Abstract
BACKGROUND
Historically, invasive hemodynamic guidance was not superior compared to clinical assessment in patients admitted with acute decompensated heart failure (ADHF). This study assessed the accuracy of clinical assessment versus. invasive hemodynamics in patients with ADHF.
METHODS AND RESULTS
We conducted a prospective cohort study of patients admitted with ADHF. Prior to RHC, physicians categorically predicted right atrial pressure (RAP), pulmonary capillary wedge pressure (PCWP), cardiac index (CI) and hemodynamic profile (Wet/Dry, Warm/Cold) based on physical exam and clinical data evaluation. “Warm”= CI > 2.2 L/min/m2; “Wet” = PCWP >18 mmHg. 218 surveys (83 cardiology fellows, 55 attending cardiologists, 45 residents, 35 interns) evaluating 97 patients were collected. 46% were receiving inotropes prior to RHC. Positive and negative predictive values of clinical assessment compared to RHC for the “Cold and Wet” subgroup were 74.7% and 50.4%. Accuracy of categorical prediction was 43.6% for RAP, 34.4% for PCWP, 49.1% for CI, and did not differ by clinician (P >0.05 for all). Interprovider agreement was 44.4%. Therapeutic changes following RHC occurred in 71.1% overall (P <0.001).
CONCLUSIONS
Clinical assessment of patients with advanced heart failure presenting with ADHF has low accuracy across all training levels, with exaggerated rates of misrecognition of the most high-risk patients.
Keywords: Heart Failure, Hemodynamics, Physical Exam
Graphical abstract

INTRODUCTION
Inpatient evaluation of patients with advanced heart failure (HF) who present with decompensation involves clinical assessment, non-invasive imaging, laboratory evaluation and occasionally right heart catheterization (RHC) to guide therapies. Though not applicable in the inpatient setting, the recent study of implantable hemodynamic monitoring devices in patients with chronic heart failure was shown to reduce HF-related hospitalizations compared to standard of care alone (1, 2).
The ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness Trial) was an unblinded, randomized controlled trial that compared invasive hemodynamic-guided therapy versus clinical assessment in patients admitted with ADHF. ESCAPE failed to demonstrate a reduction in hospitalization or mortality at six months when invasive hemodynamics were added to clinical assessment (3). However, subgroup analyses from ESCAPE showed that residual elevated filling pressures post-treatment were associated with higher rates of adverse outcomes (4). This data questions whether more routine invasive hemodynamic assessments should be considered to better risk stratify advanced heart failure patients who present with ADHF.
Treatment of patients presenting with ADHF is based on patient symptomology and comprehensive clinical assessment, which are used to predict intracardiac filling pressures and adequacy of cardiac output. However, data on the accuracy of physician clinical assessments in patient with advanced HF is not well studied, especially in the setting on existing inotropic support. In the present study, we evaluated the accuracy of physician clinical assessment of patients admitted with ADHF, using invasive measurements from RHC as the gold standard for comparison. Our secondary aim was to determine whether knowledge of hemodynamic measurements had an impact on treatment strategy.
METHODS
Study design and patient selection:
We conducted a single-center, prospective cohort study of a convenience sample consisting of 97 patients at the University of Chicago Medical center between January 2016 and January 2018. Patients were eligible for inclusion if they had a diagnosis of heart failure (reduced or preserved ejection fraction), were > 18 years of age, and presented in either the outpatient or inpatient setting with suspicion of ADHF, as defined by worsening clinical symptoms or objective markers consistent with worsening congestion or perfusion. Patients were excluded if they were mechanically ventilated or presented following cardiac arrest. Acute HF was defined as new onset (< 1 month) HF with normal LV cavity end-diastolic dimensions (5) (5.3 cm for female; 5.9 cm for male) in the setting of reduced left-ventricular ejection fraction by history. Outpatients were screened for study inclusion if they were evaluated in the clinic setting and sent directly for RHC if deemed necessary by the treatment team. Inpatients were enrolled in the emergency room, on the cardiac telemetry floor, or in the cardiac care unit if they were being referred for RHC. Decision to proceed with RHC was for suspicion of ADHF. Members of the team that initially evaluated the patient including attending cardiologists, cardiology fellows, medical residents or interns were eligible to complete a pre-RHC survey. The treatment team alone decided if RHC was necessary to guide management. Patients were not excluded if they were on existing inotropes or vasoactive agents, but were excluded if they were on temporary mechanical circulatory support (MCS) or had a left ventricular assist device. Patients were excluded if RHC was performed more than 48 hours after admission. This study was approved by the center institutional review board, with all patients providing informed consent.
