Abstract
Given the imminent threat of influenza pandemics and continuing emergence of new drug-resistant influenza virus strains, novel strategies for preventing and treating influenza disease are urgently needed. Herbal medicine, used for thousands of years in combinational therapies (Herb Formula), plays a significant role in stimulating the host immune system in vivo, and meanwhile, in fighting against the pandemic by directly inhibiting influenza virus in vitro. Such potential Janus functions may spark interest in therapeutic manipulation of virus diseases. Unfortunately, the molecular mechanism of the Janus functions of the medicinal herbs in the treatment of influenza remains unclear. In this work, to illustrate the therapeutic concept of Janus functions in the treatment of influenza, we have introduced a novel systems pharmacology model that integrates pharmacokinetic screening, targeting and network analysis of two representative herbs Lonicera japonica and Fructus Forsythiae that are efficient in the treatment of influenza, inflammation and other diseases. 50 Chemicals with favorable pharmacokinetic profiles have been identified for the two herbs, and the ligand–target network was constructed by complementing the literature-based experimental data deposited in DrugBank. The annotation of these chemicals was assigned using a novel drug targeting approach, and mapped to target–disease and drug–target–pathway networks. The overall data suggest that the medicinal herbs function by indirectly suppressing the virus proliferation via regulating the immune systems in hosts, and also, by directly inhibiting virus proliferation through targeting viral proteins essential for the viral life cycle. For the first time, we have demonstrated the mechanism of medicinal herbs in prevention and treatment of virus diseases via the Janus functions on a systematic level.
Insight, innovation, integration
Understanding how the diverse chemical components in medicinal herbs contribute to the overall pharmacological effect is a major challenge for current studies. Additionally, some components play dual, even multiple roles, during fighting against influenza virus which added another layer of complexity to pharmacological researches. Here, we introduced a novel systems pharmacology model which integrates pharmacokinetic screening, targeting and network analysis. By using this model, two representative herbs Lonicera japonica and Fructus Forsythiae were analyzed regarding their pharmacological effect on influenza, inflammation and other diseases. Of noted, Janus-function is a striking pharmacological characteristic of these chemical compounds in both herbs: directly inhibiting virus replication and simultaneously promoting host immune response.
1. Introduction
Influenza pandemics, defined as epidemics of influenza viruses, are responsible for acute contagious respiratory infection, and finally result in substantial morbidity and mortality nearly every year.1,2 As an important treatment option, adamantane and neuraminidase (NA) as part of pandemic-preparedness plans enable us to inhibit the activities of the membrane proteins of influenza viruses.3,4 However, such therapy is constrained by the emergence of resistant strains.5,6 With the aim of reducing the drug resistance and increasing the efficacy of the therapeutic effects, the combination therapy, the use of more than one medication, has been proposed as an alternative to control the influenza diseases.7 As the leading choice for treating the most dreadful diseases, such as cancer and infectious diseases,8,9 the combination drugs impact multiple targets, and are more efficacious and less vulnerable to adaptive resistance than monotherapies.10,11
The combination therapy has been advocated for thousands of years in herbal medicines to combat health problems,12,13 and more than one hundred of medicinal herbs have been found to be effective against microbes.14 For example, the two herbs Lonicera japonica (Jinyinhua) and Fructus Forsythiae (Lianqiao) possess excellent anti-SARS coronavirus and anti-H1N1 flu virus activities.15,16 They are widely used as the principal components in many different formulae, such as shuanghuanglian and yinqiaosan, to clear away the heat evil and toxin, and cure fever, cough, and the swell of throat, etc.14 As the main group of ingredients in both the herbs, flavonoids enable us to target the NA protein, and thus exhibit antiviral effects against influenza A virus.17,18 Also, Shuangkangsu, isolated from Lonicera japonica, displays significant inhibition to influenza B and A3 viruses.19
In fact, for a promising influenza treatment, in addition to the antiviral defense, the antiviral agents should also enable us to eliminate/alleviate inflammation since an excessive proinflammatory response is associated with fatality following infection with highly pathogenic avian influenza viruses.20 Fortunately, both the herbs Lonicera japonica and Fructus Forsythiae were demonstrated to enable us to regulate the host's immune system, and meanwhile, stimulate the non-specific/specific immune responses to allothigene. For instance, luteolin isolated from the flowers of Lonicera japonica can suppress inflammatory mediator release by blocking the NF-κB and MAPKs activation pathways in HMC-1 cells;21Arctigenin from Fructus Forsythiae significantly decreases carrageenan-induced paw edema, arachidonic acid-induced ear edema, and acetic acid-induced writhing response and capillary permeability accentuation, respectively.22 This evidence implies that the two herbs display Janus functions, i.e., treating virus infection in a two-faced fashion: inhibiting virus, and meanwhile, stimulating host's immune system. This suggests that the combination of anti-viral and immunomodulatory agents will be more than passing interest for the anti-viral agents singly used in patients with influenza. However, despite the attractive therapy for influenza, it is still unclear what the underlying molecular mechanism of the Janus functions in the treatment of virus disease is?
To solve the problem, attempts have been made to document research data from the medicinal herbs according to orthodox pharmacological actions. However, the wide range of compounds involved in the herbs significantly complicate the pharmacological research, because multiple active ingredients in them might have synergistic effects.10,23 Although several experimental methods have recently been launched to screen the favorable drug combinations by disease-relevant phenotypic assays,24 the systematic search for synergistic agent combinations arising from the large number of ingredients in medicinal herbs is still an unresolved issue,11 let alone identifying the precise targets of herbs and the mechanism of their interactions experimentally.
Alternative to experiments, systems pharmacology is an emerging area of pharmacology that integrates knowledge about pharmacokinetics (the adsorption, distribution, metabolism, excretion and toxicity (ADME/T) characteristics of a drug) to understand drug actions across multiple scales of complexity ranging from molecular and cellular levels to tissue and organism levels by using network analysis.25 The study of the ADME/T properties of herbal medicines would help understanding of molecular mechanisms of herbal medicine pharmacology, toxicity and drug–drug interactions. By considering drug actions and side effects in the context of the regulatory networks within which the drug targets and disease gene products function, network analysis promises to greatly increase our knowledge of the mechanisms underlying the multiple actions of drugs.25 Thus the application of systems pharmacology to herbal medicine affords new possibilities for investigating the explicit targets of medicinal herbs' active ingredients and their interactions in the context of molecular networks,25 and thereby highlights the molecular mechanism of their Janus functions. In the previous work, we have developed a robust model based on systems pharmacology to evaluate the efficiency of medicinal herbs and identified a series of bioactive ingredients and possible targets from these herbs. By mapping them onto functional ontologies, the resulting biologically meaningful networks for those herbs offer a deep understanding of their action mechanisms.26,27
In this work, we aim to verify whether medicinal herbs have Janus functions in combating virus infection, including (1) inactivating or restraining the virus directly by targeting viral proteins and pathways unique to the viral life cycle; and (2) suppressing the virus indirectly by enhancing or regulating immune system in host cells. If so, what is the underlying molecular basis? To address this, we take the two herbs Lonicera japonica and Fructus Forsythiae as examples. We firstly explored their active compound cohorts and potential targets, and then mapped these compounds and protein targets onto functional ontologies such as the compound–target associations, compound–pathway connections and target–disease interactions, which offered a means to infer details on the specific mechanisms of the herbs that led to the pathologic transition. The obtained results provided a new window over the Janus functions underlying the medicinal herbs and were beneficial for designing of compounds or combination strategies for treating influenza diseases.
2. Materials and methods
2.1. Database construction
All ingredients of both medicinal herbs Lonicera japonica (227 compounds) and Fructus Forsythiae (146 compounds) (Table S1 in ESI†) were derived from our own database: TcmSP: Traditional Chinese Medicines for Systems Pharmacology Database and Analysis Platform (http://tcmspnw.com ). The structures of these ingredients were saved as mol2 format for further analysis. Sybyl 6.9 (Tripos Associates, St. Louis, MO) was employed to optimize these molecules with the same parameters as described in our previous work.28 Considering the deglycosylation process of the glucosides in the intestinal tract by enteric bacteria,29 the glycosyls of 76 molecules were removed according to the rule of glycosidase hydrolysis reaction and their products were added into the database for further analysis.
2.2. Oral bioavailability (OB) prediction
To predict the OB of medicinal herbs' ingredients, we have developed a novel and robust in-house system OBioavail 1.1.30 The details of predicting the compounds' OB have been described in our previous work.27,30 The prediction of OB is very challenging due to the fact that bioavailability is a complex function of many biological and physicochemical factors, such as dissolution in the gastrointestinal tract, intestinal membrane permeation, intestinal and hepatic first-pass metabolism, and even the dosage form. Thanks to this software, we can discard the compounds with poor OB, thus discovering the bioactive compounds or lead compounds in herbal medicine for further development.
Compounds with OB ≥ 50% were regarded as candidate compounds, while several compounds with OB < 50% were also studied in this work due to their therapeutic effects on influenza diseases, such as the adhyperforin and hyperforin in Fructus Forsythiae. The criteria used here is mainly for: (1) extracting information from the herbs as much as possible with the least number of components; (2) reasonably explaining the obtaining model by the reported pharmacological data.
2.3. Predicting and validating the potential targets of human and viral origin
Based on a systematic model developed in our previous work, we predicted the potential targets of human and viral origins. Such model has been developed in our previous work that efficiently integrated the chemical, genomic and pharmacological information for drug targeting and discovery on a large scale, based on the two powerful methods: Random Forest (RF) and Support Vector Machine (SVM).26 This model shows an impressive performance of prediction for drug–target interaction, with a concordance of 86%, a sensitivity of 80% and a specificity of 93%, respectively. According to the possibilities of compounds interacting with candidate targets in the RF and SVM models, respectively, both sets of top 100 high-ranking proteins were selected in this work and their overlap between them was treated as potential targets.
To validate the predicted targets, we have performed molecular docking analysis using the AutoDock software31 on active substances in both herbs to compare their binding modes with co-crystallized ligands involved in the predicted targets. This approach performs the docking of small, flexible ligand to a set of grids describing the target protein.32 Prior to docking, the three dimensional (3D) structures of protein targets are prerequisite. To this end, the predicted targets of human and viral origin that link to active substances in either herb were reserved, then their 3D structures were directly derived from RCSB Protein Data Bank (http://www.pdb.org/ ) or modeled using the Swiss-Model Automated Protein Modeling Server (http://swissmodel.expasy.org/ ) with default parameters if the 3D structures are unavailable. During the docking process, the protein was considered as rigid and the molecules flexible.
2.4. Network construction
To characterize the multicomponent therapeutic mechanisms of herbal medicine in the treatment of influenza from a network target perspective, we constructed three kinds of visualized networks: compound–target network (C–T network), target–disease network (T–D network), and compound–target–pathway network (C–T–P network), to afford new possibilities for understanding of the holistic, complementary and synergic essence of herbal medicine.33
C–T network
All active substances in both the herbs and their targets were used to generate a bipartite graph of drug–protein interactions in which a drug and a protein were connected to each other if the protein was a target of the drug, giving rise to a C–T network. Since all potential targets were distributed in both human and virus, we divided all targets into two parts according to their origin (human or virus), then each part was used to construct C–T network, respectively, that was, the C–T network with protein targets of human origin was referred to as C–Th network, while the C–T network with protein targets of viral origin was referred to as C–Tv network.
