Abstract
Dengue virus infection in the central nervous system (CNS) of immunized mice results in a strong influx of CD8 T cells into the brain. Whereas the kinetics of the splenic antiviral response are conventional, i.e. expansion followed by a rapid drop in the frequency of specific CD8 T cells, dengue virus‐specific CD8 T cells are retained in the CNS at a high frequency. These CD8 T cells display a partially activated phenotype (CD69high, Ly‐6A/Ehigh, CD62Llow), characteristic for effector‐memory T cells. CD43 expression, visualized by staining with the 1B11 mAb, decreased in time, suggesting that these persisting CD8 T cells differentiated into memory cells. These data add to the growing evidence implicating the CNS as a non‐lymphoid tissue capable of supporting prolonged T cell survival/maintenance.
Keywords: effector‐memory CD8 T cell, lymphocyte retention, viral infection
Introduction
Most of our understanding of immunological memory comes from the analysis of memory cells in lymphoid tissues. However, it is becoming increasingly clear in mouse models that long‐lived memory T cells are also present in non‐lymphoid tissues (1–8). Several studies have indicated that non‐lymphoid memory cells exhibit an activated phenotype (1–3), leading to the proposition that these cells represent an in situ rapid response force. Consistent with these ideas, two subsets of human memory T cells have recently been described, central and effector‐memory cells, that differ in the expression of the chemokine receptor CCR7 and the lymphoid homing marker L‐selectin (CD62L) (8). Whereas central memory cells (CCR7high/CD62Lhigh) represent classical lymphoid memory cells, effector‐memory cells (CCR7low/CD62Llow) circulate outside the lymphoid organs and were found to maintain various effector functions (8). It should be noted though that phenotypic classification of CD8 T cells during chronic human infection may be more complex: memory CD8 T cells appear to accumulate at different points along the differentiation pathway in different infections (9). At least three different phenotypes can be distinguished based on CD27 and CD28 expression (9). From these studies it was concluded that human memory CD8 T cells would be better defined on the basis of their activation status (9). Thus, comparing phenotypes between human and murine infections can be problematic.
In the present study, we have found evidence for long‐lived effector‐memory T cells in the brains of dengue virus‐challenged mice. Previously, evidence for persisting antiviral CD8 T cells in the central nervous system (CNS) has come from experiments in which immunized mice were intranasally challenged with a neurotropic strain of influenza virus (1). In the brains of these mice, influenza‐specific CD8 T cells persisted for up to 320 days in a partially activated state (CD62Llow, CD25low, CD69high) (1). Additionally, primary infection of mice with a neurotropic strain of the coronavirus mouse hepatitis virus (MHV) also leads to prolonged presence of antiviral T cells in the CNS (10–12).
In our study, we have analyzed persisting CD8 T cells in the brains of immunized mice surviving dengue challenge. Intracranial challenge of naive mice with dengue virus induces lethal encephalitis (13,14), accompanied by a weak antiviral CD8 T cell response (15). Only low frequencies of dengue virus‐specific CD8 T cells can be detected in the spleens and in the brains of encephalitic mice (15). In contrast, immunized mice control the dengue virus challenge (13–15): immunization primes a cellular immune response that is effectively recruited in the CNS after intracranial challenge, similar to that described for influenza virus encephalitis (1). A detailed phenotypic analysis of these CNS‐resident T cells indicates that they should be categorized as effector‐memory T cells.
Methods
Cells and virus
The chimeric YF/DEN virus (15) was grown on SW‐13 cells, which were propagated in minimal essential medium supplemented with 10% FCS, 2 mM l‐glutamine, antibiotics and non‐essential amino acids. The vaccinia virus recombinant expressing pre‐membrane (prM) and envelope (E) from dengue‐2 virus (16) was provided by Dr Ching‐Juh Lai (National Institutes of Health, Bethesda, MD). A20 cells were propagated in RPMI supplemented with 10% FCS, 2 mM l‐glutamine and antibiotics.
Immunization and challenge of mice
Three‐week‐old BALB/c mice (Jackson Laboratory, Bar Harbor, ME) were immunized by s.c. injection of 5 × 105 p.f.u. of the chimeric YF/DEN virus. Results of control immunizations with PBS or with the parental yellow fever virus (YFV)‐17D strain have been described previously (15). Mice were challenged 2 weeks after immunization by intracranial injection of 1.5 × 104 p.f.u. (100 50% lethal doses) of mouse‐adapted, neurovirulent dengue‐2 virus (strain New Guinea C; kindly provided by Dr Kenneth Eckels, Walter Reed Army Institute for Research, Rockville, MD) (13,14).
Isolation of lymphocytes from mouse brain
Isolation of lymphocytes from the brains of YF/DEN immunized and dengue‐2 virus‐challenged mice was done as described previously (10,11,12,15). Briefly, mouse brains were homogenized using a Tenbroeck homogenizer and lymphocytes were isolated by centrifugation over a Percoll cushion.
Lymphocyte staining and flow cytometry
Lymphocytes from the CNS and splenocytes were stained using FITC‐labeled mAb against CD62L, CD69, CD25 and CD11a (LFA‐1) (clones MEL‐14, H1.2F3, 7D4 and 2D7 respectively), R‐phycoerythrin (PE)‐conjugated antibodies against CD43, Ly6A/E, CD122 and CD80 (B7.1) (clones 1B11, D7, TM‐b1 and 16‐10A1 respectively), and allophycocyanin (APC)‐labeled anti‐CD8 (clone 53‐6.7). Intracellular staining to detect IFN‐γ‐producing T cells was done as described (15). Briefly, A20 stimulator cells were infected with the prM‐E‐expressing vaccinia virus recombinant (15) at a m.o.i. = 1. Infected cells (2 × 105) were used at 8 h post‐infection, at which time they were incubated with 8 × 105 splenocytes or CNS lymphocytes, in the presence of Brefeldin A (PharMingen, San Diego, CA). As a control, cells were left unstimulated. After a 6‐h incubation, cells were surface stained with FITC‐labeled anti‐CD4 (clone GK1.5) and PE‐conjugated anti‐CD8 (clone 53‐6.7) antibodies. Follow ing fixation and permeabilization (Cytofix/Cytperm kit; PharMingen), intracellular staining was performed using an APC‐conjugated antibody against IFN‐γ (clone XMG1.2). Samples were acquired using a FACSCalibur flow cytometer and analyzed using CellQuest software (Becton Dickinson, San Jose, CA). All mAb were obtained from PharMingen.
Results
Antiviral CD8 T cells persist in the brains, but not in the spleens, of dengue virus‐infected mice
In our vaccination/challenge model, BALB/c mice were first immunized using a YFV/dengue chimeric virus (15). As described before, this recombinant virus expresses the prM and E genes from dengue‐2 virus in a YFV‐17D genetic background (15), and will therefore induce responses specific for the dengue virus prM‐E proteins. Based on the rules of prime‐boost immunization (17), it can be expected that the dengue‐specific T cell response after challenge will be predominantly directed against the two dengue proteins which were used for priming, i.e. prM and E. Thus, we have measured dengue virus prM‐ and E‐specific T cell responses in the spleen and in the CNS, both after immunization and after the challenge (15). To present the dengue virus prM and E proteins we used a recombinant vaccinia virus expressing prM and E (16) to infect syngeneic A20 cells (15). Responses were quantitated by intracellular IFN‐γ staining.
After immunization, dengue virus prM‐E‐specific CD8 T cell responses were readily detected in the spleen at a frequency of 0.5% of the total CD8 T cell population (day 14 post‐immunization, Fig. 1). We did observe some migration of CD8 T cells into the CNS, with 5–6% of these cells responding to dengue virus prM and E antigens (Fig. 1). However, it should be noted that the number of CD8+IFN‐γ+ events was very small and that the brains had not been perfused. Therefore, it cannot be rigorously excluded that we observed responses from peripheral blood. At 14 days post‐immunization, mice were challenged intracranially with a mouse‐adapted, neurovirulent strain of dengue‐2 virus (13). Whereas 13 out of 15 non‐immunized control mice succumbed to the ensuing dengue encephalitis, only one out of 12 immunized mice developed symptoms (15). In the spleens of immunized and challenged mice, we found that up to 4% of CD8 T cells were prM‐E specific at day 5 post‐challenge (Fig. 1) (15). In addition, we measured a strong influx of T cells into the CNS and 34% of CD8 T cells in the brain were prM‐E specific at day 5 post‐challenge (Fig. 1). Thus, as expected, virus‐specific T cells migrated to the site of infection, i.e. the CNS, during the acute response. Alternatively, it could be argued that a small population of CNS‐resident prM‐E‐specific CD8 T cells, primed by the immunization, expanded after the challenge. Clearly, prM‐E‐specific effector T cells, both in the spleen and in the CNS, displayed CD8 down‐regulation (Fig. 1 and Table 1).
