Abstract
Statin medications [3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors] are generally used to treat hypercholesterolemia. Lichenoid drug eruptions are a potential cutaneous side effect of medications including antibiotics, antimalarials, and statins. This drug eruption can mimic features of idiopathic lichen planus in clinical presentation and pathology. We describe the case of a 73-year-old man who developed a lichenoid drug eruption secondary to atorvastatin. His clinical features, in addition to histological findings, helped to establish the diagnosis. The cutaneous eruption resolved one month after the cessation of atorvastatin and with corticosteroid therapy. Statins have been associated with adverse events including bullous dermatosis, eosinophilic fasciitis, lichenoid drug eruption, and phototoxicity. Lichenoid drug eruption associated with statin therapy requires discontinuation of the statin medication; an alternative class of medication for the treatment of hypercholesterolemia is usually necessary.
Keywords: adverse, atorvastatin, cutaneous, drug, lichen, lichenoid, eruption, planus, skin, statin
Introduction
Atorvastatin, a 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor, is commonly used to manage hypercholesterolemia. Atorvastatin usually prevents the production of cholesterol and other sterol products, including corticosteroids, vitamin D, and sex steroids, in the mevalonate pathway. However, statins can have a diverse array of effects beyond lowering the risk of cardiovascular disease [1]. Statins have been associated with various adverse cutaneous side effects including alopecia, bullous dermatosis, and lichenoid drug eruptions [1-18]. Lichenoid drug eruptions clinically mimic idiopathic lichen planus [19].
We report the case of a man with atorvastatin-induced lichenoid drug eruption. In addition, we describe the clinical and histopathologic characteristics of idiopathic lichen planus and lichenoid drug eruptions as well as cutaneous adverse reactions observed with statin medications.
Case presentation
A 73-year-old man presented with a pruritic rash of two months' duration on his arms, chest, and neck. His past medical history was significant for asthma, erectile dysfunction, gastroesophageal reflux disease, and hypercholesterolemia. His current medications included atorvastatin, omeprazole, ranitidine, sildenafil, and Singulair (Merck & Co, Kenilworth, NJ). He had previously been seen by another physician who had topically treated him for eczema with betamethasone dipropionate 0.05% cream and crisaborole 2% ointment twice daily. His dermatitis had persisted despite therapy and he subsequently obtained a second opinion.
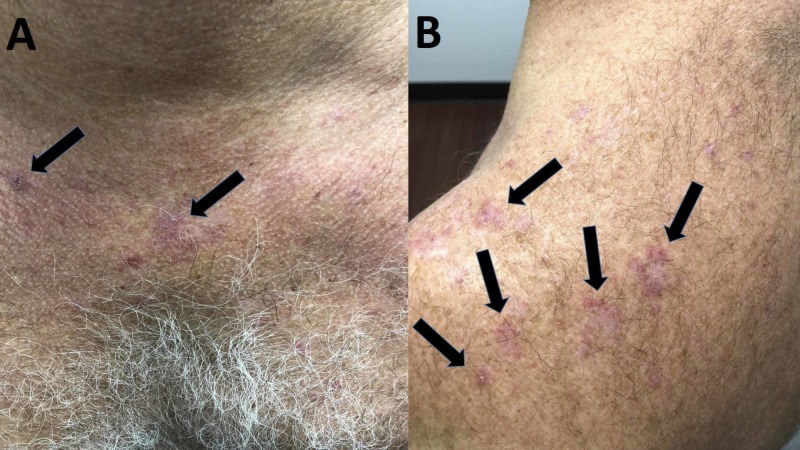
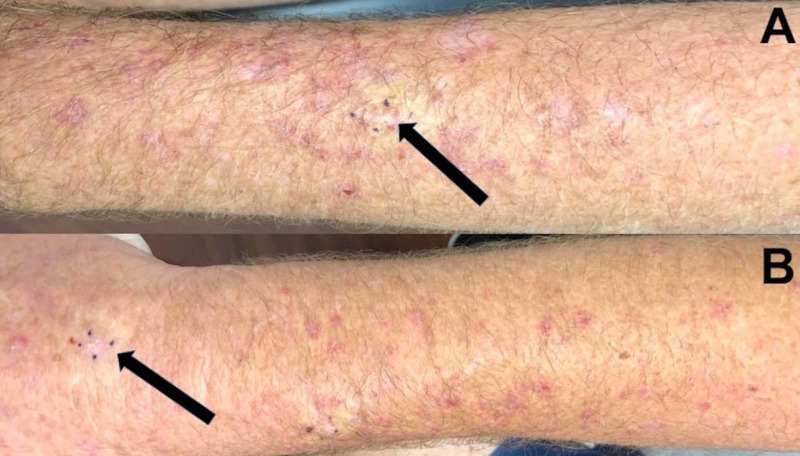
Cutaneous examination revealed erythematous to purple scaly plaques on the bilateral forearms, chest, upper back, and neck (Figure 1). A shave biopsy of skin eruptions on both the left and right forearm was performed (Figure 2).
