Abstract
Brefeldin A reversibly disassembles the Golgi complex, causing mixing of the Golgi cisternae with the ER while the trans Golgi network persists as part of a separate endosomal membrane system. Because of this compartmental separation, Brefeldin A treatment has been used to map the sub-Golgi locations of several Golgi enzymes including GM2 synthase. We previously proposed that GM2 synthase might be located in a distal portion of the Golgi complex which in the presence of Brefeldin A would be separated from the substrate ganglioside GM3 present in the mixed ER-Golgi membrane system. In the present study we show using GM2 synthase chimeras that GM2 synthesis was blocked by Brefeldin A when GM2 synthase was distributed throughout all Golgi subcompartments or even when it was restricted to the medial Golgi. Because these findings opposed our speculation regarding a distal location of this enzyme, we sought an alternative explanation for the inhibition of ganglioside synthesis by Brefeldin A. However, Brefeldin A did not degrade GM2 synthase, prevent its homodimerization, or inhibit its in vitro activity. Brefeldin A did result in the conversion of a portion of membrane bound GM2 synthase into a soluble form which has minimal capability to produce GM2 in whole cells. However, this conversion was not sufficient to explain the nearly total loss of GM2 production in intact cells in the presence of Brefeldin A. Nevertheless, the results of this study indicate that Brefeldin A-induced inhibition of ganglioside synthesis cannot be used to deduce the location of GM2 synthase.
Keywords: Brefeldin A, gangliosides, glycosphingolipid, glycosyltransferase, GM2 synthase, Golgi
Introduction
The spatial organization of glycosyltransferases and other enzymes of the Golgi apparatus is poorly understood. Historically the subdivision of the Golgi stack into cis, medial, and trans portions was based primarily on the locations of key enzymes required for N-linked glycosylation (Kornfeld and Kornfeld, 1985). This distribution gave rise to an assembly line model for the arrangement of these enzymes, i.e., that the enzymes are distributed across the Golgi stack in the order in which they act. However, it was later shown that the subcompartmentation of these enzymes varies between cell types (Roth et al., 1986) and, furthermore, that there is considerable overlap in the distributions of these enzymes (Nilsson et al., 1993). Our understanding of the arrangement of enzymes involved in synthesis of O-linked chains and glycosphingolipids (GSL) is even less clear. Evidence had been accumulating, based on subcellular fractionation and use of inhibitors (reviewed in Young, 1993), that the steps of GSL synthesis might be arranged in an assembly line manner. A modified version of an assembly line model was proposed recently in which synthesis of lactosylceramide, GM3, and GD3 occurs in the trans Golgi whereas synthesis of more complex gangliosides occurs in the trans Golgi network (TGN) (Lannert et al., 1998).
We (Young et al., 1990) and others (Van Echten et al., 1990) previously reported that Brefeldin A (BFA) blocked the synthesis of GM2, GD2, and GA2, the products of GM2 synthase. BFA inhibits protein secretion by causing the breakdown of the Golgi complex and the microtubule-dependent retrograde transport of Golgi components back to the endoplasmic reticulum (ER); similarly, BFA causes fusion of early endosomal compartments and the trans-Golgi network (TGN; reviewed in Hunziker et al., 1992). Therefore, we speculated that the inhibition of GM2 synthase product synthesis indicated that GM2 synthase was located late in the secretory pathway and that BFA physically separated the GSL substrates in the ER-Golgi mix from GM2 synthase in the TGN-endosome membrane system. Using a similar rationale, BFA has been used to provide indirect evidence for the location of other enzymes of the Golgi complex including several glycosyltransferases (Shite et al., 1990; Locker et al., 1992; Sampath et al., 1992; Sherwood and Holmes, 1992; Holmes and Greene, 1993; Uhlin-Hansen and Yanagishita, 1993; Farrer et al., 1995; Rosales Fritz and Maccioni, 1995).
Subsequent to our BFA study, GM2 synthase was cloned (Nagata et al., 1992). Upon stable transfection of epitope tagged GM2 synthase into CHO cells, we localized the enzyme to all cisternae of the Golgi as well as to the TGN by immunoelectronmicroscopy (Jaskiewicz et al., 1996b). This finding appeared at odds with our previous speculation described above for a TGN location for GM2 synthase (Young et al., 1990). However, Lannert et al. discounted our localization results in their modified assembly line model for GSL biosynthesis (Lannert et al., 1998), by suggesting that our results could have been artefactual due to either overexpression of GM2 synthase or mislocalization caused by the presence of the epitope tag. Because of these discrepancies, in the present report we sought to clarify the effect of BFA on ganglioside synthesis. The results indicate that BFA inhibited GM2 synthesis even if GM2 synthase was located in the medial Golgi. We conclude that, although BFA treatment results in the separation of early and late compartments of the secretory pathway, additional effects must contribute to the BFA-induced inhibition of ganglioside synthesis. Therefore, the location of GM2 synthase cannot be deduced from the inhibition of GM2 synthesis by BFA.
Fig. 1.
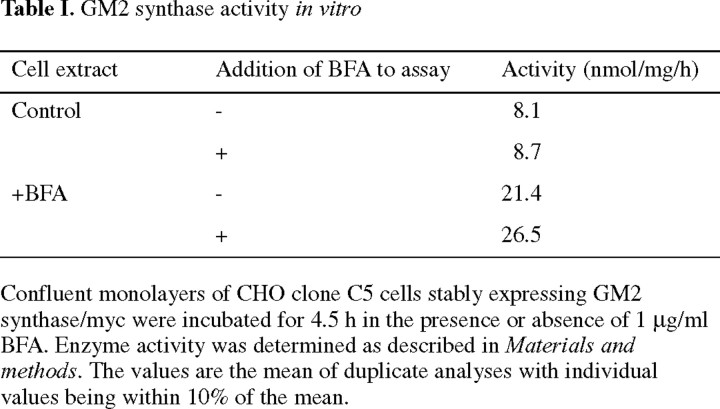
GM2 synthase and hybrid proteins. GM2 synthase/myc: the sequence encoding the ten amino acid myc epitope recognized by the 9E10 MoAb (Evan et al., 1985) was fused to the carboxy terminal sequence of GM2 synthase. GNT/GM2 synthase/myc: the sequence for the cytoplasmic and transmembrane domains plus the first 12 lumenal amino acids of N-acetylglucosaminyltransferase I (GNT) were fused to the lumenal domain of GM2 synthase/myc. Iip33/GM2 synthase/myc: the sequence for the cytoplasmic domain of the Iip33 form of human invariant chain was fused to the transmembrane and lumenal domains of GM2 synthase/myc. TM, Transmembrane domain.