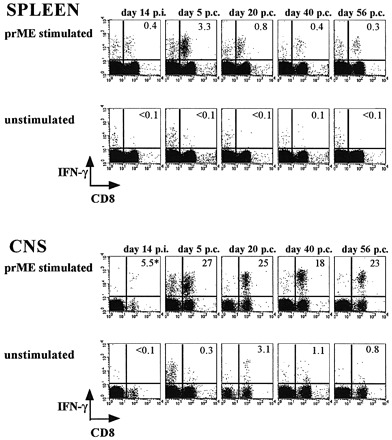
Fig. 1. Quantitation of prM‐E‐specific CD8 T cell responses in the spleen and in the CNS. Mice were challenged at 14 days post‐immunization. Splenic and CNS T cell responses were measured at 14 days post‐immunization, and 5, 20, 40 and 56 days post‐challenge. Cells were stimulated with recombinant vaccinia virus‐infected A20 stimulator cells (prME) or were left unstimulated, and were then stained with FITC‐conjugated anti‐CD4, PE‐conjugated anti‐CD8 and APC‐conjugated anti‐IFN‐γ mAb. Frequencies of IFN‐γ+ of CD8 cells are shown. Populations of CD8–IFN‐γ+ cells are CD4+ T cells (23). The asterisk indicates a very low number of events.
Table 1.
Expression levels of IFN‐γ and CD8, on the population of IFN‐γ+ and CD8+ dengue prM‐E specific cells, expressed as mean fluoresence intensity
| Tissue | Marker | Days post‐challenge | |||
| 5 | 20 | 40 | 56 | ||
| Spleen | IFN‐γ | 135 | 140 | 160 | 184 |
| CD8 | 36 | 28 | 48 | 53 | |
| CNS | IFN‐γ | 114 | 240 | 350 | 335 |
| CD8 | 52 | 73 | 66 | 130 | |
Values were determined using CellQuest software, based on the data shown in Fig. 1.
Strikingly, however, we still detected high frequencies of prM‐E‐specific CD8 T cells in the CNS at 20, 40 and 56 days after the dengue virus challenge: 18–28% of CD8 T cells residing in the brain were specific for the dengue prM‐E proteins at these timepoints (Fig. 1). In addition, the absolute number of CD8 T cells in the brain did not decrease. Phenotypically, however, these cells did change: CD8 expression and the level of IFN‐γ production increased from day 5 to 56 (Table 1). In contrast, the kinetics of the splenic T cell response were more conventional: at day 56 post‐challenge, only 0.4% of CD8 T cells were prM‐E specific (Fig. 1). This 10‐fold decrease in the frequency of specific T cells in the transition from the effector phase to memory (the ‘death phase’) corresponds well to observations in other (acute infection) models (17). From these findings, we concluded that virus‐specific memory CD8 T cells preferentially persisted in the CNS.
Expression of CD43: kinetics of the cytolytic phenotype
As a first phenotypic characterization, we analyzed the expression patterns of cell‐surface O‐glycans using the 1B11 mAb. This antibody recognizes the O‐glycans on an activation‐associated form of CD43 (19,20). Positive 1B11 staining identifies the population of cytotoxic effector T cells that display direct ex vivo cytolytic activity (cytotoxic T lymphocytes) (19,20). Memory cells, in contrast, are 1B11low (19,20). Figure 2 shows the CD43/1B11 expression levels on dengue virus prM‐E‐specific CD8 T cells in the brains and spleens, before and after immunization. As can be deduced from Fig. 2, the prM‐E specific T cells (i.e. IFN‐γ+ cells) in the spleen and in the CNS were all 1B11high at day 14 post‐immunization, indicating that these cells are effector cytotoxic T lymphocytes. Although these cells may not yet have acquired a full memory phenotype (18,19), we consistently found solid protection and recruitment of prM‐E‐specific CD8 T cells after challenge at 14 days post‐infection (14) (Fig. 1). At days 20 and 56 post‐challenge, we found that prM‐E‐specific CD8 T cells, in the spleen as well as in the CNS, expressed decreasing amounts of the 1B11 determinant (Fig. 2). This suggests that these cells were in the process of differentiating into memory cells (19,20). The kinetics of CD43 down‐regulation were somewhat slower in the CNS as compared to the spleen. Alternatively, the loss of CD43high CD8 T cells from the spleen at day 20 post‐challenge could reflect migration of activated cells into the CNS. Nevertheless, our data suggest that the majority of prM‐E‐specific CD8 T cells retained in the CNS at day 56 post‐challenge are non‐cytolytic, based on their 1B11 expression patterns (19).