Figure 1. Cutaneous presentation of atorvastatin-induced lichenoid drug eruption.
Erythematous, pruritic plaques (black arrows) on the chest (A), neck, and the upper back (B)
Figure 2. Skin biopsy sites of statin-induced lichenoid drug eruption on forearms.
A horizontal view of the biopsy sites (black arrows) of lichenoid drug eruption that presented as red, planar plaques on the left (A) and the right (B) forearms are each outlined by four small purple dots
Microscopic examination revealed orthokeratosis, acanthosis, and spongiosis. A dense, band-like inflammatory infiltrate composed predominantly of lymphocytes was present in the upper dermis and along the dermoepidermal junction. In addition, apoptotic cells, eosinophils, and histiocytes were observed.
Pathologic findings pointed to lichenoid dermatitis with eosinophils. Correlation of the clinical history, lesion morphology, and pathologic findings established a diagnosis of a lichenoid drug eruption. We suspected that the causative agent was atorvastatin, which the patient had begun taking two months prior to the onset of his eruption.
Management included discontinuing the atorvastatin and treatment with prednisone, initially 40 mg daily with a gradual tapering of the dosage over 20 days. Additionally, a topical betamethasone dipropionate 0.05% cream to be applied twice daily for three weeks was also prescribed. His symptoms and skin eruption completely resolved and had not recurred at a one-month follow-up.
Discussion
Adverse cutaneous events are a consequence of various medications including antibiotics, anticonvulsants, and statins. Earlier studies have observed that the majority of lichenoid drug eruptions were caused by either antimalarial agents or oral gold therapy [19].
The duration and onset of lichenoid drug eruptions are often dependent on the causative agent and dosage. Lichenoid drug eruptions occur most often in individuals between the age of 57 to 66 years and can have an average latent period of one year between the beginning of the medication treatment and the onset of an eruption [19]. This medication-induced eruption should be considered when an individual receiving statin treatment develops new lesions akin to lichen planus.
The clinical presentation and pathology of lichenoid drug eruptions can mimic those of lichen planus (Table 1) [15-16,19-20]. Both conditions present as erythematous to purple papules and plaques; however, lichenoid drug eruptions may be scaly, more pruritic, and resolve with greater residual hyperpigmentation [15,19]. In addition, Wickham’s striae (a lacy, white network of streak often located bilaterally on the buccal mucosa) and involvement of other mucosal areas are observed less frequently in drug-induced lesions [15,19]. Compared to the flexor surface distribution on extremities seen with idiopathic lichen planus, lichenoid drug eruptions may present in a photodistributed or symmetric pattern [15].
Table 1. Comparison between lichen planus and lichenoid drug eruption.
| Characteristic | Lichen planus | Lichenoid drug eruption | Reference |
| Morphology | Erythematous, planar, and polygonal papules are commonly described | Similar to lichen planus but can be scaly and more pruritic; alopecia, desquamation, eczematous papules, and greater residual hyperpigmentation may also occur | [15,19] |
| Pathology | A band-like lymphocyte infiltrate along the dermoepidermal junction is present along with apoptotic keratinocytes (Civatte bodies) | Similar to lichen planus but can also present with an infiltrate containing eosinophils. Focal parakeratosis, more prominent perivascular inflammation, and irregular granular layers may be present | [19,20] |
| Onset | Variable | Can appear one year after starting the causative medication; onset can vary based on the medication and dosage | [19] |
| Dermatology (primary lesion location) | Extremities | Arms, legs, and trunk | [15,19] |
| Distribution | Flexor surface | Symmetric, photodistributed pattern | [15,19] |
| Wickham’s striae | Commonly present | Typically not present | [15] |
| Oral/mucosal involvement | Majority of cases | Less common | [19] |
| Associated conditions | Diabetes mellitus, dyslipidemia, hepatitis B virus infection, hepatitis C virus infection, and thyroid dysfunction | Antimalarials, beta-blockers, oral gold therapy, penicillamine, statins, and thiazides | [16,19] |
| Prognosis | May spontaneously resolve | Less likely to spontaneously resolve and may not regress for months even after stopping the causative agent | [19] |
| Treatment | Can resolve spontaneously; however, oral and topical corticosteroids usually expedite resolution | May resolve after discontinuing the causative drug; however, oral and/or topical corticosteroids are usually needed to resolve the eruption | [19] |
Microscopically, both lichenoid drug eruptions and idiopathic lichen planus exhibit a band-like lymphocytic infiltrate along the dermal-epidermal junction and apoptotic keratinocytes. Both conditions also show acanthosis, hypergranulosis, and hyperkeratosis [20]. However, an infiltrate with eosinophils in the dermis can help delineate lichenoid drug eruption from lichen planus [20].