Survey Assessment
Once a patient was screened, the study survey was provided to the clinical team for completion prior to RHC. The clinical team typically consisted of an attending cardiologist specializing in HF, advanced HF or general cardiology fellow, senior (2nd or 3rd year post-graduate) internal medicine residents, and junior resident physician (1st year post-graduate or intern) specializing in internal medicine or emergency medicine. Physicians were only able to complete a pre-RHC survey if they directly interviewed and physically examined the patient. Patients were included even if only a single survey was filled out prior to RHC. All laboratory values, prior documentation and diagnostic tests were available to the physician prior to survey completion. Survey assessments were completed in a blinded fashion and were only included if the RHC took place within 12 hours of survey completion. The study team instructed physicians on the study protocol at the beginning of every clinical rotation period to ensure protocol compliance. Surveys were collected in a secure folder on a twice-a-day basis by the study team. Additionally, the study team audited the clinical course of the patient between time of survey completion and time of RHC. Patients were excluded if significant clinical changes occurred following survey completion and prior to RHC, including hemodynamic deterioration or improvement, alteration in vasoactive medication or initiation of MCS. Data was audited continuously to adjudicate patient outcomes during and following admission.
The survey assessments consisted of four questions. Physicians were asked to classify patients into one of four hemodynamic profiles first described by Nohria and Stevenson (Figure 1) (6). “Cold” perfusion status was defined as a cardiac index (CI) ≤ 2.2 L/min/m2 while elevated filling pressures or “Wet” was defined as pulmonary capillary wedge pressure (PCWP) > 18 mmHg. Thereafter, physicians were asked to categorically predict right atrial pressure (RAP) (mmHg; <6, 6–12, 13–18 and >18), PCWP (mmHg; 7–12, 13–18, 19–24 and >24) and CI (L/min/m2; <1.5, 1.5–2.2, and > 2.2). Regarding categorical prediction of hemodynamic variables, concordance was defined as correct prediction of the range for a given hemodynamic variable compared to the actual hemodynamics from RHC. When the hemodynamic categorical prediction differed by one category between the survey and RHC hemodynamics directionality notwithstanding, this would be classified as a one category error. Discordance of two or more categories were grouped together.
Figure 1.

Hemodynamic Profiles in Patients With Heart Failure
Abbreviations: CI, cardiac index; PCWP, pulmonary capillary wedge pressure.
Right Heart Catheterization
Advanced HF (n=92 patients) and interventional (n=5 patients) cardiologists performed the RHCs for patients enrolled. Supine RHC was performed, with the most common access for indwelling pulmonary artery catheter (PAC) placement occurring in the right internal jugular vein. Hemodynamics collected included RAP, right ventricular pressure, pulmonary artery pressure, PCWP, estimated Fick cardiac output and index, systemic and pulmonary vascular resistances. Thermodilution cardiac output and index measurements were collected at the discretion of primary operator performing the procedure. Measurements were obtained at end-expiration. Formula for estimated oxygen uptake was: VO2 (ml/min) = 125 (ml/min/m2) x body surface area (m2)(7). Body surface area was calculated according to the formula of Dubois (8). Estimated Fick cardiac index was then calculated by the following equation: [125 (ml/min/m2) x body surface area]/[(13.6*hemoglobin (g/L)* (ambient oxygen saturation – mixed venous saturation)]/body surface area. Post-procedure, the PAC was kept in place with subsequent transfer to the cardiac care unit.
Statistical Analyses
Descriptive statistics are reported as median (interquartile range), mean (standard deviation) or as number (%). Accuracy of clinical assessment for correct selection of hemodynamic classification (Figure 1), was assessed by positive and negative predictive values (PPV and NPV). Comparison of accuracy between physicians for categorical hemodynamic variables was completed by using Pearson’s Chi-Square Method. Fischer’s exact test was used to determine if significant changes to treatments were made following RHC. Univariate linear/logistic regression analysis was used to determine if any covariates affected the accuracy of prediction of overall hemodynamic classification or individual hemodynamic variables. Statistical significance was set at 0.05, and all tests were 2-tailed. Statistical analyses were conducted using Stata version 15 (StataCorp, Inc., College Station, TX).
RESULTS
Study Sample Characteristics
Patient characteristics are shown in Table 1. 97 patients were included, with 218 physician surveys completed. 74.2% (n=72) of the cohort had ≥1 survey (range 2–5) completed. Median patient age was 60 (51, 67.5) years, 80.4% were male. Median RAP was 15 (9.5, 21) mmHg, PCWP 26 (21, 30.5) mmHg and estimated Fick CI 1.9 (1.6, 2.5) L/min/m2. Approximately 46% of patients were on inotropes and/or vasoactive medications prior to RHC. At one-year, all-cause mortality was 36.1%, 14.4% underwent heart transplantation, 20.6% underwent left ventricular assist device implantation, and 28.9% survived with medical therapy alone.
Table 1.
Baseline Descriptive Characteristics of the Overall Cohort (n=97)
| Age, median (IQR), y | 60 (51, 67.5) |
| Male, No. (%) | 78 (80.4) |
| BMI, median (IQR), kg/m2 | 27.9 (23.9, 32.4) |
| Ischemic cardiomyopathy, No. (%) | 46 (47.4) |
| Race/Ethnicity, No. (%) | |
| White | 47 (48.5) |
| Black | 40 (41.2) |
| Other | 10 (9.3) |
| Left ventricular ejection fraction, median (IQR), % | 21 (17.5, 28) |
| LVEDD, mean (SD), cm | 6.6 (5.6, 7.4) |
| Heart failure classification, No. (%) | |
| Chronic | 87 (89.7) |
| Acute | 10 (10.3) |
| Implantable defibrillator, No. (%) | |
| Single/Dual Chamber | 39 (40.2) |
| Biventricular | 29 (29.9) |
| None | 29 (29.9) |
| HFpEF, No. (%) | 6 (6.2) |
| Cardiac amyloidosis | 2 (2.1) |
| Unknown etiology | 4 (4.1) |
| Mode of admission, No. (%) | |
| Outside hospital transfer | 56 (57.7) |
| Clinic presentation | 22 (22.7) |
| Emergency room presentation | 19 (19.6) |
| Sodium, median (IQR), mmol/L | 138 (133.5, 141) |
| Renal function on admission | |
| Creatinine, median (IQR) mg/dl | 1.5 (1.1, 2.1) |
| Glomerular filtration rate, median (IQR) ml/min/1.73 m2 | 46 (31, 66.5) |
| Admission serum lactic acid, median (IQR), mmol/L* | 1.6 (1.2, 2.1) |
| Admission NT-proBNP, median (IQR), pg/mL† | 5,647 (2,570, 13,765) |
| Pre-pulmonary artery catheterization therapy, No. (%) | |
| Standard medical therapy | 52 (53.6) |
| Inotropes and/or vasoactives | 45 (46.4) |
| One-year outcomes, No. (%) | |
| All-cause mortality | 35 (36.1) |
| Progression to heart transplantation | 14 (14.4) |
| Progression to LVAD | 20 (20.6) |
| Survival with medical therapy | 28 (28.9) |
| Systolic blood pressure, median (IQR), mmHg | 103 (91, 118) |
| Diastolic blood pressure, median (IQR), mmHg | 68 (61, 77) |
| Mean arterial pressure, median (IQR), mmHg | 83 (71, 91) |
| Heart rate at time of evaluation, median (IQR), mmHg | 85 (76.5, 98.5) |
| Right atrial pressure, median (IQR), mmHg | 15 (9.5, 21) |
| Pulmonary capillary wedge pressure, median (IQR) mmHg | 26 (21, 30.5) |
| Pulmonary artery systolic pressure, median (IQR), mmHg | 59 (46, 65.5) |
| Pulmonary artery diastolic pressure, median (IQR), mmHg | 30 (22.5, 36.5) |
| Estimated Fick cardiac index, median (IQR), L/min/m2 | 1.9 (1.6, 2.5) |
| Home Medications, No. (%) | |
| ACE Inhibitors or ARBs | 36 (37.1) |
| Beta-Blockers | 68 (70.1) |
| Aldosterone antagonist | 34 (35.1) |
| Loop diuretics | 55 (56.7) |
| Digoxin | 16 (16.5) |
| Antiarrhythmics | 31 (40) |
| Aspirin | 28 (28.9) |
| Statin | 40 (41.2) |
| Systemic blood thinners (Warfarin or Direct anticoagulant) | 27 (27.8) |
| Clopidogrel/Ticagrelor | 16 (16.5) |
Abbreviations: ACE; angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; BMI, body mass index (calculated as weight in kilogram divided by height in meters squared); cm, centimeter; HFpEF, heart failure with preserved ejection fraction; IQR, interquartile range; kg/m2, kilogram per meters squared; LVEDD, left-ventricular end-diastolic dimension; LVAD, left-ventricular assist device; No, number; NT-proBNP, N-terminal pro brain natriuretic peptide; mmol/L, millimoles per liter; mg/dl, milligrams per deciliter; ml/min/m2, milliliters per minute per meters squared; mmHg, millimeters of mercury; SD, standard deviation; y; year.