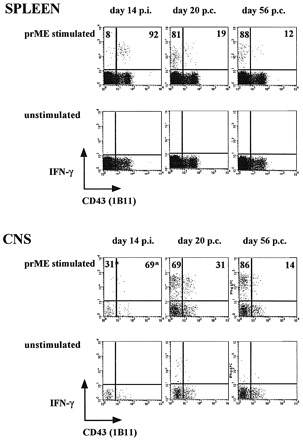
Fig. 2. CD43/1B11 expression levels on CD8 T cells in the spleen and in the CNS. Cells were stimulated with recombinant vaccinia virus‐infected A20 stimulator cells (prME) or were left unstimulated, and were then stained with FITC‐conjugated anti‐CD43 (1B11), PE‐conjugated anti‐CD8 and APC‐conjugated anti‐IFN‐γ. Cells were gated on CD8+. The background of CD8+1B11high cells was 4–5% of CD8 T cells. Frequencies of IFN‐γ+/1B11+ and IFN‐γ+/1B11– of CD8 cells are shown. The asterisk indicates a very low number of events.
Phenotypic characterization of CNS‐resident CD8 T cells
We then characterized the entire population of CNS‐resident T cells, by measuring the expression of several different activation makers. Analysis of this population (which includes the IFN‐γ+ prM‐E‐specific CD8 T cells, ∼20–25%, but also other cells), shown in Fig. 3, revealed that all CD8 T cells in the CNS expressed the 1B11 determinant at day 5 post‐infection (Fig. 3A) and were therefore most likely true cytotoxic T cells. Clearly, two 1B11high CD8 T cell populations can be distinguished, and, as shown in Fig. 4, these populations appear to differ in the expression level of CD69, and, therefore, possibly in their activation status. Reflecting the differentiation pattern of the IFN‐γ+ prM‐E‐specific CD8 T cells, CD43 expression levels were decreasing in the entire population of CNS‐resident CD8 T cells at day 56 post‐infection, although 1B11high CD8 T cells were still present. Thus, CNS‐resident CD8 T cells were heterogeneous in their 1B11 expression patterns. On the other hand, the expression patterns of several activation and homing markers (CD69, Ly6A/E and CD62L) suggested an activated phenotype. A large majority of CD8 T cells in the CNS had down‐regulated L‐selectin (CD62Llow), both at day 5 (85%) and day 56 (89%) post‐infection (Fig. 3B), consistent with their non‐lymphoid localization. In addition, the vast majority of the CD8 T cells in the brain were CD69high (89%) and Ly‐6A/Ehigh (80%) at day 56 post‐infection (Fig. 3C and D). CD69 and Ly‐6A/E were also up‐regulated in day 5 effector cells (Fig. 3C and D), although expression levels at day 5 were more heterogeneous than at day 56. The differences between the CD8 T cell populations at days 5 and 56 are most clearly illustrated by double‐staining for CD69 and 1B11/CD43: at day 56, more cells have up‐regulated CD69 expression and down‐regulated CD43/1B11 expression (Fig. 4). As a control, expression levels of CD43, CD62L, CD69 and Ly‐6A/E in day 5 and 56 spleen cells are shown in Fig. 3: these CD8 T cells are 1B11low, CD62Lhigh, CD69low and Ly‐6A/Elow. No changes in the expression levels of CD25 (IL‐2Rα), CD122 (IL‐2Rγ), B7.1 and CD11a (LFA‐1) were observed when comparing splenic and CNS‐derived lymphocytes at days 5 and 56 post‐challenge (data not shown). Expression levels of CD25, CD122 and B7.1 were uniformly low, and LFA‐1 expression was high on all CD8 T lymphocytes.