Lichenoid drug eruptions are associated with medications. In contrast, lichen planus can be associated with systemic conditions such as diabetes mellitus and hepatitis B or hepatitis C viral infections. Lichenoid drug eruptions are also less likely to spontaneously resolve and may require discontinuation of the causative agent in addition to topical and/or oral corticosteroid therapy.
Several cutaneous adverse events have been described in patients who have received statins (Table 2) [1-18]. Among these, bullous dermatosis, cutaneous lupus erythematosus, dermatomyositis, eosinophilic fasciitis, and photosensitivity are the most common [1,3,5-6]. Acute generalized exanthematous pustulosis, alopecia, cheilitis, chronic actinic dermatitis, dermatographism, eczema, erythema multiforme, pityriasis lichenoides chronica, pityriasis rubra pilaris, porphyria cutanea tarda, purpuric lesions, and skin ulcers have also been associated with statin use [1-2,4,7-12].
Table 2. Cutaneous adverse events observed with statin medications.
CR: current report
| Statin-associated adverse skin effects | Reference |
| Acute generalized exanthematous pustulosis | [1] |
| Alopecia | [2] |
| Angioedema | [1] |
| Bullous dermatosis | [3] |
| Cheilitis | [4] |
| Chronic actinic dermatitis | [1] |
| Cutaneous lupus erythematosus | [5] |
| Dermatographism | [1] |
| Dermatomyositis | [6] |
| Eczema | [1] |
| Eosinophilic fasciitis | [1] |
| Erythema multiforme | [7] |
| Ichthyosis | [1] |
| Lichenoid drug eruptions | [13-18, CR] |
| Lichen planus pemphigoides | [1] |
| Phototoxicity | [1] |
| Pityriasis lichenoides chronica | [8] |
| Pityriasis rubra pilaris | [9] |
| Porphyria cutanea tarda | [10] |
| Purpuric lesions | [11] |
| Skin ulcers | [12] |
| Toxic epidermal necrolysis | [1] |
Lichenoid drug eruptions have historically been associated with antimalarials, gold, and penicillamine. More recently, they have been observed with antineoplastics, beta-blockers, and thiazides [16]. Our patient developed a lichenoid drug eruption secondary to atorvastatin. In addition to atorvastatin, other statin medications have also been implicated with lichenoid drug eruptions (Table 3) [13-18].
Table 3. Characteristics of patients with statin-induced lichenoid drug eruptions.
CR: current report
| Drug, dosage | Age, race, and sex of patient | Location and onset | Morphology | Pathology | Treatment and result | Reference |
| Atorvastatin, 40 mg/day | 73-year-old Caucasian male | Bilateral arms, chest, back, and neck; onset after two months on atorvastatin | Erythematous to purple, scaly patches | Lymphocytic infiltrate along the dermoepidermal junction with eosinophils and histiocytes | Discontinued atorvastatin; betamethasone and prednisone treatment; remission in one month | [CR] |
| Fluvastatin, 20 mg/day and lovastatin, 20 mg/day | 59-year-old woman of unknown ethnicity | Extremities; onset after four weeks on fluvastatin. Redeveloped after two weeks on lovastatin | Papules and plaques with Wickham’s striae on papules. Some oral involvement was reported | A band-like lymphocytic infiltrate with apoptotic keratinocytes, hyperkeratosis, and vacuolar alteration | Discontinued fluvastatin use and treatment with mometasone-furoate resolved the initial eruption in three weeks; later treatment with lovastatin resulted in similar eruptions. Discontinued lovastatin; remission in three weeks | [13] |
| Pravastatin, unknown dosage | 64-year-old woman of unknown ethnicity | Face and upper back; onset three months after beginning statin treatment | Dense freckling with no rash | Lymphocytic inflammation found along the dermoepidermal junction with basal cell damage and Civatte bodies | Discontinued statin; pigmentation resolved after nine months | [14] |
| Pravastatin, 10 mg/day | 75-year-old Black man | Photodistributed, symmetric fashion on arms and hands; onset three weeks after beginning statin treatment. Reappeared after two weeks with pravastatin rechallenge | Erythematous plaques and papules with shiny scales | Focal hypergranulosis, hyperkeratotic stratum corneum, lymphocytic infiltrate, and vacuolar degeneration | Treatment with fluocinonide 0.05% gel and mupirocin 2% ointment was not effective. