Abstract
Mesostigmatid mites are blood-sucking parasitic mites found in wild rodent populations. Periodically they can also become a problem for laboratory rodent colonies, particularly when building construction or renovations disturb colonies of commensal (building) rodents that had been acting as hosts. Mesostigmatid mites infest both rats and mice and, unlike the more common rodent fur mites (Myobia, Myocoptes, and Radfordia sp.), can survive for long periods in the environment and travel considerable distances in search of new hosts. They easily penetrate barrier caging systems, including individually ventilated cages, thus circumventing the usual precautions to protect rodents from infection. The two mites reported in laboratory rodent colonies, Ornithonyssus bacoti and Laelaps echidnina, also bite humans and have the potential to transmit zoonotic diseases. Once the mites gain access to a colony, eradication requires elimination of commensal rodent reservoirs in addition to insecticide treatment of both the laboratory rodents and the environment. In view of the undesirability of insecticide use in the animal facility, it is advisable to investigate the effectiveness of preventive treatments, such as environmental application of insect growth regulators or silica-based products. This article summarizes available information on mesostigmatid mites and their laboratory incursions, and provides suggestions for diagnosis, treatment, and control based on the author’s experience with several outbreaks at a large academic institution.
Keywords: rodent, Laelaps echidnina, mice, Ornithonyssus bacoti, parasite, rats, spiny rat mite, tropical rat mite
Introduction
The overall health status of laboratory rodent colonies has improved over the last 20 years such that organisms prevalent in the past—such as Sendai virus, mouse cytomegalovirus, mouse adenovirus 1 and 2, reovirus 3, polyomavirus, and pneumonia virus of mice—are increasingly rare in modern barrier facilities (Clifford 2007a,b; Jacoby 1998; Livingston 2003; Schoondermarkvan de Ven et al. 2006; Zenner and Regnault 2000). Pathogen-free rodents are readily available from vendors, and widespread use of barrier caging systems in concert with sterilized equipment, high-efficiency particulate air (HEPA)– filtered air cabinets, protective clothing, and disinfection help keep pathogens out (Brielmeier et al. 2006; Dillehay et al. 1990; Lipman et al. 1993; NRC 1991; Otto and Tolwani 2002; Stakutis 2003). Regular health surveillance (Livingston 2003) helps to detect excluded organisms, and should there be an outbreak, mites and pinworms are variously susceptible to treatment with parasiticides such as avermectins, pyrethroids, and benzimidazoles (Baumans et al. 1988; Bean-Knudsen et al. 1986; Bornstein et al. 2006; Coghlan et al. 1993; Flynn et al. 1989; Hill et al. 2005, 2006; Huerkamp et al. 2000, 2004, 2005; Klement et al. 1996; Pullium et al. 2005; Santora et al. 2002; Sueta et al. 2002). Also in the event of an outbreak, the rederivation of valuable genetic lines is possible through embryo transfer (Baker 1988; Reetz et al. 1988; Van Keuren and Saunders 2004), cesarean rederivation (Nagy et al. 2003), and neonatal transfer (Hickman and Thompson 2004; Huerkamp et al. 2005; Singletary et al. 2003; Truett et al. 2000; Watson et al. 2005).
But despite the technological and therapeutic tools available, it would be a mistake to become complacent. Mice carry pathogens that may remain undetected in barrier housing due to low prevalence and inefficient transmission to bedding sentinels (Besselsen et al. 2000, 2007; Compton et al. 2004b; Dillehay et al. 1990; Gaertner 2004; Smith et al. 2007; Thigpen et al. 1989; Whary et al. 2000). In addition, there is the threat of contamination from wild rodent populations, which harbor a wide variety of rodent pathogens (Becker et al. 2007; Easterbrook et al. 2007, 2008; Morita et al. 1996; Singleton et al. 1993, 2000; Smith et al. 1993; Van Vuuren et al. 1990), including some that may not be recognized because they are infrequently encountered in laboratory rodent facilities.
In this article I review published information on mesostigmatid mites, which have a wild rodent reservoir and cause sporadic problems in laboratory animal facilities, particularly during building renovations. I also provide additional comments and suggestions based on personal experience with several mesostigmatid mite outbreaks at a large academic institution.
Characteristics
Mesostigmatid mites are a large order of highly mobile mites, class Arachnida, subclass Acari, order Mesostigmata. Most are predators of other mites, but three genera are ectoparasites of mammals and birds: Liponyssoides, Laelaps (syn. Haemogamasus, Echinolaelaps), and Ornithonyssus (Baker 1999; Flynn 1973). Among laboratory rodents, they usually parasitize the Norwegian rat (Rattus norvegicus) and black rat (Rattus rattus), but they also readily infest mice.
Wild rodents are hosts to Liponyssoides sanguineus (house mouse mite), Laelaps echidnina (spiny rat mite), Laelaps pontiger (syn. Haemogamasus pontiger), and Ornithonyssus bacoti (tropical rat mite). Rodents may also be accidental hosts to O. sylviarum (northern fowl mite), O. bursa (tropical fowl mite), and Androlaelaps pontiger (poultry litter mite). O. bacoti (syn. Bdellonyssys bacoti, Liponyssys bacoti) (Flynn 1973) has the widest host range, parasitizing a variety of domestic and wild mammals and birds, and is the most commonly reported mesostigmatid mite both in laboratory rodent colonies (Chu and Couto 2005; Cole et al. 2005; Coons 2005; Fox 1982; Harris and Stockton 1960; Hill et al. 2005; Keefe et al. 1964; Kelaher et al. 2005; Ram et al. 1986) and in human rat mite dermatitis cases (Baumstark et al. 2007; Beck and Pfister 2004, 2006; Creel et al. 2003; Fishman 1988; Fox et al. 2004; Pizzi et al. 2004; Skirnisson 2001; Theis et al. 1981).