Results
Effect of BFA on GM2 synthase distribution
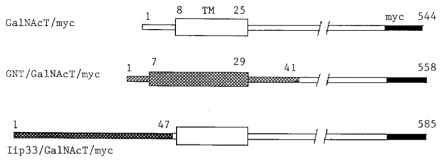
We first compared the immunofluorescence staining patterns of GM2 synthase/myc (Figure 1) with those of the well characterized medial Golgi enzyme a-mannosidase II (Dunphy and Rothman, 1983) to verify that BFA produced the redistribution of transfected GM2 synthase expected of a Golgi enzyme. In control medium the staining patterns produced by anti-mannosidase II (Figure 2a) and anti-myc (Figure 2b) were very similar. Both consisted mainly of a cluster of punctate, perinuclear structures characteristic of the Golgi of CHO cells, as we have described previously (Jaskiewicz et al., 1996b). In the presence of BFA the staining by anti-mannosidase II (Figure 2c) was largely redistributed into a weak focal staining of the nuclear envelope and a diffuse reticular pattern characteristic of the ER. Anti-myc staining in the presence of BFA (Figure 2d) also consisted of the diffuse reticular pattern plus nuclear membrane staining expected for relocation of a Golgi membrane protein to the ER. In addition there was also a focal, perinuclear staining similar to Golgi staining in many cells (Figure 2d). This latter pattern probably reflected GM2 synthase/myc molecules in the TGN because other residents of the TGN have been shown to redistribute in the presence of BFA into a stable structure surrounding the microtubule organizing center (Ladinsky and Howell, 1992; Miller et al., 1992). Thus, GM2 synthase in control and BFA-containing media exhibited the behavior expected for a membrane protein of the Golgi cisternae and TGN, consistent with the distribution we reported previously (Jaskiewicz et al., 1996b).
Effect of BFA on GM2 biosynthesis
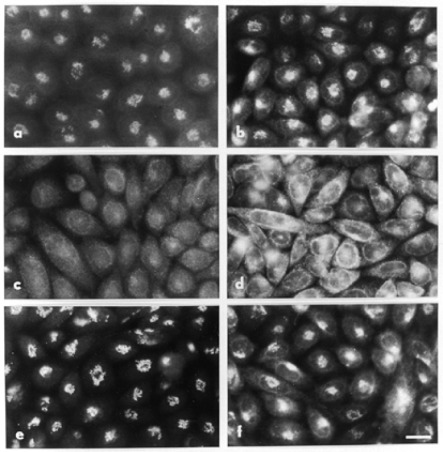
To determine the effect of BFA on GM2 synthase activity in transfected cells, we first tested CHO clone C5 which stably expresses GM2 synthase/myc (Jaskiewicz et al., 1996b). C5 cell monolayers metabolically labeled with [3H] palmitate in control medium produced doublets of both gangliosides GM3 and GM2 (Figure 3) as we reported previously (Jaskiewicz et al., 1996b). In striking contrast, the labeling of GM2 was almost totally inhibited by incubation in the presence of BFA (Figure 3). Thus, the block in GM2 synthesis that we previously observed in cells endogenously expressing GM2 synthase (Young et al., 1990) was reproduced in cells expressing cloned GM2 synthase/myc. However, in the case of C5 cells we showed previously by immunoelectronmicroscopy that GM2 synthase/myc was present in all Golgi cisternae as well as the TGN (Jaskiewicz et al., 1996b). Therefore, our previous hypothesis that BFA separated GM3 substrate in an early compartment from GM2 synthase in a late compartment could not explain the nearly complete inhibition of GM2 synthesis in C5 cells.
Fig. 2.
BFA-induced disruption and recovery of the Golgi apparatus. Clone C5 cells stably expressing GM2 synthase/myc were grown on coverslips and incubated in the presence (c-f) or absence (a-b) of 1 µg/ml BFA for 2 h at 37°. In the case of (e) and (f), BFA was washed out by rinsing with serum containing medium three times and then incubating for 2 h at 37° in BFA-free medium. Cells were then fixed and permeabilized as described in Materials and methods. Cells were incubated in succession with primary antibodies (a, c, e: rabbit anti-mannosidase II; b, d, f: MoAb 9E10 anti-myc) and then with secondary antisera (a, c, e: FITC-goat anti-rabbit immunoglobulin; b, d, f: FITC-goat anti-mouse immunoglobulin). Scale bar, 20 µm.
Fig. 3.
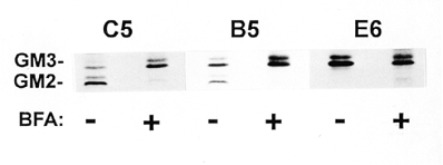
Effect of BFA on the synthesis of GM2 ganglioside. Cells were labeled with [3H] palmitate in the presence or absence of 1 µg/ml of BFA as described in Materials and methods. The purified GSL were analyzed by high performance thin layer chromatography (HPTLC) in solvent A.
BFA inhibition of ganglioside synthesis
Previously we reported that GM2 synthase/myc was capable of synthesizing GM2 in intact cells when the enzyme was restricted to the medial Golgi by using the GNT/GM2 synthase/ myc construct (Figure 1), as in transfected CHO cell clone B5 (Jaskiewicz et al., 1996b). We next asked if GM2 synthesis in clone B5 cells could be blocked by BFA. Clone B5 expresses a chimeric protein consisting of the transmembrane domain and flanking regions of GlcNAc transferase I (GNT) fused to the lumenal domain of GM2 synthase/myc. GlcNAc transferase I is generally regarded as a medial Golgi enzyme (Dunphy and Rothman, 1985; Roth, 1987), and in fact we previously localized the GNT/GM2 synthase/myc chimera primarily to the medial Golgi of clone B5 cells (Jaskiewicz et al., 1996b). In control medium clone B5 cells produced both GM3 and GM2 (Figure 3) as we previously reported (Jaskiewicz et al., 1996b). In the presence of BFA, only a trace of GM2 was synthesized in clone B5 cells (Figure 3). Thus, BFA blocked GM2 synthesis when GM2 synthase was located in the medial Golgi, in stark opposition to our previous hypothesis that BFA inhibition of GM2 synthesis in vivo indicated a TGN location of GM2 synthase.