n=77 patients with available admission serum lactic acid
n=85 patients with available admission NT-proBNP
Overall Physician Accuracy
Cardiology fellows completed the most surveys (38.1%, n=83), followed by attending cardiologists (25.2%, n=55), senior internal medicine resident physicians (20.6%, n=45) and interns (16.1%, n=35). Overall diagnostic accuracy of hemodynamic classification is shown in Figure 2. Patients defined as “Cold and Wet” by RHC were most prevalent (59.6%, n=130). PPV for this category was 74.7% and NPV was 50.4%. “Warm and Wet” profiles were the next most prevalent hemodynamic classifications (20.2%, n=44), followed by “Warm and Dry” (13.8%, n=30) and “Cold and Dry” (6.4%, n=14). PPV’s and NPV’s were comparable for “Warm and Wet” and “Warm and Dry”, with lower PPV and higher NPV seen in patients identified as “Cold and Dry”. 73 patients (75.3%) had thermodilution-derived cardiac outputs measured, which did not significantly change accuracy of hemodynamic classification (Supplemental Figure 1). This was also the case when hemodynamic classifications were predicted only by attending cardiologists (Supplemental Figure 2).
Figure 2.
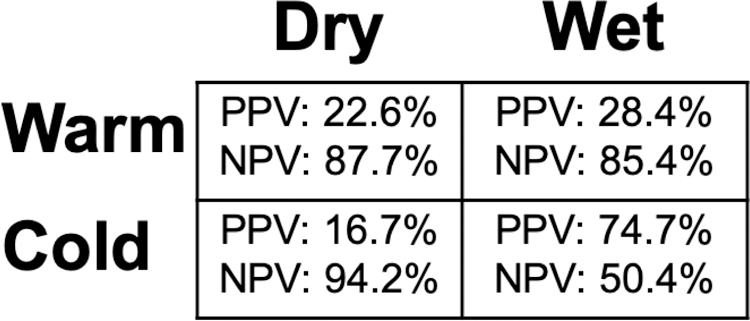
Positive Predictive And Negative Predictive Values by Hemodynamic Profile for the Overall Cohort
Abbreviations: PPV, positive predictive value; NPV, negative predictive value.
Overall physician accuracy for categorical hemodynamic variable prediction is shown in Figure 3A. RAP prediction was accurate in 43.6% of total surveys. PCWP prediction was more inaccurate overall, with one category error occurring in 41.3% of surveys, and at least two category error occurring in 24.3% of surveys. Accurate CI prediction occurred in 49.1% of surveys, with a total of 37.2% (n=81) of surveys incorrectly predicting perfusion status as “Warm” or “Cold”. Out of the 47 patients evaluated by both an attending physician and at least one other physician of differing training level, 37 (78.3%) patients had incorrect categorical hemodynamic predictions by both the attending physician and the other physician.
Figure 3.
Categorical Hemodynamic Variable Accuracy For A) All Physicians and by B) RA Pressure C) PCWP And D) CI
Abbreviations: CI, cardiac index; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure
RAP Prediction
Accuracy of RAP prediction by level of physician experience is shown in Figure 3B. Accounting for sample size differences by physician experience, there was no significant difference in the proportion of accurate predictions of RAP when stratified by physician experience (chi-square = 0.35, P=0.95). Interns had the highest proportion of severely inaccurate predictions (≥2 category error) compared to other physicians (chi-square = 10.1, P=0.02). No overall difference was found in the directionality of inaccurate RAP predictions (overestimation, n=57 vs. underestimation n=66; chi-square = 0.41, P=0.52) Out of the 33 surveys classified as ≥2 category error in prediction, 6 (17.6%; 3 residents, 2 fellows, 1 intern) severely underestimated the prediction of actual RAP (when measured as ≥ 24 mmHg).
PCWP Prediction
Accuracy of PCWP prediction by physician experience is shown in Figure 3C. There was no significant difference in proportion of accurate predictions of PCWP when stratified by physician experience (chi-square = 4.02, P = 0.26). The proportion of severely inaccurate predictions (≥2 category error) did not differ by experience (chi square = 5.98, P=0.11). Overall, inaccurate predictions (≥ 1 category error) of PCWP collectively underestimated the actual value by RHC (underestimation, n=98 vs. overestimation, n=45; chi-square = 11.73, P=0.0006). Out of the 53 surveys classified as ≥2 category error in prediction, 24 (43.6%; 9 fellows, 6 attendings, 5 interns; 4 residents) severely underestimated the actual PCWP (when measured as ≥ 30 mmHg).