Mites are distinguishable from insects as the adults have eight legs and never have wings. All mesostigmatid mites are dorsoventrally flattened in the body (idiosome) and head (gnathostome), and have four pairs of jointed legs attached to the anterior half of the body. Both males and females have a single dorsal shield (thickened chitinous area); males usually have one ventral shield and females three: sternal, genital, and anal. Females are readily identifiable by the characteristic arrangement of their ventral shields together with the number and arrangement of setae (hair-like extensions) (Figure 1).
Figure 1.
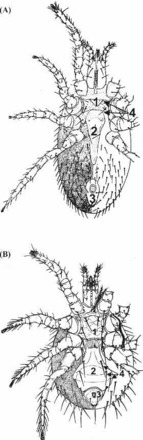
Ventral aspect of female Ornithonyssus bacoti (top) and Laelaps echidnina (bottom). The three chitinized shields—sternal (1), genital (2), and anal (3)—and setae (4) are often used for identification. Diagram reproduced, with modifications, from Baker EW, Evans TM, Gould DJ, Hull WB, Keegan HL. 1956. A Manual of Parasitic Mites of Medical and Economic Importance, with permission from the publisher, National Pest Management Association, formerly the National Pest Control Association, NY.
Laelaps spp. are easiest to distinguish because they are large (0.75 to 1 mm) and heavily sclerotized with sucker-like ambulacra (foot pads) on all tarsae (legs). The female L. echidnina has an unmistakable ventral shield-shaped genital plate with a rounded posterior concavity that perfectly matches the closely opposed anterior convex margin of the anal shield.
Liponyssoides sp. and Ornithonyssus sp. are smaller in the unengorged state (∼0.5 mm), although Ornithonyssus females can reach 1.0 mm when engorged. They are only weakly sclerotized and semitransparent, appearing white when unengorged and red-brown when engorged. Compared with Ornithonyssus, Liponyssoides has much sparser and shorter setae and its genital shield is broader and rounder than the narrow tapering genital shield of Ornithonyssus sp. (Baker et al. 1956; Baker 1999; Flynn 1973; Green and Baker 1996; Hirst 1913; Sudd 1952). O. bacoti is distinguishable from O. sylviarum and O. bursa because its dorsal shield setae are as long as or slightly longer than those on the surrounding cuticle (Baker 1998).
The mesostigmatid mite life cycle consists of an egg, six-legged larva, and two eight-legged nymph stages before adulthood. Only the first nymphal and adult stages feed on blood and serum (Laelaps sp. also feed on lacrimal secretions). Under laboratory conditions (65-75% relative humidity and 25°C) the life cycle is completed in as little as 6 to 8 days (Baker 1999; Flynn 1973).
Mesostigmatid Mites in the Animal Facility
Infestations in laboratory animal colonies most likely originate with wild rodents that transmit their mite populations to laboratory mice via commensal mice living in the building. Pigeon or chicken colonies may also harbor Ornithonyssus bursa or O. sylviarum that can make their way to laboratory rodents.
At our institution the first report of an infestation was from a laboratory that housed experimental mice for short periods and that showed evidence of commensal rodents. The experimental mice transferred the infection back to their housing facility and, despite apparently successful treatment of the affected mice and facility, four other housing facilities were subsequently infested over a period of 2 years (JW personal observation).
Building demolition and renovations or removal of commensal rodents are particularly high-risk periods and can result in new laboratory rodent mite infestations as the mites travel in search of new hosts. Renovations therefore require coordinated treatment to prevent infestation of adjacent rodent facilities when sites harboring commensal rodents are demolished. Treatment of the environment for mites should precede vermin eradication, which should in turn be coordinated with demolition.
Limitations of Cage Barrier Systems
Cage barrier systems (NRC 1991) are commonly used in research institutions as a means to exclude unwanted pathogens. Unlike barriers at the room or facility level that depend on limiting access to a few highly trained individuals, barriers at the cage level allow facility access by multiple personnel while still protecting the laboratory rodents. Whether the institution uses individually ventilated or static caging with filter tops, the effect is the same: barrier cages protect the rodents inside from commonly encountered rodent pathogens while they remain closed (Brielmeier et al. 2006; Compton et al. 2004a,b; Dillehay et al. 1990; Kraft 1958; Lipman et al. 1993; Otto and Tolwani 2002; Sedlacek and Mason 1977; Wescott et al. 1976), and the use of aseptic procedures in biosafety cabinets can prevent infection when the cages are opened.
In the case of mesostigmatid mites, however, the cage barrier system breaks down. These blood-sucking mites live in the environment adjacent to their rodent hosts and are freely mobile (Baker 1999; Flynn 1973). Indeed, we have observed them leaving occupied, closed cages on individually ventilated racks after cage treatments (JW personal observation). Disturbances to existing rodent hosts (e.g., during vermin eradication efforts or building renovations) may cause the mites to travel long distances in search of new hosts (Baker 1999; Flynn 1973). Once established, they colonize the environment adjacent to the rodent and, due to their small size (0.55 to 1.0 mm) (Baker 1999; Green and Baker 1996), may escape detection until they reach large numbers or bite personnel who handle the laboratory rodents or their caging.