We previously constructed a third GM2 synthase chimera in which we replaced the cytoplasmic domain of GM2 synthase with that of Iip33 (Figure 1), a mutant form of human invariant chain which has an ER retention signal (Jaskiewicz et al., 1996b). In clone E6 cells expressing this chimera, GM2 synthase was retained in the ER, was unable to produce GM2 in intact cells, but was active when assayed in vitro (Jaskiewicz et al., 1996b). Since synthesis of GM3 occurs in the Golgi, we reasoned that treatment of E6 cells with BFA could result in GM3 substrate from the Golgi being brought into contact with GM2 synthase in the ER, possibly resulting in GM2 synthesis. Precedents for other GalNAc transferases being able to function not only in the ER but also in the mixed ER-Golgi membrane system created by BFA support the feasibility of this approach; namely, O-linked specific GalNAc transferase 1 and 2 were capable of initiating protein O-glycosylation when they were relocated to the ER (Rottger et al., 1998), and the ribophorins, which are restricted to the ER, were shown to be O-glycosylated in the presence of BFA (Ivessa et al., 1992). In fact a weak doublet of GM2, representing 6% of the labeled GM3, was produced by E6 cells when they were labeled with palmitate in the presence of BFA (Figure 3). In contrast no GM2 was produced by E6 cells in control medium (Figure 3) as we reported previously (Jaskiewicz et al., 1996b).
The preceding results with E6 cells indicate that BFA-induced redistribution of intracellular compartments can lead to GM2 synthesis. However, conversion of GM3 to GM2 was inefficient as it also was when C5 and B5 cells were treated with BFA (Figure 3). This inefficiency suggested the possibility that GM2 synthase might simply be inhibited by the conditions in the mixed ER-Golgi membrane system produced by BFA. To explore this possibility we assayed for GM2 synthase activity in vitro. Surprisingly, the in vitro GM2 synthase activity of extracts of BFA treated cells was more than twice as great as that from control cell extracts (Table I). This effect was not the result of the presence of BFA in the assay (Table I), which produced a slight increase in activity, but was consistent with the accumulation of GM2 synthase in the BFA treated cells due to the BFA-induced block in secretion as seen in Western blots (see Figure 4A, lanes 2 and 4 below). Thus, the block of GM2 synthase activity in intact cells was not due to simple degradation or irreversible inhibition of GM2 synthase activity in the mixed ER-Golgi membrane system.
Fig. 4.
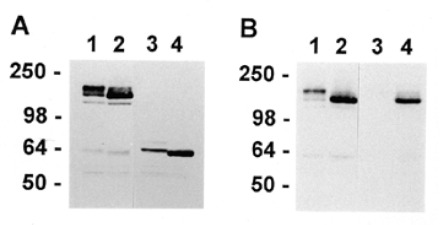
Effect of BFA on GM2 synthase/myc homodimer formation and cleavage to a soluble form. A. Clone C5 cells expressing GM2 synthase/myc were grown in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of 1 µg/ml BFA for 4.5 h and extracted with detergent. The extracts were fractionated by SDS-PAGE in the presence (lanes 3 and 4) or absence (lanes 1 and 2) of 5% β-mercaptoethanol (β-ME), Western blots stained with anti-myc 9E10, and bands visualized by enhanced chemiluminescence. B. Clone C5 cells were grown in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of 1 µg/ml of BFA and harvested, and membrane pellets (lanes 1 and 2) and supernatants (lanes 3 and 4) were prepared by freeze-thaw as described in Materials and methods. Aliquots were run under nonreducing conditions on SDS-PAGE gels followed by Western blotting with anti-myc.
Effects of BFA on the life cycle of GM2 synthase
We have shown that GM2 synthase forms a disulfide bonded homodimer in the ER (Zhu et al., 1997) which then moves to the Golgi and is proteolytically cleaved to produce a soluble catalytic domain that is released from the cell (Jaskiewicz et al., 1996a). Also, we have found that this soluble form is very inefficient at producing GM2 product in intact cells as compared to its membrane bound counterpart despite the fact that the soluble form retains activity in vitro (Zhu et al., 1998). In the following experiments we tested if BFA altered some aspect of this normal life cycle of GM2 synthase. Western blotting under non-reducing conditions with anti-myc demonstrated that GM2 synthase continued to be produced as homodimers in the presence of BFA (Figure 4A, lane 2) similar to GM2 synthase from cells grown in control medium (Figure 4A, lane 1). Under reducing conditions GM2 synthase grown in control medium produced a doublet which we have shown to consist of an upper band containing N-linked chains that are sialylated and endo-glycosidase H resistant and a lower band that is nonsialylated and endo-H sensitive (Figure 4A, lane 3; Jaskiewicz et al., 1996a). In the presence of BFA GM2 synthase consisted of a single band running at or slightly faster than the lower band of the control sample (Figure 4A, lane 4). A similar shift to faster migrating species was visible under nonreducing conditions as well (compare Figure 4A, lanes 1 and 2). The faster migration was the result of the inhibition of sialylation of N linked chains by BFA as shown previously (Chege and Pfeffer, 1990). Also, the greater intensity of the band in the presence of BFA was expected because we have shown elsewhere that retention of GM2 synthase in the ER led to an increase in the intracellular content because the rapid turnover due to proteolysis in the Golgi was prevented (Zhu et al., 1997).
As mentioned above, the lumenal domain of GM2 synthase that is released as a soluble form as a result of proteolytic cleavage possesses minimal GM2 synthesizing activity in intact cells (Zhu et al., 1998). Therefore, it was possible that in the ER-Golgi membrane system produced by BFA GM2 synthase might be cleaved to a soluble catalytic domain which would be non-functional in vivo. As a first step to testing this possibility, we looked at the recovery of the Golgi following washout of BFA by immunofluorescence. For both a-mannosidase II and GM2 synthase/myc, recovery of the Golgi staining pattern was partial at 30 min (data not shown) and virtually fully restored at 2 h (Figure 2, e and f, respectively). Thus, GM2 synthase/myc appeared to be behaving similarly to the prototypical Golgi marker a-mannosidase II, suggesting that GM2 synthase/myc might not be cleaved to a soluble form by BFA.
