Abstract
The reproductive biology of wild Canis species is often described as unique among mammals because an unusual combination of behavioral and physiological characteristics including a seasonally monestrous cycle, copulatory lock or tie, obligatory pseudopregnancy, social monogamy, and biparental care of the young. We investigated social behavior, endocrine profiles, and vaginal cytology of female coyotes (Canis latrans) during 4 breeding seasons, 2000–2003. Blood levels of estradiol, progesterone, prolactin, and relaxin were measured, and mating behavior and changes in vaginal epithelium were documented. After aligning the data from each individual to her estimated day of ovulation, we compared pregnant coyotes with nonpregnant females and evaluated temporal relationships among hormone levels, behavior, and vaginal cytology. We found that patterns of proceptive and receptive behaviors correlated with the secretion of steroid hormones, as did vaginal epithelial cytomorphosis. In addition, although progesterone levels of pregnant and pseudopregnant coyotes were indistinguishable, prolactin demonstrated a discernible intergroup difference and relaxin was only detectable in pregnant females. Although this study included characteristics not previously published for this species, it also showed how key aspects of reproduction were correlated temporally, and emphasized the importance of an integrated perspective when addressing the reproductive biology of coyotes, or other wild species of canids.
Keywords: Canis latrans, coyote, mating behavior, ovarian cycle, pseudopregnancy, reproductive hormones, vaginal cytology, wild canid
Coyotes (Canis latrans) are medium-sized wild canids indigenous to North America. They are seasonally monestrus (Gier 1968; Hamlett 1938; Kennelly and Johns 1976; Stellflug et al. 1981), socially monogamous, and territorial (Andelt 1985; Bekoff and Wells 1986; Bromley and Gese 2001; Camenzind 1978; Gese 2001). Once bonded, a coyote pair remains together for an indefinite number of years, sharing responsibility for territory maintenance. Litters averaging 3–7 pups are typically born March–May in most North American latitudes after a gestation of 60–63 days (Gier 1968; Hamlett 1938; Kennelly et al. 1977; Knowlton 1972) and both parents participate in the care and rearing of young (Andelt 1985; Camenzind 1978; Gier 1968; Hatier 1995; Mengel 1971; Silver and Silver 1969).
Mature offspring may disperse or remain within their natal territories, assisting in the defense of resources and infant pups, but typically only the dominant male and female breed (Andelt 1985; Bekoff and Wells 1986; Gese 2001; Gese et al. 1989, 1996). Juvenile coyotes < 12 months of age can be reproduc-tively active in their 1st winter, but available evidence suggests that juvenile and yearling (12–24 months) females are less fecund than adult females ≥ 2 years of age (Gier 1968; Green et al. 2002; Hamlett 1938; Kennelly and Johns 1976; Sacks 2005; Windberg 1995). Older females ≥ 10 years of age gradually pass into reproductive senescence (Green et al. 2002; Sacks 2005), whereas a male coyote was reported to have sired pups when ≥12 years of age (Gese 1990). Older coyotes may continue to maintain territory residency or revert to a transient lifestyle (Gese 1990; Windberg 1995).
The reproductive tracts of adult coyotes experience extensive remodeling during the breeding season, and histological evidence suggests a female coyote is incapable of serial ovulations, even if she is not impregnated during her 1st estrus (Gier 1968; Hamlett 1938; Kennelly and Johns 1976). Ovulation is spontaneous, synchronous, and bilateral. The subsequent corpora lutea crowd other ovarian tissue to such a degree that the existence of additional tertiary follicles appears improbable. Furthermore, ovarian retrogression is protracted, the corpora lutea taking >9 months to degenerate, thereby inhibiting a new wave of follicular recruitment (Hamlett 1938).
Hypertrophy of the uterus and vagina are also remarkable with gross morphological differences between sexually mature and immature females (Gier 1968; Kennelly and Johns 1976). Juvenile females may experience up-regulation of reproductive hormones (specifically estradiol) and concomitant physical signs such as vulvar edema and a serosanguineous vaginal discharge. However, they may not ovulate (Hodges 1990; Stellflug et al. 1981), with follicular development arresting before, or at, the tertiary stage (Kennelly and Johns 1976). Alternatively, subordinate females may ovulate, but proestrus and estrus in these individuals appears delayed relative to the estrous phases of dominant female pack-mates, and typically subordinate females fail to breed (Hodges 1990).
Among wild species, not dependent on human intervention, successful reproduction relies on a progression of key elements (Asa and Valdespino 1998; Kleiman and Eisenberg 1973). Mate acquisition, conception, gestation, and parental care rely upon effective synchronization of physiological processes, anatomical modifications, and social behaviors. During 4 consecutive breeding seasons (2000–2003) we measured the levels of estradiol, progesterone, prolactin, and relaxin in coyote sera and plasma. Concurrently, samples of exfoliated vaginal epithelium were collected in proestrus, estrus, and diestrus, and examined microscopically; observations of mating behavior also were documented. The data were then aligned to each individual female's estimated day of ovulation. Females housed with their mates (and that became pregnant) were compared to a nonpregnant cohort (sequestered females). Herein, we describe our observations and findings, comparing pregnant coyotes with pseudopregnant females, but also examine associations among behavior, endocrine patterns, and vaginal cytology. Examination of our data suggests that important relationships exist between these factors, and integrated examinations of complex systems can increase our understanding to an extent that might not be accrued from simple experimental constructions.
Materials and Methods
Animals.—Coyotes were captive born or wild caught as pups, and reared at the National Wildlife Research Center facility in Millville, Utah (41°68′N, 111°82′W). All animals were housed in outdoor enclosures with natural lighting. Adult (≥2 years of age) coyotes were randomly assigned an unrelated mate before winter, and whenever possible, an established pair remained together for several years. Mated pairs resided in individual 0.1-ha pens with access to sheltered den boxes. Three pens formed a clover-shaped cluster separated by double fencing and concrete barriers. Although physically separated, all pairs were within visual and audible range of other coyotes. As required, females were sequestered from their mates during the breeding season and served as nonpregnant controls. In these cases, the coyotes were housed individually in sheltered outdoor kennels with attached den boxes for privacy, and pair-mates were kept near each other in adjacent kennels during their separation.
Mean (± SD) ages for female and male coyotes in this study were 4.7 ± 2.0 and 5.6 ± 3.2 years, respectively; and females weighed an average 11.0 ± 1.3 kg (weights of males were not collected). The coyotes were fed a commercially prepared carnivore diet (Fur Breeders Agricultural Cooperative, Sandy, Utah) once daily, and fasted 1 day per week. Water was provided ad libitum. Vaccinations were given annually against canine distemper, hepatitis, leptospirosis, parvovirus, para-influenza, type 2 coronavirus, adenovirus, and rabies. Routine parasite control was administered as indicated. Research protocols were approved by the Institutional Animal Care and Use Committees at Utah State University and National Wildlife Research Center, and were in compliance with guidelines of the American Society of Mammalogists (Gannon et al. 2007).
Study design.—During 2000–2003 breeding seasons (January–March), mating behavior of 32 pairs of coyotes was observed and recorded. In 2000–2002, serial blood and vaginal cytology samples were collected from a subset of 18 females, in 2 cohort groups—8 sequestered during estrus and 10 that remained with their mates.
Females were considered “pregnant” in years when they resided with their mates, and were observed with live pups after parturition, or tested positive for relaxin. Alternatively, sequestered females were considered “nonpregnant.” Hormone profiles of 2 coyotes were excluded when they experienced idiopathic spontaneous abortions in mid- and late-term gestation. In the 1st case, 2 expelled fetuses were recovered; in the 2nd case, 1 fetus was seen but later was consumed by the female. So although their pregnancies were confirmed, cause of the abortions was unknown, and the hormone data were considered potentially misrepresentative of a normal pregnancy. However, behavioral and vaginal cytology data for these individuals were included with those of the pregnant cohort.
