10.1042/bj2830531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchemin N., Kunath T., Robitaille J., Chow B., Turbide C., Daniels E., Veillette A. Association of biliary glycoprotein with protein tyrosine phosphatase SHP-1 in malignant colon epithelial cells. Oncogene. 1997;14:783–790. doi: 10.1038/sj.onc.1200888. [DOI] [PubMed] [Google Scholar]
- Becker A., Neumeier R., Heidrich C., Loch N., Hartel S., Reutter W. Cell surface glycoproteins of hepatocytes and hepatoma cells identified by monoclonal antibodies. Biol. Chem. Hoppe Seyler. 1986;367:681–688. doi: 10.1515/bchm3.1986.367.2.681. [DOI] [PubMed] [Google Scholar]
- Becker A., Neumeier R., Park C.S., Gossrau R., Reutter W. Identification of a transformation-sensitive 110-kDa plasma membrane glycoprotein of rat hepatocytes. Eur. J. Cell. Biol. 1985;39:417–423. [PubMed] [Google Scholar]
- Becker A., Gossrau R., Hoffmann C., Reutter W. Localization of a putative cell adhesion molecule (gp110) in Wistar and Fischer rat tissues. Histochemistry. 1989;93:55–61. doi: 10.1007/BF00266847. [DOI] [PubMed] [Google Scholar]
- Becker A., Lucka L., Kilian C., Kannicht C., Reutter W. Characterization of the ATP-dependent taurocholate-carrier protein (gp110) of the hepatocyte canalicular membrane. Eur. J. Biochem. 1993;214:539–48. doi: 10.1111/j.1432-1033.1993.tb17952.x. [DOI] [PubMed] [Google Scholar]
- Bierhuizen M.F., Hansson M., Odin P., Debray H., Obrink B., van Dijk W. Structural assessment of the N-linked oligosaccharides of cell-CAM 105 by lectin-agarose affinity chromatography. Glycoconj. J. 1989;6:195–208. doi: 10.1007/BF01050648. [DOI] [PubMed] [Google Scholar]
- Blikstad I., Wikstrom T., Aurivillius M., Obrink B. C-CAM (Cell-CAM 105) is a calmodulin binding protein. FEBS Lett. 1992;302:26–30. doi: 10.1016/0014-5793(92)80276-m. [DOI] [PubMed] [Google Scholar]
- Brummer J., Neumaier M., Gopfert C., Wagener C. Association of pp60c-src with biliary glycoprotein (CD66a), an adhesion molecule of the carcinoembryonic antigen family downregulated in colorectal carcinomas. Oncogene. 1995;11:1649–1655. [PubMed] [Google Scholar]
- Cheung P.H., Luo W., Qiu Y., Zhang X., Earley K., Millirons P., Lin S.H. Structure and function of C-CAM1. The first immunoglobulin domain is required for intercellular adhesion. J. Biol. Chem. 1993;268:24303–24310. [PubMed] [Google Scholar]
- Cumming D.A., Hellerqvist C.G., Harris-Brandts M., Michnick S.W., Carver J.P., Bendiak B. Structures of asparagine-linked oligosaccharides of the glycoprotein fetuin having sialic acid linked to N-acetylglucosamine. Biochemistry. 1989;28:6500–6512. doi: 10.1021/bi00441a051. [DOI] [PubMed] [Google Scholar]
- Drzeniek R. Substrate specificity of neuraminidases. Histochem.J. 1973;5:271–290. doi: 10.1007/BF01004994. [DOI] [PubMed] [Google Scholar]
- Dveksler G.S., Dieffenbach C.W., Cardellichio C.B., McCuaig K., Pensiero M.N., Jiang G.S., Beauchemin N., Holmes K.V. Several members of the mouse carcinoembryonic antigen-related glycoprotein family are functional receptors for the coronavirus mouse hepatitis virus-A59. J. Virol. 1993;67:1–8. doi: 10.1128/jvi.67.1.1-8.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge C.J., Rademacher T.W., Wormald M.R., Parekh R.B., Butters T.D., Wing D.R., Dwek R.A. Fast sequencing of oligosaccharides: the reagent-array analysis method. Proc. Natl. Acad. Sci. USA. 1992;89:6338–6342. doi: 10.1073/pnas.89.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund M., Obrink B. Evidence for calmodulin binding to the cytoplasmic domains of two C-CAM isoforms. FEBS Lett. 1993;327:90–94. doi: 10.1016/0014-5793(93)81046-3. [DOI] [PubMed] [Google Scholar]