T–D network
The associated disease information was mined by mapping the terminology of our potential targets to the relevant targeted disease conditions in the database TTD (as of April 1st, 2012).34 The obtained 219 diseases were then projected into the medical subject headings (MeSH, http://www.nlm.nih.gov ) whose terms are assigned according to the analysis of expert indexers to best reflect article content, and finally, 20 disease categories were probed. For example, herpes virus, hepatitis C, and influenza virus infections belong to virus diseases; inflammation, inflammatory diseases, and chronic inflammatory diseases are grouped together as pathological conditions, signs and symptoms; asthma, cough, and chronic obstructive pulmonary diseases are categorized as respiratory tract diseases; acute lymphoblastic leukemia, nasopharyngeal cancer, and colorectal cancer are classified as neoplasms; pain and vascular headache are affiliated to nervous system diseases; and autoimmune diseases, immunodeficiency and immunosuppression are placed together as immune system diseases.
C–T–P network
The information about pathways were initially collected as defined by the KEGG (Kyoto Encyclopedia of Genes and Genomes),35 and then the T–P network was built by connecting the targets and their related signaling pathways. Targets and their related signaling pathways were represented as nodes and their interactions as links. To examine the global relationships between compounds and pathways, the C–T–P network was constructed by overlaying the C–T network onto T–P network.
All visualized networks were generated by Cytoscape 2.8.1, a popular bioinformatics package for biological network visualization and data integration.36 The quantitative properties of these networks were analyzed by two plugins including NetworkAnalyzer and CentiScaPe 1.2. The degree of a node was the number of edges associated with the node.
3. Results and discussion
Thousands of years' clinical practices in herbal medicine have proven the in vivo efficacy and safety of herbal medicines.14,37 However, despite the promising therapy of such herbs for the treatment of influenza, their molecular mechanisms responsible for the Janus functions remain unclear. Fortunately, recent advances in systems biology and medicine have allowed the application of systems pharmacology approach in the study of the botanic drugs. In this work, we have carried out our investigation as follows: first, OB prediction and analysis of pharmacological mechanisms; second, target identification and validation; and finally, network construction and analysis. Such successful prediction of the potential targets and understanding of the mechanism of action of medicinal herbs will facilitate the development of novel drugs for the treatment of influenza disease, and improve the efficacy of both herbal medicine and modern medicine interventions.
3.1. OB prediction and analysis of pharmacological mechanisms
Herbal medicines are often administered by the oral route, and thereby the ingredients with poor aqueous solubility might exhibit low efficiency in entering blood, and provide few beneficial therapeutic effects in patients. Generally, the failure/success of multiple chemicals in the developmental stage is evaluated by ADME properties.38 Among the properties, good OB is one of the most desirable attributes of a new drug. To increase the probability of success of compounds as an oral drug in the subsequent development, we have thus developed a robust in-house system30 to evaluate human OB for structurally diverse drugs. In this section, we use this OB prescreening model to screen out the ingredients of Lonicera japonica and Fructus Forsythiae with favorable pharmacokinetic properties.
3.1.1. Lonicera japonica
For Lonicera japonica, 41 of 227 chemicals (19%) show OB ≥ 50% (Table 1), and most of them (OB ≥ 50%) are essential oils, organic acids, flavonoids and saponins with good pharmacological effects.16 For α-cedrol, this essential oil possesses an OB of 89%, and shows anti-inflammatory and antifungal activities against a broad spectrum of plant pathogenic fungi.39,40 While the cis-jasmone with an OB of 87% shows antiviral activity by inducing selective production of secondary metabolites that can directly reduce the development of pests and diseases.41 As for linalool, this essential oil exhibits a dose-dependent analgesic action on the central nervous system (CNS), and possesses good anti-inflammatory activity.42,43
Table 1.
62 Compounds from Fructus Forsythiae and Lonicera japonica and corresponding predicted oral bioavailability (OB) and drug-likeness (DL)
| No. | Molecule (before deglycosylation) | Molecule (after deglycosylation) | OB(%) | DL |
|---|---|---|---|---|
| LM294a |
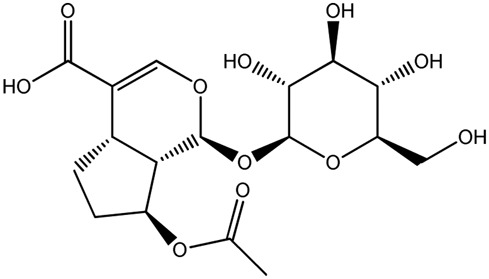
|
|
100b | 0.17 |
| LM387a |
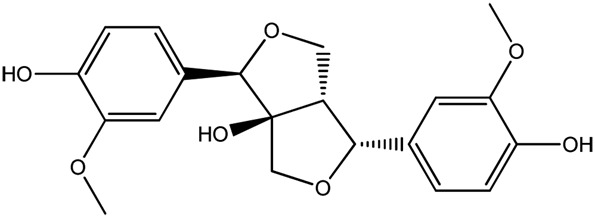
|
— | 94.8 | 0.51 |
| LM394a |
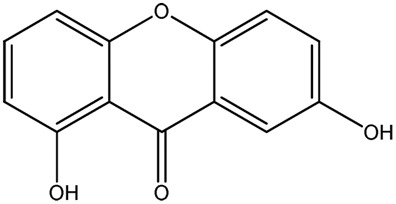
|
— | 93.8 | 0.23 |
| LM289a |
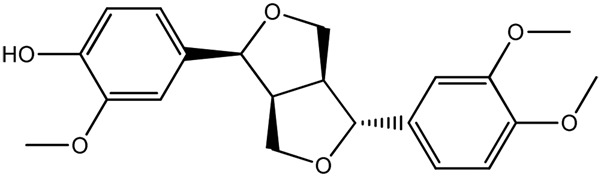
|
— | 92.9 | 0.47 |
| LM285a |
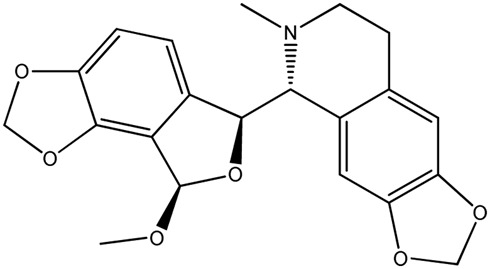
|
— | 90.3 | 0.92 |
| LM298a |
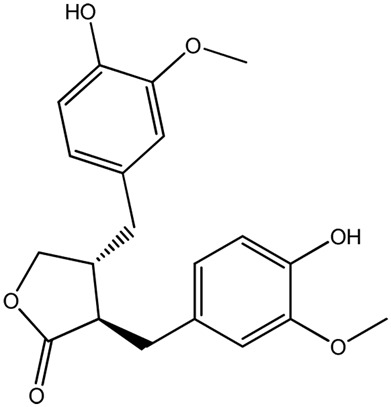
|
— | 89.6 | 0.38 |
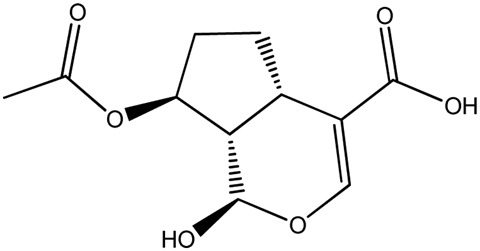
| JM77a |
|
|
89.4b | 0.22 |
| JM63a |
|
— | 89.3 | 0.28 |
| JM1a |
|
|
87.5b | 0.30 |
| LM304a |
|
|
85.1b | 0.47 |
| JM57a |
|
|
85.1b | 0.16 |
| LM398a |
|
— | 83.3 | 0.17 |
| LM287a |
|
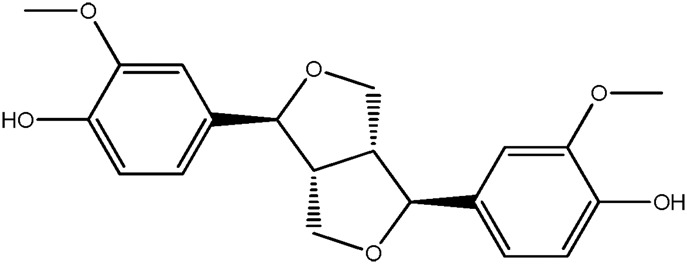
— | 82.8 | 0.45 |
| LM356a |
|
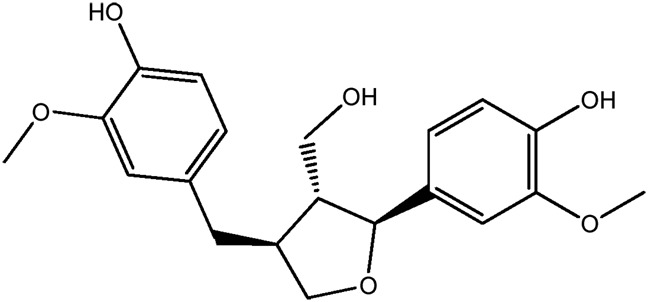
— | 80.0 | 0.37 |
| JM170 |
|
— | 79.7 | 0.26 |
| LM414a |
|
— | 76.0 | 0.21 |
| LM386a |
|
— | 75.9 | 0.51 |
| LM428a |
|
— | 75.9 | 0.35 |
| LM345a |
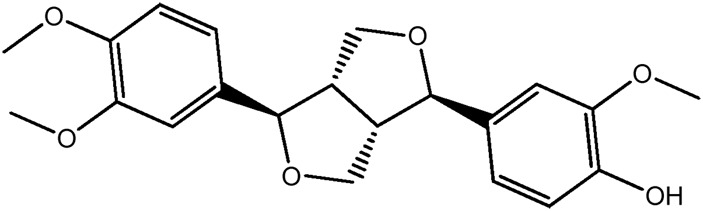
|
— | 74.4 | 0.47 |
| LM305a |
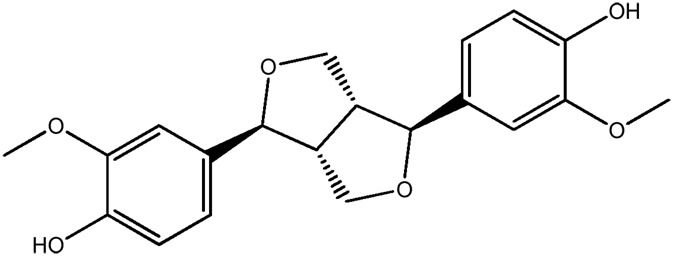
|
— | 74.1 | 0.45 |
| JM113a |
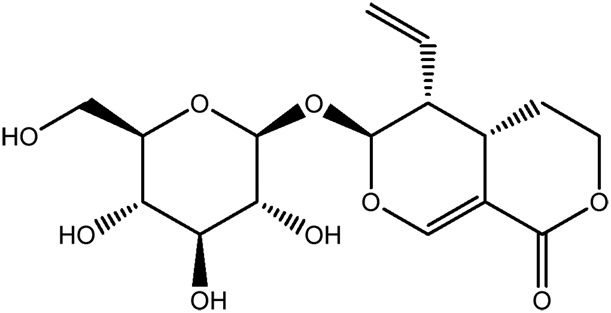
|
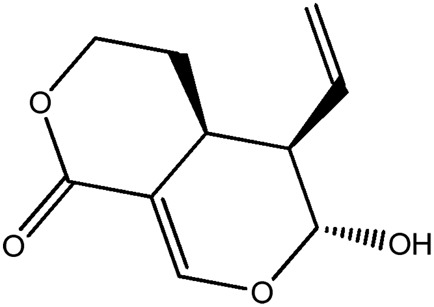
|
73.6b | 0.13 |
| JM107a |
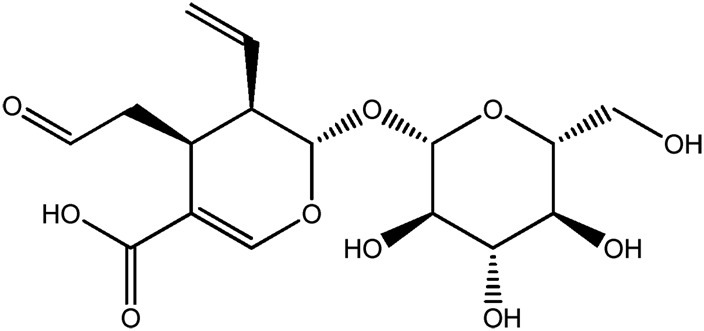
|
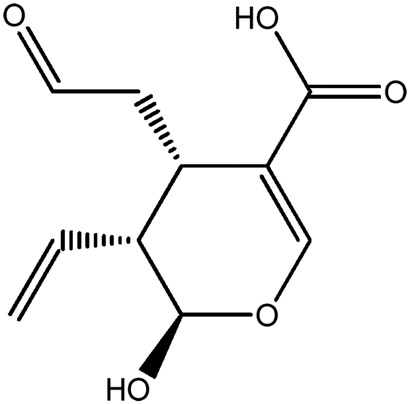
|
73.3b | 0.12 |
| LM309a |
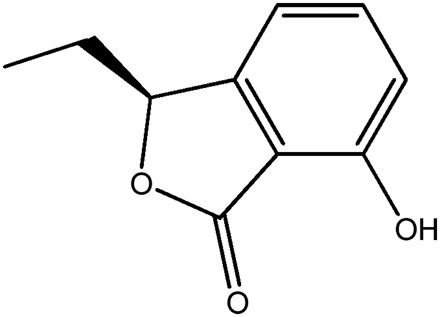
|
— | 70.5 | 0.12 |
| JM250a |
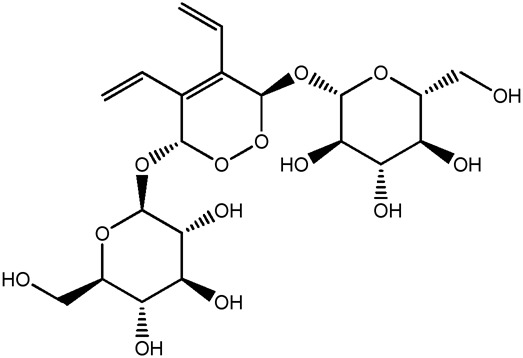
|
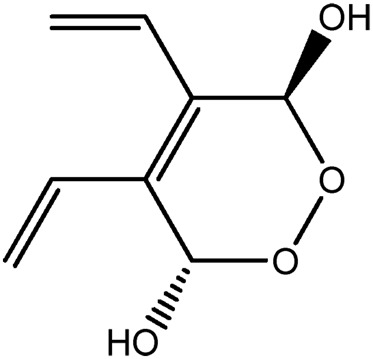
|
70.1b | 0.09 |
| LM274a |
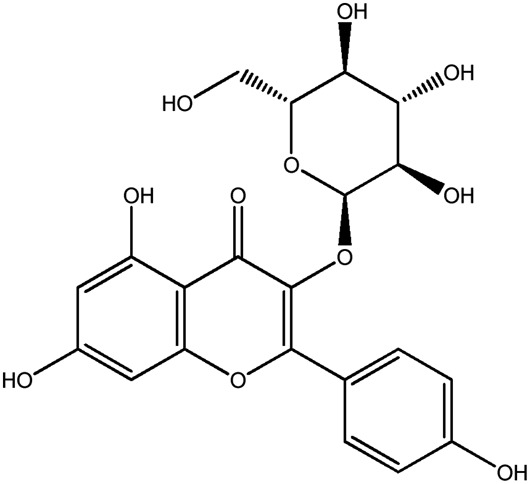
|
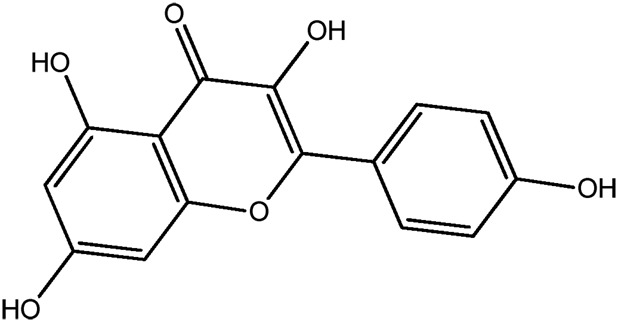
|
68.6b | 0.30 |
| LM299a |
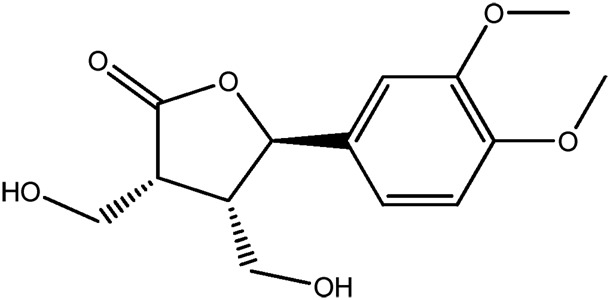
|
— | 67.5 | 0.21 |
| LM399a |
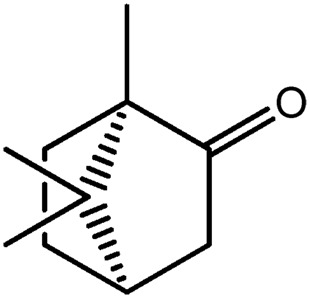
|
— | 67.4 | 0.17 |
| LM338a |
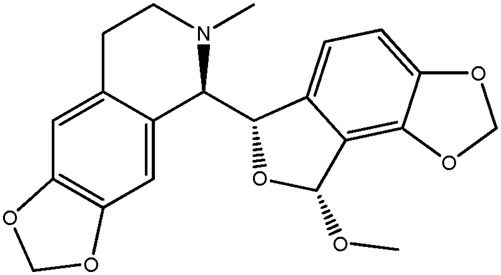
|
— | 67.1 | 0.92 |
| LM279a |
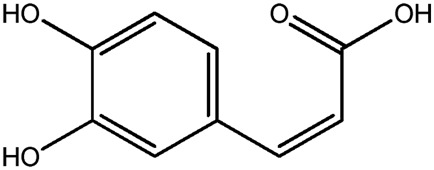
|
— | 65.7 | 0.08 |
| JM217a |
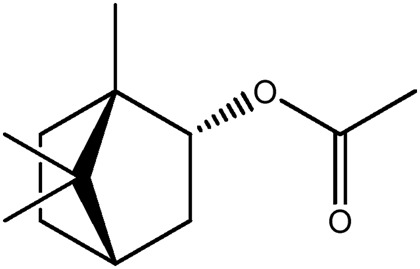
|
— | 65.5 | 0.19 |
| LM384a |
|
— | 65.4 | 0.07 |
| LM409 |
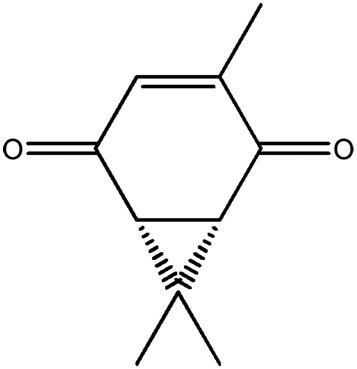
|
— | 64.2 | 0.18 |
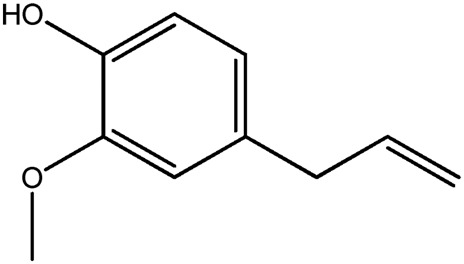
| LM349a |
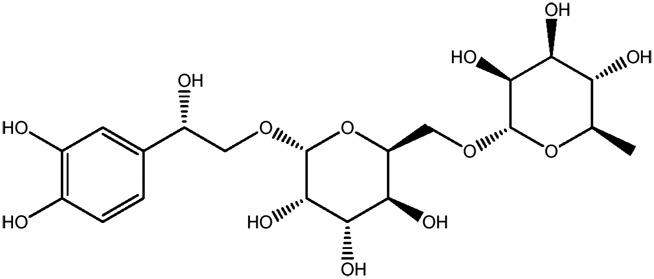
|
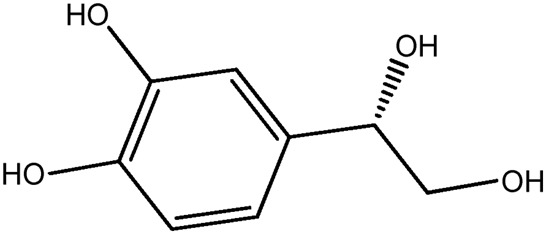
|
63.7b | 0.08 |
| LM306a |
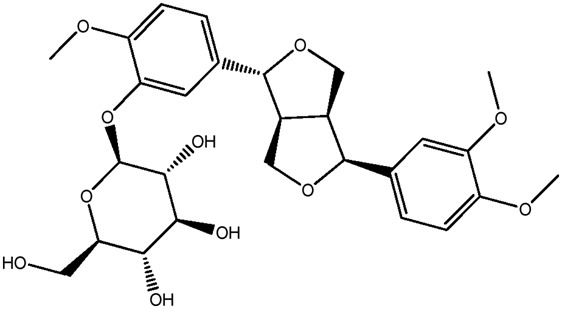
|
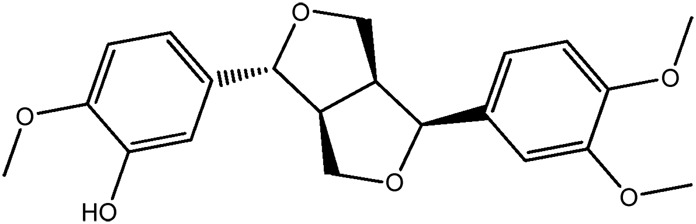
|
61.2b | 0.47 |
| JM74a |
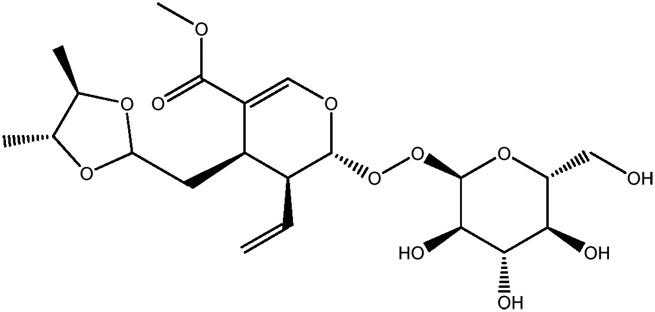
|
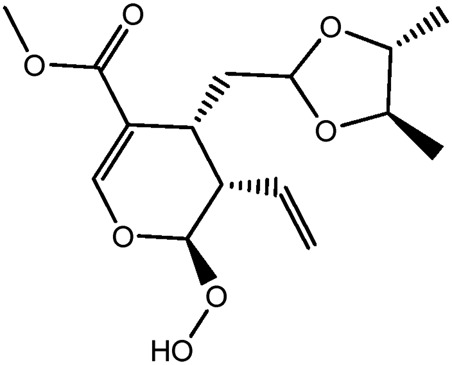
|
61.2b | 0.25 |
| JM116a |
|
|
60.9b | 0.16 |
| LM273 |
|
— | 60.2 | 0.41 |
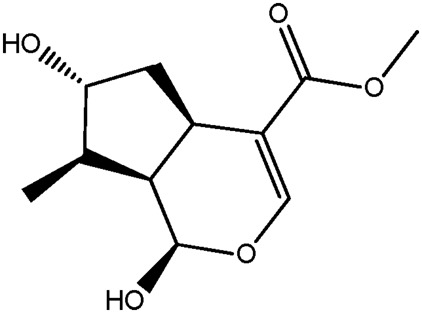
| JM53a |
|
— | 59.9 | 0.36 |
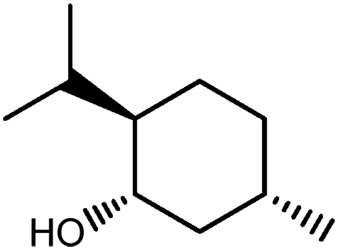
| JM130a |
|
— | 59.4 | 0.07 |
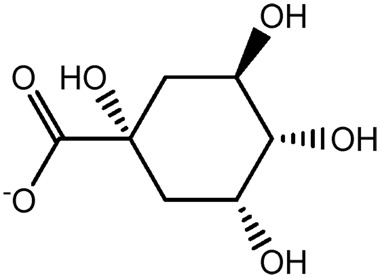
| JM249a |
|
— | 59.2 | 0.13 |
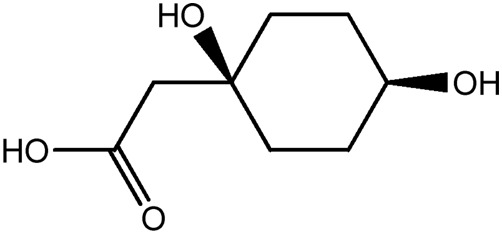
| LM344a |
|
— | 57.6 | 0.08 |
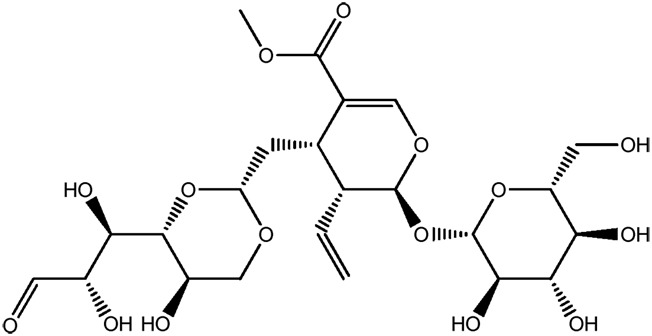
| JM65 |
|
|
55.6b | 0.85 |
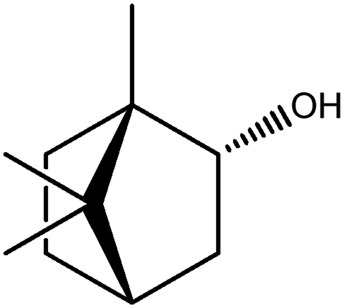
| LM358a |
|
|
55.4b | 0.15 |
| LM424 |
|
— | 55.3 | 0.13 |
| JM108a |
|
|
53.6b | 0.29 |
| LM363a |
|
— | 53.6 | 0.12 |
| JM205 |
|
— | 53.5 | 0.22 |
| JM251a |
|
|
53.4b | 0.16 |
| JM258a |
|
— | 52.9 | 0.41 |
| LM374 |
|
— | 52.5 | 0.05 |
| LM284a |
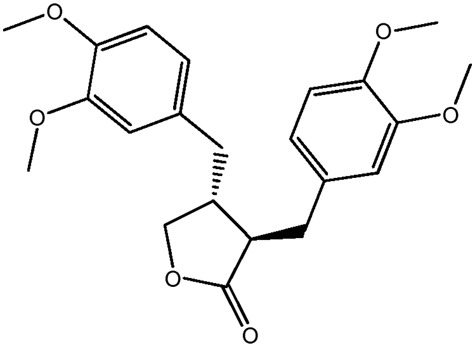
|
— | 52.3 | 0.43 |
| JM111a |
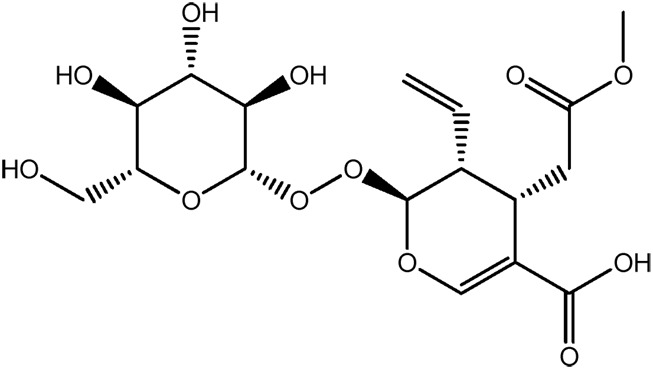
|
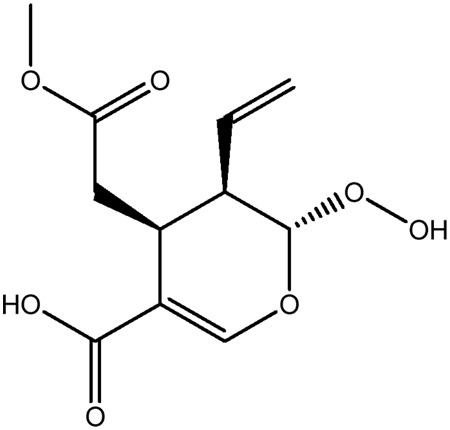
|
51.4b | 0.53 |
| LM332a |
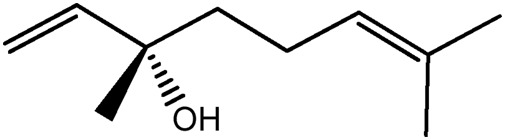
|
— | 51.2 | 0.05 |
| JM190a |
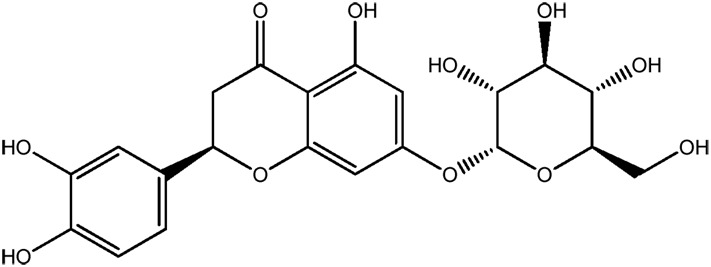
|
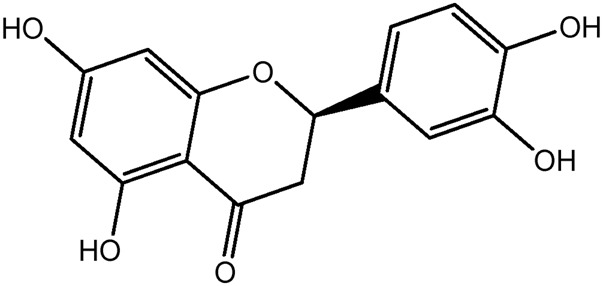
|
51.0b | 0.29 |
| LM401a |
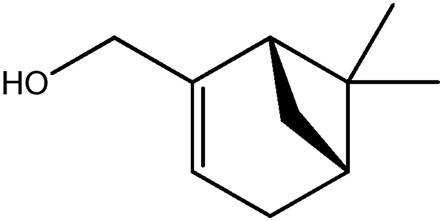
|
— | 50.5 | 0.18 |
| JM202 |
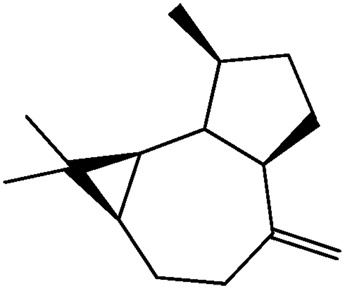
|
— | 50.5 | 0.22 |
| LM377a |
|
— | 49.0 | 0.30 |
| LM310a |
|
— | 46.8 | 0.34 |
| LM323 |
|
— | 46.2 | 0.18 |
| LM371 |
|
— | 44.0 | 0.78 |
| LM370 |
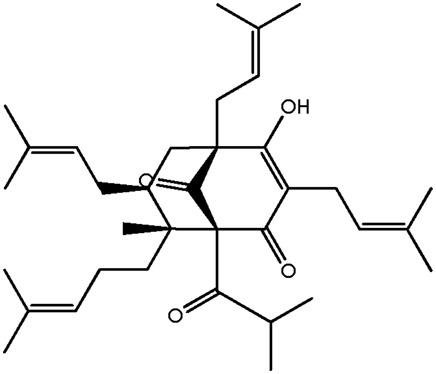
|
— | 44.0 | 0.77 |
| LM364 |
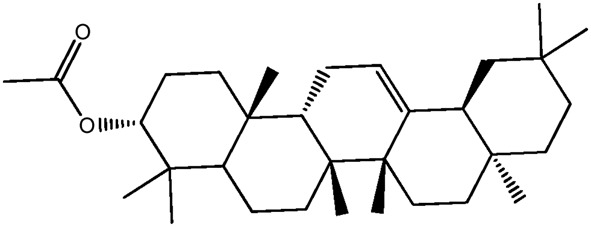
|
— | 42.1 | 0.87 |
LM represents the molecule of Fructus Forsythiae, JM represents the molecule of Lonicera japonica.
The active ingredients of the herbs bind to 48 potential targets associated with influenza.
The predicted OB after deglycosylation.
For the organic acids, caffeic acid (OB = 66%), quinic acid (OB = 59%), and 3-O-caffeoyl quinic acid methyl ester (OB = 53%), they are regarded as the major antibacterial and antiviral44 ingredients of Lonicera japonica. It is worth to note that chlorogenic acid (3-caffeoylquinic acid) exhibits very poor OB as low as 11%, although this compound enable us to display antivirus, anticancer, anti-inflammation activities,45,46 and has been officially used as the indicator compound to characterize the quality of this herb.16 This fact, combined with the evidence that other chlorogenic acids also have low OB less than 30%, suggests that the chlorogenic acids cannot be well absorbed and metabolized by human. In fact, we note that chlorogenic acid is a conjugate of quinic and caffeic acids which have good OBs (66% and 59%, respectively) and exert biological activities including antioxidant, anti-thrombosis, anti-hypertension, antivirus and antitumor properties.47 This implies that the chlorogenic acid may play its role mainly in the form of its catabolites.
For flavonoids, a class of secondary metabolites in Lonicera japonica, they are most commonly known for their antiviral, antimicrobial, and antioxidant activities in vitro. Quercetin, a flavonoid present in a wide variety of fruits and vegetables, has OB of about 50%, which can reduce the susceptibility to influenza infection following stressful exercise.48 Also, cell culture studies have illustrated that quercetin can exert cytoprotective effects against various viruses, inflammation, and oxidation.49
As for the major saponin constituents sweroside and 7-epi-loganin, they exhibit significantly low OBs (4% and 12%, respectively), although recent studies have reported their pharmacological effects, such as antiprotozoal, antiallergic and antiviral activities.15 The reason is that both the chemicals are triterpene glycoside compounds which fail to permeate membrane, and thus show poor oral bioavailability. Indeed, after intestinal deglycosylation, sweroside and 7-epi-loganin can display good OBs (74% and 85%, respectively), which suggests that they play their bioactivities mainly in the form of their catabolites, and probably act as prodrugs that are metabolized to the active form.29 Similarly, some other compounds known as hederageninglycosides also show good OB and present certain bioactivities after deglycosylation. For example, the OB of loniceroside A, a glycoside with significant anti-inflammatory activity,50 increases from 23% to 89% as a result of glycosylhydrolysis. This implies that loniceroside A may exhibit its biological activity primarily in the form of a non-carbohydrate moiety.