CI Prediction
Accuracy of CI prediction by physician experience is shown in Figure 3D. There was no significant difference in proportion of accurate predictions of CI when stratified by experience (chi-square = 2.35, P=0.50). 50.9% (n=111) of surveys incorrectly characterized CI. The majority of predictions overestimated CI systematically, with 34.2% of surveys (n=38) overestimating CI when CI was ≤ 2.2 L/min/m2 and 22.5% of surveys (n=25) overestimating CI in setting of very low CI (predicted CI 1.5–2.2 L/min/m2, when CI was < 1.5 L/min/m2). 38.8% (n=43) of surveys underestimated CI when CI was > 2.2 L/min/m2, and 4.5% of surveys (n=5) predicted CI was < 1.5 L/min/m2 when CI was 1.5–2.2 L/min/m2.
Predictors of Accuracy
Linear and logistic regression analysis was performed to determine covariates associated with accurate hemodynamic profile prediction (Table 2). Improved RAP accuracy was modestly associated with presence of pulse pressure ≤25% (OR 1.92, 95% CI 1.01–3.63). Accurate CI prediction decreased by 53% when systolic blood pressure was > 90 mmHg at the time of RHC, (OR 0.47, 95% CI 0.25–0.89; P = 0.02). There was a trend towards more accurate hemodynamic profile predictions by attending physicians (OR 1.80, 95% CI 0.97–3.35; P = 0.06). Conversely, the odds of correct hemodynamic classification decreased by 58% when performed by interns (OR 0.42, 0.19–0.93; P =0.03).
Table 2.
Univariate predictors of hemodynamic profile and variable accuracy.
| Hemodynamic Profile Prediction Accuracy (OR 95% CI) | RA Pressure Prediction Accuracy (OR 95% CI) | PCWP Prediction Accuracy (OR 95% CI) | CI Prediction Accuracy (OR 95% CI) | |
|---|---|---|---|---|
| Attending Cardiologist | 1.80 (0.97–3.35) P=0.06 | 1.10 (0.60–2.05) P=0.75 | 1.53 (0.82–2.88) P=0.18 | 1.48 (0.80–2.74) P=0.21 |
| Cardiology Fellow | 1.02 (0.59–1.77) P=0.93 | 1.07 (0.62–1.85) P=0.82 | 1.23 (0.70–2.18) P=0.47 | 0.94 (0.55–1.63) P=0.84 |
| Senior Resident | 0.95 (0.49–1.84) P=0.88 | 0.83 (0.43–1.62) P=0.59 | 0.63 (0.31–1.32) P=0.22 | 0.99 (0.51–1.91) P=0.98 |
| Intern | 0.42 (0.19–0.93) P=0.03 | 0.97 (0.47–2.00) P=0.93 | 0.61 (0.27–1.39) P=0.24 | 0.65 (0.31–1.35) P=0.24 |
| Acute Kidney Injury | 1.47 (0.81–2.65) P=0.20 | 0.55 (0.30–1.01) P=0.05 | 1.41 (0.77–2.61) P=0.27 | 1.27 (0.71–2.28) P=0.28 |
| Chronic Heart Failure History | 1.75 (0.68–4.53) P=0.25 | 0.68 (0.27–1.67) P=0.39 | 0.54 (0.22–1.34) P=0.19 | 0.86 (0.35–2.13) P=0.78 |
| SBP > 90 mmHg | 0.78 (0.42–1.45) P=0.44 | 1.29 (0.69–2.42) P=0.42 | 0.69 (0.37–1.31) P=0.26 | 0.47 (0.25–0.89) P=0.02 |
| BMI | 0.98 (0.94–1.02) P=0.33 | 0.98 (0.98–1.06) P=0.27 | 1.04 (1.00–1.09) P=0.05 | 0.98 (0.94–1.03) P=0.24 |
| Inotropes/Vasoactives Pre-RHC | 1.73 (1.00–2.97) P=0.05 | 1.44 (0.84–2.49) P=0.19 | 0.92 (0.52–1.63) P=0.78 | 1.15 (0.67–1.97) P=0.62 |
| Lactic Acid > 2.1 | 2.02 (0.79–5.21) P=0.14 | 0.83 (0.33–2.10) P=0.70 | 1.08 (0.42–2.81) P=0.87 | 0.62 (0.25–1.57) P=0.39 |
| Outpatient Admission | 1.16 (0.63–2.14) P=0.64 | 1.16 (0.62–2.15) P=0.64 | 1.44 (0.76–2.71) P=0.26 | 1.56 (0.84–2.90) P=0.16 |
| Pulse Pressure ≤25% | 1.41 (0.75–2.66) P=0.29 | 1.92 (1.01–3.63) P=0.0.05 | 1.53 (0.80–2.92) P=0.20 | 0.95 (0.50–1.78) P=0.86 |
Abbreviations: BMI, body mass index (calculated as weight in kilogram divided by height in meters squared); CI, confidence interval; MCS, mechanical circulatory support; RHC, OR, odds ratio; right heart catheterization; SBP, systolic blood pressure.
Interprovider Agreement and Therapy Shift
Interprovider agreement for clinical assessment was analyzed for patients who had multiple survey completed (n=72). We defined disagreement as differences in hemodynamic variable category prediction by at least one category, for at least two hemodynamic variables. Discordant clinical assessments occurred in 44.4% of patents with multiple surveys (4A).
Following RHC, significant changes in therapies were classified as addition of a new inotrope/vasoactive agent, placement of MCS +/− inotrope, addition of MCS +/− inotrope for those on existing inotrope, or weaning of inotrope/MCS occurred in 71.1% of the cohort (Figure 4B, P <0.001). The addition of new inotropes/vasopressors and adjustment of inotrope/vasopressor dosing or addition of MCS to those on existing parenteral therapies were the treatment shifts which occurred most often overall (Supplemental Table 1).
Figure 4.
A) Interprovider Agreement in Clinical Assessment For Patients With Multiple Survey Assessments (n=72) B) Percentage of Patients With Significant Changes in Therapy Post-RHC in the Overall Study Population (n=97)
Abbreviations: RHC, right heart catheterization
DISCUSSION
We analyzed the accuracy of physician clinical assessments of hemodynamic profiles compared with invasive measurements in consecutive advanced HF patients presenting with ADHF. Our main findings are: (1) The “Cold and Wet” subgroup is identified at an alarmingly low rate by clinical assessment; (2) Overall clinical assessments of RAP, PCWP, and CI are inaccurate regardless of clinical experience, with clinical assessments by interns the least accurate; (3) Interobserver agreement in clinical assessment was low (4) RHC-guided therapy altered clinical decision-making in a majority of patients.
Filling pressures are assessed by examination of jugular venous pressure, lower extremity edema, auscultation of rales, presence of ascites, and a positive hepatojugular reflux. Assessment of N-terminal pro-B type natriuretic peptide, mitral valve annular tissue Doppler velocities (9) and inferior vena diameter/distensibility may also further elucidate filling pressures (10). Narrow pulse pressure (<25%) is commonly seen in advanced HF patients and may correlate with low CI (11, 12). Although a robust clinical marker in patients with sepsis (13), elevated lactic acid levels may not be present in high-risk patient subsets (“Cold and Wet”) with ADHF (14). Furthermore, identification of cool extremities as a marker of impaired systemic perfusion can be subjective with reported sensitivity of just 20% in patients with low CI (12, 15).
In this cohort, the accuracy of hemodynamic profile classification was exceedingly low for the most high-risk subgroup (“Cold and Wet). Sustained elevated filling pressures in HF patients are associated with increased long-term mortality (16), though the relationship between CI and outcome is less established. This does not diminish the importance of identifying those with low CI (16) as this often guides prioritization for heart transplantation listing(17).
Overall physician clinical assessment of hemodynamics by categorical selection showed < 50% accuracy in predicting RAP, PCWP and CI. Other than worse prediction of RAP by interns, no overall difference in accuracy across all levels of provider experience was observed. A non-negligible degree of severely inaccurate predictions of PCWP (23% overall ≥ 2 category error) and inability to correctly classify a patient as warm or cold by CI highlight the potential impact of managing patients with advanced HF without hemodynamic data. We observed a systematic underestimation of PCWP and overestimation of CI, reflecting the high prevalence of inaccurately identified “Cold and Wet” patients. These findings may reflect the challenges of assessing central congestion along with clinical adaptation to abnormal filling pressures and inadequate perfusion in the advanced heart failure patient. Furthermore, decoupling of right and left-sided filling pressures, as well as an absence of clinically detectable pulmonary congestion that can further cloud accurate clinical assessment, despite provider experience (18, 19). This is reflected in the high prevalence of observed clustering of inaccurate clinical assessments performed by both attending physicians and other clinicians evaluating the same patient. All but four surveys completed by attending physicians were done by HF cardiologists, suggesting that even the most highly trained clinicians can misclassify hemodynamic profiles.