To obtain independent evidence for cleavage of GM2 synthase in the presence of BFA, we separated the soluble and membrane fractions of clone C5 cells lysed by repeated freeze-thaw cycles and analyzed their content of GM2 synthase/myc by Western blotting. In control medium the membrane fraction (Figure 4B, lane 1) contained the doublet of GM2 synthase/myc bands characteristic of C5 cells (compare Figure 4A, lane 1). The soluble fraction of the cell lysate from control medium contained at most a trace of soluble GM2 synthase/myc (Figure 4B, lane 3) as expected because the soluble enzyme would be secreted from the cells shortly after cleavage (Jaskiewicz et al., 1996a).
The membrane fraction of BFA-treated cells contained an increased amount of GM2 synthase/myc as compared to control (compare Figure 4B, lanes 2 and 1, respectively). As expected for material retained in the BFA-induced ER-Golgi system, the majority of this reactivity comigrated with the endo H sensitive, neuraminidase resistant lower band of control cells which we have shown is present in the ER (Jaskiewicz et al., 1996a). The soluble fraction from BFA-treated cells contained soluble GM2 synthase/myc which, like the corresponding membrane fraction, comigrated primarily with the lower band from control cells (Figure 4B, lane 4). However, the amount of soluble GM2 synthase/myc constituted only a small portion of the total GM2 synthase/myc, and therefore conversion of the enzyme to a soluble form could not account for the total inhibition of GM2 production in BFA treated cells. In conclusion although we could not discern the cause of the BFA-induced inhibition of GM2 synthesis, these results indicate that the effect must be the result of other factors in addition to the compartmental separation of GM2 synthase from its substrate ganglioside GM3.
Discussion
In the present study we have shown that BFA inhibited GM2 synthesis when GM2 synthase was distributed in all cisternae of the Golgi stacks as well as the TGN in CHO transfected clone C5 cells or when GM2 synthase was restricted to the medial Golgi in clone B5 (Figure 3). Therefore, our earlier speculation that such inhibition implied a distal location for this enzyme is no longer tenable. Clearly, it is well documented that BFA can lead to separation of early from late portions of the secretory pathway (Hunziker et al., 1992); therefore, in clone C5 and perhaps in cells expressing endogenous GM2 synthase that portion of the enzyme located in the TGN would be blocked by BFA from coming in contact with GM3. However, GM2 synthase located in the Golgi cisternae in clone C5 and clone B5 would not be expected to be separated by BFA from the site of GM3 synthesis; therefore, additional effects besides compartmental separation must contribute to the BFA-induced inhibition of GM2 synthesis in these cells.
BFA-induced redistribution of compartments has the potential to result in the fusion of compartments containing enzymes and substrates that do not normally contact each other in control medium; such appears to be the case for the BFA-induced production of a modest amount of GM2 in clone E6 cells in which GM2 synthase is restricted to the ER (Figure 3). However, the inefficiency of GM3 to GM2 conversion in those cells is similar to the trace of GM2 synthesis remaining in both clone C5 and B5 cells treated with BFA (Figure 3); this similarity in all three clones suggests some common feature(s) of the mixed ER-Golgi membrane system produced by BFA that prevents efficient GM2 synthesis. The more basic pH of the ER as compared to the Golgi is unlikely to be a factor in the BFA-induced inhibition of GM2 synthesis because the pH optimum of GM2 synthase of 7.4-7.9 (Iber et al., 1990; Hashimoto et al., 1993) is closer to the pH of the ER than that of the Golgi (Llopis et al., 1998). A direct effect on GM2 synthase itself also appears unlikely. Assay of GM2 synthase activity in extracts of BFA-treated cells indicated that the enzyme was neither degraded nor irreversibly inhibited by BFA treatment (Table I). The only effect of BFA on the life cycle of this enzyme that we could detect was the cleavage of a portion of membrane bound GM2 synthase to a soluble form which is inactive in intact cells (Figure 4). However, because only a minor percentage of the enzyme was cleaved, this conversion could not explain the nearly total inhibition of GM2 production in BFA treated cells.
BFA disrupts the Golgi secretory pathway by preventing the binding of the small GTP-binding protein ADP-ribosylation factor (ARF) and the coatomer coat proteins to Golgi membranes (Donaldson et al., 1992; Helms and Rothman, 1992). Uncoating of Golgi membranes is followed by tubulation and absorption of Golgi membranes into the ER (Sciaky et al., 1997). However, remnants of the Golgi membranes remain as permanent, distinct structures separated from the ER cisternae (De Lemos-Chiarandini et al., 1992; Hendricks et al., 1992; Hidalgo et al., 1992). Incomplete mixing of GM2 synthase in such remnants with ganglioside GM3 may contribute to the inefficient synthesis of GM2 observed in the presence of BFA. Alternatively, one function of ARF that is blocked by BFA is the activation of phospholipase D on Golgi membranes, resulting in the conversion of phosphatidylcholine to phosphatidic acid (Ktistakis et al., 1995). It is possible that this change in phospholipid metabolism within the Golgi could effect the biosynthesis of gangliosides, perhaps by altering enzyme interactions with its GSL substrates.
Our understanding of the details of GSL biosynthesis is incomplete. Clearly, the ceramide backbone is produced in the ER (Mandon et al., 1992; Rother et al., 1992) while glucosylceramide is synthesized on the cytoplasmic face of both early and late Golgi membranes (Coste et al., 1986; Futerman and Pagano, 1991; Jeckel et al., 1992; Schweizer et al., 1994a). Based on earlier subcellular fractionation data (Trinchera and Ghidoni, 1989; Trinchera et al., 1990), the next steps of synthesis of lactosylceramide and ganglioside GM3 were thought to occur in the early Golgi while GM2 synthase was proposed to be a component of the TGN, based in part on our speculation (Young et al., 1990) arising from BFA results (Van Echten et al., 1990; Young et al., 1990). A recent revision of this model based on subcellular fractionation of rat liver Golgi proposes that biosynthesis of lactosylceramide, GM3 and GD3 occurs in the trans Golgi while all further steps of ganglioside synthesis, including GM2 synthesis, occur in the TGN (Lannert et al., 1998). That revised model conflicted with our immunoelectron microscopic data which indicated that GM2 synthase was located in all Golgi cisternae as well as the TGN (Jaskiewicz et al., 1996b). However, Lannert et al. discounted our results by suggesting that they could have been artefactual due to either overexpression of transfected GM2 synthase or mislocalization due to the presence of the epitope tag. Our present results suggest that the inhibition of ganglioside synthesis by BFA is not informative about the location of GM2 synthase and that such BFA inhibition results can no longer be used to support the revised assembly line model proposed by Lannert et al (Lannert et al., 1998).