3.1.2. Fructus Forsythiae
For Fructus Forsythiae, 28% (44 of 146, Table 1) of ingredients in this herb show good OB (≥50%), and most of them have profound pharmacological effects. For example, the two alkaloids egenine and (−)-7′-O-methylegenine with OB of 90% and 67% show anti-inflammatory activities, with inhibition rates of the release of β-glucuronidase from polymorphonuclear leukocytes of rats.51 Also, (±)-car-3-ene-2,5-dione with OB of 64% displays potent antibacterial activity against a wide range of bacteria.52 For matairesinol, this compound shows an OB of 90%, which enables us to inhibit HIV replication in H9 lymphocyte cells.53 As for rengynic acid, this chemical shows an OB of 58%, and has been assessed for potent antiviral effect on RSV in vitro by cell morphology methods.54
It is worth to note that phillyrin (forsythin) and forsythiaside show rather poor OBs (<35%), although they are two major effective constituents of Fructus Forsythiae that possess anti-inflammatory,55 anti-viral,56antioxidant and antibacterial activities,57 and are commonly used as chemical markers for quality control of Fructus Forsythiae raw material.58,59 Interestingly, after deglycosylation, both the aglycones of phillyrin and forsythiaside show high OB of 66% and 74%. Additionally, some compounds with acceptable OB (between 40% and 50%) including hyperforin (OB = 44%), adhyperforin (OB = 44%) and luteolin (OB = 49%) are also demonstrated to possess remarkable pharmacodynamic effects. It has been reported that hyperforin has the antibacterial activity against multiresistant Staphylococcus aureus and gram-positive bacteria,60 while adhyperforin and luteolin have anti-inflammatory activity.61 Finally, we yield 65 candidate compounds derived from the OB, drug-likeness (≥0.1), and drug metabolism prescreening in vivo.
3.2. Target identification and validation
Generally, herbal medicine contains numerous pharmacological compounds, which offer bright prospects for the control of complex diseases in a synergistic manner. To understand the underlying mechanism of such synergistic effect, it is important to search the knowledge about the therapeutic targets of drugs. Traditionally, such information have been generated experimentally including the sequencing of expressed-sequence tags (ESTs), serial analysis of gene expression (SAGE) differential display, homology cloning, and related approaches.62 However, this process is challenging and time-consuming,63 and many drugs may not be predicted simply by sequence or structural homology due to their multiple physiological targets63
To overcome this bottleneck, a new and robust approach is urgently needed to be developed that is capable of systematically analyzing protein–drug interactions. Thanks to the recent development in ‘omics’ technology and systems biology, we have developed a systematic approach that efficiently integrates the chemical, genomic and pharmacological information for drug targeting and discovery on a large scale, based on the RF and SVM methods.26
Using this approach, we have searched 248 candidate targets (Table S2 in ESI†) for all the 62 candidate compounds. The candidate targets are further validated by molecular docking method, and 91% targets are reserved (named as potential targets). The results show that many candidate compounds have promiscuous actions. For example, menthol (from Lonicera japonica) acts as an inducer of transient receptor potential cation channels (subfamily M member 8, subfamily A member 1, and subfamily V member 3)64,65 and also as an agonist of kappa-type opioid receptor;66hyperforin (from Fructus Forsythiae) has dual specificity for canalicular multispecific organic anion transporter 1 (acting as an inducer)67 and solute carrier organic anion transporter family member 1B1 (acting as an inhibitor);68 Quercetin (sharing compounds from both Lonicera japonica and Fructus Forsythiae) can interact with tens of proteins such as cytochrome P450 2C869 and multidrug resistance-associated protein 1.70 These confirm that herbal treatment is a multicomponent therapeutics, in which two or more active ingredients simultaneously target multiple proteins.
Furthermore, we retrieve 48 potential targets associated with influenza (Table S2, ESI†) which link with 50 active ingredients of the herbs. A significant target overlap is found between the two herbs Lonicera japonica and Fructus Forsythiae (38 potential targets), which suggests the similar therapeutic efficiency of both herbs. Interestingly, all these potential targets are distributed in both virus (5) and human (43), implying that these medicinal herbs probably protects human against virus infection in two ways: limiting replication of viruses, and enhancing innate and adaptive antiviral defenses in hosts to restrain viral infection (for details, see section “Network construction and analysis” below).
3.3. Network construction and analysis
3.3.1. C–Th network: medicinal herbs regulating human defense system
C–Th network is comprised of 93 nodes (50 potential compounds and 43 potential targets) and 565 edges. The triangles and circles represent the potential compounds and targets for Lonicera japonica (red) and Fructus Forsythiae (yellow), respectively, while cyan and purple show the overlapped compounds and targets between the two herbs, respectively. The global view of the network in Fig. 1 shows that the targets display different number of interactions with drugs with a mean number of potential compounds per target of 12. For example, cell division protein kinase 2 (CDK2) exhibits the highest number of drug interactions (degree distribution DD = 38), while epidermal growth factor receptor (EGFR) has only one drug interaction.
Fig. 1.
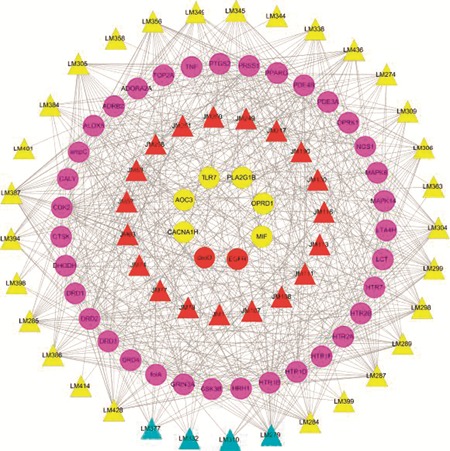
C–Th network. A compound and a protein of human origin are connected to each other if the protein is a target of the compound. The triangles and circles, respectively, represent potential compounds and targets for both herbs Lonicera japonica (red) and Fructus Forsythiae (yellow), and the overlapped compounds and targets between two herbs are shown in cyan and purple, respectively.
Influenza is often viewed as a complex disease since it is usually an acute, self-limited respiratory tract infection that begins with the sudden onset of high fever, followed by inflammation of the upper respiratory tree and trachea, with coryza, cough, headache, prostration, malaise and other signs.71 Indeed, further observation of the C–Th network shows that for most of the targets with high degree (>12), such as the peroxisome proliferator-activated receptors (PPARs), and mitogen-activated protein kinase (MAPK), they are key effectors of inflammation, which confirms the efficiency of our network. Since inflammation is one of the first responses of the immune system of human body to infection, it is reasonable to believe that Lonicera japonica and Fructus Forsythiae probably resist viral infection by regulating hosts' immune function.
Further analysis of the T–D network (Fig. 2) shows that there are high degrees of correlations of targets with the respiratory tract diseases (22/346), nervous system diseases (45/346), and pathological conditions, signs and symptoms (32/346). Since the symptoms of influenza are roughly classified into respiratory tract diseases (e.g. respiratory tract infection and cough), nervous system diseases (e.g. headache), and pathological conditions, signs and symptoms (e.g., inflammation), the above result further confirms the effectiveness of the medicinal herbs for treating the influenza disease. For instance, as one of the hallmarks of influenza, acute respiratory distress syndrome is attributed to the hyperproduction of cytokines and chemokines in the lung, including tumor necrosis factor (TNF)-α, granulocyte macrophage colony-stimulating factor, interleukin (IL)-6, and IL-8.72 These cytokines are mainly released by the macrophages enriched in lung and their production is mediated by the MAPK pathway. Thus inhibition of MAPK can lower the production of these inflammatory cytokines and thereby may have a therapeutic benefit.73 In fact, both MAPK and TNF are predicted to be targets shared by the both herbs. This result thus suggests that Lonicera japonica and Fructus Forsythiae attaches great importance to the immunity and self-adjusting functions of human beings in the treatment of influenza.
Fig. 2.
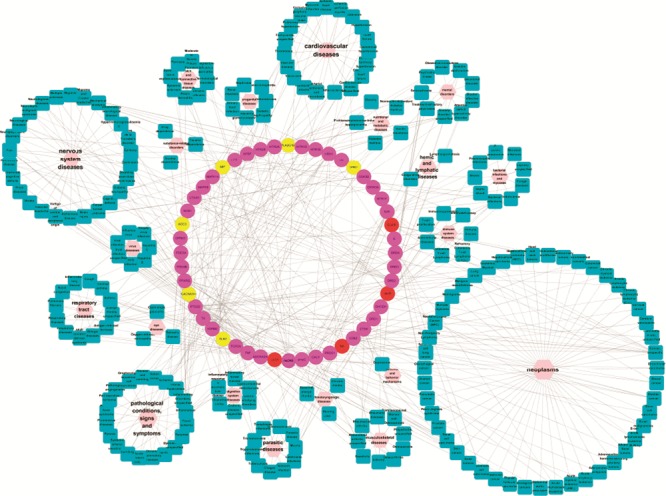
T–D network. T–D link was generated by mapping a target's terminology to relevant terms from the medical subject headings. In this way, a total of 219 disease terms (cyan squares) which can be organized into 20 categories (pink hexagons) according to MeSH were obtained. The colors of target nodes are the same as Fig. 2.
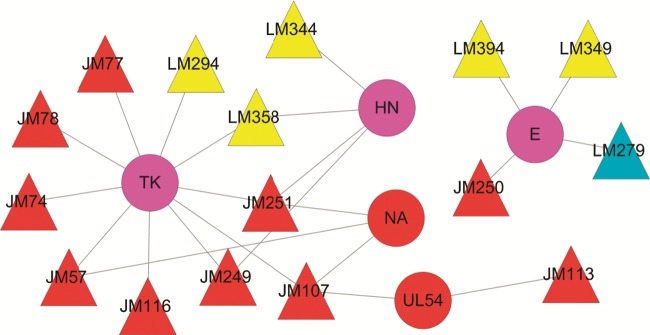
3.3.2. C–Tv network: medicinal herbs directly inhibiting virus infection
Fig. 3 shows that there are five targets of viral origin in the C–Tv network modulated by 15 potential molecules. Among these targets, thymidine kinase (TK) displays the highest degree distribution (DD = 9), followed by the lysozyme (E, DD = 4), hemagglutinin–neuraminidase (HN, DD = 4), neuraminidase (NA, DD = 3), and DNA polymerase (UL54, DD = 2). This result suggests that Lonicera japonica and Fructus Forsythiae can directly target viral proteins. For example, neuraminidase (NA) and hemagglutinin are antigenic glycoproteins on the surface of influenza virions. Both of them recognize the carbohydrate structures and bind to the sialic acid receptor on the surface of the host cell, which aids the entry of the virus.74 Then the NA antigen cleaves the terminal linkage of the sialic acid receptor, thereby leading to the release of the progeny virions from the infected host cells. Due to the essential role of NA in influenza viral replication, spread and pathogenesis, NA and HN are considered to be attractive targets for combating influenza.75 By analyzing the T–D network as described above, we suggest that the two medicinal herbs can limit the virus infection by targeting the viral protein directly.
Fig. 3.
C–Tv network. A compound and a protein of virus origin are connected to each other if the protein is a target of the compound. The node has the same color as Fig. 2.
3.3.3. C–Th network vs. C–Tv network: medicinal herbs' Janus effects on the treatment of influenza
The above C–Th and C–Tv networks show that the herbs, Lonicera japonica and Fructus Forsythiae, possess Janus functions—regulating human immune system to resist virus activity and meanwhile, directly inhibiting the viral protein. Although the mechanisms of actions of the herbs seem to have little in common, they are reconcilable in the treatment of influenza. After being threatened by viruses such as influenza, the innate immune system of human provides a first line of defense against pathogens. Thus many proteins in immune system, such as TLRs, MAPKs and PPARs, become attractive targets for limiting the infection with influenza virus. For instances, (1) TLRs, germline encoded pattern-recognition receptors (PRRs), recognize virion glycoproteins (hemagglutinin)76 and then rapidly initiate a cascade of events that results in the expression of a variety of genes involved in the inflammatory and immune responses.77 The inhibition of TLRs pathway can effectively suppress the influenza A virus-induced upregulation of proinflammatory cytokines, and viral replication.78 Similarly, euxanthone from Fructus Forsythiae, which is predicted to inhibit TLRs, also probably inactivates the influenza; (2) p38 MAPK activation has been shown to contribute to the hyperinduction of TNF-α in response to influenza virus infection, which is a critical step in influenza virus replication. Thus it has been suggested that p38 MAPK inhibitors could be used to limit the viral replication.76 Here, we find that molecule quercetin (involved in both herbs) is able to inhibit MAPK, and thus reduce the TNF-α-induced chemokine secretion,79 verifying that both herbs have important implications for the host resistance against influenza virus infection; (3) PPARs are ligand-dependent transcription factors that regulate the gene expression.80 Their activation can dampen the proinflammatory cytokine secretion by inhibiting the activation of NF-κB, thereby reducing the inflammation in response to influenza virus infection.81 Thus PPAR agonists like hyperforin82 and caffeic acid83 would hold a great promise as influenza treatments. Intriguingly, these molecules as agonist have been identified in herbs Lonicera japonica and/or Fructus Forsythiae, further highlighting the efficiency of medicinal herbs in treatment of influenza. Combined, these results indicate that both herbs can help hosts to restrain viral infection by enhancing the antiviral defenses.
On the other hand, viruses have to counter these defenses by incorporating tools that can cripple or overcome the antiviral host defense. Therefore, the viral proteins associated with viral replication, such as NA and HN, are also attractive drug targets.75 Notably, we find 15 molecules in two herbs can modulate five potential targets in virus, and some of them, such as quinic acid from Lonicera japonica84 and luteolin from Fructus Forsythiae,85 have been experimentally identified as inhibitors of NA.
Taken together, these results explain that the Janus functions of medicinal herbs, Lonicera japonica and Fructus Forsythiae, are reconcilable, that is, medicinal herbs can exert influenza antiviral effects by inhibiting the viral protein directly and regulating the immune-mediated resistance to viral infection. This synergistic effect achieved by the multiple ingredients that inhibit the virus at different stages, strengthen the impaired immune system and improve the overall symptoms will make the herb medicine promising to become the therapeutics of choice in the future.
3.3.4. Characteristics of feature patterns enriched in both herbs
To identify the similarities and compare the differences between Lonicera japonica and Fructus Forsythiae in the treatment of influenza, we further analyze the potential compounds and targets involved in the C–Th and C–Tv networks. For the C–Th network, 9 of 50 compounds show high degree distributions and each of them hits more than 22 potential targets, and 8 of the 9 compounds come from Fructus Forsythiae, i.e. LM387 (7′-epi-8-hydroxypinoresinol), LM304 (phillyrin), LM279 (caffeic acid), LM428 (onjixanthone I), LM356 ((+)-lariciresinol), LM299 ((3R,4S,5R)-5-(3,4-dimethoxyphenyl)-3,4-bis(hydroxymethyl) dihydrofuran-2(3H)-one), LM305 ((+)-pinoresinol), LM287 (epipinoresinol). This result shows a bias toward the specific drug compounds of Fructus Forsythiae, suggesting that this herb primarily regulates the human defense system that is probably more efficient than Lonicera japonica.
As for the 43 potential targets in the C–Th network, 35 of them are shared by both herbs, implying similar therapeutic mechanisms of both medicinal herbs in the treatment of influenza. While the rest 8 targets are specifically hit by Lonicera japonica and Fructus Forsythiae, that is, dihydrofolate reductase (deoD), and epidermal growth factor receptor (EGFR) for Lonicera japonica, macrophage migration inhibitory factor (MIF), delta-type opioid receptor (OPRD1), phospholipase A2 (PLA2G1B), membrane copper amine oxidase (AOC3), toll-like receptor 7 (TLR7), and voltage-dependent T-type calcium channel subunit alpha-1H (CACNA1H) for Fructus Forsythiae. This result indicates that both herbs probably possess different binding properties with the active substances. Combined, these data suggest that one medicinal herb enables us to constitute a formula with different herbs for the treatment of various diseases to some extent.
While in the C–Tv network, three targets, i.e., TK, E, and HN, are shared by both herbs, whereas the NA and UL54 are specifically hit by Lonicera japonica. More importantly, we observed that, different from C–Th network, the molecules bound to all five targets in virus mainly come from Lonicera japonica (11/15) rather than Fructus Forsythiae (6/15), suggesting that Lonicera japonica directly controls the influenza virus infection more effectively than Fructus Forsythiae. Taken together, both C–T networks suggest that the combination of both herbs probably explores a wider biological space and work effects at low concentration, and is evidently safer than single-component drugs. For example, yinqiaosan, with Lonicera japonica and Fructus Forsythiae acts as its principal active components, has been clinically identified as effective therapy as the NA inhibitoroseltamivir for patients with H1N1 influenza,86 which implies that the herbs involved in a formula probably possess combination effects.
3.3.5. Illuminating medicinal herbs in the influenza treatment with biological pathways
The biological pathway, from the simplest cascades to the highly intertwined networks of protein kinases, contributes extensively to the diversity of developmental programs and adaptation responses in animals.87 Diseases including influenza are characterized by dysregulation of biological pathways88 that is caused by infections, environmental factors, genetic mutations, or lifestyle. Such dysregulation changes the expression of proteins in multiple cellular pathways, resulting in alteration in growth, differentiation, or apoptosis.89 Thus identification of molecular pathways targeted by a compound is crucial for drug discovery, and also for new clinical applications of already existing drugs. The above C–T networks suggest that herb drugs have many physiological targets in the treatment of influenza infection, indicating that this ‘polypharmacology’ is probably therapeutically essential. Here, we have constructed the C–T–P network to provide more information on the mechanisms of action of medicinal herbs and potential side effects.
We have chosen 13 signal pathways closely associated with the influenza and its associated symptoms in KEGG as shown in Fig. 4. We find that there are 3 pathways significantly enriched for these target proteins: (1) Of all 32 target proteins, 13 participate in the neuroactive ligand–receptor interaction pathway. This pathway is directly related to the neurodevelopment, which utilizes the neurotransmittersglutamate, dopamine, serotonin, noradrenaline as its starters and regulates certain crucial pathways that maintain mood and regulate stress such as, long-term potentiation, GnRH (Gonadotrophin Releasing Hormone) signaling and synthesis of gap junction. More importantly, the neuroactive ligand–receptor interaction pathway can be activated early after virus infection.90 This result indicates that herbal medicine is a response-oriented treatment and emphasizes restoring the self-regulatory ability of the body; (2) the calcium signaling pathway also stood out in the enriched pathway list. This pathway can be modulated by small messenger molecule nitric oxide (NO) which was produced by NOS (the enzyme nitric oxide synthase).91 Hence, NO displays various potent physiological effects, including the vascular headache pathophysiology probably associated with this pathway. Furthermore, this pathway has also been demonstrated to be associated with influenza A virus-induced apoptosis;92 (3) the nucleotide metabolism pathways including “pyrimidine metabolism” and “purine metabolism” are crucial to the survival of the virus and bacteria. The common target DHODH is an enzyme that catalyzes the rate limiting step in de novo biosynthesis of pyrimidine in different cell lines,93 and its inhibition can suppress the cell growth and antibody production including the immunoglobulin production.94 Thus, these pathways including DHODH would be an attractive target for antiviral and antibacterial therapies. Additionally, TLR and MAPK pathways, as described above, are directly relevant to influenza virus infection.
Fig. 4.
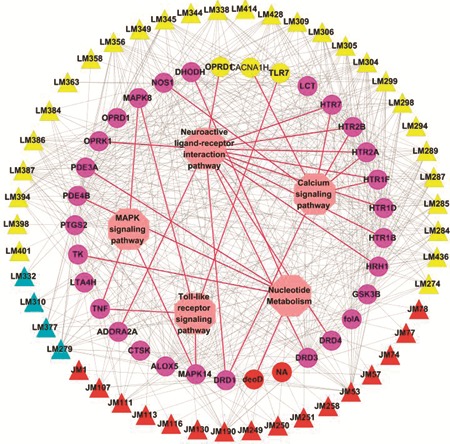
C–T–P network. The information about the pathways as defined by the KEGG was collected and then the T–P network was built by connecting the targets and their related signaling pathways. The C–T–P network was constructed by overlaying the C–T network onto T–P network. The colors of compound and target nodes are the same as Fig. 2, and the pathway node is represented as pink hexagon.
Furthermore, the common targets shared by both herbs link with all pathways, implying that similar mechanisms are shared by both herbs as anti-flu agents. Moreover, several protein targets belong to more than one signaling pathways, indicating that a single protein can function in more than one signaling pathways. For example, the common target TNF is a cytokine that participates in systemic inflammation and stimulates acute phase reaction. It is accumulated in macrophages infected by influenza A virus95 and can initiate several pathways such as T cell receptor, TLR and cytokine–cytokine receptor interaction pro-inflammatory signaling pathways during the innate defense,96 suggesting the potential role of cross-talking proteins in drug target discovery. This further verifies that as anti-influenza agents medicinal herbs probably act through several pathways which enable us to treat influenza and its associated symptoms efficiently.
4. Conclusion
The threat of influenza pandemics and the emergence of drug-resistant viral strains to existing drugs mean that the development of novel, effective and affordable drug treatments is an urgent priority. Natural products and their derivatives, such as Chinese herb medicine, have historically been invaluable as a source of therapeutic agents for the treatment of diseases, including especially the influenza and concomitant infections. However, the molecular mechanism of medicinal herbs exerting therapeutic effects on the special disease is still unknown. Here, we used a systems pharmacology approach by integrating the OB screening, targets prediction and validation, and network analysis to probe the molecular mechanisms of action of the representative medicinal herbs Lonicera japonica and Fructus Forsythiae for the influenza disease treatment. Our results show that:
(1) 50 active ingredients of both herbs have been identified, and the systematic use of these ingredients to explore the biological systems on a proteome-wide scale will offer valuable clues on the molecular basis of cellular functions, and also the results of target prediction will shift the conventional one-drug–one-target drug discovery process to a new multi-drug–multi-target paradigm.
(2) The potential targets of the medicinal herbs Lonicera japonica and Fructus Forsythiae have been identified in both human and virus, harnessing the potential of the medicinal herbs as anti-influenza agents that act in two ways: inactivating or restraining the virus directly by targeting the viral proteins and pathways unique to the viral life cycle; and suppressing the virus indirectly by enhancing or regulating the immune function in host cells.
(3) The T–D network displays that both herbs have great efficiency for the control of not only the influenza but also of other diseases such as neoplasms, nervous system diseases, and nutritional and cardiovascular diseases, suggesting that multiple diseases can be treated with the same herbal medicine.
(4) The integrated C–T–P network constructed in our work demonstrates that active compounds in both herbs can impact diverse clinically relevant signal pathways, confirming the efficiency of the medicinal herbs for the treatment of influenza.
(5) The present work provides a novel strategy for network-based herbal medicine pharmacology study, which will promote botanical drug discovery. The approach developed in this work is expected to bridge the existing gap between traditional medicine and modern medicine.
Supplementary Material
Acknowledgements
The research is financially supported by the Fund of Northwest A & F University, the National Natural Science Foundation of China (Grant No. 31170796) and the Fundamental Research Funds of China Academy of Chinese Medical Sciences (ZZ0608).
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ib20204b
References
- 1. Tumpey T. M., Basler C. F., Aguilar P. V., Zeng H. and Solorzano A., et al. , Characterization of the reconstructed 1918 Spanish influenza pandemic virus, Science, 2005, 310, 77–80. [DOI] [PubMed] [Google Scholar]
- 2. Simonsen L., Clarke M. J., Schonberger L. B., Arden N. H. and Cox N. J., et al. , Pandemicversus epidemic influenza mortality: a pattern of changing age distribution, J. Infect. Dis., 1998, 178, 53–60. [DOI] [PubMed] [Google Scholar]
- 3. Wang C., Takeuchi K., Pinto L. H. and Lamb R. A., Ion channel activity of influenza A virus M2 protein: characterization of the amantadine block, J. Virol., 1993, 67, 5585–5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moscona A., Neuraminidase inhibitors for influenza, N. Engl. J. Med., 2005, 353, 1363–1373. [DOI] [PubMed] [Google Scholar]
- 5. Deyde V. M., Xu X., Bright R. A., Shaw M. and Smith C. B., et al. , Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide, J. Infect. Dis., 2007, 196, 249–257. [DOI] [PubMed] [Google Scholar]
- 6. Daar E. S. and Richman D. D., Confronting the emergence of drug-resistant HIV type 1: impact of antiretroviral therapy on individual and population resistance, AIDS Res. Hum. Retroviruses, 2005, 21, 343–357. [DOI] [PubMed] [Google Scholar]
- 7. Chou T. C., Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies, Pharmacol. Rev., 2006, 58, 621–681. [DOI] [PubMed] [Google Scholar]
- 8. Feldmann G., Dhara S., Fendrich V., Bedja D. and Beaty R., et al. , Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers, Cancer Res., 2007, 67, 2187–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lennox J. L., DeJesus E., Lazzarin A., Pollard R. B. and Madruga J. V., et al. , Safety and efficacy of raltegravir-basedversus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial, Lancet, 2009, 374, 796–806. [DOI] [PubMed] [Google Scholar]
- 10. Wang L., Zhou G. B., Liu P., Song J. H. and Liang Y., et al. , Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 4826–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zimmermann G. R., Lehár J. and Keith C. T., Multi-target therapeutics: when the whole is greater than the sum of the parts, Drug Discovery Today, 2007, 12, 34–42. [DOI] [PubMed] [Google Scholar]
- 12. Anonymous, Formularies for 52 Kinds of Disorders,Culture Relics Press,Beijing,1979. [Google Scholar]
- 13. Anonymous, The Inner Canon of Emperor Huang,Chinese Medical Ancient Books Publishing House,Beijing,2003. [Google Scholar]
- 14. Chinese Pharmacopoeia Commission, Pharmacopoeia of the People's Republic of China,China Medical Science Press,Beijing,2011. [Google Scholar]
- 15. Wang X., Jia W. and Zhao A., Anti-influenza agents from plants and traditional Chinese medicine, Phytother. Res., 2006, 20, 335–341. [DOI] [PubMed] [Google Scholar]
- 16. Shang X., Pan H., Li M., Miao X. and Ding H., Lonicera japonica Thunb.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine, J. Ethnopharmacol., 2011, 138, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jeong H. J., Ryu Y. B., Park S. J., Kim J. H. and Kwon H. J., et al. , Neuraminidase inhibitory activities of flavonols isolated fromRhodiola rosea roots and theirin vitro anti-influenza viral activities, Bioorg. Med. Chem., 2009, 17, 6816–6823. [DOI] [PubMed] [Google Scholar]
- 18. Miki K., Nagai T., Suzuki K., Tsujimura R. and Koyama K., et al. , Anti-influenza virus activity of biflavonoids, Bioorg. Med. Chem. Lett., 2007, 17, 772–775. [DOI] [PubMed] [Google Scholar]
- 19. Yu D. Q., Chen R. Y., Huang L. J., Xie F. Z. and Ming D. S., et al. , The structure and absolute configuration of Shuangkangsu: a novel natural cyclic peroxide fromLonicera japonica (Thunb.), J. Asian Nat. Prod. Res., 2008, 10, 851–856. [DOI] [PubMed] [Google Scholar]
- 20. Alleva L. M., Gualano R. C. and Clark I. A., Current work and future possibilities for the management of severe influenza: using immunomodulatory agents that target the host response, Future Virol., 2011, 6, 843–854. [Google Scholar]
- 21. Kang O. H., Choi J. G., Lee J. H. and Kwon D. Y., Luteolin isolated from the flowers ofLonicera japonica suppresses inflammatory mediator release by blocking NF-kappaB and MAPKs activation pathways in HMC-1 cells, Molecules, 2010, 15, 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang H. S., Lee J. Y. and Kim C. J., Anti-inflammatory activity of arctigenin fromForsythiae Fructus, J. Ethnopharmacol., 2008, 116, 305–312. [DOI] [PubMed] [Google Scholar]
- 23. Kan W. L., Cho C. H., Rudd J. A. and Lin G., Study of the anti-proliferative effects and synergy of phthalides fromAngelica sinensis on colon cancer cells, J. Ethnopharmacol., 2008, 120, 36–43. [DOI] [PubMed] [Google Scholar]
- 24. Borisy A. A., Elliott P. J., Hurst N. W., Lee M. S. and Lehar J., et al. , Systematic discovery of multicomponent therapeutics, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 7977–7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berger S. I. and Iyengar R., Network analyses in systems pharmacology, Bioinformatics, 2009, 25, 2466–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu H., Chen J., Xu X., Li Y., Zhao H., Fang Y., Li X., Zhou W., Wang W. and Wang Y., A systematic prediction of multiple drug–target interactions from chemical, genomic, and pharmacological data, PLoS One, 2012, 7, e37608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li X., Xu X., Wang J., Yu H., Wang X., Yang H., Xu H., Tang S., Li Y., Yang L., Huang L., Wang Y. and Yang S., A System-Level Investigation into the Mechanisms of Chinese Traditional Medicine: Compound Danshen Formula for Cardiovascular Disease Treatment, PLoS One, 2012, 7, e43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang X., Yang W., Xu X., Zhang H. and Li Y., et al. , Studies of benzothiadiazine derivatives as hepatitis C virus NS5B polymerase inhibitors using 3D-QSAR, molecular docking and molecular dynamics, Curr. Med. Chem., 2010, 17, 2788–2803. [DOI] [PubMed] [Google Scholar]
- 29. Nemeth K., Plumb G. W., Berrin J. G., Juge N. and Jacob R., et al. , Deglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans, Eur. J. Nutr., 2003, 42, 29–42. [DOI] [PubMed] [Google Scholar]
- 30. Xu X., Zhang W., Wang C., Li Y., Yu H., Huang Y., Duan J. and Ling Y., A novel chemometric method for the prediction of human oral bioavailability, Int. J. Mol. Sci., 2012, 13, 6964–6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sousa S. F., Fernandes P. A. and Ramos M. J., Protein–ligand docking: current status and future challenges, Proteins, 2006, 65, 15–26. [DOI] [PubMed] [Google Scholar]
- 32. Cosconati S., Forli S., Perryman A. L., Harris R. and Goodsell D. S., et al. , Virtual Screening with AutoDock: Theory and Practice, Expert Opin. Drug Discovery, 2010, 5, 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao J., Jiang P. and Zhang W., Molecular networks for the study of TCM pharmacology, Briefings Bioinf., 2010, 11, 417–430. [DOI] [PubMed] [Google Scholar]
- 34. Zhu F., Han B., Kumar P., Liu X. and Ma X., et al. , Update of TTD: Therapeutic Target Database, Nucleic Acids Res., 2010, 38, D787–D791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kanehisa M., Goto S., Furumichi M., Tanabe M. and Hirakawa M., KEGG for representation and analysis of molecular networks involving diseases and drugs, Nucleic Acids Res., 2010, 38, D355–D360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smoot M. E., Ono K., Ruscheinski J., Wang P. L. and Ideker T., Cytoscape 2.8: new features for data integration and network visualization, Bioinformatics, 2011, 27, 431–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang W. Y., Therapeutic wisdom in traditional Chinese medicine: a perspective from modern science, Trends Pharmacol. Sci., 2005, 26, 558–563. [DOI] [PubMed] [Google Scholar]
- 38. Hou T. and Xu X., Recent development and application of virtual screening in drug discovery: an overview, Curr. Pharm. Des., 2004, 10, 1011–1033. [DOI] [PubMed] [Google Scholar]
- 39. Chang H. T., Cheng Y. H., Wu C. L., Chang S. T. and Chang T. T., et al. , Antifungal activity of essential oil and its constituents fromCalocedrus macrolepis var. formosana Florin leaf against plant pathogenic fungi, Bioresour. Technol., 2008, 99, 6266–6270. [DOI] [PubMed] [Google Scholar]
- 40. Chao L. K., Hua K. F., Hsu H. Y., Cheng S. S. and Liu J. Y., et al. , Study on the antiinflammatory activity of essential oil from leaves ofCinnamomum osmophloeum, J. Agric. Food Chem., 2005, 53, 7274–7278. [DOI] [PubMed] [Google Scholar]
- 41. Moraes M. C., Birkett M. A., gordon-Weeks R., Smart L. E. and Martin J. L., et al. , cis-Jasmone induces accumulation of defence compounds in wheat,Triticum aestivum, Phytochemistry, 2008, 69, 9–17. [DOI] [PubMed] [Google Scholar]
- 42. Peana A. T., D'Aquila P. S., Panin F., Serra G. and Pippia P., et al. , Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils, Phytomedicine, 2002, 9, 721–726. [DOI] [PubMed] [Google Scholar]
- 43. Jirovetz L., Jager W., Buchbauer G., Nikiforov A. and Raverdino V., Investigations of animal blood samples after fragrance drug inhalation by gas chromatography/mass spectrometry with chemical ionization and selected ion monitoring, Biol. Mass Spectrom., 1991, 20, 801–803. [DOI] [PubMed] [Google Scholar]
- 44. Ma S. C., Paul But P. H., Vincent Ooi E. C., Spencer Lee H. S. and Lee S. F., Determination of the antiviral caffeoyl quinic acids isolated fromL. japonica thunb, Chin. J. Pharm. Anal., 2005, 25, 751–753. [Google Scholar]
- 45. Wu H. Z., Luo J., Yin Y. X. and Wei Q., Effects of chlorogenic acid, an active compound activating calcineurin, purified fromFlos Lonicerae on macrophage, Acta Pharmacol. Sin., 2004, 25, 1685–1689. [PubMed] [Google Scholar]
- 46. Boon A. C., Vos A. P., Graus Y. M., Rimmelzwaan G. F. and Osterhaus A. D., In vitro effect of bioactive compounds on influenza virus specific B- and T-cell responses, Scand. J. Immunol., 2002, 55, 24–32. [DOI] [PubMed] [Google Scholar]
- 47. Jiang R. W., Lau K. M., Hon P. M., Mak T. C. and Woo K. S., et al. , Chemistry and biological activities of caffeic acid derivatives fromSalvia miltiorrhiza, Curr. Med. Chem., 2005, 12, 237–246. [DOI] [PubMed] [Google Scholar]
- 48. Davis J. M., Murphy E. A., McClellan J. L., Carmichael M. D. and Gangemi J. D., Quercetin reduces susceptibility to influenza infection following stressful exercise, Am. J. Physiol.: Regul., Integr. Comp. Physiol., 2008, 295, R505–R509. [DOI] [PubMed] [Google Scholar]
- 49. Erlund I., Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology, Nutr. Res., 2004, 24, 851–874. [Google Scholar]
- 50. Lee S., Shin E., Son K., Chang H. and Kang S., et al. , Anti-inflammatory activity of the major constituents ofLonicerajaponica, Arch. Pharmacal Res., 1995, 18, 133–135. [Google Scholar]
- 51. Dai S., Ren Y., Shen L. and Zhang D., New alkaloids fromForsythia suspensa and their anti-inflammatory activities, Planta Med., 2009, 75, 375–377. [DOI] [PubMed] [Google Scholar]
- 52. Oh J., Hwang I. H., Kim D. C., Kang S. C. and Jang T. S., et al. , Anti-listerial compounds fromAsari Radix, Arch. Pharmacal Res., 2010, 33, 1339–1345. [DOI] [PubMed] [Google Scholar]
- 53. Ishida J., Wang H. K., Oyama M., Cosentino M. L. and Hu C. Q., et al. , Anti-AIDS agents. 46. Anti-HIV activity of harman, an anti-HIV principle fromSymplocos setchuensis, and its derivatives, J. Nat. Prod., 2001, 64, 958–960. [DOI] [PubMed] [Google Scholar]
- 54. Zhang G. G., Song S. J., Ren J. and Xu S. X., A new compound fromForsythia suspensa (Thunb.) Vahl with antiviral effect on RSV, J. Herb. Pharmacother., 2002, 2, 35–40. [PubMed] [Google Scholar]
- 55. Calixto J. B., Otuki M. F. and Santos A. R., Anti-inflammatory compounds of plant origin. Part I. Action on arachidonic acid pathway, nitric oxide and nuclear factor kappa B (NF-kappaB), Planta. Med., 2003, 69, 973–983. [DOI] [PubMed] [Google Scholar]
- 56. Chen X., Zhang J., Xue C., Chen X. and Hu Z., Simultaneous determination of some active ingredients in anti-viral preparations of traditional Chinese medicine by micellar electrokinetic chromatography, Biomed. Chromatogr., 2004, 18, 673–680. [DOI] [PubMed] [Google Scholar]
- 57. Qu H., Zhang Y., Wang Y., Li B. and Sun W., Antioxidant and antibacterial activity of two compounds (forsythiaside and forsythin) isolated fromForsythia suspensa, J. Pharm. Pharmacol., 2008, 60, 261–266. [DOI] [PubMed] [Google Scholar]
- 58. Qu H., Li B., Li X., Tu G. and Lü J., et al. , Qualitative and quantitative analyses of three bioactive compounds in different parts ofForsythia suspensa by high-performance liquid chromatography-electrospray ionization-mass spectrometry, Microchem. J., 2008, 89, 159–164. [Google Scholar]
- 59. Wang G. N., Pan R. L., Liao Y. H., Chen Y. and Tang J. T., et al. , An LC-MS/MS method for determination of forsythiaside in rat plasma and application to a pharmacokinetic study, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2010, 878, 102–106. [DOI] [PubMed] [Google Scholar]
- 60. Schempp C. M., Pelz K., Wittmer A., Schopf E. and Simon J. C., Antibacterial activity of hyperforin from St John's wort, against multiresistantStaphylococcus aureus and gram-positive bacteria, Lancet, 1999, 353, 2129. [DOI] [PubMed] [Google Scholar]
- 61. Sosa S., Pace R., Bornancin A., Morazzoni P. and Riva A., et al. , Topical anti-inflammatory activity of extracts and compounds fromHypericum perforatum L, J. Pharm. Pharmacol., 2007, 59, 703–709. [DOI] [PubMed] [Google Scholar]
- 62. Fryer R. M., Randall J., Yoshida T., Hsiao L. L. and Blumenstock J., et al. , Global analysis of gene expression: methods, interpretation, and pitfalls, Exp. Nephrol., 2002, 10, 64–74. [DOI] [PubMed] [Google Scholar]
- 63. Lomenick B., Olsen R. W. and Huang J., Identification of direct protein targets of small molecules, ACS Chem. Biol., 2011, 6, 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen X., Ji Z. L. and Chen Y. Z., TTD: Therapeutic Target Database, Nucleic Acids Res., 2002, 30, 412–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Macpherson L. J., Hwang S. W., Miyamoto T., Dubin A. E. and Patapoutian A., et al. , More than cool: promiscuous relationships of menthol and other sensory compounds, Mol. Cell. Neurosci., 2006, 32, 335–343. [DOI] [PubMed] [Google Scholar]
- 66. Galeotti N., Cesare Di, Mannelli L., Mazzanti G., Bartolini A. and Ghelardini C., Menthol: a natural analgesic compound, Neurosci. Lett., 2002, 322, 145–148. [DOI] [PubMed] [Google Scholar]
- 67. Kast H. R., Goodwin B., Tarr P. T., Jones S. A. and Anisfeld A. M., et al. , Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor, J. Biol. Chem., 2002, 277, 2908–2915. [DOI] [PubMed] [Google Scholar]
- 68. Tirona R. G., Leake B. F., Wolkoff A. W. and Kim R. B., Human organic anion transporting polypeptide-C (SLC21A6) is a major determinant of rifampin-mediated pregnane X receptor activation, J. Pharmacol. Exp. Ther., 2003, 304, 223–228. [DOI] [PubMed] [Google Scholar]
- 69. Walsky R. L., Gaman E. A. and Obach R. S., Examination of 209 drugs for inhibition of cytochrome P450 2C8, J. Clin. Pharmacol., 2005, 45, 68–78. [DOI] [PubMed] [Google Scholar]
- 70. Nguyen H., Zhang S. and Morris M. E., Effect of flavonoids on MRP1-mediated transport in Panc-1 cells, J. Pharm. Sci., 2003, 92, 250–257. [DOI] [PubMed] [Google Scholar]
- 71. Taubenberger J. K. and Morens D. M., The pathology of influenza virus infections, Annu. Rev. Pathol., 2008, 3, 499–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kawada J., Kimura H., Ito Y., Hara S. and Iriyama M., et al. , Systemic cytokine responses in patients with influenza-associated encephalopathy, J. Infect. Dis., 2003, 188, 690–698. [DOI] [PubMed] [Google Scholar]
- 73. Conze D., Lumsden J., Enslen H., Davis R. J. and Gros Leg, et al. , Activation of p38 MAP kinase in T cells facilitates the immune response to the influenza virus, Mol. Immunol., 2000, 37, 503–513. [DOI] [PubMed] [Google Scholar]
- 74. Takeda M., Leser G. P., Russell C. J. and Lamb R. A., Influenza virus hemagglutinin concentrates in lipid raft microdomains for efficient viral fusion, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 14610–14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Grienke U., Schmidtke M., Grafenstein von S., Kirchmair J. and Liedl K. R., et al. , Influenza neuraminidase: a druggable target for natural products, Nat. Prod. Rep., 2012, 29, 11–36. [DOI] [PubMed] [Google Scholar]
- 76. Marchant D., Singhera G. K., Utokaparch S., Hackett T. L. and Boyd J. H., et al. , Toll-like receptor 4-mediated activation of p38 mitogen-activated protein kinase is a determinant of respiratory virus entry and tropism, J. Virol., 2010, 84, 11359–11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Akira S., Uematsu S. and Takeuchi O., Pathogen recognition and innate immunity, Cell, 2006, 124, 783–801. [DOI] [PubMed] [Google Scholar]
- 78. Pan H. Y., Yano M. and Kido H., Effects of inhibitors of Toll-like receptors, protease-activated receptor-2 signalings and trypsin on influenza A virus replication and upregulation of cellular factors in cardiomyocytes, J. Med. Invest., 2011, 58, 19–28. [DOI] [PubMed] [Google Scholar]
- 79. Min Y. D., Choi C. H., Bark H., Son H. Y. and Park H. H., et al. , Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-kappaB and p38 MAPK in HMC-1 human mast cell line, Inflammation Res., 2007, 56, 210–215. [DOI] [PubMed] [Google Scholar]
- 80. Kersten S., Desvergne B. and Wahli W., Roles of PPARs in health and disease, Nature, 2000, 405, 421–424. [DOI] [PubMed] [Google Scholar]
- 81. Crisafulli C. and Cuzzocrea S., The role of endogenous and exogenous ligands for the peroxisome proliferator-activated receptor alpha (PPAR-alpha) in the regulation of inflammation in macrophages, Shock, 2009, 32, 62–73. [DOI] [PubMed] [Google Scholar]
- 82. Krusekopf S. and Roots I., St. John's wort and its constituent hyperforin concordantly regulate expression of genes encoding enzymes involved in basic cellular pathways, Pharmacogenet. Genomics, 2005, 15, 817–829. [DOI] [PubMed] [Google Scholar]
- 83. Oh M., Kim J., Lee S., Kim B. and Kim H., et al. , Caffeic acid improves skin barrier function as Peroxisome proliferator-activated receptor (PPAR)-α agonist, J. Invest. Dermatol., 2012, 132, S51–S64. [Google Scholar]
- 84. Kim C. U., Lew W., Williams M. A., Liu H. and Zhang L., et al. , Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity, J. Am. Chem. Soc., 1997, 119, 681–690. [DOI] [PubMed] [Google Scholar]
- 85. Liu A. L., Liu B., Qin H. L., Lee S. M. and Wang Y. T., et al. , Anti-influenza virus activities of flavonoids from the medicinal plantElsholtzia rugulosa, Planta Med., 2008, 74, 847–851. [DOI] [PubMed] [Google Scholar]
- 86. Wang C., Cao B., Liu Q. Q., Zou Z. Q. and Liang Z. A., et al. , Oseltamivir compared with the Chinese traditional therapy maxingshigan-yinqiaosan in the treatment of H1N1 influenza: a randomized trial, Ann. Intern. Med., 2011, 155, 217–225. [DOI] [PubMed] [Google Scholar]
- 87. daSilva Pires-A. and Sommer R. J., The evolution of signalling pathways in animal development, Nat. Rev. Genet., 2003, 4, 39–49. [DOI] [PubMed] [Google Scholar]
- 88. Baylin S. B. and Ohm J. E., Epigenetic gene silencing in cancer – a mechanism for early oncogenic pathway addiction?, Nat. Rev. Cancer, 2006, 6, 107–116. [DOI] [PubMed] [Google Scholar]
- 89. Aggarwal B. B., Sethi G., Baladandayuthapani V., Krishnan S. and Shishodia S., Targeting cell signaling pathways for drug discovery: an old lock needs a new key, J. Cell. Biochem., 2007, 102, 580–592. [DOI] [PubMed] [Google Scholar]
- 90. Huang Y. C., Li Z., Hyseni X., Schmitt M. and Devlin R. B., et al. , Identification of gene biomarkers for respiratory syncytial virus infection in a bronchial epithelial cell line, Genomic Med., 2008, 2, 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hare J. M. and Stamler J. S., NOS: modulator, not mediator of cardiac performance, Nat. Med., 1999, 5, 273–274. [DOI] [PubMed] [Google Scholar]
- 92. Cherry Schultz-S., Krug R. M. and Hinshaw V. S., Induction of Apoptosis by Influenza Virus, Semin. Virol., 1998, 8, 491–495. [Google Scholar]
- 93. Fairbanks L. D., Bofill M., Ruckemann K. and Simmonds H. A., Importance of ribonucleotide availability to proliferating T-lymphocytes from healthy humans. Disproportionate expansion of pyrimidine pools and contrasting effects ofde novo synthesis inhibitors, J. Biol. Chem., 1995, 270, 29682–29689. [PubMed] [Google Scholar]
- 94. Deguchi M., Kishino J., Hattori M., Furue Y. and Yamamoto M., et al. , Suppression of immunoglobulin production by a novel dihydroorotate dehydrogenase inhibitor, S-2678, Eur. J. Pharmacol., 2008, 601, 163–170. [DOI] [PubMed] [Google Scholar]
- 95. Gong J. H., Sprenger H., Hinder F., Bender A. and Schmidt A., et al. , Influenza A virus infection of macrophages. Enhanced tumor necrosis factor-alpha (TNF-alpha) gene expression and lipopolysaccharide-triggered TNF-alpha release, J. Immunol., 1991, 147, 3507–3513. [PubMed] [Google Scholar]
- 96. Ozinsky A., Underhill D. M., Fontenot J. D., Hajjar A. M. and Smith K. D., et al. , The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors, Proc. Natl. Acad. Sci. U. S. A., 2003, 97, 13766–13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.