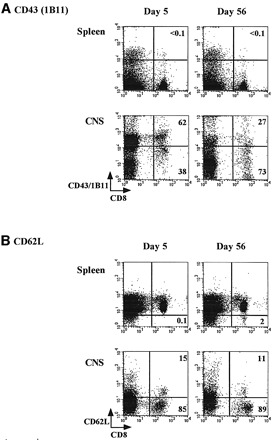
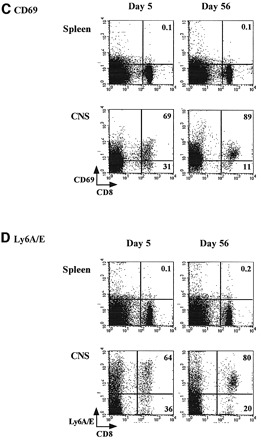
Fig. 3. Lymphocytes were harvested from the spleen and the CNS (21) at days 5 and 56 post‐challenge, and stained with APC‐conjugated anti‐CD8 mAb and combinations of FITC‐conjugated antibodies against CD62L and CD69 and PE‐conjugated antibodies against CD43 (1B11) and Ly6A/E. Expression patterns of (A) CD43, (B) CD62L (L‐selectin), (C) CD69 and (D) Ly6A/E are shown. Numbers shown represent the percentages of CD8 cells in each quadrant.
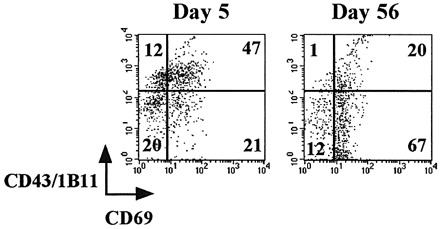
Fig. 4. Lymphocytes harvested from the CNS at days 5 and 56 were stained with APC‐conjugated anti‐CD8 mAb and FITC‐conjugated anti‐CD69 and PE‐conjugated anti‐CD43. Cells were gated on CD8 expression.
Discussion
In the present study, we have found that virus‐specific CD8 T cells persisted at the site of infection and that these cells displayed a unique phenotype, featuring traits from both memory (CD43/1B11low) and activated T cells (CD62Llow, CD69high, Ly6A/Ehigh). The CD43 expression pattern of the population of CNS‐resident CD8 T cells changes from (biphasic) CD43high at day 5 post‐challenge towards a more heterogeneous pattern at day 56 post‐challenge Thus, it appears that the majority of CD8 T cells are differentiating into memory cells, as judged by CD43 expression (19,20). Based on the strong correlation between CD43 (1B11) expression and direct ex vivo cytotoxicity (19), this would suggest that fewer CD8 cells would display direct ex vivo cytotoxicity at day 56. Clearly, dengue virus‐specific CD8 T cells expressed low levels of the 1B11/CD43 determinant at late timepoints. It should be noted, however, that cytolytic clearance may not play a major role in the CNS, especially in the case of infected neurons. Instead, it has been shown in several viral infection models that T cells use IFN‐γ to clear virus from the brain (21–23). In this respect, an activated phenotype may be more important than direct ex vivo cytotoxicity in the brain.
The expression patterns of CD69 and Ly6A/E were striking: expression levels of these activation markers were higher and more homogenous at day 56 post‐challenge than immediately after challenge. This same pattern (i.e. heterogeneous CD69 expression during acute encephalitis and uniform high expression late after infection) was seen in mice infected with a neurovirulent strains of influenza virus (1) or MHV (10). As pointed out by Bergmann et al., CD69 expression is a common hallmark of CD8 T cells retained in the CNS (10,11). The CNS‐resident CD8 T cells after dengue virus infection were CD69high and also CD25low, similar to antiviral CD8 T cells in the brains of MHV‐infected rats and mice (10,24). It has been suggested that the phenotype of these cells reflects a state of ‘anergy post‐activation’ (10,25). Alternatively, as discussed below, CD69 may play a role in the regulation of T cell trafficking (26) and persistent CD69 up‐regulation could be involved in T cell retention.
Our study raises three important questions. The first question relates to the specificity of the majority of CNS‐resident CD8 T cells that are not prM‐E specific. In our view, there are three explanations. (i) We may be observing cells with unknown specificities that migrated into the brain through bystander activation. (ii) The remaining CD8 T cells could recognize other dengue virus epitopes, outside the prM‐E proteins. It is possible that other epitopes, cross‐reactive in the YFV vaccine and the dengue challenge virus (e.g. from the conserved NS5 polymerase protein), are responsible for the expansion of T cells. (iii) There may be a population of prM‐E‐specific CD8 T cells that failed to produce IFN‐γ upon antigenic stimulation. Inactivated CD8 T cells have been detected previously in the context of antiviral responses (27–29). The second question is perhaps the most important issue raised by our experiments and by several earlier studies (1,10,11): why are virus‐specific CD8 T cells retained in the brain and what keeps them in a partially activated state? There has been some debate on the role of persisting antigen in retaining T cells in the brain. Hawke et al. observed persisting antiviral CD8 T cells in the absence of antigen after challenge of immunized mice with influenza virus (1). However, as proposed by these authors (1) and given the extreme sensitivity of CD8 T cells (30), it is possible that CD8 T cells are retained by persisting MHC class I–peptide complexes on neuronal cells that are below the threshold of detection. Marten et al., in contrast, reported that retention of both CD4 and CD8 T cells in the brains of MHV‐infected mice strictly coincided with the presence of viral RNA (11). These authors have provided two explanations for the observed differences (11). (i) T cells recruited during secondary influenza virus infection are memory cells, whereas primary cells are induced during MHV infection. (ii) Influenza virus infects neurons, which express low levels of MHC class I molecules (31,32), whereas MHV predominantly infects microglia and astrocytes, which express higher levels of both MHC class I and class II molecules (11). Clearly, our dengue virus infection model is more similar to secondary influenza virus infection: the intracranial dengue virus infection recruits memory T cells (induced by s.c. vaccination) and dengue virus has a neuronal tropism in mice (33). These differences should also be interpreted in the light of the complex phenotypic differences seen in human chronic infections (9): MHV does establish a persistent infection in mice (10,11), whereas influenza virus evidently does not (1). It is unclear whether dengue virus persists after the acute encephalitis and this will be a subject for further investigation. Importantly, however, the CD4 T cell response in the brain (the CD8– IFN‐γ+ cells in Fig. 1) (14) strongly decreased from day 5 to 56. This decreasing CD4 T cell response is inconsistent with persistence of antigen, as shown by Marten et al. (11) in the case of persisting T cell responses against MHV. Thus, in the absence of antigen, CD4 T cells efflux from the CNS (11,34), whereas CD8 T cells can persist (1). A third issue that needs further investigation is the homing potential of the CNS‐resident CD8 T cells [reviewed in (35)]. It is currently unclear whether these cells express homing markers specific for the brain or a set of markers that merely specifies non‐lymphoid localization, irrespective of the tissue. One intriguing possibility is that CD69 plays a role in the retention of T cells in tissues. Recent results using CD69 transgenic mice indicate that thymocytes that constitutively express CD69 are retained in the thymus (26). This may implicate CD69 in the regulation of T cell trafficking, as suggested by the authors (26). In addition, CD43 (i.e. the high mol. wt form detected by the 1B11 antibody) may play a role in CNS localization: antigen‐specific CD8 T cells do not migrate into the brains of CD43 knockout mice that are intracerebrally infected with lymphocytic choriomeningitis virus (20).
In conclusion, we hypothesize that the prM‐E‐specific CD8 T cells form a pool of local, activated memory cells that is independent and phenotypically different from the peripheral (lymphoid) memory T cell pool.
Acknowledgements
We thank Dr Kenneth H. Eckels for providing dengue‐2 virus, Dr Ching‐Juh Lai for the prME‐expressing vaccinia virus recombinant, Dr Jacob J. Schlesinger for helpful suggestions on the dengue challenge model and Madhavi Krishna for technical assistance. This work was supported by NIH grant RO1 AI49532 to R. A. and R. v. d. M.
Abbreviations
APC—allophycocyanin
CNS—central nervous system
E—envelope
MHV—mouse hepatitis virus
prM—pre‐membrane
PE—R‐phycoerythrin
YFV—yellow fever virus
References
- 1.Hawke, S., Stevenson, P. G., Freeman, S. and Bangham, C. R. 1998. Long‐term persistence of activated cytotoxic T lymphocytes after viral infection of the central nervous system. J. Exp. Med. 187:1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostler, T., Hussell, T., Suhr, C. D., Openshaw, P. and Ehl, S. 2001. Long‐term persistence and reactivation of T cell memory in the lung of mice infected with respiratory syncytial virus. Eur. J. Immunol. 31:2574. [DOI] [PubMed] [Google Scholar]
- 3.Hogan, R. J., Usherwood, E. J., Zhong, W., Roberts, A. A., Dutton, R. W., Harmsen, A. G. and Woodland, D. L. 2001. Activated antigen‐specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J. Immunol. 166:1813. [DOI] [PubMed] [Google Scholar]
- 4.Hogan, R. J., Zhong, W., Usherwood, E. J., Roberts, A. D., Cookenham, T. and Woodland, D. L. 2001. Protection from respiratory virus infections can be mediated by antigen‐specific CD4+ T cells that persist in the lungs. J. Exp. Med. 193:981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall, D. R., Turner, S. J., Belz, G. T., Wingo, S., Andreansky, S., Sangster, M. Y., Riberdy, J. M., Liu, T., Tan, M. and Doherty, P. C. 2001. Measuring the diaspora for virus‐specific CD8+ T cells. Proc. Natl Acad. Sci. USA 98:6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masopust, D., Vezys, V., Marzo, A. L. and Lefrancois, L. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413. [DOI] [PubMed] [Google Scholar]
- 7.Reinhardt, R. L., Khoruts, A., Merica, R., Zell, T. and Jenkins, M. K. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410:101. [DOI] [PubMed] [Google Scholar]
- 8.Sallusto, F., Lenig, D., Forster, R., Lipp, M. and Lanzavecchia, A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708. [DOI] [PubMed] [Google Scholar]
- 9.Appay V., Dunbar, P. R., Callan, M., Klenerman, P., Gillespie, G. M., Papagno, L., Ogg, G. S., King, A., Lechner, F., Spina, C. A., Little, S., Havlir, D. V., Richman, D. D., Gruener, N., Pape, G., Waters, A., Easterbrook, P., Salio, M., Cerundolo, V., McMichael, A. J. and Rowland‐Jones, S. L. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379. [DOI] [PubMed] [Google Scholar]
- 10.Bergmann, C. C., Altman, J. D., Hinton, D. and Stohlman, S. A. 1999. Inverted immuno‐dominance and impaired cytolytic function of CD8+ T cells during viral persistence in the CNS. J. Immunol. 163:3379. [PubMed] [Google Scholar]
- 11.Marten, N. W., Stohlman, S. A. and Bergmann, C. C. 2000. Role of viral persistence in retaining CD8+ T cells within the central nervous system. J. Virol. 74:7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marten, N. W., Stohlman, S. A., Atkinson, R. D., Hinton, D. R., Fleming, J. O. and Bergmann, C. C. 2000. Contributions of CD8+ T cells and viral spread to demyelinating disease. J. Immunol. 164:3905. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman, B. M., Summers, P. L., Dubois, D. R. and Eckels, K. H. 1987. Monoclonal antibodies against dengue‐2 virus E‐glycoprotein protect mice against lethal dengue infection. Am. J. Trop. Med. Hyg. 36:427. [DOI] [PubMed] [Google Scholar]
- 14.Schlesinger, J. J., Brandiss, M. W. and Walsh, E. E. 1987. Protection of mice against dengue‐2 encephalitis by immunization with the dengue‐2 virus non‐structural glycoprotein NS1. J. Gen. Virol. 68:853. [DOI] [PubMed] [Google Scholar]
- 15.Van der Most, R. G., Murali‐Krishna, K., Ahmed, R. and Strauss, J. H. 2000. Chimeric yellow fever/dengue virus as a candidate dengue vaccine: characterization of the dengue virus specific CD8 T cell response. J. Virol. 74:8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bray, M. and Lai, C. J. 1991. Dengue virus pre‐membrane and membrane proteins elicit a protective immune response. Virology 185:505. [DOI] [PubMed] [Google Scholar]
- 17.Schneider, J., Gilbert, S. C., Hannan, C. M., Degano, P., Prieur, E., Sheu, E. G., Plebanski, M. and Hill, A. V. 1999. Induction of CD8+ T cells using heterologous prime‐boost immunisation strategies. Immunol. Rev. 170:28. [DOI] [PubMed] [Google Scholar]
- 18.Murali‐Krishna, K. Altman, J. D., Suresh, M., Sourdive, D. J. D., Zajac, A. J., Miller, J. D., Slansky, J. and Ahmed, R. 1998. Counting antigen‐specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177. [DOI] [PubMed] [Google Scholar]
- 19.Harrington, L. E., Galvan, M., Baum, L. G., Altman, J. D. and Ahmed, R. 2000. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O‐glycans. J. Exp. Med. 191:1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onami, T. M., Harrington, L. E., Williams, M. A., Galvan, M., Larsen, C. P., Pearson, T. C., Manjunath, N., Baum, L. G., Pearce, B. D. and Ahmed, R. 2002. Dynamic regulation of T cell immunity by CD43. J. Immunol. 168:6022. [DOI] [PubMed] [Google Scholar]
- 21.Binder, G. K. and Griffin, D. E. 2001. Interferon‐γ‐mediated site‐specific clearance of alphavirus from CNS neurons. Science 293:303. [DOI] [PubMed] [Google Scholar]
- 22.Kundig, T. M., Hengartner, H. and Zinkernagel, R. M. 1993. T cell‐dependent IFN‐γ exerts an antiviral effect in the central nervous system but not in peripheral solid organs. J. Immunol. 150:2316. [PubMed] [Google Scholar]
- 23.Parra, B., Hinton, D. R., Marten, N. W., Bergmann, C. C., Lin, M. T., Yang, C. S. and Stohlman, S. A. 1999. IFN‐γ is required for viral clearance from central nervous system oligodendroglia. J. Immunol. 162:1641. [PubMed] [Google Scholar]
- 24.Hein, A., Schwender, S., Imrich, H., Sopper, S., Czub, M. and Dorries, R. 1995. Phenotypic and functional characterization of CD8+ T lymphocytes from the central nervous system of rats with coronavirus JHM induced demyelinating encephalomyelitis. J. Neurovirol. 1:340. [DOI] [PubMed] [Google Scholar]
- 25.Craston, R., Koh, M., McDermott, A., Ray, N., Prentice, H. G. and Lowdell, M. W. 1997. Temporal dynamics of CD69 expression on lymphoid cells. J. Immunol. Methods 209:37. [DOI] [PubMed] [Google Scholar]
- 26.Feng, C., Woodside, K. J., Vance, B. A., El‐Khoury, D., Canelles, M., Lee, J., Gress, R., Fowlkes, B. J., Shores, E. W. and Love, P. E. 2002. A potential role for CD69 in thymocyte emigration. Int. Immunol. 14:535. [DOI] [PubMed] [Google Scholar]
- 27.Chang, J. and Braciale, T. J. 2001. Respiratory syncytial virus infection suppresses lung CD8+ T‐cell effector activity and peripheral CD8+ T‐cell memory in the respiratory tract. Nat. Med. 8:54. [DOI] [PubMed] [Google Scholar]
- 28.Gruener, N. H., Lechner, F., Jung, M. C., Diepolder, H., Gerlach, T., Lauer, G., Walker, B., Sullivan, J., Phillips, R., Pape, G. R. and Klenerman, P. 2001. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 75:5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zajac, A. J., Blattman, J. N., Murali‐Krishna, K., Sourdive, D. J. D., Suresh, M., Altman, J. D. and Ahmed, R. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sykulev, Y., Joo, M., Vturina, I., Tsomides, T. and Eisen, H. 1996. Evidence that a single peptide–MHC complex on a target cell can elicit a CTL response. Immunity 4:565. [DOI] [PubMed] [Google Scholar]
- 31.Horwitz, M. S., Evans, C. F., Klier, F. G. and Oldstone, M. B. A. 1999. Detailed in vivo analysis of interferon‐γ induced major histocompatibility complex expression in the central nervous system: astrocytes fail to express major histocompatibility complex class I and II molecules. Lab. Invest. 79:235. [PubMed] [Google Scholar]
- 32.Massa, P. T., Ozato, K. and McFarlin, D. E. 1993. Cell type‐specific regulation of major histocompatibility complex (MHC) class I expression in astrocytes, oligodendrocytes, and neurons. Glia 8:201. [DOI] [PubMed] [Google Scholar]
- 33.Johnson, R. T. 1987. The pathogenesis of acute viral encephalitis and postinfectious encephalomyelitis. J. Infect. Dis. 155:359. [DOI] [PubMed] [Google Scholar]
- 34.Irani, D. N. and Griffin, D. E. 1996. Regulation of lymphocyte homing into the brain during viral encephalitis at various stages of infection. J. Immunol. 156:3850. [PubMed] [Google Scholar]
- 35.Irani, D. N. and Griffin, D. E. 2002. Regulation of T cell responses during central nervous system viral infection. Adv. Virus Res. 56:175. [DOI] [PMC free article] [PubMed] [Google Scholar]