A second model for the synthesis of complex GSL is that the responsible enzymes are organized in functional complexes or hetero-oligomers. This possibility was speculated upon by Roseman as early as 1970 (Roseman, 1970). Initial data for the functional association of enzymes involved in glycoprotein synthesis came in 1981 from Ivatt who suggested that such complexes would provide a mechanism for the preferential use of substrates when enzymes of potentially competing synthetic pathways coexisted in the same Golgi cisterna(e) (Ivatt, 1981). Based on precursor-product relationships, Caputto's group proposed the existence of functional complexes of ganglioside specific enzymes in 1976 (Caputto et al., 1976). Similarly, Makita's group suggested that the GalNAc transferases required for synthesis of globoside and Forssman glycolipid existed in a functional complex (Kijimoto-Ochiai et al., 1980). Solid evidence for functional complexes was provided recently by the report that in yeast Golgi the enzymes involved in the mannosylation of glycoproteins exist in two distinct physical complexes which reflect functional associations (Jungmann and Munro, 1998). This landmark paper represents a solid precedent for the possibility that such complexes may exist in other organisms and in other glycosylation pathways. At present there is no firm experimental data supporting the existence of complexes of GSL synthetic enzymes. Nevertheless, it is possible that BFA could disrupt the organization of such complexes by indirect means.
Although the focus of GSL metabolism has been on de novo biosynthesis via the classical Golgi pathway described above, in fact in recent years it has become increasingly clear that recycling pathways are of considerable importance at least in certain cell types. In addition to de novo synthesis there are two other pathways for incorporation of sugar into GSL (Gillard et al., 1996, and references therein). First, catabolism of sphingomyelin and GSL in the lysosomes will produce sphingosine which can be reutilized in the ER to produce ceramide and in turn GSL in the Golgi. Second, intact or partially hydrolyzed GSL from the plasma membranes can recycle via the endosomes to the Golgi for resynthesis of more complex GSL. Tettamanti's group has shown that both of these pathways are effected by BFA in cerebellar granule cells (Riboni et al., 1994). At present, however, the relative importance of these recycling pathways to overall GSL synthesis in CHO cells remains to be determined. With regard to GSL recycling, it is of interest to note that in clone E6 cells (Figure 3) in which GM2 synthase is restricted to the ER GM2 was not produced in control medium. The fact that a modest amount of GM3 was converted to GM2 in the presence of BFA indicated that GM2 synthase was capable of producing GM2 when located in the ER. These findings suggest that in control medium the reaction did not occur probably due to lack of GM3 in the ER. Thus, the retrograde traffic of GM3 from the cell surface to earlier compartments of the secretory pathway (Riboni et al., 1997) does not appear to extend to the ER at least in CHO cells.
We conclude that the inhibition of ganglioside synthesis by BFA cannot be used to deduce the location of GM2 synthase. The mechanisms by which BFA inhibits GM2 synthase activity may be clarified by studies with a set of BFA-resistant mutant CHO cell lines (Yan et al., 1994). Finally, the present report should provide a note of caution about using the results of studies with BFA (Shite et al., 1990; Locker et al., 1992; Sampath et al., 1992; Sherwood and Holmes, 1992; Holmes and Greene, 1993; Uhlin-Hansen and Yanagishita, 1993; Farrer et al., 1995; Rosales Fritz and Maccioni, 1995) to draw conclusions about the locations of other Golgi enzymes. This is not to suggest, however, that the findings or conclusions of these other reports are incorrect. The findings reported here may be selective for glycosyltransferases involved in GSL synthesis or could even be unique to GM2 synthase.
Materials and methods
Chimeric constructs, cell culture, and transfection
GM2 synthase/myc, GNT/GM2 synthase/myc, and Iip33/GM2 synthase/myc constructs have been described elsewhere (Jaskiewicz et al., 1996b) and are illustrated in Figure 1. Cells were stably transfected, and clones were isolated and grown in a-minimal essential medium containing 10% (v/v) fetal calf serum plus glutamine and 0.4 mg/ml of active geneticin (G418; GIBCO, Grand Island, NY) at 37° as previously described (Jaskiewicz et al., 1996b).
Antibodies
Hybridoma cells producing monoclonal IgG1 anti-myc 9E10 (Evan et al., 1985) were obtained from the American Type Culture Collection. Rabbit serum against rat liver Golgi a-mannosidase II was obtained from Dr. Kelley Moremen (University of Georgia).
Immunofluorescence
Indirect immunofluorescence was performed as described previously (Zhu et al., 1997). Cells were grown on ethanol sterilized cover slips for 48 h, fixed for 15 min at room temperature in freshly prepared 2% p-formaldehyde (EM Science, Gibbstown, NJ) in PBS pH 7.4, rinsed in serum-free medium, and permeabilized at room temperature for 20 min in 0.1% Triton X-100 in PBS. Cells were incubated in succession, each step for 45 min at room temperature with intervening washing, with 1% BSA in PBS, primary antibody, and secondary antibody. Primary antibodies were the undiluted culture supernatant of anti-myc 9E10 (Evan et al., 1985) and rabbit anti-mannosidase II diluted 1:1000. Secondary antisera were FITC-goat anti-mouse immunoglobulin (Cappel, Durham, NC) and FITC-goat anti-rabbit immunoglobulin (Cappel). Coverslips were mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA), sealed with nail polish, and examined with an Optiphot fluorescence microscope (Nikon, Melville, NY).
GSL metabolic labeling and GSL analysis
Confluent cell monolayers were precultured in normal growth medium in the presence or absence of BFA (1 µg/ml) for 30 min at 37° and labeled in medium containing 25 µCi of [3H] palmitic acid per ml with or without BFA for 4 h at 37°. Cell monolayers were rinsed with ice-cold saline, harvested with a rubber policeman, and frozen at -80°. GSL were extracted, purified, and analyzed on high performance TLC plates (E. Merck, Darmstadt, Germany) in solvent A (CHCl3:MeOH: 0.25% KCl, 50:40:10, v/v) as described previously (Jaskiewicz et al., 1996b). Radiolabeled TLC bands were visualized by autoradiography with fluorographic enhancement (EN3HANCE spray; NEN, Boston, MA) and quantitated by scraping into scintillation vials and assaying in the presence of 10 ml of Budget-Solv (Research Products International Corp., Mt. Prospect, IL) scintillation fluid.
GM2 synthase enzyme assay
Confluent monolayers of CHO clone C5 cells were incubated in the presence or absence of BFA (1 µg/ml) for 4.5 h at 37°. After cell harvest, cell extracts were prepared in Zwittergent 3-14 lysis buffer as described previously (Jaskiewicz et al., 1996b). The in vitro assay for GM2 synthase activity was performed as described previously (Jaskiewicz et al., 1996b), except that where indicated the assay tubes contained 1 µg/ml BFA or the equivalent volume of MeOH as vehicle control. Protein concentration was determined by the BCA method (Smith et al., 1985).
Western blotting
Details have been described previously (Jaskiewicz et al., 1996b). Briefly, samples were separated by SDS-PAGE on 4% stacking, 10% separating gels, and proteins transferred to PVDF membranes (Millipore, Marlborough, MA). Blots were blocked and then incubated in succession with intervening washing with anti-myc 9E10 hybridoma cell culture medium diluted 1:10 and then with horseradish peroxidase-coupled sheep anti-mouse Ig at a dilution of 1:5000. After washing, bands were visualized using the LumiGLO detection system (KPL, Gaithersburg, MD).
Freeze thaw
To distinguish membrane bound from soluble GM2 synthase, cells were grown in the presence or absence of BFA (1 µg/ml) for 4.5 h. Cell monolayers were rinsed in ice cold PBS and harvested with a rubber policeman. The cell pellet was resuspended in PBS containing a protease inhibitor cocktail (Schweizer et al., 1994b), frozen in liquid nitrogen, and thawed at 37°. This freeze-thaw cycle was repeated for a total of three times after which the suspension was centrifuged at 200,000 × g for 5 min in an Airfuge (Beckman, Fullerton, CA). The pellet was resuspended in SDS-PAGE sample buffer, and aliquots of pellets and supernatants were tested by Western blotting with anti-myc.
Acknowledgments
This work was supported by NIH Grant GM42698.
References
- Caputto R., Maccioni H.J., Arce A., Cumar F.A. Biosynthesis of brain gangliosides. Adv. Exp. Med. Biol. 1976;71:27–44. doi: 10.1007/978-1-4614-4614-9_3. [DOI] [PubMed] [Google Scholar]
- Chege N.W., Pfeffer S.R. Compartmentation of the Golgi complex: Brefeldin A distinguishes trans-Golgi cisternae from the trans-Golgi network. J. Cell. Biol. 1990;111:893–899. doi: 10.1083/jcb.111.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste H., Martel M.B., Got R. Topology of glucosylceramide synthesis in Golgi membranes from porcine submaxillary glands. Biochim. Biophys. Acta. 1986;858:6–12. doi: 10.1016/0005-2736(86)90285-3. [DOI] [PubMed] [Google Scholar]
- De Lemos-Chiarandini C, Ivessa N.E., Black V.H., Tsao Y.S., Gumper I., Kreibich G. A Golgi-related structure remains after the Brefeldin A-induced formation of an ER-Golgi hybrid compartment. Eur. J. Cell. Biol. 1992;58:187–201. [PubMed] [Google Scholar]
- Donaldson J.G., Cassel D., Kahn A., Klausner R.D. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein beta-COP to Golgi membranes. Proc. Natl. Acad. Sci. USA. 1992;89:6408–6412. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W.G., Rothman J.E. Compartmentation of asparagine-linked oligosaccharide processing in the Golgi apparatus. J. Cell. Biol. 1983;97:270–275. doi: 10.1083/jcb.97.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W.G., Rothman J.E. Compartmental organization of the Golgi stack. Cell. 1985;42:13–21. doi: 10.1016/s0092-8674(85)80097-0. [DOI] [PubMed] [Google Scholar]
- Evan G.I., Lewis G.K., Ramsay G., Bishop J.M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer R.G., Warden M.P., Quarles R.H. Effects of Brefeldin A on galactosphingolipid synthesis in an immortalized Schwann cell line: evidence for different intracellular locations of galactosylceramide sulfotransferase and ceramide galactosyltransferase activities. J. Neurochem. 1995;65:1865–1873. doi: 10.1046/j.1471-4159.1995.65041865.x. [DOI] [PubMed] [Google Scholar]
- Futerman A.H., Pagano R.E. Determination of the intracellular sites and topology of glucosylceramide synthesis in rat liver. Biochem. J. 1991;280:295–302. doi: 10.1042/bj2800295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard B.K., Harrell R.G., Marcus D.M. Pathways of glycosphingolipid biosynthesis in SW13 cells in the presence and absence of vimentin intermediate filaments. Glycobiology. 1996;6:33–42. doi: 10.1093/glycob/6.1.33. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Sekine M., Iwasaki K., Suzuki A. Purification and characterization of UDP-N-acetylgalactosamine GM3/GD3 N-acetylgalactosaminyltransferase from mouse liver. J. Biol. Chem. 1993;268:25857–25864. [PubMed] [Google Scholar]
- Helms J.B., Rothman J.E. Inhibition by Brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature. 1992;360:352–354. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- Hendricks L.C., McClanahan S.L., McCaffery M., Palade G.E., Farqu-har M.G. Golgi proteins persist in the tubulovesicular remnants found in Brefeldin A-treated pancreatic acinar cells. Eur. J. Cell. Biol. 1992;58:202–213. [PubMed] [Google Scholar]
- Hidalgo J., Garcia-Navarro R., Gracia-Navarro F., Perez-Vilar J., Velasco A. Presence of Golgi remnant membranes in the cytoplasm of Brefeldin A-treated cells. Eur. J. Cell. Biol. 1992;58:214–227. [PubMed] [Google Scholar]
- Holmes E.H., Greene T.G. De novo synthesis of type 1 lacto-series glycolipids in human colonic adenocarcinoma cells: Efficient synthesis of the Lea antigen and absence of Brefeldin A-induced inhibition of its synthesis in Colo 205 cells. Arch. Biochem. Biophys. 1993;305:328–340. doi: 10.1006/abbi.1993.1430. [DOI] [PubMed] [Google Scholar]
- Hunziker W., Whitney J.A., Mellman I. Brefeldin A and the endocytic pathway: possible implications for membrane traffic and sorting. FEBS Lett. 1992;307:93–96. doi: 10.1016/0014-5793(92)80908-y. [DOI] [PubMed] [Google Scholar]
- Iber H, Van Echten G., Klein R.A., Sandhoff K. pH-dependent changes of ganglioside biosynthesis in neuronal cell culture. Eur. J. Cell. Biol. 1990;52:236–240. [PubMed] [Google Scholar]
- Ivatt R.J. Regulation of glycoprotein biosynthesis by formation of specific glycosyltransferase complexes. Proc. Natl. Acad. Sci. USA. 1981;78:4021–4025. doi: 10.1073/pnas.78.7.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivessa N.E., De Lemos-Chiarandini C., Tsao Y.S., Takatsuki A., Adesnik M., Sabatini D.D, Kreibich G. O-Glycosylation of intact and truncated ribophorins in Brefeldin A-treated cells: newly synthesized intact ribophorins are only transiently accessible to the relocated glycosyltransferases. J. Cell. Biol. 1992;117:949–958. doi: 10.1083/jcb.117.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskiewicz E., Zhu G., Bassi R., Darling D.S., Young W.W., Jr. β1.4 N-Acetylgalactosaminyltransferase (GM2 synthase) is released from Golgi membranes as a neuraminidase sensitive, disulfide-bonded dimer by a cathepsin D-like protease. J. Biol. Chem. 1996;271:26395–26403. [PubMed] [Google Scholar]
- Jaskiewicz E., Zhu G., Taatjes D.J., Darling D.S., Zwanzig G.E., Jr., Young W.W., Jr. Cloned β1,4 N-acetylgalactosaminyltransferase: subcellular localization and formation of disulfide bonded species. Glycoconjugate J. 1996;13:213–223. doi: 10.1007/BF00731496. [DOI] [PubMed] [Google Scholar]
- Jeckel D., Karrenbauer A., Burger K.N.J., Van Meer G., Wieland F. Glucosylceramide is synthesized at the cytosolic surface of various Golgi subfractions. J. Cell. Biol. 1992;117:259–267. doi: 10.1083/jcb.117.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann J., Munro S. Multi-protein complexes in the cis Golgi of Saccharomyces cerevisiae with a-1^6-mannosyltransferase activity. EMBO J. 1998;17:423–434. doi: 10.1093/emboj/17.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijimoto-Ochiai S., Yokosawa N., Makita A. Mechanism for the biosynthesis of Forssman glycolipid from trihexosylceramide. J. Biol. Chem. 1980;255:9037–9040. [PubMed] [Google Scholar]
- Kornfeld S., Kornfeld R. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Ktistakis N.T., Brown H.A., Sternweis P.C, Roth M.G. Phospholipase D is present on Golgi-enriched membranes and its activation by ADP ribosylation factor is sensitive to Brefeldin A. Proc. Natl Acad. Sci. USA. 1995;92:4952–4956. doi: 10.1073/pnas.92.11.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladinsky M.S., Howell K.E. The trans-Golgi network can be dissected structurally and functionally from the cisternae of the Golgi complex by Brefeldin A. Eur. J. Cell Biol. 1992;59:92–105. [PubMed] [Google Scholar]
- Lannert H., Gorgas K., Meissner I., Wieland F.T., Jeckel D. Functional organization of the Golgi apparatus in glycosphingolipid biosynthesis—lactosylceramide and subsequent glycosphingolipids are formed in the lumen of the late Golgi. J. Biol. Chem. 1998;273:2939–2946. doi: 10.1074/jbc.273.5.2939. [DOI] [PubMed] [Google Scholar]
- Llopis J., McCaffery J.M, Miyawaki A., Farquhar M.G., Tsien R.Y. Measurement of cytosolic, mitochondrial and Golgi pH in single living cells with green fluorescent proteins. Proc. Natl. Acad. Sci. USA. 1998;95:6803–6808. doi: 10.1073/pnas.95.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker J.K., Griffiths G., Horzinek M.C., Rottier P.J.M. O-Glycosylation of the coronavirus M protein. J. Biol. Chem. 1992;267:14094–14101. doi: 10.1016/S0021-9258(19)49683-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandon E.C., Ehses I., Rother J., Van Echten G., Sandhoff K. Subcellular localization and membrane topology of serine palmitoyltransferase, 3-dehydrosphinganine reductase and sphinganine N-acyltransferase in mouse liver. J. Biol. Chem. 1992;267:11144–11148. [PubMed] [Google Scholar]
- Miller S.G., Carnell L., Moore H.-P.H. Post-Golgi membrane traffic: Brefeldin A inhibits export from distal Golgi compartments to the cell surface but not recycling. J. Cell. Biol. 1992;118:267–283. doi: 10.1083/jcb.118.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y., Yamashiro S., Yodoi J., Lloyd K.O., Furukawa K. Expression cloning of beta 1,4 N-acetylgalactosaminyltransferase cDNAs that determine the expression of GM2 and GD2 gangliosides. J. Biol. Chem. 1992;267:12082–12089. [PubMed] [Google Scholar]
- Nilsson T., Pypaert M., Hoe M.H., Slusarewicz P., Berger E.G., Warren G. Overlapping distribution of two glycosyltransferases in the Golgi apparatus of HeLa cells. J. Cell. Biol. 1993;120:5–13. doi: 10.1083/jcb.120.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riboni L., Bassi R., Tettamanti G. Effect of Brefeldin A on ganglioside metabolism in cultured neurons: implications for the intracellular traffic of gangliosides. J. Biochem. (Tokyo) 1994;116:140–146. doi: 10.1093/oxfordjournals.jbchem.a124486. [DOI] [PubMed] [Google Scholar]
- Riboni L., Viani P., Bassi R., Prinetti A., Tettamanti G. The role of sphingolipids in the process of signal transduction. Prog. Lipid Res. 1997;36:153–195. doi: 10.1016/s0163-7827(97)00008-8. [DOI] [PubMed] [Google Scholar]
- Rosales Fritz V.M.R., Maccioni H.J.F. Effects of Brefeldin A on synthesis and intracellular transport of ganglioside GT3 by chick embryo retina cells. J. Neurochem. 1995;65:1859–1864. doi: 10.1046/j.1471-4159.1995.65041859.x. [DOI] [PubMed] [Google Scholar]