Prevalence
Reports of mesostigmatid mite infestations in laboratory rodent facilities have generally been uncommon in the last 2 decades. Recently, however, several institutions have reported infestations with O. bacoti (tropical rat mite) and Laelaps echidnina (spiny rat mite) (Chu and Couto 2005; Cole et al. 2005; Coons 2005; Hill et al. 2005; Kelaher et al. 2005). Both O. bacoti and L. echidnina have recently been found on wild urban Rattus norvegicus captured in alleys in Baltimore, Maryland (Easterbrook et al. 2007, 2008), and both mites were also identified during repeated outbreaks of mesostigmatid mites in the rodent colonies in my institution between 2005 and 2007 (JW personal observation).
When laboratory rodents are infested, personnel who have contact with materials or equipment used in the animal facility or who handle the infested animals may be bitten (Kelaher et al. 2005).
Pathogenesis and Clinical Effects
There is considerable evidence that rat mites carry and have the potential to transmit a number of pathogens. Experiments have shown that Ornithonyssus bacoti transmits Rickettsia akari (rickettsialpox; Phillip 1948), Francisella pestis (plague; Yamada 1932), Coxsackie virus (Schwab et al. 1952), Francisella tularensis (tularemia; Hopla 1951), and Trypanosoma cruzi (Chagas disease; Cortez-Jimenez and Aguilar 1994). Researchers have also documented O. bacoti specimens with Coxiella burnetii (Q fever; Zemskaya 1968), hantavirus (Zhuge et al. 1998), Borrelia sp. (Lopatina Iu et al. 1999), Bartonella sp. (Kim et al. 2005), and Rickettsia sp. (Reeves et al. 2007). L. echidnina specimens have been found carrying Junin virus (Argentine hemorrhagic fever; Parodi et al. 1959), L. sanguineus is a vector of Rickettsia akari (human rickettsialpox; Saini et al. 2004), and Coxiella burnetii (Q fever) has been isolated from L. sanguineus specimens feeding on laboratory animals (Zemskaya 1968). In addition, O. bacoti is the intermediate host of the rodent filarial worm Litosomoides carinii (Bertram et al. 1946; Renz and Wenk 1981), and L. echidnina transmits Hepatozoon muris (Weinrich 1949).
Rat mites thus can transmit several zoonotic diseases, and both O. bacoti and L. echidnina readily bite humans, but the status of rat mites as vectors in naturally occurring human infections has not been proven (Baker 1998). There are, however, sporadic but persistent reports of cases of rat bite dermatitis caused by O. bacoti from rodent-infested buildings and yards or from pet rodents (Baumstark et al. 2007; Beck and Pfister 2004, 2006; Creel et al. 2003; Fishman 1988; Fox et al. 2004; Pizzi et al. 2004; Skirnisson 2001; Theis et al. 1981).
Severe rat mite infestations cause debility and anemia in rodents (French 1987; Harris and Stockton 1960; Keefe et al. 1964; Olsen 1946), but in my laboratory we have observed no clinical effects in lightly infested cages (JW personal observation).
Detection, Eradication, and Prevention
Detection
The first sign of a mesostigmatid mite infestation is usually a complaint of bites among personnel who work with the laboratory rodents. Follow-up investigation reveals the presence of mites as tiny moving black dots on the animal or in the cage, most easily visible against a pale background, such as a cage filter; indeed, where cages have filter tops, their close examination proves to be a good way to detect an infestation.
In our institution, sticky insect traps were inefficient as a diagnostic tool; instead, in each of our outbreaks the diagnosis of mites came from animal handlers who had either been bitten or had observed the mites on themselves or on cage tops. Sticky traps placed on or adjacent to the racks remained negative even after confirmation of the presence of mites in the cages (presumably because the mites did not venture far from their rodent hosts). The traps proved more useful for determining the extent of an outbreak after the fact: trapping mites from previously undiagnosed positive cages after administering mite-repelling permethrin cage treatments (JW personal observation).
Eradication
Permanent elimination of mesostigmatid mite infestations requires a coordinated and sustained effort to eliminate both mites and rodent vermin reservoirs. Mites have been reported to survive 2 weeks to several months without feeding (Baker 1998), necessitating repeat treatments and/or residual environmental treatments to prevent reinfestation.
In our experience, although individual facility treatments were successful, further outbreaks subsequently occurred in other rodent facilities. These were likely due to the transfer of infested rodents before discovery of the outbreak, undiscovered commensal rodent reservoirs, or mites that escaped during early treatments before we learned to sequence our environmental and cage treatments (JW personal observation).
Cage Treatments
The use of insecticides is contraindicated in animal facilities, but the zoonotic potential of mesostigmatid mites requires extraordinary measures. Recent reports of successful eradication from laboratory vivaria describe the use of permethrin-impregnated cotton balls (MITEARREST®, Eco-Health, Inc., Boston, MA) in the mouse cages for 5 to 8 weeks together with environmental treatment with various sustained-release pyrethrin (Chu and Couto 2005; Cole et al. 2005; Hill et al. 2005) or dichlorvos (Coons 2005) products. We used MITEARREST for 8 weeks in combination with sustained-release deltamethrin 0.06% (Suspend®SC, Bayer Environmental Science, Montvale, NJ) fan-sprayed on floors and baseboards monthly for three treatments, timed to precede cage sanitation (JW personal observation).
Permethrin is often chosen for use in laboratory animal facilities because of its exceptionally high (>1000) selectivity ratio (mammalian oral LD50/insect topical LD50) (Valentine 1990), poor skin absorption, and rapid inactivation in the body; the skin LD50 for rats is >4000 mg/kg, and the oral LD50 for rats is 430 to 4000 mg/kg and for mice, 540 to 2700 mg/kg (WHO 2006). Permethrin is a more stable synthetic analog of pyrethrum, which was originally derived from chrysanthemum cinerariaefolium flowers; it acts as a neurotoxin, prolonging sodium channel activation and causing repetitive firing of peripheral nerves and eventual paralysis in insects (Narahashi 1982). It is also an insect repellent (Mencke 2006) and is available impregnated into clothing for that purpose (Faulde et al. 2003).