There were few clinical characteristics associated with improved clinical assessment accuracy. Systolic blood pressure > 90 mmHg was associated with reduced accuracy of CI prediction suggesting that low cardiac index can often be present in the setting of relatively preserved blood pressure. Poor interprovider agreement was observed, emphasizing the challenges in the clinical assessment of advanced HF patients. Multiple providers care for HF patients during a given hospitalization, and potential mismanagement due to flawed clinical assessment cannot be understated. In this cohort, invasive hemodynamics led to significant alterations of therapy in ~68% of the cohort. While we do not have data to support whether therapeutic decisions following RHC altered patient outcomes, it is clear that decision-making was altered by hemodynamic data. Prior studies evaluating the concordance and association of clinical assessment with both invasive hemodynamics and outcomes in patients with ADHF excluded patients on inotropic support and do not assess interprovider variabilities in clinical assessment (12, 20)—two important and unique aspects of this study.
Although no difference in outcomes were shown with or without the use of RHC-guidance in the primary trial, subanalyses from ESCAPE showed that post-treatment RAP + PCWP ≥ 30 mmHg was associated with significant mortality (4). Presence of these high-risk subtypes further underscores the potential consequence of hemodynamic misclassification. ADHF may present with a diversity of phenotypes, and the idea that hemodynamic assessment should play a limited role in the management of these patients may lead poor risk-stratification (21), a magnified effect of variability in clinical assessment, and misguided tailoring of medical therapies.
LIMITATIONS
The findings of the present study need to be interpreted within the context of several limitations. The number of patients analyzed was moderate in size and limited primarily to an advanced HF cohort. The imbalance of number of physicians in each group surveyed may limit comparisons in clinical assessment accuracy between physicians with varying experience. The study was a prospective convenience sample of patients undergoing RHC due to suspicion of ADHF. Patients that may have been eligible for enrollment may have been missed due to an inability of physicians to complete survey assessments in a timely manner. In addition, some patients in the study had assessments completed by one provider, precluding interobserver agreement analysis. The maximum time allowed between completion of survey assessment for a given patient and RHC was 12 hours. We audited all surveys and disqualified patients from study inclusion if significant clinical changes occurred during that period, though subtle changes may have occurred which theoretically could alter a physician’s assessment if completed at different times prior to RHC. We calculated an estimated Fick CI according to the Dehmer formula (7) to establish cutoffs for “Warm” and “Cold”. Although estimated Fick CI has its limitations compared to directly measured Fick CI (22), we chose this over thermodilution CI due to the high proportion of regurgitant valvular lesions present making thermodilution estimates of CI susceptible to inaccurate calculation.
CONCLUSIONS
Clinical prediction of hemodynamics in patients with advanced HF presenting ADHF has low accuracy independent of a physician’s clinical experience and can be highly variable between physicians evaluating the same patient. Moreover, there was a surprisingly high rate of misdiagnosis of high-risk patients with low perfusion status and elevated filling pressures. Knowledge of hemodynamics impacted choices of medical therapies. However, whether those decisions impact any aspect of clinical outcomes remains to be determined. In the years since the ESCAPE trial, medical therapies have advanced and there is greater appreciation of how to use hemodynamic information (21, 23). The trial did not prescribe a protocol on how to use the hemodynamic information, which has been an important criticism of all negative studies of hemodynamic measurements. This study cohort overall was more ill compared to that of the ESCAPE trial, with both a high utilization of inotropes and number of patient-comorbidities. This may preclude applying the finding of comparable efficacy in clinical assessment and invasive hemodynamics in the management of HF patients. Accordingly, a reevaluation of the impact of hemodynamic monitoring on clinical outcomes may be warranted. This study supports more routine use of RHC in advanced HF patients given the low accuracy of clinical assessment.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to acknowledge the clinicians who contributed to their time in completing survey assessments during patient care.
FUNDING
Dr. Narang is supported by the Ruth L. Kirschstein Institutional National Research Service Award T32 Training Grant (T32HL007381) from the National Heart Lung and Blood Institute.
DISCLOSURES
Dr. Uriel receives grant support from Abbott Healthcare and Medtronic. Dr. Burkhoff has received an unrestricted educational grant from Abiomed. Dr. Jeevanandam receives consulting fees from Abbott Healthcare. There are no other disclosures to report. Dr. Laffin receives consulting fees from Vascular Dynamics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Givertz MM, Stevenson LW, Costanzo MR, Bourge RC, Bauman JG, Ginn G, et al. Pulmonary Artery Pressure-Guided Management of Patients With Heart Failure and Reduced Ejection Fraction. J Am Coll Cardiol 2017;70(15):1875–86. doi: 10.1016/j.jacc.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 2.Abraham J, Bharmi R, Jonsson O, Oliveira GH, Artis A, Valika A, et al. Association of Ambulatory Hemodynamic Monitoring With Clinical Outcomes in a Concurrent Matched Control Analysis. JAMA Cardiol 2019. doi: 10.1001/jamacardio.2019.1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA 2005;294(13):1625–33. doi: 10.1001/jama.294.13.1625 [DOI] [PubMed] [Google Scholar]
- 4.Ma TS, Paniagua D, Denktas AE, Jneid H, Kar B, Chan W, et al. Usefulness of the Sum of Pulmonary Capillary Wedge Pressure and Right Atrial Pressure as a Congestion Index that Prognosticates Heart Failure Survival (from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness Trial). Am J Cardiol 2016;118(6):854–9. doi: 10.1016/j.amjcard.2016.06.040 [DOI] [PubMed] [Google Scholar]
- 5.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography 2015;28(1):1–39. e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 6.Nohria A, Tsang SW, Fang JC, Lewis EF, Jarcho JA, Mudge GH, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol 2003;41(10):1797–804. [DOI] [PubMed] [Google Scholar]
- 7.Dehmer GJ, Firth BG, Hillis LD. Oxygen consumption in adult patients during cardiac catheterization. Clin Cardiol 1982;5(8):436–40. [DOI] [PubMed] [Google Scholar]
- 8.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989;5(5):303–11; discussion 12–3. [PubMed] [Google Scholar]
- 9.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29(4):277–314. doi: 10.1016/j.echo.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28(1):1–39 e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 11.Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA 1989;261(6):884–8. [PubMed] [Google Scholar]
- 12.Drazner MH, Hellkamp AS, Leier CV, Shah MR, Miller LW, Russell SD, et al. Value of clinician assessment of hemodynamics in advanced heart failure: the ESCAPE trial. Circ Heart Fail 2008;1(3):170–7. doi: 10.1161/CIRCHEARTFAILURE.108.769778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunnerson KJ, Saul M, He S, Kellum JA. Lactate versus non-lactate metabolic acidosis: a retrospective outcome evaluation of critically ill patients. Crit Care 2006;10(1):R22. doi: 10.1186/cc3987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narang N, Laffin L, Kalathiya R, Chung B, Nguyen A, Costanzo MR, et al. (149) - Normal Serum Lactic Acid is Discordant with Shock in Advanced Heart Failure Patients. The Journal of Heart and Lung Transplantation 2018;37(4, Supplement):S67–S8. doi: 10.1016/j.healun.2018.01.151 [DOI] [Google Scholar]
- 15.Thibodeau JT, Drazner MH. The Role of the Clinical Examination in Patients With Heart Failure. JACC Heart Fail 2018;6(7):543–51. doi: 10.1016/j.jchf.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 16.Cooper LB, Mentz RJ, Stevens SR, Felker GM, Lombardi C, Metra M, et al. Hemodynamic Predictors of Heart Failure Morbidity and Mortality: Fluid or Flow? J Card Fail 2016;22(3):182–9. doi: 10.1016/j.cardfail.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adult Heart Allocation: Allocation of Hearts and Heart-Lungs: Organ Procurement and Transplant Network; 2018. [Available from: https://optn.transplant.hrsa.gov/governance/public-comment/modify-adult-heart-allocation-2016-2nd-round/.
- 18.Drazner MH, Velez-Martinez M, Ayers CR, Reimold SC, Thibodeau JT, Mishkin JD, et al. Relationship of right- to left-sided ventricular filling pressures in advanced heart failure: insights from the ESCAPE trial. Circ Heart Fail 2013;6(2):264–70. doi: 10.1161/CIRCHEARTFAILURE.112.000204 [DOI] [PubMed] [Google Scholar]
- 19.Mahdyoon H, Klein R, Eyler W, Lakier JB, Chakko SC, Gheorghiade M. Radiographic pulmonary congestion in end-stage congestive heart failure. Am J Cardiol 1989;63(9):625–7. [DOI] [PubMed] [Google Scholar]
- 20.Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J 2013;34(11):835–43. doi: 10.1093/eurheartj/ehs444 [DOI] [PubMed] [Google Scholar]
- 21.Tehrani BN, Truesdell AG, Sherwood MW, Desai S, Tran HA, Epps KC, et al. Standardized Team-Based Care for Cardiogenic Shock. J Am Coll Cardiol 2019;73(13):1659–69. doi: 10.1016/j.jacc.2018.12.084 [DOI] [PubMed] [Google Scholar]
- 22.Narang N, Thibodeau JT, Levine BD, Gore MO, Ayers CR, Lange RA, et al. Inaccuracy of estimated resting oxygen uptake in the clinical setting. Circulation 2014;129(2):203–10. doi: 10.1161/CIRCULATIONAHA.113.003334 [DOI] [PubMed] [Google Scholar]
- 23.Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv 2019. doi: 10.1002/ccd.28329 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