- Roseman S. The synthesis of complex carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem. Phys. Lipids. 1970;48:270–297. doi: 10.1016/0009-3084(70)90024-1. [DOI] [PubMed] [Google Scholar]
- Roth J. Subcellular organization of glycosylation in mammalian cells. Biochim. Biophys. Acta. 1987;906:405–436. doi: 10.1016/0304-4157(87)90018-9. [DOI] [PubMed] [Google Scholar]
- Roth J, Taatjes D.J., Weinstein J., Paulson J.C., Greenwell P., Watkins W.M. Differential subcompartmentation of terminal glycosylation in the Golgi apparatus of intestinal absorptive and goblet cells. J. Biol. Chem. 1986;261:14307–14312. [PubMed] [Google Scholar]
- Rother J., Van Echten G., Schwarzmann G., Sandhoff K. Biosynthesis of sphingolipids: dihydroceramide and not sphinganine is desaturated by cultured cells. Biochem. Biophys. Res. Commun. 1992;189:14–20. doi: 10.1016/0006-291x(92)91518-u. [DOI] [PubMed] [Google Scholar]
- Rottger S., White J., Wandall H., Olivo J.C, Stark A., Bennett E., Whitehouse C., Berger E., Clausen H., Nilsson T. Localization of three human polypeptide GalNAc-transferases in HeLa cells suggests initiation of O-linked glycosylation throughout the Golgi apparatus. J. Cell. Sci. 1998;111:45–60. doi: 10.1242/jcs.111.1.45. [DOI] [PubMed] [Google Scholar]
- Sampath D., Varki A., Freeze H.H. The spectrum of incomplete N-linked oligosaccharides synthesized by endothelial cells in the presence of Brefeldin A. J. Biol. Chem. 1992;267:4440–4455. [PubMed] [Google Scholar]
- Schweizer A., Clausen H., Van Meer G., Hauri H.-P. Localization of O-glycan initiation, sphingomyelin synthesis and glucosylceramide synthesis in Vero cells with respect to the endoplasmic reticulum-Golgi intermediate compartment. J. Biol. Chem. 1994;269:4035–4041. [PubMed] [Google Scholar]
- Schweizer A., Rohrer J., Hauri H.-P., Kornfeld S. Retention of p63 in an ER-Golgi intermediate compartment depends on the presence of all three of its domains and on its ability to form oligomers. J. Cell. Biol. 1994;126:25–39. doi: 10.1083/jcb.126.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciaky N., Presley J., Smith C., Zaal K.J.M., Cole N., Moreira J.E., Terasaki M., Siggia E., Lippincott-Schwartz J. Golgi tubule traffic and the effects of Brefeldin A visualized in living cells. J. Cell. Biol. 1997;139:1137–1155. doi: 10.1083/jcb.139.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood A.L, Holmes E.H. Brefeldin A induced inhibition of de novo globo- and neolacto-series glycolipid core chain biosynthesis in human cells. Evidence for an effect on β1.4galactosyltransferase activity. J. Biol. Chem. 1992;267:25328–25336. [PubMed] [Google Scholar]
- Shite S, Seguchi T, Mizoguchi H, Ono M, Kuwano M. Differential effects of Brefeldin A on sialylation of N- and O-linked oliggosaccharides in low density lipoprotein receptor and epidermal growth factor receptor. J. Biol. Chem. 1990;265:17385–17388. [PubMed] [Google Scholar]
- Smith P.K., Krohn R.I., Hermanson G.T., Mallic A.K., Gartner F.H., Provenzano M.D., Fujimoto E.K., Goeke N.M., Olson B.J., Klenk D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Trinchera M., Ghidoni R. Two glycosphingolipid sialyltransferases are localized in different sub-Golgi compartments in rat liver. J. Biol. Chem. 1989;264:15766–15769. [PubMed] [Google Scholar]
- Trinchera M., Pirovano B., Ghidoni R. Sub-Golgi distribution in rat liver of CMP-NeuAc GM3 and CMP-NeuAc:GT1b alpha2-8 sialyltransferases and comparison with the distribution of the other glycosyltransferase activities involved in ganglioside biosynthesis. J. Biol. Chem. 1990;265:18242–18247. [PubMed] [Google Scholar]
- Uhlin-Hansen L., Yanagishita M. Differential effect of Brefeldin A on the biosynthesis of heparan sulfate and chondroitin/dermatan sulfate proteoglycans in rat ovarian granulosa cells in culture. J. Biol. Chem. 1993;268:17370–17376. [PubMed] [Google Scholar]
- Van Echten G., Iber H., Stotz H., Takatsuki A, Sandhoff K. Uncoupling of ganglioside biosynthesis by Brefeldin A. Eur. J. Cell Biol. 1990;51:135–139. [PubMed] [Google Scholar]
- Yan J.-P., Colon M.E., Beebe L.A., Melançon P. Isolation and characterization of mutant CHO cell lines with compartment-specific resistance to Brefeldin A. J. Cell. Biol. 1994;126:65–75. doi: 10.1083/jcb.126.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W.W., Lutz M.S., Mills S.E., Lechler-Osborn S. Use of Brefeldin A to define sites of glycosphingolipid synthesis: GA2/GM2/GD2 synthase is trans to the Brefeldin A block. Proc. Natl. Acad. Sci. USA. 1990;87:6838–6842. doi: 10.1073/pnas.87.17.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W.W. Molecular approaches to studying the intracellular trafficking of glycosphingolipids. Adv. Lipid Res. 1993;26:161–179. [PubMed] [Google Scholar]
- Zhu G., Allende M.L., Jaskiewicz E., Qian R., Darling D.S., Worth C.A., Colley K.J., Young W.W., Jr. Two soluble glycosyltransferases glycosylate less efficiently in vivo than their membrane bound counterparts. Glycobiology. 1998;8:831–840. doi: 10.1093/glycob/8.8.831. [DOI] [PubMed] [Google Scholar]
- Zhu G., Jaskiewicz E., Bassi R., Darling D.S., Young W.W., Jr. β1,4 N-Acetylgalactosaminyltransferase (GM2/GD2/GA2 synthase) forms homodimers in the endoplasmic reticulum: a strategy to test for dimerization of Golgi membrane proteins. Glycobiology. 1997;7:987–996. doi: 10.1093/glycob/7.7.987. [DOI] [PubMed] [Google Scholar]