Environmental Treatments
Any treatment plan should start with environmental treatments. As mentioned above, we have observed mites exiting ventilated rack cages after permethrin was placed inside to treat infested mice (JW personal observation), so treatment of cages without first treating the environment can result in the establishment of escaped mites on rodents outside the treated area.
Unlike animal treatments, environmental treatments that can result in personnel exposure usually require application by a licensed pest control professional. Operatives should work inward from the periphery of the risk area toward the center to prevent mites escaping to untreated areas. To avoid reinfestation, they should treat all areas occupied by rodents or rodent equipment, including support areas, investigator laboratories, and procedure areas. In addition, treatments should address potential locations for colonies of escaped rodents, such as inside poorly sealed animal facility doors, biosafety cabinets, and ventilation ducts.
Prevention
Prevention, particularly in laboratory housing areas or during high-risk periods such as building renovation, is unquestionably preferable to treatment. Given the undesirability of insecticides, alternatives include physical methods such as silica-based products and biological methods such as insect growth regulators.
Insect growth regulators fall into two categories, nonsteroidal ecdysteroid agonists that mimic the action of molting hormones and cause accelerated and incomplete molting, and juvenile hormone chemical analogs that inhibit maturation (Dhadialla et al. 1998). Juvenile hormone analogs are registered with the Environmental Protection Agency (EPA) as biopesticides and approved for indoor use (they are also effective against the German cockroach) (Atkinson et al. 1992). The EPA reports no evidence of toxicity in nontarget species (EPA 2001), but it is advisable to avoid exposure of Drosophila melanogaster colonies and mice or cell lines that use ecdysone-inducible gene expression systems (No et al. 1996).
There is no published information on the use of insect growth regulators (IGRs) to prevent mesostigmatid mite infestations in animal facilities. We chose to add them to our pest control program for a number of reasons, not least of which was our previous inability to prevent further infestations in distant areas despite apparently successful cage and environment insecticide treatments. Product literature suggested IGRs would have no impact on research and were effective against many insect and mite species, and applications could take place during regularly scheduled visits, which made them relatively inexpensive. We selected baseboard applications every 4 months of (S)-hydroprene (Gentrol® IGR Concentrate, Wellmark International, Bensenville, IL), a juvenile hormone analog, because it was already approved for animal facility use against cockroaches. In the 12 months since we added it to our pest control program, we have remained free of rat mites and have observed no untoward effects (JW personal observation).
Environmental application of silica aerogel has been effective for controlling rat mite infestations in homes (Ebeling 1971) and, as it is not likely to have a research impact, could also be a candidate for environmental control of insect and mobile mite pests in laboratory animal facilities.
Conclusion
Mesostigmatid mites are present on wild rodents and thus may migrate to commensal rodents. Laboratory rodent colonies are at increased risk of infestation if they are exposed to infested commensal rodents (e.g., in laboratories with inadequate pest control programs) or if the latter are disturbed (e.g., during renovations or aggressive vermin control efforts). Once an infestation is diagnosed in laboratory rodents, eradication requires removal of commensal rodent reservoirs and widespread treatment of both the environment and the mice. Pyrethrins appear to be safe and effective for both cage and environmental use. Failure to eliminate the commensal rodent reservoir or to prevent mite migration during treatments may result in repeated infestations or in new infestations at sites distant from the original problem. Noninsecticidal preventive methods, such as environmental application of insect growth regulators or silica-based sprays, may be appropriate under high-risk conditions such as during building renovations or ongoing mite infestations.
References
- Atkinson TH, Koehler PG, Patterson RS. 1992. Volatile effects of insect growth regulators against the German cockroach (Dictyoptera: Blattellidae). J Med Entomol 29:364–367. [DOI] [PubMed] [Google Scholar]
- Baker AS. 1999. Mites and Ticks of Domestic Animals, 1st ed London: The Stationery Office. [Google Scholar]
- Baker DG. 1998. Natural pathogens of laboratory mice, rats, and rabbits and their effects on research. Clin Microbiol Rev 11:231–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker EW, Evans TM, Gould DJ, Hull WB, Keegan HL. 1956. A Manual of Parasitic Mites of Medical or Economic Importance. New York: National Pest Control Association. [Google Scholar]
- Baker HJ. 1988. Rederivation of inbred strains of mice by means of embryo transfer. Lab Anim Sci 38:661–662. [PubMed] [Google Scholar]
- Baumans V, Havenaar R, van Herck H, Rooymans TP. 1988. The effectiveness of Ivomec and Neguvon in the control of murine mites. Lab Anim 22:243–245. [DOI] [PubMed] [Google Scholar]
- Baumstark J, Beck W, Hofmann H. 2007. Outbreak of tropical rat mite (Ornithonyssus bacoti) dermatitis in a home for disabled persons. Dermatology 215:66–68. [DOI] [PubMed] [Google Scholar]
- Bean-Knudsen DE, Wagner JE, Hall RD. 1986. Evaluation of the control of Myobia musculi infestations on laboratory mice with permethrin. Lab Anim Sci 36:268–270. [PubMed] [Google Scholar]
- Beck W, Pfister K. 2004. [Occurrence of a house-infesting tropical rat mite (Ornithonyssus bacoti) on murides and human beings in Munich: 3 case reports]. Wien Klin Wochenschr 116 Suppl 4:65–68. [PubMed] [Google Scholar]
- Beck W, Pfister K. 2006. [Mites as a cause of zoonoses in human beings]. Wien Klin Wochenschr 118:27–32. [DOI] [PubMed] [Google Scholar]
- Becker SD, Bennett M, Stewart JP, Hurst JL. 2007. Serological survey of virus infection among wild house mice (Mus domesticus) in the UK. Lab Anim 41:229–238. [DOI] [PubMed] [Google Scholar]
- Bertram DS, Unsworth K, Gordon RM. 1946. The biology and maintenance of Liponyssus bacoti Hirst 1913, and an investigation into its role as a vector of Litosomoides carinii to cotton rats and white rats, together with some observations on the white rats. Ann Trop Med 34: 124–131. [DOI] [PubMed] [Google Scholar]
- Besselsen DG, Becker MD, Henderson KS, Wagner AM, Banu LA, Shek WR. 2007. Temporal transmission studies of mouse parvovirus 1 in BALB/c and C.B-17/Icr-Prkdc(SCID) mice. Comp Med 57:66–73. [PubMed] [Google Scholar]
- Besselsen DG, Wagner AM, Loganbill JK. 2000. Effect of mouse strain and age on detection of mouse parvovirus 1 by use of serologic testing and polymerase chain reaction analysis. Comp Med 50:498–502. [PubMed] [Google Scholar]
- Bornstein DA, Scola J, Rath A, Warren HB. 2006. Multimodal approach to treatment for control of fur mites. JAALAS 45:29–32. [PubMed] [Google Scholar]
- Brielmeier M, Mahabir E, Needham JR, Lengger C, Wilhelm P, Schmidt J. 2006. Microbiological monitoring of laboratory mice and biocontainment in individually ventilated cages: A field study. Lab Anim 40:247–260. [DOI] [PubMed] [Google Scholar]
- Chu DK, Couto MA. 2005. Arthropod infestation in a colony of mice. Lab Anim (NY) 34:25–27. [DOI] [PubMed] [Google Scholar]
- Clifford C. 2007. Diseases of Mice. In: 50th Pathology of Laboratory Animals (POLA), Bethesda, MD. [Google Scholar]
- Clifford C. 2007. Diseases of Rats. In: 50th Pathology of Laboratory Animals (POLA), Bethesda, MD. [Google Scholar]
- Coghlan LG, Lee DR, Psencik B, Weiss D. 1993. Practical and effective eradication of pinworms (Syphacia muris) in rats by use of fenbendazole. Lab Anim Sci 43:481–487. [PubMed] [Google Scholar]
- Cole JS, Sabol-Jones M, Karolewski B, Byford T. 2005. Ornithonyssus bacoti infestation and elimination from a mouse colony. Contemp Top Lab Anim 44:27–30. [PubMed] [Google Scholar]
- Compton SR, Homberger FR, MacArthur Clark J. 2004. Microbiological monitoring in individually ventilated cage systems. Lab Anim (NY) 33:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton SR, Homberger FR, Paturzo FX, Clark JM. 2004. Efficacy of three microbiological monitoring methods in a ventilated cage rack. Comp Med 54:382–392. [PubMed] [Google Scholar]
- Coons O. 2005. P35 Infestation of spiny rat mites in vivarium. Abstract. Contemp Top Lab Anim 44:79. [Google Scholar]
- Cortez Jimenez M, Aguilar RA. 1994. Transmisión experimental de Trypanosoma cruzi por Ornithonyssys bacoti. Veterinaria, Mexico 25:61–63. [Google Scholar]
- Creel NB, Crowe MA, Mullen GR. 2003. Pet hamsters as a source of rat mite dermatitis. Cutis 71:457–461. [PubMed] [Google Scholar]
- Dhadialla TS, Carlson GR, Le DP. 1998. New insecticides with ecdysteroidal and juvenile hormone activity. Annu Rev Entomol 43:545–569. [DOI] [PubMed] [Google Scholar]
- Dillehay DL, Lehner ND, Huerkamp MJ. 1990. The effectiveness of a microisolator cage system and sentinel mice for controlling and detecting MHV and Sendai virus infections. Lab Anim Sci 40:367–370. [PubMed] [Google Scholar]
- Easterbrook JD, Kaplan JB, Vanasco NB, Reeves WK, Purcell H, Kosoy MY, Glass GE, Watson J, Klein SL. 2007. A survey of zoonotic pathogens carried by Norway rats in Baltimore, Maryland, USA. Epidemiol Infect:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterbrook J, Kaplan JB, Glass GE, Watson J, Klein SL. 2008. A survey of rodent-borne pathogens carried by wild-caught Norway rats: A potential threat to laboratory rodent colonies. Lab Anim 42:92–98. [DOI] [PubMed] [Google Scholar]
- Ebeling W. 1971. Sorptive dusts for pest control. Annu Rev Entomol 16:123–158. [DOI] [PubMed] [Google Scholar]
- EPA [US Environmental Protection Agency]. 2001. Insect Growth Regulators: S-Hydroprene (128966), S-Kinoprene (107502), Methoprene (105401), S-Methoprene (105402) Fact Sheet. Available online at http://www.epa.gov/pesticides/biopesticides/ingredients/factsheets/factsheet_igr.htm(accessed 1/10/08).
- Faulde MK, Uedelhoven WM, Robbins RG. 2003. Contact toxicity and residual activity of different permethrin-based fabric impregnation methods for Aedes aegypti (Diptera: Culicidae), Ixodes ricinus (Acari: Ixodidae), and Lepisma saccharina (Thysanura: Lepismatidae). J Med Entomol 40:935–941. [DOI] [PubMed] [Google Scholar]
- Fishman HC. 1988. Rat mite dermatitis. Cutis 42:414–416. [PubMed] [Google Scholar]
- Flynn BM, Brown PA, Eckstein JM, Strong D. 1989. Treatment of Syphacia obvelata in mice using ivermectin. Lab Anim Sci 39:461–463. [PubMed] [Google Scholar]
- Flynn RJ. 1973. Parasites of Laboratory Animals. Ames: Iowa State University Press. [Google Scholar]
- Fox JG. 1982. Outbreak of tropical rat mite dermatitis in laboratory personnel. Arch Dermatol 118:676–678. [DOI] [PubMed] [Google Scholar]
- Fox MT, Baker AS, Farquhar R, Eve E. 2004. First record of Ornithonyssus bacoti from a domestic pet in the United Kingdom. Vet Rec 154: 437–438. [DOI] [PubMed] [Google Scholar]
- French AW. 1987. Elimination of Ornithonyssus bacoti in a colony of aging mice. Lab Anim Sci 37:670–672. [PubMed] [Google Scholar]
- Gaertner DJ. 2004. Speculations on why some lab rodent pathogens continue to be prevalent. Contemp Top Lab Anim 43:8. [PubMed] [Google Scholar]
- Green ED, Baker C. 1996. Observations on the micromorphology of the tropical rat mite Ornithonyssus bacoti (Hirst) as revealed by scanning electron microscopy. J S Afr Vet Assoc 67:128–132. [PubMed] [Google Scholar]
- Harris JM, Stockton JJ. 1960. Eradication of the tropical rat mite Ornithonyssus bacoti (Hirst, 1913) from a colony of mice. Am J Vet Res 21:316–318. [PubMed] [Google Scholar]
- Hickman DL, Thompson KJ. 2004. Multi-phase approach to eradicate enzootic mouse coronavirus infection. Contemp Top Lab Anim 43:22–28. [PubMed] [Google Scholar]
- Hill WA, Randolph MM, Boyd KL, Mandrell TD. 2005. Use of permethrin eradicated the tropical rat mite (Ornithonyssus bacoti) from a colony of mutagenized and transgenic mice. Contemp Top Lab Anim 44:31–34. [PubMed] [Google Scholar]
- Hill WA, Randolph MM, Lokey SJ, Hayes E, Boyd KL, Mandrell D. 2006. Efficacy and safety of topical selamectin to eradicate pinworm (Syphacia spp.) infections in rats (Rattus norvegicus) and mice (Mus musculus). JAALAS 45:23–26. [PubMed] [Google Scholar]
- Hirst S. 1913. Leiognathus bacoti. Bull Ent Res 4:122. [Google Scholar]
- Hopla CE. 1951. Experimental transmission of tularemia by the tropical rat mite. Am J Trop Med Hyg 31:768–783. [DOI] [PubMed] [Google Scholar]
- Huerkamp MJ, Benjamin KA, Zitzow LA, Pullium JK, Lloyd JA, Thompson WD, Webb SK, Lehner ND. 2000. Fenbendazole treatment without environmental decontamination eradicates Syphacia muris from all rats in a large, complex research institution. Contemp Top Lab Anim 39: 9–12. [PubMed] [Google Scholar]
- Huerkamp MJ, Benjamin KA, Webb SK, Pullium JK. 2004. Long-term results of dietary fenbendazole to eradicate Syphacia muris from rat colonies. Contemp Top Lab Anim 43:35–36. [PubMed] [Google Scholar]
- Huerkamp MJ, Zitzow LA, Webb S, Pullium JK. 2005. Cross-fostering in combination with ivermectin therapy: A method to eradicate murine fur mites. Contemp Top Lab Anim 44:12–16. [PubMed] [Google Scholar]
- Jacoby RO, Lindsey JR. 1998. Risks of infection among laboratory rats and mice at major biomedical research institutions. ILAR J 39:266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe TJ, Scanlon JE, Wetherald LD. 1964. Ornithonyssus bacoti (Hirst) infestation in mouse and hamster colonies. Lab Anim Care 14:366–369. [PubMed] [Google Scholar]
- Kelaher J, Jogi R, Katta R. 2005. An outbreak of rat mite dermatitis in an animal research facility. Cutis 75:282–286. [PubMed] [Google Scholar]
- Kim CM, Kim JY, Yi YH, Lee MJ, Cho MR, Shah H, Klein TA, Kim HC, Song JW, Chong ST, O’Guinn ML, Lee JS, Lee IY, Park JH, Chae JS. 2005. Detection of Bartonella species from ticks, mites and small mammals in Korea. J Vet Sci 6:327–334. [PubMed] [Google Scholar]
- Klement P, Augustine JM, Delaney KH, Klement G, Weitz JI. 1996. An oral ivermectin regimen that eradicates pinworms (Syphacia spp.) in laboratory rats and mice. Lab Anim Sci 46:286–290. [PubMed] [Google Scholar]
- Kraft LM. 1958. Observations on the control and natural history of epidemic diarrhea of infant mice (EDIM). Yale J Biol Med 31:121–137. [PMC free article] [PubMed] [Google Scholar]
- Lipman NS, Corning BF, Saifuddin M. 1993. Evaluation of isolator caging systems for protection of mice against challenge with mouse hepatitis virus. Lab Anim 27:134–140. [DOI] [PubMed] [Google Scholar]
- Livingston R. 2003. Diagnostic testing of mouse and rat colonies for infectious agents. Lab Anim (NY) 32:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatina Iu V, Vasil’eva IS, Gutova VP, Ershova AS, Burakova OV, Naumov RL, Petrova AD. 1999. [An experimental study of the capacity of the rat mite Ornithonyssus bacoti (Hirst, 1913) to ingest, maintain and transmit Borrelia]. Med Parazitol (Mosk)2:26–30. [PubMed] [Google Scholar]
- Mencke N. 2006. Acaricidal and repellent properties of permethrin: Its role in reducing transmission of vector-borne pathogens. Parassitologia 48: 139–140. [PubMed] [Google Scholar]
- Morita C, Tsuchiya K, Ueno H, Muramatsu Y, Kojimahara A, Suzuki H, Miyashita N, Moriwaki K, Jin ML, Wu XL, Wang FS. 1996. Seroepidemiological survey of lymphocytic choriomeningitis virus in wild house mice in China with particular reference to their subspecies. Microbiol Immunol 40:313–315. [DOI] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K, Behringer R. 2003. Manipulating the Mouse Embryo: A Laboratory Manual, 3rd ed Cold Spring Harbor NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Narahashi T. 1982. Modification of nerve membrane sodium channels by the insecticide pyrethroids. Comp Biochem Physiol C 72:411–414. [DOI] [PubMed] [Google Scholar]
- No D, Yao TP, Evans RM. 1996. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc Natl Acad SciUSA 93:3346–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC [National Research Council]. 1991. Infectious Diseases of Mice and Rats. Washington: National Academy Press. [Google Scholar]
- Olsen TA, Dahms RG. 1946. Observations on the tropical rat mite, Liponyssus bacoti, as an ectoparasite of laboratory animals and suggestions for its control. J Parasitol 32:56–60. [PubMed] [Google Scholar]
- Otto G, Tolwani RJ. 2002. Use of microisolator caging in a risk-based mouse import and quarantine program: A retrospective study. Contemp Top Lab Anim 41:20–27. [PubMed] [Google Scholar]
- Parodi AS, Rugiero HR, Greenway DJ, Mettler N, Martinez A, Boxaca M, De La Barrera JM. 1959. [Isolation of the Junin virus (epidemic hemorrhagic fever) from the mites of the epidemic area (Echinolaelaps echidninus, Barlese)]. Prensa Med Argent 46:2242–2244. [PubMed] [Google Scholar]
- Phillip CB, Hughes LE. 1948. The tropical rat mite, Liponyssus bacoti,as an experimental vector of rickettsialpox. Am J Trop Med 28:697–705. [DOI] [PubMed] [Google Scholar]
- Pizzi R, Meredith A, Thoday KL, Walker A. 2004. Ornithonyssus bacoti infestation on pets in the UK. Vet Rec 154:576. [PubMed] [Google Scholar]
- Pullium JK, Brooks WJ, Langley AD, Huerkamp MJ. 2005. A single dose of topical moxidectin as an effective treatment for murine acariasis due to Myocoptes musculinus. Contemp Top Lab Anim 44:26–28. [PubMed] [Google Scholar]
- Ram SM, Satija KC, Kaushik RK. 1986. Ornithonyssus bacoti infestation in laboratory personnel and veterinary students. Int J Zoonoses 13:138–140. [PubMed] [Google Scholar]
- Reetz IC, Wullenweber-Schmidt M, Kraft V, Hedrich HJ. 1988. Rederivation of inbred strains of mice by means of embryo transfer. Lab Anim Sci 38:696–701. [PubMed] [Google Scholar]
- Reeves WK, Loftis AD, Szumlas DE, Abbassy MM, Helmy IM, Hanafi HA, Dasch GA. 2007. Rickettsial pathogens in the tropical rat mite Ornithonyssus bacoti (Acari: Macronyssidae) from Egyptian rats (Rattus spp.). Exp Appl Acarol 41:101–107. [DOI] [PubMed] [Google Scholar]
- Renz A, Wenk P. 1981. Intracellular development of the cotton-rat filaria Litomosoides carinii in the vector mite Ornithonyssus bacoti. T Roy Soc Trop Med H 75:166–168. [DOI] [PubMed] [Google Scholar]
- Saini R, Pui JC, Burgin S. 2004. Rickettsialpox: Report of three cases and a review. J Am Acad Dermatol 51:S137–S142. [DOI] [PubMed] [Google Scholar]
- Santora KA, Zakson-Aiken M, Rasa C, Shoop W. 2002. Development of a mouse model to determine the systemic activity of potential flea-control compounds. Vet Parasitol 104:257–264. [DOI] [PubMed] [Google Scholar]
- Schoondermark-van de Ven EM, Philipse-Bergmann IM, van der Logt JT. 2006. Prevalence of naturally occurring viral infections, Mycoplasma pulmonis and Clostridium piliforme in laboratory rodents in Western Europe screened from 2000 to 2003. Lab Anim 40:137–143. [DOI] [PubMed] [Google Scholar]
- Schwab M, Allen R, Sulkin SE. 1952. The tropical rat mite (Liponyssus bacoti) as an experimental vector of Coxsackie virus. Am J Trop Med Hyg 1:982–986. [DOI] [PubMed] [Google Scholar]
- Sedlacek RS, Mason KA. 1977. A simple and inexpensive method for maintaining a defined flora mouse colony. Lab Anim Sci 27:667–670. [PubMed] [Google Scholar]
- Singletary KB, Kloster CA, Baker DG. 2003. Optimal age at fostering for derivation of Helicobacter hepaticus-free mice. Comp Med 53:259–264. [PubMed] [Google Scholar]
- Singleton GR, Smith AL, Shellam GR, Fitzgerald N, Muller WJ. 1993. Prevalence of viral antibodies and helminths in field populations of house mice (Mus domesticus) in southeastern Australia. Epidemiol Infect 110:399–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton GR, Smith AL, Krebs CJ. 2000. The prevalence of viral antibodies during a large population fluctuation of house mice in Australia. Epidemiol Infect 125:719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirnisson K. 2001. [The tropical rat mite Ornithonyssus bacoti attacks humans in Iceland]. Laeknabladid 87:991–993. [PubMed] [Google Scholar]
- Smith AL, Singleton GR, Hansen GM, Shellam G. 1993. A serologic survey for viruses and Mycoplasma pulmonis among wild house mice (Mus domesticus) in southeastern Australia. J Wildl Dis 29:219–229. [DOI] [PubMed] [Google Scholar]
- Smith PC, Nucifora M, Reuter JD, Compton SR. 2007. Reliability of soiled bedding transfer for detection of mouse parvovirus and mouse hepatitis virus. Comp Med 57:90–96. [PubMed] [Google Scholar]
- Stakutis RE. 2003. Cage rack ventilation options for laboratory animal facilities. Lab Anim (NY) 32:47–52. [DOI] [PubMed] [Google Scholar]
- Sudd JH. 1952. Laboratory studies of adult female Bdellonyssus bacoti (Hirst, 1916) (Acarina, parasitiformes). Ann Trop Med Parasit 46:158–164. [DOI] [PubMed] [Google Scholar]
- Sueta T, Miyoshi I, Okamura T, Kasai N. 2002. Experimental eradication of pinworms (Syphacia obvelata and Aspiculuris tetraptera) from mice colonies using ivermectin. Exp Anim 51:367–373. [DOI] [PubMed] [Google Scholar]
- Theis J, Lavoipierre MM, LaPerriere R, Kroese H. 1981. Tropical rat mite dermatitis: Report of six cases and review of mite infestations. Arch Dermatol 117:341–343. [PubMed] [Google Scholar]
- Thigpen JE, Lebetkin EH, Dawes ML, Amyx HL, Caviness GF, Sawyer BA, Blackmore DE. 1989. The use of dirty bedding for detection of murine pathogens in sentinel mice. Lab Anim Sci 39:324–327. [PubMed] [Google Scholar]
- Truett GE, Walker JA, Baker DG. 2000. Eradication of infection with Helicobacter spp. by use of neonatal transfer. Comp Med 50:444–451. [PubMed] [Google Scholar]
- Valentine WM. 1990. Toxicology of selected pesticides, drugs, and chemicals: Pyrethrin and pyrethroid insecticides. Vet Clin N Am–Small 20: 375–382. [DOI] [PubMed] [Google Scholar]
- Van Keuren ML, Saunders TL. 2004. Rederivation of transgenic and gene-targeted mice by embryo transfer. Transgenic Res 13:363–371. [DOI] [PubMed] [Google Scholar]
- Van Vuuren M, De Klerk WA, De Beer MC, Van Niekerk TA. 1990. Survey for antibodies to selected viruses in laboratory mice in South Africa. J S Afr Vet Assoc 61:174–175. [PubMed] [Google Scholar]
- Watson J, Thompson KN, Feldman SH. 2005. Successful rederivation of contaminated immunocompetent mice using neonatal transfer with iodine immersion. Comp Med 55:465–469. [PubMed] [Google Scholar]
- Weinrich D. 1949. Protozoan parasites of the rat. In: Farris EJ, Griffith JQ. eds. The Rat in Laboratory Investigation, 2nd ed Philadelphia: JP Lippincott; p 486–501. [Google Scholar]
- Wescott RB, Malczewski A, Van Hoosier GL. 1976. The influence of filter top caging on the transmission of pinworm infections in mice. Lab Anim Sci 26:742–745. [PubMed] [Google Scholar]
- Whary MT, Cline JH, King AE, Hewes KM, Chojnacky D, Salvarrey A, Fox JG. 2000. Monitoring sentinel mice for Helicobacter hepaticus, H rodentium, and H bilis infection by use of polymerase chain reaction analysis and serologic testing. Comp Med 50:436–443. [PubMed] [Google Scholar]
- WHO [World Health Organization]. 2006. Datasheet on pesticides no. 51: Permethrin. Available on the WHO website under “Inventory of IPCS and other WHO pesticide evaluations and summary of toxicological evaluations performed by the Joint Meeting on Pesticide Residues (JMPR),” at http://www.who.int/ipcs/publications/jmpr/pesticide_inventory.pdf(accessed 1/10/08).
- Yamada S. 1932. Observations of a house-infesting mite (Liponyssus nagayoi, n.sp.) which attacks human beings, rats, and other domestic mammals, with brief notes of experiments regarding the possibility of the plague transmission by means of this mite. In: Far East Assoc Trop Med, Trans 8th Congr, vol 2 p 358–372. [Google Scholar]
- Zemskaya AA, Pchelkina AA. 1968. Infection of some species of Gamasidae with R. burneti in natural Q fever foci. Zh Mikrob Epid Immun 45:130–132. [PubMed] [Google Scholar]
- Zenner L, Regnault JP. 2000. Ten-year long monitoring of laboratory mouse and rat colonies in French facilities: A retrospective study. Lab Anim 34:76–83. [DOI] [PubMed] [Google Scholar]
- Zhuge H, Meng Y, Wu J, Zhu Z, Liang W, Yao P. 1998. [Studies on the experimental transmission of Rattus-borne Hantavirus by Ornithonyssus bacoti]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 16:445–448. [PubMed] [Google Scholar]