Ovarian cycle.—The reproductive cycle of a female is partitioned into phases based upon changes in physiology or behavior. For coyotes in this study, estrus was the phase during which the female was receptive and cooperative with her mate's attempts to copulate, and the beginning of estrus was demarcated by the 1st observed copulatory tie (or sperm in the vaginal smear). Proestrus precedes estrus and is characterized by the presence of red blood cells in vaginal exudate. Diestrus immediately follows estrus and includes pregnancy and the protracted luteal phase of nonpregnant females. Luteal activity is typically prolonged in nonpregnant canids; and because the expression of progesterone is indistinguishable between pregnant and nonpregnant females, the latter are commonly described as pseudopregnant (Concannon et al. 1989; Feldman and Nelson 2004). Accordingly, a coyote was described as pseudopregnant if there was a marked and sustained elevation of serum progesterone after ovulation. Pseudopregnancy was assumed to be covert and subclinical (i.e., no mammary development, lactation, or denning behavior) unless otherwise noted. After parturition, or regression of the corpora lutea, hormone synthesis diminishes and anestrus follows diestrus.
Specimen collection and handling.—Peripheral blood samples were collected weekly by venipuncture of the cephalic or saphenous veins, or daily from an indwelling catheter in the external jugular vein. Samples were collected between 0700 and 0900 h, before the animals were fed and without sedation or anesthesia. In some cases sampling began as early as 4 weeks before a female was receptive to her mate's attempts to copulate, or 6 weeks before a sequestered female ovulated. Blood sampling continued throughout estrus and diestrus with the latest samples collected 3 weeks after the birth of pups. To minimize investigator disturbance of mating activity, blood collection from paired females was restricted to weekly sampling until 3 weeks after the 1st copulatory tie.
For quantitative analyses of progesterone, estradiol, and prolactin, whole blood was collected in an evacuated tube and allowed to clot at room temperature (20–24°C) for 30–120 min. The serum was separated from the blood cells, divided into aliquots, and stored at ≤ −20°C until testing. Progesterone levels were determined from 727 specimens, and sample subsets also were used to measure estradiol (n = 405) and prolactin (n = 205).
Specimens for qualitative assay of relaxin were collected as whole blood in sodium or lithium heparin. Plasma was separated as soon as possible and stored at ≤ −20°C until testing. Samples for relaxin were collected from the 32 coyote females included in this study but also from additional animals enrolled in other studies at the research facility. Collectively, these data were used to validate relaxin as a diagnostic marker of pregnancy in coyotes and the results have been discussed elsewhere (Carlson and Gese 2007).
Exfoliated vaginal epithelial cells were collected weekly (typically the same day as a blood sample) using a sterile swab premoistened with normal saline. The swab was gently passed into the vaginal vault, carefully avoiding the clitoral fossa, and rotated against the lining of the vaginal lumen (Feldman and Nelson 2004). Once withdrawn the swab was immediately rolled along a clean glass slide in 2 or 3 rows. The sample was allowed to air dry at room temperature then fixed and stained as soon as possible.
Mating behavior.—Continuous scanning observations were conducted daily throughout available daylight hours, January–March, 2000–2003. The animals were habituated to low-level human activity before data collection although all enclosures could be viewed through binoculars or a spotting scope from observation sites 100–500 m away. The observer would view a pen, document any interactive behavior occurring between pair-mates, then scan the next pen. Because this process rarely took more than 30 s per pen, all pens were viewed at least once every 5–10 min.
Characterization of mating behavior was standardized between observers and documented (Bekoff and Diamond 1976; Golani and Mendelssohn 1971; Schenkel 1967). Behaviors recorded included courtship (nonantagonistic play-wrestling or play-chases; allogrooming face, ears, or back; body-bumps; hip-pushes; or sleeping curled against each other); olfactory sampling (sniff or lick of the female's anogenital region by the male, solicitation by females with diverted tail, and sniff or lick of the male's inguinal area by the female); overt sexual activity (attempted mount usually preceded by the male standing perpendicular to the female with his head or bent foreleg on her shoulders or back, male mounting the female, and copulatory tie or lock); and mate guarding (male shadowing the female around the pen, or when in view of neighbors the male would stand on the female with stiff forelegs on her back, or stand over her as she lay on the ground).
Observers avoided redundant documentation by recording a mating behavior only once even if a pair of coyotes continued the behavior for an extended period of time (e.g., playing might last 15 min and through several scanning passes). Exceptions were any behavior that was terminated then reinitiated (e.g., precopulatory mounts).
Reproductive hormone assays.—Quantitative measurement of progesterone in coyote sera was performed by competitive binding enzyme immunoassay according to the manufacturer's instructions (Progesterone EIA, DSL-10-3900; Diagnostic Systems Laboratories, Inc., Webster, Texas). By this method, horseradish peroxidase–labeled progesterone competed with free progesterone in coyote sera for a fixed quantity of rabbit anti-progesterone. Microtiter wells coated with goat anti-rabbit immunoglobulin G captured the antibody-bound progesterone. Extraneous material was rinsed from the well, and addition of tetramethylbenzidine chromogenic solution permitted photometric measurement (Benchmark microplate reader; Bio-Rad Laboratories, Hercules, California) of reagent standards, controls, and unknown samples. Unknown coyote samples were compared to a standard curve generated for each run using Microplate Manager/PC software (version 4.0; Bio-Rad Laboratories), with the quantity of progesterone being inversely proportional to the intensity of color development. Stated level of sensitivity for progesterone was 0.13 ng/ml.
Quantitative measurement of estradiol was performed by competitive binding enzyme immunoassay (3rd Generation Estradiol EIA, DSL-10-39100; Diagnostic Systems Laboratories). In this assay, estradiol–biotin conjugate competed with free estradiol in coyote sera for available rabbit anti-estradiol sites fixed to microtiter wells. Streptavidin–horseradish peroxidase was added, binding to the biotinylated estradiol, and tetramethylbenzidine precipitated color development in the reagent standards, controls, and unknown coyote samples. Color development measured with a photometer (Benchmark microplate reader) was inversely proportional to the quantity of estradiol captured in each well, and unknown coyote samples were compared to a standard curve generated for each run using Microplate Manager/PC software (version 4.0). Level of sensitivity for this assay was 1.5 pg/ml.
Coyote samples were not pretreated or extracted before testing for progesterone or estradiol. Validation procedures including linearity and recovery assessments were performed and the assays were found acceptable for use in this species (Carlson 2008). When possible, samples from the same cohort were tested together to reduce reagent lot-to-lot variability. Unknown samples were tested in duplicate and intra-assay coefficient of variation (CV) was ≤10% for all results included in the data set. For progesterone reagent standards and controls, within-lot interassay mean CV was 9.6%, and interlot CV was 20.2%. For estradiol (single lot only) interassay mean CV was 11.2%.
Prolactin was quantitatively measured by double-antibody prolactin radioimmunoassay at the Colorado State University Endocrine Laboratory (Colorado State University, Fort Collins, Colorado). In this assay, coyote prolactin competed with 125I canine prolactin for a fixed amount of rabbit anti-canine prolactin antibodies. Anti-rabbit immunoglobulin G was added and radioactivity of the precipitated pellet was measured. Unknown coyote samples were compared to a standard curve, with the amount of iodinated antibody–antigen complexes detected being inversely proportional to the quantity of prolactin in the coyote sera. Lowest detectable limit for prolactin was 2.33 ng/ml.
Qualitative measurement of relaxin in heparinized coyote plasma was performed by enzyme-linked immunoassay for canine relaxin (ReproCHEK; Synbiotics Corporation, San Diego, California). Free relaxin in an unknown sample was captured between polyclonal anti-relaxin antibodies in solid phase (microtiter wells) and monoclonal anti-relaxin antibodies conjugated to horseradish peroxidase. Subsequent color development was directly associated with presence or absence of relaxin in the sample. Absorbance was measured photometrically, and an optical density > 0.050 (minimum threshold for a positive result) was adopted to distinguish between pregnancy and pseudopregnancy in the coyote. It was also important to note that relaxin persisted in peripheral blood after parturition and could not reliably predict abortion in coyotes (Carlson and Gese 2007).
Vaginal exfoliative cytology.—Air-dried samples of vaginal epithelial cells and uterine exudate on glass slides were fixed with methanol and stained with a modified Wright–Giemsa stain (Diff-Quik; Jorgensen Laboratories, Loveland, Colorado). Slides were then examined under high dry magnification (400 ×) with at least 5 fields per row (≥10 fields per slide) of stained material viewed. The observed epithelial cells were characterized as parabasal, small intermediate, large intermediate, superficial, and keratinized (anuclear) superficial (Shutte classification—Christie et al. 1972; Feldman and Nelson 2004), and their relative representations in the sample were graded on a semiquantitative scale of 1–5 (Bradley and Benson 1974). In addition, inclusions such as white blood cells, red blood cells, mucus, amorphous debris (degenerating blood and epithelial cells), and spermatozoa also were noted.
Data analysis.—Data were aligned by the estimated day of ovulation for an individual before it was compiled by study group. This estimate was either back-calculated from the day of parturition, or based upon changes in serum progesterone levels. Examination of data presented by Kennelly and Johns (1976) suggested that coyotes ovulate immature (primary) oocytes, similar to domestic dogs (Canis familiaris). If true, then fertilization probably does not occur until 2–3 days after ovulation (domestic dog—Tsutsui 1989). In this study, there-fore, gestation was standardized and assumed to be 62 days from fertilization (Gier 1968; Hamlett 1938; Kennelly et al. 1977) or 64 days after ovulation. Alternatively, the day of ovulation for nonpregnant females was inferred from the change in daily progesterone levels; specifically, the day on which progesterone concentrations approximately doubled from previous samples.
Hormone and vaginal cytology data are presented herein as weekly mean values of all females within a cohort, and results obtained on the estimated day of ovulation are included in “Week 1.” In circumstances in which multiple hormone assay results were available for an individual within a given week, a weekly mean value for that individual was calculated in order to normalize the influence of each individual's contribution to the cohort mean. Behavioral observations were categorized, aligned by the day of ovulation for each individual, then reported as cumulative daily or weekly data for the cohort.
Multivariate analysis of variance and repeated-measures statistical procedures were used to analyze endocrine hormone profiles and detect differences between study groups, and between successive weeks (Statistical Analysis System, SAS, version 8.2; SAS Institute Inc., Cary, North Carolina). Pearson correlation coefficient and multiple regression procedures were used to analyze relationships between hormones and behavior, and between hormones and vaginal cytology. Unless otherwise noted, we assumed a level of statistical significance to be <0.05.
Results
Behavior.—Females in this captive population were naturally synchronized; each individual began behavioral estrus within a 4-week period, mid-January to mid-February. Before estrus, intrapair activity consisted of courtship behavior including allogrooming, play-wrestling, play-chases, body-bumps, and hip-pushes (Fig. 1). Olfactory sampling (i.e., anogenital and inguinal sniff or lick) by both the male and female, as well as mate guarding, also increased during proestrus. Males began mounting attempts; however, the overtures were usually rejected by the female using gentle admonition, aggressive rebuff, or passive avoidance tactics (such as sitting, lying down, or running away).
Fig. 1.
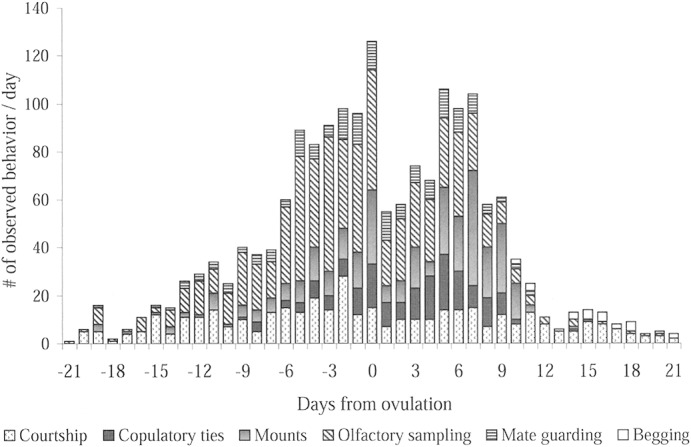
Social and mating behaviors of coyotes (Canis latrans) shown as daily cumulative data (n = 1,757) from 32 mated pairs during breeding seasons in 2000–2003. Observations represent 21 days before and after the estimated day of ovulation (day 0 on the chart). Behavioral estrus ranged from day −8 to day 10 (as shown by the solid bar).
Mounting attempts became more frequent, and the 1st copulatory tie marked the start of estrus. In contrast to the response observed in proestrus, females were tolerant and receptive to the males' mounting attempts in estrus, and often the female would solicit attention by positioning herself in front of the male and diverting her tail. Also, an increased frequency of mounting was expected during estrus because this was the antecedent posture to copulation, and it was common for a male to mount and dismount several times before achieving intromission and coital lock.
Copulatory ties generally lasted 5–45 min, with ties occurring early in estrus lasting longer than those observed later toward the end. The earliest a copulatory tie was observed during this study was on day –9, and the latest on day 15; however, 98.4% (179/182) of all ties occurred between day –8 and day 10. At the individual level, behavioral estrus lasted (mean ± SD) 7.6 ± 6.0 days, with 59.4% (19/32) of pairs of coyotes beginning estrus before ovulation (day −2.2 ± 3.9 days).
During preovulatory estrus, physical contact such as body-bumps and hip-pushes continued to rise. Mate guarding postures such as a stand-over within view of neighbors and shadowing became more frequent. Olfactory sampling (male and female) increased almost 3-fold; specifically, vaginal sniff or lick by males doubled on day −6 from the previous day, continued to increase, then peaked on the estimated day of ovulation.
Immediately after the periovulatory pulse of activity there was a brief quiescence before the sexual activities of the cohort pairs peaked again on day 5, particularly copulations (Fig. 1). Sixty percent (203/341) of mounts and 65% (119/182) of ties occurred during postovulation estrus. When pairs of coyotes were observed in >1 tie per day, the multiple ties most frequently occurred on days 4–6. Mate guarding also showed a postovulatory surge on days 5–6. Mounting attempts peaked on day 7 although this was associated with a decline in the number of successful copulations.
As estrus waned, females began to reject some (but not yet all) of the males' attempts to copulate. Physical bodily contact remained high in late estrus, but play behavior was only sporadically observed until the transition into diestrus. On day 11 postovulation, sexual activities abruptly declined and observation of copulatory ties (2/182), mounting attempts (4/341), olfactory sampling (14/667), or mate guarding (2/163) were relatively rare (1.1%, 1.2%, 2.1%, and 1.2%, respectively) during the next 10 days (Fig. 1). In 2001 a pair was observed in a single tie 17 days after the previously recorded copulation; similarly in 2002, another pair tied 15 days after their previous mating. Back-calculation from parturition suggested an earlier date of fertilization in both cases, and because neither copulation was associated with other sexual behaviors (the tie lasted <2 min), these events were considered to have had some other unexplained intrapair social function rather than a sign of extended or split estrus.
Although there was a dearth of sexual activity, the coyotes in diestrus continued to engage in physical contact such as allogrooming, body-rubs, play-wrestling, and chasing. In addition, a previously unseen behavior emerged, begging (Fig. 1). Beginning on day 10 and continuing through the end of the observations, females were periodically observed in a juvenile-like submissive behavior that sometimes provoked regurgitation of food by the male. Specifically, the female would approach her mate with her tail held low and wagging, then she would lick his mouth or gently bite his lower jaw, matching his movement if he tried to turn away. Sometimes the male would admonish the female and escape. At other times, however, the female would cease the behavior and appear to be eating off the ground. In 7 of 55 cases line of sight allowed the observer to confirm that the food being consumed by the female had just been regurgitated by the male.
Reproductive hormones.—During proestrus and estrus, spontaneous up-regulation of ovarian steroid synthesis was evident in changes in peripheral blood levels of estradiol and progesterone, regardless of a coyote's social status (Fig. 2). Sequestered females (Fig. 2A) experienced a pattern of steroid expression (estradiol followed by progesterone) and periodicity similar to that of mated females (Fig. 2B), and distinction between groups was not discernible until pregnancy was established.
Fig. 2.
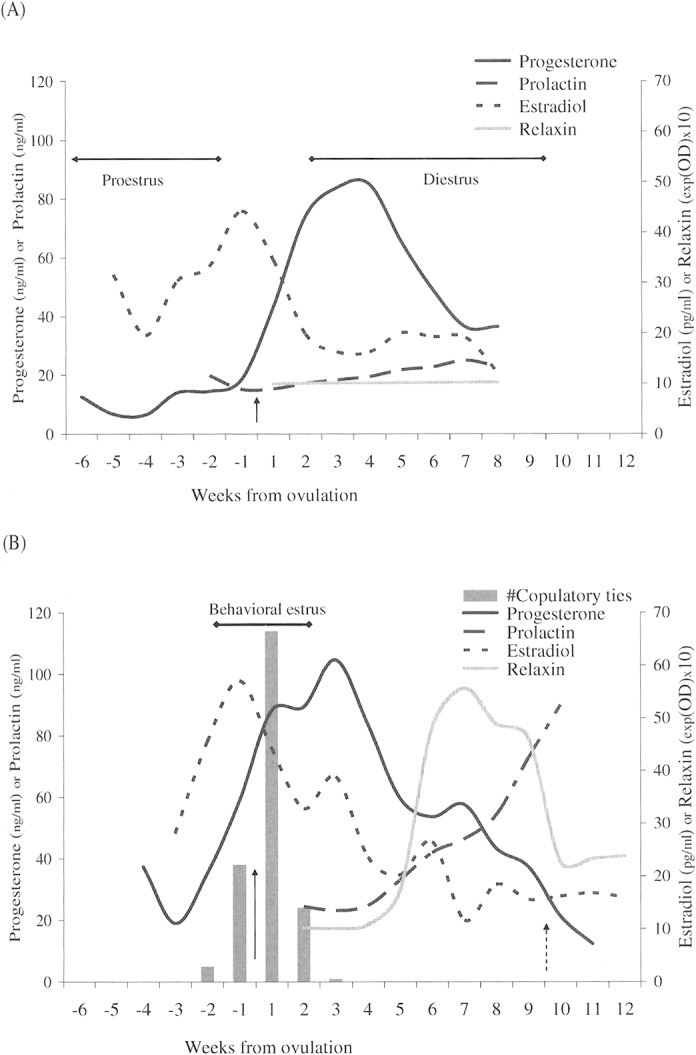
Temporal relationship of weekly mean blood levels of estradiol (pg/ml), progesterone (ng/ml), prolactin (ng/ml), and relaxin (exp(optical density) × 10; for graphical representation only), aligned to the estimated day of ovulation (day 0). A) Pseudopregnant females; B) pregnant coyotes. Solid arrow indicates ovulation. Columns indicate weekly number of copulatory ties observed during the breeding seasons in 2000–2003; dashed arrow indicates day of parturition (day 64 postovulation). Solid bars indicate phases of ovarian cycle studied.
In both study groups of coyotes, fluctuating serum estradiol levels generally increased during proestrus (Fig. 3), and an interweek comparison, week −3 to week −2, suggested a significant incremental rise in mean values (F = 20.93, d.f. = 1, 8, P = 0.002). Also during this time, the rate of change among females residing with their mates appeared different in contrast to the sequestered females (F = 7.84, d.f. = 1, 8, P = 0.023), although the between-group weekly means remained statistically indistinct (week −3, P = 0.917; week −2, P = 0.245). In estrus, weekly preovulation (week −1) estradiol levels peaked at (mean ± SD) 57.1 ± 16.3 pg/ml (n = 5) among mated females (pregnant cohort) and 44.2 ±21.1 pg/ml (n = 5) in the nonmated (nonpregnant cohort), whereas postovulation (week 1) levels subsequently declined in both groups.
Fig. 3.
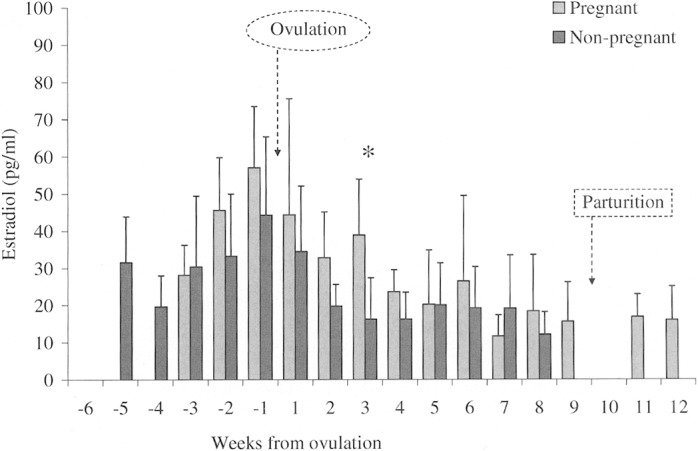
Weekly mean (± SD) serum levels of estradiol (pg/ml) from 5 pregnant (n = 117) and 5 nonpregnant (n = 288) coyotes during the breeding seasons in 2000–2002, aligned to the estimated day of ovulation (day 0). An asterisk (*) indicates statistical difference detected between study groups. Missing columns are weeks for which there were insufficient data available.
Estradiol levels continued to fall from late estrus to early diestrus (week 1 to week 2). Although the decremental change was similar between groups (F = 0.07, d.f. = 1, 8, P = 0.801), comparison of the between-group mean difference was borderline (week 2, P|t|0.05(2),8 ≥ 2.16 = 0.063, F = 4.45, d.f. = 4, 4). From week 2 to week 3, however, the study groups demonstrated a notable divergence (F = 6.41, d.f. = 1, 8, P = 0.035) in estradiol levels. Specifically, the pregnant cohort experienced a transient spike in week 3 (38.9 ± 15.0 pg/ml, n = 5) that was different (P|t|0.05(2),8 ≥ 2.70 = 0.027, F = 1.80, d.f. = 4, 4) from the nonpregnant group (16.2 ± 11.2 pg/ml, n = 5; Fig. 3). Nevertheless, serum estradiol levels continued to fall in both cohort groups, and fluctuations appeared to dampen as pregnant females approached parturition and nonpregnant females entered anestrus (Fig. 3).
Although estradiol was the predominant ovarian hormone in proestrus, progesterone synthesis was detectable in female coyotes during this period (Fig. 4). Immediately before estrus, the incremental change in mean progesterone levels, from week –2 to week –1, was significant (F = 6.24, d.f. = 1, 14, P = 0.026). Furthermore, while the most notable change observed was periovulation (week –1 to week 1: F = 27.94, d.f. = 1, 14, P < 0.001), successive weekly levels rose or fell significantly (P < 0.05) from week –2 through week 7; exceptions were week 1 to week 2 (F = 3.22, d.f. = 1, 14, P = 0.094), and week 3 to week 4 (F = 0.93, d.f. = 1, 14, P = 0.352).
Fig. 4.
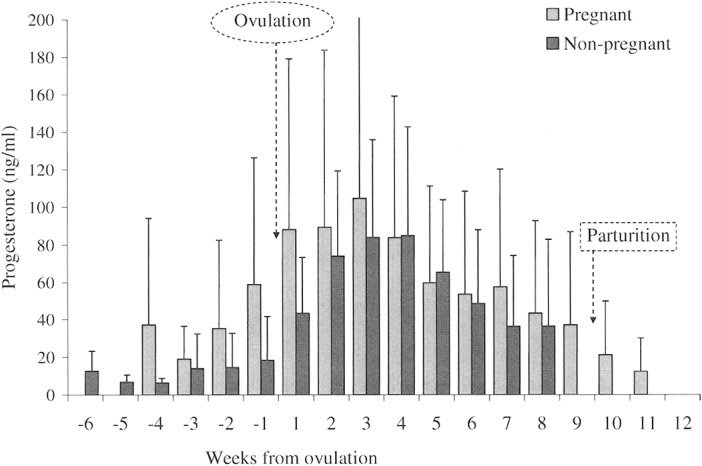
Weekly mean (± SD) serum progesterone (ng/ml) levels from 8 pregnant (n = 245) and 8 nonpregnant in = 482) coyotes during the breeding seasons in 2000–2002, aligned to the estimated day of ovulation (day 0). No intergroup statistical difference was detected. Missing columns are weeks for which there were insufficient data available.
During estrus, progesterone levels in the mated females (pregnant cohort) rose from (weekly mean ± SD) 58.9 ± 67.5 ng/ml (n = 7) to 89.3 ± 94.4 ng/ml (n = 8), whereas levels in the nonmated females rose from 18.5 ± 23.2 ng/ml (n = 8) to 74.1 ± 45.2 ng/ml (n = 8). However, the mean levels were not different between groups (week –1,P = 0.179; week 1, P = 0.208; and week 2, P = 0.687). In fact, no overall effect of status was detected throughout the study period (week –2 to week 7: F = 2.34, d.f. = 9, 6, P = 0.157), possibly because of the degree of individual variability observed among the coyotes. For example; among females in estrus and residing with their mates (pregnant cohort), the progesterone minimum, maximum, and CV, respectively, were: week –1 =2.8 ng/ml, 181.4 ng/ml, 1.1; week 1 = 6.7 ng/ml, 266.5 ng/ml, 1.0; and week 2 = 10.3 ng/ml, 305.5 ng/ml, 1.1. Among sequestered females in estrus (nonpregnant cohort) the same hormone parameters were: week –1: 5.4 ng/ml, 75.1 ng/ml, 1.3; week 1: 13.3 ng/ml, 108.9 ng/ml, 0.7; and week 2: 15.8 ng/ml, 147.5 ng/ml, 0.6.
Regardless of the variation, the secretion pattern of progesterone was generally consistent between study groups. Progesterone levels (mean ± SD) peaked between week 3 (pregnant, 104.6 ± 97.8 ng/ml, n = 7) and week 4 (nonpregnant, 85.0 ± 57.8 ng/ml, n = 8) in pregnancy and diestrus. Subsequently, levels declined in both groups. The pregnant cohort appeared to experience a transient surge in week 7, but the groups remained statistically indistinct until parturition and the end of sample collection (Fig. 4).
In contrast to progesterone, there was a distinctive overall effect of status (F = 6.03, d.f. = 6, 6, P = 0.023) on coyote prolactin blood levels. During early pregnancy and diestrus, week 2 through week 4, mean prolactin levels did not markedly change within either cohort group; however, subsequent weeks showed a pronounced elevation among pregnant females (Fig. 5). Specifically, a significant change occurred between weeks 4 and 5, both in mean prolactin levels (F = 41.26, d.f. = 1, 11, P < 0.001) and intercohort rate of change (F = 13.34, d.f. = 1, 11, P = 0.004). Levels in pregnant coyotes rose from 24.8 ± 4.5 ng/ml (week 4, n = 5) to 33.0 ± 7.8 ng/ml (week 5, n = 5), whereas among nonpregnant coyotes prolactin increased from 19.6 ± 5.5 ng/ml (week 4, n = 8) to 21.8 ± 6.2 ng/ml (week 5, n = 8). Thereafter, prolactin levels remained elevated throughout pregnancy, parturition, and the 1st week of lactation in those coyotes observed with live pups. Non-pregnant females, meanwhile, continued to synthesize prolactin but at lower levels (P < 0.006).
Fig. 5.
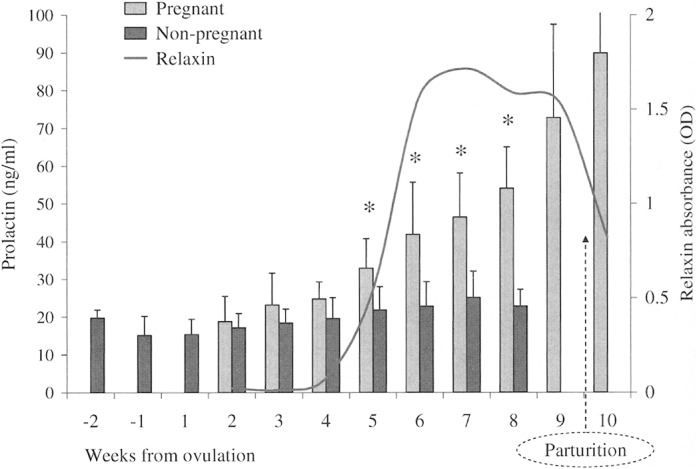
Weekly mean (± SD) serum levels of prolactin (ng/ml) from 5 pregnant (n = 85) and 8 nonpregnant (n = 120) female coyotes during the breeding seasons in 2000–2002. Data are aligned to the estimated day of ovulation (day 0). An asterisk (*) indicates statistical difference detected between study groups. Also shown are plasma relaxin (OD = optical density) readings from 82 pregnant coyotes (n = 209); seasons 2000–2003. Relaxin was <0.100 OD for nonpregnant coyotes (data not shown).
Relaxin was detectable in plasma of pregnant coyotes within 4 weeks after ovulation (Fig. 5); specifically, 10 of 11 pregnant coyotes tested positive (optical density > 0.050) on day 27 postovulation, and 20 of 21 females with pups were positive on days 28–30. By comparison, relaxin was not detected (optical density < 0.033) in samples collected from 2 females residing with castrated mates, 7 nonmated females, or 8 male coyotes. In addition, from week 5 through parturition, relaxin remained detectable in all samples collected from pregnant females; and although absorbance intensity weakened, postpartum females did not revert to negative until several weeks after whelping.
Vaginal exfoliative cytology.—A serosanguineous discharge from the vagina was not always apparent upon gross examination of a female coyote in proestrus, but red blood cells were typically observed microscopically when the vaginal smear was examined (Fig. 6A). Epithelial cells on these smears were primarily of parabasal and intermediate cell types but gradually, as the female progressed through proestrus, the exfoliated epithelial cells appeared larger and samples presented as admixtures of parabasal, small and large intermediate, and superficial epithelial cells (Fig. 7). Red blood cells, amorphous debris, and mucus remained grossly obvious throughout proestrus (Fig. 8). However, leukocytes were only occasionally seen on smears from this stage, and their occurrence was likely due to secondary passage with the high number of red blood cells rather than by diapedesis.
Fig. 6.
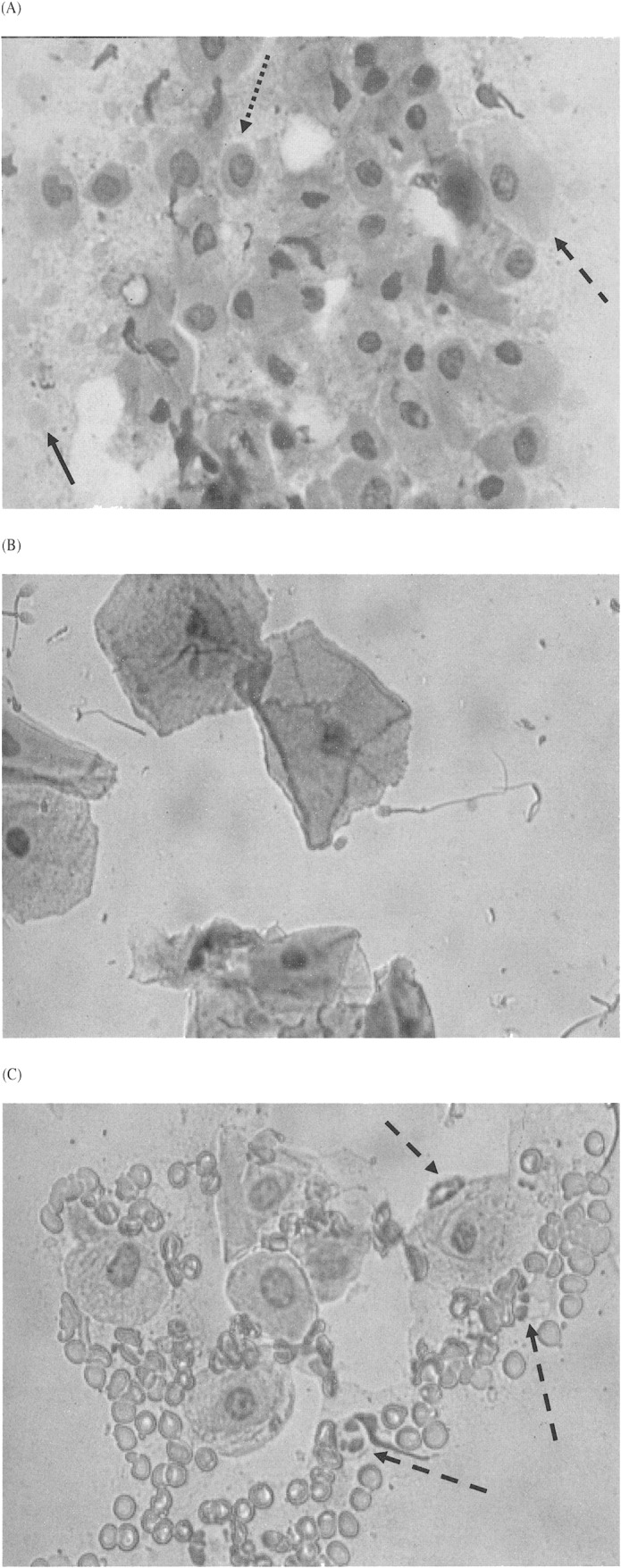
Representative examples of exfoliated epithelium and other inclusions commonly seen on a coyote vaginal smear (480 ×). A) Proestrus, dotted arrow indicates a parabasal cell, dashed arrow indicates an intermediate cell, and solid arrow indicates a red blood cell. Amorphous debris and mucus is conspicuous in the background. B) Estrus, superficial cells with pyknotic nuclei and spermatozoa are shown; note the relatively clear background as compared to A and C. C) Diestrus, dashed arrows indicate intact white blood cells (neutrophilic leukocytes). Also, parabasal and intermediate epithelial cells have reemerged, and red blood cells, mucus, and amorphous debris are abundant.
Fig. 7.
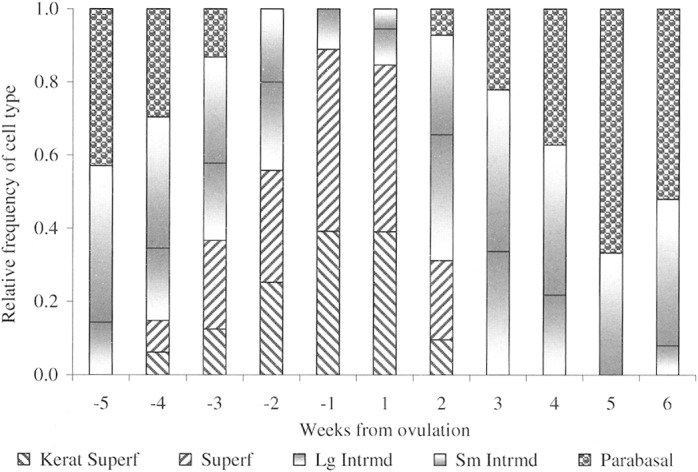
Relative proportion of exfoliated vaginal epithelial cells viewed on vaginal smears (n = 133) collected weekly from 18 coyotes during the breeding seasons in 2000–2002. Data are aligned to the estimated day of ovulation (day 0) including 5 weeks preovulation to 6 weeks postovulation. Kerat Superf = keratinized (anuclear) superficial, Superf = nucleated superficial, Lg Intrmd = large intermediate, Sm Intrmd = small intermediate, Parabasal = parabasal cells.
Fig. 8.
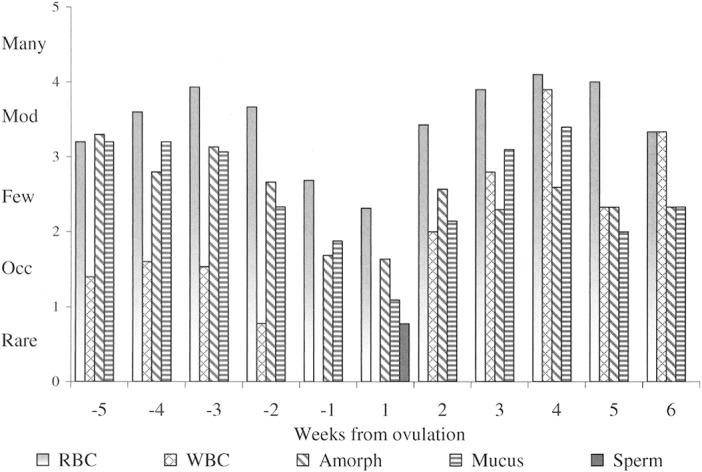
Inclusions other than epithelial cells viewed on weekly vaginal smears (n = 133) from 18 coyotes during the breeding seasons in 2000–2002. RBC = red blood cells, WBC = white blood cells, Amorph = amorphous debris, Mucus = mucus, and Sperm = spermatozoa. Data are aligned to the estimated day of ovulation (day 0) including 5 weeks preovulation to 6 weeks postovulation.
A coyote's vulva appeared turgid but relaxed in estrus, and passing a swab into the vagina for collection of exudate was easier than in proestrus. On vaginal smears, superficial epithelial cells were the predominant cell type from approximately day –4 through ovulation to day 7; these cells were either keratinized (anuclear) or retained pyknotic nuclei (Fig. 7). Concurrently, appearance of red blood cells, mucus, and amorphous debris were diminished, and white blood cells were rarely seen (Fig. 8). Thus, the appearance of superficial cells (nuclear and anuclear) against a clear background represented the characteristic vaginal smear during estrus in the coyotes (Fig. 6B), particularly after ovulation.
Spermatozoa were sometimes viewed in the vaginal smears (Fig. 6B), confirming that mating had occurred, but they were an unpredictable and erratic element during estrus. In several circumstances spermatozoa were not recovered although the females were known to be actively breeding. Sperm deposition in the coyote was assumed to be transcervical, as in the domestic dog, and if true, would thus explain the inconsistent findings. Nevertheless, among those samples that did contain spermatozoa, most were collected during the period of frequent copulations, days 3–6 postovulation.
Toward the end of estrus (days 7–10), vaginal epithelial cells regressed to intermediate forms; eventually returning to a preponderance of parabasal cells as the coyotes entered diestrus (Fig. 7). Also during diestrus, red blood cells, amorphous debris, and mucus once again became abundant (Fig. 8). Most notable in this phase, however, was the reappearance and disproportionate number of leukocytes (Fig. 6C), particularly in relationship to the number of red blood cells (Fig. 8). Thereafter, a fluctuating mix of blood cells and cellular debris persisted into anestrus. We observed no discernible difference between the vaginal smears from pregnant and pseudopregnant coyotes.
Interrelationships among behavior, endocrine patterns, and vaginal cytology.—During proestrus, significant relationships between olfactory sampling and other behaviors were detected. Specifically, there was a strong positive correlation between olfactory sampling and mate-guarding (r = 0.739, P = 0.0039). Also, relationships between olfactory sampling and mounting attempts (r = 0.581, P = 0.0374), as well as with courtship (r = 0.567, P = 0.0432), suggested that olfactory sampling may stimulate other behavior, or inform coyotes (both male and female) of their mate's physiological status.
Eighty-four percent (558/667) of olfactory investigations occurred during estrus; with the apparent peak in activity on day –3 (56 events) reflecting an increase in vulva sniff or lick and female solicitations. Meanwhile, 88% (143/163) of mate-guarding events also occurred during estrus, and as in proestrus, mate-guarding and olfactory sampling maintained a positive relationship (r = 0.550, P = 0.0146).
Twenty-three percent (42/182) of all copulatory ties occurred between day –8 and day – 1; a time when progesterone synthesis was increasing and estradiol was reaching its preovulation peak on day –4 (Fig. 9). Throughout estrus, however, copulatory ties appeared to have a significant relationship with progesterone (r = 0.554, P = 0.0139) yet a poor correlation with estradiol (r = –0.084, P = 0.7324). Furthermore, an initial spike in the daily number of ties (18 events) was observed on the estimated day of ovulation, a day when mean progesterone levels experienced the greatest incremental change (CV(day –1 to day 0) = 0.275); and another major peak in copulations (23 events, day 5) immediately followed a secondary surge in progesterone (CV(day 3 to day 4) = 0.247; Fig. 9).
Fig. 9.
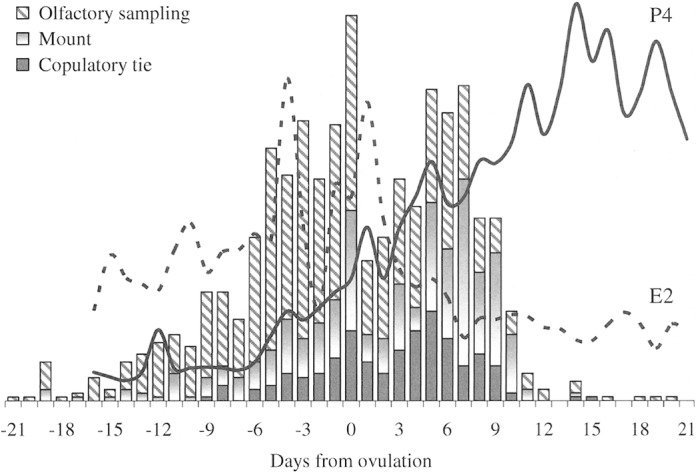
Daily mean blood levels of estradiol (pg/ml) and progesterone (ng/ml) overlaying sexually specific behaviors in coyote pairs (olfactory sampling, mounts, and copulatory ties) observed during the breeding seasons in 2000–2003. Data are aligned to the estimated day of ovulation (day 0).
We noted peaks in mate-guarding (11–13 events per day) on day –5, day –2 through day 1, and day 5; days immediately adjacent to peaks in steroid hormone activity and sexual behavior. However, although mate-guarding and copulatory ties appeared to be positively correlated (r = 0.521, P = 0.0221), mate-guarding did not appear to be statistically related to estradiol (r = 0.248, P = 0.3054) or progesterone (r = –0.032, P = 0.8964) levels, either singly or as covariables (AdjR2 = 0.047, P = 0.5629).
As estradiol levels diminished, superficial cells disappeared (Fig. 10), and a strong association was detected between estradiol blood levels and the appearance of superficial cells (r = 0.897, P = 0.0004). Although this evidence suggests that vaginal smears might be used as a surrogate measure of breeding condition, defining the specific day of ovulation was not possible and changes in cytology did not appear to be correlated with progesterone levels (r = –0.340, P = 0.3358).
Fig. 10.
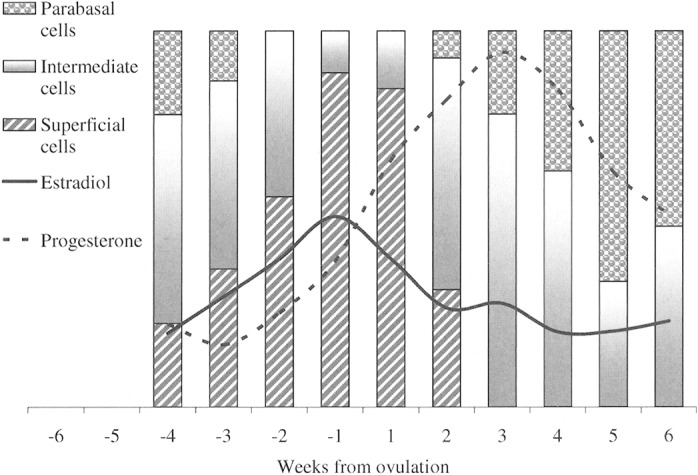
Weekly mean blood levels of estradiol (pg/ml) and progesterone (ng/ml) and the relative proportion of epithelial cells observed on vaginal smears from coyotes during the breeding seasons in 2000–2002, 4 weeks preovulation to 6 weeks postovulation. Data are aligned to the estimated day of ovulation.
Discussion
This study describes for the coyote the secretion pattern and temporal relationship between estradiol, progesterone, pro-lactin, and relaxin, from late proestrus through estrus and diestrus; mating behaviors observed during those same estrous periods; and changes in vaginal cytology. Furthermore, it compares reproductive endocrine patterns of females residing with their mates with those of females sequestered during estrus, thereby providing contrasting profiles of pregnancy and pseudopregnancy in the coyote. Graphic and statistical comparisons also provided insight into relationships between various elements of coyote reproduction, emphasizing the importance of viewing reproductive biology as a spectrum and integration of physiology and behavior.
Proestrus.—Proestrus appears to be a crucial period of preparation and staging as levels of estradiol and progesterone rise coincidentally with alterations in coyote behavior and reproductive tissue. Appearance of red cells in vaginal exudate, and transformation of epithelial cells, suggested progressive remodeling of the reproductive tract for the ensuing rigors of mating and pregnancy. Meanwhile, physical and social interactions between pair-mates increased, as well as defensive displays. Positive relationships between olfactory sampling and other behaviors suggested a possible mechanism by which either sex might assess the physiological status of their mate, and ritualized agonistic behaviors may reinforce the pair-bond or inform a coyote about its mate's readiness to breed, defend territory, or provide parental care.
Estrus.—As previously noted, the majority of copulations occurred after ovulation, suggesting that the coyotes may have concentrated their reproductive effort to coincide with the optimal time for viable sperm to encounter mature ova. Yet interestingly, most 1st ties were observed before ovulation. Because coyotes ovulate spontaneously, there is no apparent physiological function that can be ascribed to exceptionally early copulations (although spermatozoa can survive for several days within the female reproductive tract). We also observed exceptionally late copulations (albeit with very short ties).
It is unclear if such sexual behavior exists among free-roaming coyotes; if so, it would presumably be costly because a pair would be especially vulnerable to predation while in a coital lock. But possible social and future benefits gained through employment of multiple copulations include mate fidelity, guaranteed paternity, advertisement of a confirmed pair-bond (thus discouraging competitors), or assured access to resources (Gier 1975; Hunter et al. 1993; Sillero-Zubiri et al. 1996). The influence of steroid hormones on preovulatory copulations also cannot be ruled out. Progesterone levels in coyotes began rising before ovulation and were positively correlated with copulations. Furthermore, estradiol followed by progesterone has been linked to expression of sexual behavior in the domestic dog (Concannon et al. 1979). Therefore, the preovulation ascendancy of progesterone described in this study may suggest a possible physiological stimulus for seemingly premature copulations among coyotes.
Ironically, mate-guarding appeared correlated to copulatory ties, but not to either steroid. However, these results are not surprising when one considers the male's role in mating behavior (and the influence testosterone certainly exerts during the breeding season). Regardless, the association between rising progesterone levels and overt sexual behavior reflects the female coyote's sexual determination. A male may shadow a female, investigate her anogenital scent, play, groom her, et cetera, but copulations will not occur without her permission and explicit cooperation.
Cytomorphosis of vaginal epithelium to keratinized or nucleated superficial cells was transient, but correlated with estradiol levels. Predominance of superficial cells was most pronounced after the preovulatory peak in estradiol, and persisted until estradiol was withdrawn in late estrus. Meanwhile, other cellular inclusions were conspicuously rare or absent. In cases when mating behavior is unobservable or protracted, vaginal smears may serve as a qualitative assessment of estradiol synthesis, thereby predicting a period of receptivity, or optimal fertility.
Diestrus.—Estradiol secretion generally dampened during diestrus although pregnant coyotes experienced an estradiol pulse during week 3—the period in which implantation occurs (Gier 1968). Meanwhile, progesterone levels were indistinguishable between pregnant coyotes and nonpregnant females.
However, prolactin and relaxin emerged as salient hormones in diestrus, differentiating pregnancy from pseudopregnancy. Relaxin was pregnancy specific and became detectable in week 4, followed shortly thereafter by an increase in prolactin. Prolactin levels in pregnant coyotes then diverged from pseudopregnant females in week 5. Both hormones remained elevated thereafter until parturition, persisting for several weeks after the pups were born. It has been hypothesized (but not yet established) that relaxin may serve as a signal between embryo and mother, possibly stimulating prolactin synthesis, which in turn facilitates the persistence of progesterone (required for the maintenance of pregnancy in canines—Concannon et al. 2001). But although the temporal associations between relaxin, prolactin, and progesterone are suggestive in this data set, under the conditions of this study we were unable establish a definitive link in the coyote.
As sexual behavior waned, food-begging immediately appeared in diestrus. Episodes of begging, and sometimes regurgitation, were seen throughout pregnancy with greater intensity occurring during weeks 4 and 5. Interestingly, begging was not restricted to pregnant females. In a tangent study, pseudopregnant females who remained housed with their mates throughout the breeding season also were observed begging food (and receiving regurgitate) from the males (Carlson 2008).
In conclusion, this study showed that key elements of reproductive behavior, endocrine profiles, and vaginal cytology are discernible in coyotes. Biologists studying wild or captive populations might find it helpful to focus on selected criteria when assessing a breeding population. The value of seeing a pair in a copulatory tie, viewing spermatozoa on a vaginal smear, or detecting pups in a den are obvious. However, in the absence of such indicators, investigators might depend instead upon the observation of affinitive and appetitive behaviors in coyotes. When blood samples can be obtained, measurement of blood hormone levels can provide evidence of ovulation, and distinguish pregnancy from pseudopregnancy. Also, the relative prevalence of exfoliated epithelial cells, red cells, leukocytes, and other inclusions on a vaginal smear can help predict the breeding status or reproductive potential of an animal. However, the use of several of these elements together will increase the overall confidence in determining the reproductive status of an individual, or population of coyotes.
Acknowledgments
We thank T. D. Bunch, F. F. Knowlton, R. T. Skirpstunas, M. L. Wolfe, and 2 anonymous reviewers for their insightful comments on an earlier version of this manuscript and their valuable suggestions for improvement. Thanks also to D. A. Wannemacher. We appreciate the following National Wildlife Research Center staff as well as undergraduate and graduate students at Utah State University for assistance in the handling and care of the study animals, behavior observations, and specimen collections: K. Anderson, R. Bartel, S. Brummer, K. Casper, P. Darrow, R. Harrison, J. Hedelius, D. Jones, R. Kikkert, S. Kirshner, L. Minier, H. Phillips, J. Robinson, A. Seglund, H. Smith, J. Tegt, K. Wenning, M. Wollbrink, and D. Zemlicka. We are grateful to T. J. DeLiberto for arranging the funding and logistical support provided by United States Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services, National Wildlife Research Center, Logan Field Station, Utah State University, Logan, Utah.
Literature Cited
- Andelt W. F. 1985. Behavioral ecology of coyotes in south Texas. Wildlife Monographs 94:1–45. [Google Scholar]
- Asa C. S., Valdespino C. 1998. Canid reproductive biology: an integration of proximate mechanisms and ultimate causes. American Zoologist 38:251–259. [Google Scholar]
- Bekoff M., Diamond J. 1976. Precopulatory and copulatory behavior in coyotes. Journal of Mammalogy 57:372–375. [Google Scholar]
- Bekoff M., Wells M. C. 1986. Social ecology and behavior of coyotes. Advances in the Study of Behavior 16:251–338. [Google Scholar]
- Bradley G. M., Benson E. S. 1974. Examination of the urine. 15th ed. Pp. 15–83 in Clinical diagnosis by laboratory methods (Davidsohn I., Henry J. B., eds.). W. B. Saunders Company, Philadelphia, Pennsylvania. [Google Scholar]
- Bromley C., Gese E. M. 2001. Effects of sterilization on territory fidelity and maintenance, pair bonds, and survival rates of free-ranging coyotes. Canadian Journal of Zoology 79:386–392. [Google Scholar]
- Camenzind F. J. 1978. Behavioral ecology of coyotes on the National Elk Refuge, Jackson, Wyoming. Pp. 267–294 in Coyotes: biology, behavior, and management (Bekoff M., ed.). Academic Press, Inc., New York. [Google Scholar]
- Carlson D. A. 2008. Reproductive biology of the coyote (Canis latrans): integration of behavior and physiology. Ph.D. dissertation, Utah State University, Logan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson D. A., Gese E. M. 2007. Relaxin as a diagnostic tool for pregnancy in the coyote. Animal Reproduction Science 101:304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie D. W., Bailey J. B., Bell E. T. 1972. Classification of cell types in vaginal smears during the canine oestrous cycle. British Veterinary Journal 128:301–310. [DOI] [PubMed] [Google Scholar]
- Concannon P. W., Weigand N., Wilson S., Hansel W. 1979. Sexual behavior in ovariectomized bitches in response to estrogen and progesterone treatments. Biology of Reproduction 20:799–809. [DOI] [PubMed] [Google Scholar]
- Concannon P. W., McCann J. P., Temple M. 1989. Biology and endocrinology of ovulation, pregnancy and parturition in the dog. Journal of Reproduction and Fertility, Supplement 39:3–25. [PubMed] [Google Scholar]
- Concannon P., Tsutsui T., Shille V. 2001. Embryo development, hormonal requirements and maternal responses during canine pregnancy. Journal of Reproduction and Fertility, Supplement 57:169–179. [PubMed] [Google Scholar]
- Feldman E. C., Nelson R. W. 2004. Canine and feline endocrinology and reproduction. 3rd ed. Saunders, St. Louis, Missouri. [Google Scholar]
- Gannon W. L., Sikes R. S., the Animal Care,Use Committee of the American Society of Mammalogists 2007. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. Journal of Mammalogy 88:809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gese E. M. 1990. Reproductive activity in an old-age coyote in southeastern Colorado. Southwestern Naturalist 35:101–102. [Google Scholar]
- Gese E. M. 2001. Territorial defense by coyotes (Canis latrans) in Yellowstone National Park, Wyoming: who, how, where, when, and why. Canadian Journal of Zoology 79:980–987. [Google Scholar]
- Gese E. M., Rongstad O. J., Mytton W. R. 1989. Population dynamics of coyotes in southeastern Colorado. Journal of Wildlife Management 53:174–181. [Google Scholar]
- Gese E. M., Ruff R. L., Crabtree R. L. 1996. Foraging ecology of coyotes (Canis latrans): the influence of extrinsic factors and a dominance hierarchy. Canadian Journal of Zoology 74:769–783. [Google Scholar]
- Gier H. T. 1968. Coyotes in Kansas. Agricultural Experiment Station, Kansas State University, Agriculture and Applied Science, Manhattan. [Google Scholar]
- Gier H. T. 1975. Ecology and behavior of the coyote (Canis latrans). Pp. 247–262 in The wild canids (Fox M. W., ed.). Van Nostrand Reinhold Company, New York. [Google Scholar]
- Golani I., Mendelssohn H. 1971. Sequences of precopulatory behavior of the jackal (Canis aureus L.). Behaviour 38:169–192. [Google Scholar]
- Green J. S., Knowlton F. F., Pitt W. C. 2002. Reproduction in captive wild-caught coyotes (Canis latrans). Journal of Mammalogy 83:501–506. [Google Scholar]
- Hamlett G. W. D. 1938. The reproductive cycle of the coyote. United States Department of Agriculture, Technical Bulletin 616:1–11. [Google Scholar]
- Hatier K. G. 1995. Effects of helping behaviors on coyote packs in Yellowstone National Park, Wyoming. M.S. thesis, Montana State University, Bozeman. [Google Scholar]
- Hodges C. M. 1990. The reproductive biology of the coyote (Canis latrans). Ph.D. dissertation, Texas A&M University, College Station. [Google Scholar]
- Hunter F. M., Petrie M., Otronen M., Birkhead T., Møller A. P. 1993. Why do females copulate repeatedly with one male? Trends in Ecology and Evolution 8:21–26. [DOI] [PubMed] [Google Scholar]
- Kennelly J. J., Johns B. E. 1976. The estrous cycle of coyotes. Journal of Wildlife Management 40:272–277. [Google Scholar]
- Kennelly J. J., Johns B. E., Breidenstein C. P., Roberts J. D. 1977. Predicting female coyote breeding dates from fetal measurements. Journal of Wildlife Management 41:746–750. [Google Scholar]
- Kleiman D. G., Eisenberg J. F. 1973. Comparisons of canid and felid social systems from an evolutionary perspective. Animal Behaviour 21:637–659. [DOI] [PubMed] [Google Scholar]
- Knowlton F. F. 1972. Preliminary interpretations of coyote population mechanics with some management implications. Journal of Wildlife Management 36:369–382. [Google Scholar]
- Mengel R. M. 1971. A study of dog–coyote hybrids and implications concerning hybridization in Canis. Journal of Mammalogy 52: 316–336. [PubMed] [Google Scholar]
- Sacks B. N. 2005. Reproduction and body condition of California coyotes (Canis latrans). Journal of Mammalogy 86:1036–1041. [Google Scholar]
- Schenkel R. 1967. Submission: its features and functions in the wolf and dog. American Zoologist 7:305–381. [Google Scholar]
- Sillero-Zubiri C., Gottelli D., Macdonald D. W. 1996. Male philopatry, extra-pack copulations and inbreeding avoidance in Ethiopian wolves (Canis simensis). Behavioral Ecology and Sociobiology 38:331–340. [Google Scholar]
- Silver H., Silver W. T. 1969. Growth and behavior of the coyote-like canid of northern New England with observations on canid hybrids. Wildlife Monographs 17:1–41. [Google Scholar]
- Stellflug J. N., Muse P. D., Everson D. O., Louis T. M. 1981. Changes in serum progesterone and estrogen of the nonpregnant coyote during the breeding season. Proceedings of the Society for Experimental Biology and Medicine 167:220–223. [DOI] [PubMed] [Google Scholar]
- Tsutsui T. 1989. Gamete physiology and timing of ovulation and fertilization in dogs. Journal of Reproduction and Fertility, Supplement 39:269–275. [PubMed] [Google Scholar]
- Windberg L. A. 1995. Demography of a high-density coyote population. Canadian Journal of Zoology 73:942–954. [Google Scholar]