- Edlund M., Blikstad I., Obrink B. Calmodulin binds to specific sequences in the cytoplasmic domain of C-CAM and down-regulates C-CAM self-association. J. Biol. Chem. 1996;271:1393–1399. doi: 10.1074/jbc.271.3.1393. [DOI] [PubMed] [Google Scholar]
- Edlund M., WikstrÖm K., Toomik R., Ek P., Öbrink B. Charakterisation of protein kinase C-mediated phosphorylation of the short cytoplasmic domain of C-CAM. FEBS Lett. 1998;425:166–170. doi: 10.1016/s0014-5793(98)00222-1. [DOI] [PubMed] [Google Scholar]
- Geilen C.C., Kannicht C., Orthen B., Heidrich C., Paul C., Grunow D., Nuck R., Reutter W. Incorporation of the hexose analogue 2-deoxy-D-galactose into membrane glycoproteins in HepG2 cells. Arch. Biochem. Biophys. 1992;296:108–114. doi: 10.1016/0003-9861(92)90551-7. [DOI] [PubMed] [Google Scholar]
- Gohlke M., Baude G., Nuck R., Grunow D., Kannicht C., Bringmann P., Donner P., Reutter W. O-Linked L-fucose is present in Desmodus rotundus salivary plasminogen activator. J. Biol. Chem. 1996;271:7381–6. doi: 10.1074/jbc.271.13.7381. [DOI] [PubMed] [Google Scholar]
- Gohlke M., Nuck R., Kannicht C., Grunow D., Baude G., Donner P., Reutter W. Analysis of site-specific N-glycosylation of recombinant Desmodus rotundus salivary plasminogen activator rDSPA alpha 1 expressed in Chinese hamster ovary cells. Glycobiology. 1997;7:67–77. doi: 10.1093/glycob/7.1.67. [DOI] [PubMed] [Google Scholar]
- Goulut-Chassaing C., Bourrillon R. Structural differences between complex-type Asn-linked glycan chains of glycoproteins in rat hepatocytes and Zajdela hepatoma cells. Biochim Biophys. Acta. 1995;1244:30–40. doi: 10.1016/0304-4165(94)00191-y. [DOI] [PubMed] [Google Scholar]
- Gray-Owen S.D., Dehio C., Haude A., Grunert F., Meyer T.F. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J. 1997;16:3435–45. doi: 10.1093/emboj/16.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E.D., Adelt G., Baenziger J.U., Wilson S., Van Halbeek H. The asparagine-linked oligosaccharides on bovine fetuin. Structural analysis of N-glycanase-released oligosaccharides by 500-megahertz 1H NMR spectroscopy. J. Biol. Chem. 1988;263:18253–18268. [PubMed] [Google Scholar]
- Hayes B.K., Varki A. The biosynthesis of oligosaccharides in intact Golgi preparations from rat liver. Analysis of N-linked and O-linked glycans labeled by UDP-[6-3H]N-acetylgalactosamine. J. Biol. Chem. 1993;268:16170–16178. [PubMed] [Google Scholar]
- Hayes B.K., Varki A. Biosynthesis of oligosaccharides in intact Golgi preparations from rat liver. Analysis of N-linked glycans labeled by UDP-[6-3H]galactose, CMP-[9-3H]N-acetylneuraminic acid and [acetyl-3H]acetyl-coenzyme A. J. Biol. Chem. 1993;268:16155–16169. [PubMed] [Google Scholar]
- Hinoda Y., Neumaier M., Hefta S.A., Drzeniek Z., Wagener C., Shively L., Hefta L.J., Shively J.E., Paxton R.J. Molecular cloning of a cDNA coding biliary glycoprotein I: primary structure of a glycoprotein immuno-logically crossreactive with carcinoembryonic antigen. Proc. Natl. Acad. Sci. USA. 1988;85:6959–6963. doi: 10.1073/pnas.85.18.6959. [published erratum appears in Proc. Natl. Acad. Sci. USA,1989, 86, 1668] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J.T., Luo W., Song W., Wang Y., Kleinerman D.I., Van N.T., Lin S.H. Tumor suppressive role of an androgen-regulated epithelial cell adhesion molecule (C-CAM) in prostate carcinoma cell revealed by sense and antisense approaches. Cancer Res. 1995;55:190–197. [PubMed] [Google Scholar]
- Kleinerman D.I., Dinney C.P., Zhang W.W., Lin S.H., Van N.T., Hsieh J.T. Suppression of human bladder cancer growth by increased expression of C-CAM1 gene in an orthotopic model. Cancer Res. 1996;56:3431–3435. [PubMed] [Google Scholar]
- Krotkiewski H., Nilsson B., Svensson S. Structural analysis of the carbohydrate chains of a mouse monoclonal IgM antibody. Eur. J. Biochem. 1989;184:29–38. doi: 10.1111/j.1432-1033.1989.tb14986.x. [DOI] [PubMed] [Google Scholar]
- Krotkiewski H., Gronberg G., Krotkiewska B., Nilsson B., Svensson S. The carbohydrate structures of a mouse monoclonal IgG antibody OKT3. J. Biol. Chem. 1990;265:20195–20201. [PubMed] [Google Scholar]
- Leusch H.G., Drzeniek Z., Markos-Pusztai Z., Wagener C. Binding of Escherichia coli and Salmonella strains to members of the carcinoembryonic antigen family: differential binding inhibition by aromatic alpha-glycosides of mannose. Infect. Immun. 1991;59:2051–2057. doi: 10.1128/iai.59.6.2051-2057.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.H., Guidotti G. Cloning and expression of a cDNA coding for a rat liver plasma membrane ecto-ATPase. The primary structure of the ecto-ATPase is similar to that of the human biliary glycoprotein I. J. Biol. Chem. 1989;264:14408–14. [PubMed] [Google Scholar]
- Lin S.H., Culic O., Flanagan D., Hixson D.C. Immunochemical characterization of two isoforms of rat liver ecto-ATPase that show an immunological and structural identity with a glycoprotein cell-adhesion molecule with Mr 105,000. Biochem. J. 1991;278:155–161. doi: 10.1042/bj2780155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucka L., Cichocka I., Bäumler K., Bechler K., Reutter W. A short isoform of carcinoembryonic-antigen-related rat liver cell-cell adhesion molecule (C-CAM/gp110) mediates intercellular adhesion. Sequencing and recombinant functional analysis. Eur. J. Biochem. 1995;234:527–535. doi: 10.1111/j.1432-1033.1995.527_b.x. [DOI] [PubMed] [Google Scholar]
- Lucka L., Sel S., Danker K., Horstkorte R., Reutter W. Level of carcinoembryonic antigen-related cell-cell adhesion molecule C-CAM is greatly increased in serum and urine of rats with liver diseases. FEBS Lett. 1998;438:37. doi: 10.1016/s0014-5793(98)01265-4. [DOI] [PubMed] [Google Scholar]
- Mizuochi T., Yamashita K., Fujikawa K., Titani K., Kobata A. The structures of the carbohydrate moieties of bovine blood coagulation factor X. J. Biol. Chem. 1980;255:3526–3531. [PubMed] [Google Scholar]
- Mizuochi T., Taniguchi T., Fujikawa K., Titani K., Kobata A. The structures of the carbohydrate moieties of bovine blood coagulation factor IX (Christmas factor) J. Biol. Chem. 1983;258:6020–6024. [PubMed] [Google Scholar]
- Müller M., Ishikawa T., Berger U., Klunemann C., Lucka L., Schreyer A., Kannicht C., Reutter W., Kurz G., Keppler D. ATP-dependent transport of taurocholate across the hepatocyte canalicular membrane mediated by a 110-kDa glycoprotein binding ATP and bile salt. J. Biol. Chem. 1991;266:18920–18926. [PubMed] [Google Scholar]
- Najjar S.M., Philippe N., Suzuki Y., Ignacio G.A., Formisano P., Accili D., Taylor S.I. Insulin-stimulated phosphorylation of recombinant pp120/HA4, an endogenous substrate of the insulin receptor tyrosine kinase. Biochemistry. 1995;34:9341–9349. doi: 10.1021/bi00029a009. [DOI] [PubMed] [Google Scholar]
- Öbrink B. CEA adhesion molecules: multifunctional proteins with signal-regulatory properties. Curr. Opin. Cell Biol. 1997;9:616–626. doi: 10.1016/S0955-0674(97)80114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odin P., Tingstrom A., Obrink B. Chemical characterization of cell-CAM 105, a cell-adhesion molecule isolated from rat liver membranes. Biochem. J. 1986;236:559–568. doi: 10.1042/bj2360559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohannesian D.W., Lotan D., Thomas P., Jessup J.M., Fukuda M., Gabius H.J., Lotan R. Carcinoembryonic antigen and other glycoconjugates act as ligands for galectin-3 in human colon carcinoma cells. Cancer Res. 1995;55:2191–2199. [PubMed] [Google Scholar]
- Olsson H., Wikstrom K., Kjellstrom G., Obrink B. Cell adhesion activity of the short cytoplasmic domain isoform of C-CAM (C-CAM2) in CHO cells. FEBS Lett. 1995;365:51–56. doi: 10.1016/0014-5793(95)00436-d. [DOI] [PubMed] [Google Scholar]
- Paulson J.C., Weinstein J., Dorland L., van Halbeek H., Vliegenthart J.F. Newcastle disease virus contains a linkage-specific glycoprotein sialidase. Application to the localization of sialic acid residues in N- linked oligosaccharides of alpha 1-acid glycoprotein. J. Biol. Chem. 1982;257:12734–12738. [PubMed] [Google Scholar]
- Paulson J.C., Weinstein J., de Souza-e-Silva U. Biosynthesis of a disialylated sequence in N-linked oligosaccharides: identification of an N-acetylglucosaminide (alpha 2-6)-sialyltransferase in Golgi apparatus from rat liver. Eur. J. Biochem. 1984;140:523–530. doi: 10.1111/j.1432-1033.1984.tb08133.x. [DOI] [PubMed] [Google Scholar]
- Schneider C., Newman R.A., Sutherland D.R., Asser U., Greaves M.F. A one-step purification of membrane proteins using a high efficiency immunomatrix. J. Biol. Chem. 1982;257:10766–10769. [PubMed] [Google Scholar]
- Sippel C.J., Suchy F.J., Ananthanarayanan M., Perlmutter D.H. The rat liver ecto-ATPase is also a canalicular bile acid transport protein. J. Biol. Chem. 1993;268:2083–2091. [PubMed] [Google Scholar]
- Spik G., Coddeville B., Strecker G., Montreuil J., Regoeczi E., Chindemi P.A., Rudolph J.R. Carbohydrate microheterogeneity of rat serotransferrin. Determination of glycan primary structures and characterization of a new type of trisialylated diantennary glycan. Eur. J. Biochem. 1991;195:397–405. doi: 10.1111/j.1432-1033.1991.tb15719.x. [DOI] [PubMed] [Google Scholar]
- Stehling P., Gohlke M., Fitzner R., Reutter W. Rapid analysis of O-acetylated neuraminic acids by matrix assisted laser desorption/ionization time-of-flight mass spectrometry. Glycoconj. J. 1998;15:339–344. doi: 10.1023/a:1006965600322. [DOI] [PubMed] [Google Scholar]
- Stocks S.C., Kerr M.A. Neutrophil NCA-160 (CD66) is the major protein carrier of selectin binding carbohydrate groups Lewis X and sialyl Lewis X. Biochem. Biophys. Res. Commun. 1993;195:478–483. doi: 10.1006/bbrc.1993.2068. [DOI] [PubMed] [Google Scholar]
- Svenberg T., Hammarstrom S., Hedin A. Purification and properties of biliary glycoprotein I (BGP I). Immunochemical relationship to carcinoem-bryonic antigen. Mol. Immunol. 1979;16:245–252. doi: 10.1016/0161-5890(79)90063-4. [DOI] [PubMed] [Google Scholar]
- Takasaki S., Yamashita K., Suzuki K., Iwanaga S., Kobata A. The sugar chains of cold-insoluble globulin. A protein related to fibronectin. J. Biol. Chem. 1979;254:8548–8553. [PubMed] [Google Scholar]
- Townsend R.R., Hardy M.R., Wong T.C., Lee Y.C. Binding of N-linked bovine fetuin glycopeptides to isolated rabbit hepatocytes: Gal/Gal-NAc hepatic lectin discrimination between Gal beta (1,4)GlcNAc and Gal beta (1,3)GlcNAc in a triantennary structure. Biochemistry. 1986;25:5716–5725. doi: 10.1021/bi00367a055. [DOI] [PubMed] [Google Scholar]
- Turbide C., Kunath T., Daniels E., Beauchemin N. Optimal ratios of biliary glycoprotein isoforms required for inhibition of colonic tumor cell growth. Cancer Res. 1997;57:2781–2788. [PubMed] [Google Scholar]
- Uchida Y., Tsukada Y., Sugimori T. Enzymatic properties of neuraminidases from Arthrobacter ureafaciens. J. Biochem. (Tokyo) 1979;86:1573–1585. doi: 10.1093/oxfordjournals.jbchem.a132675. [DOI] [PubMed] [Google Scholar]
- Virji M., Makepeace K., Ferguson D.J., Watt S.M. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol. Microbiol. 1996;22:941–50. doi: 10.1046/j.1365-2958.1996.01551.x. [DOI] [PubMed] [Google Scholar]
- Weinstein J., de Souza-e-Silva U., Paulson J.C. Purification of a Gal beta 1 to 4GlcNAc alpha 2 to 6 sialyltransferase and a Gal beta 1 to 3 (4)GlcNAc alpha 2 to 3 sialyltransferase to homogeneity from rat liver. J. Biol. Chem. 1982;257:13835–13844. [PubMed] [Google Scholar]
- Weinstein J., de Souza-e-Silva U., Paulson J.C. Sialylation of glycoprotein oligosaccharides N-linked to asparagine. Enzymatic characterization of a Gal beta 1 to 3 (4)GlcNAc alpha 2 to 3 sialyltransferase and a Gal beta 1 to 4GlcNAc alpha 2 to 6 sialyltransferase from rat liver. J. Biol. Chem. 1982;257:13845–13853. [PubMed] [Google Scholar]
- Williams R.K., Jiang G.S., Holmes K.V. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc. Natl. Acad. Sci USA. 1991;88:5533–5536. doi: 10.1073/pnas.88.13.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlasichuk K.B., Kashem M.A., Nikrad P.V., Bird P., Jiang C., Venot A.P. Determination of the specificities of rat liver Gal (beta 1–4)GlcNAc alpha, 2,6-sialyltransferase and Gal (beta 1–3/4)GlcNAc alpha, 2,3- sialyl-transferase using synthetic modified acceptors. J. Biol. Chem. 1993;268:13971–13977. [PubMed] [Google Scholar]
- Yoshima H., Matsumoto A., Mizuochi T., Kawasaki T., Kobata A. Comparative study of the carbohydrate moieties of rat and human plasma alpha 1-acid glycoproteins. J. Biol. Chem. 1981;256:8476–8484. [PubMed] [Google Scholar]


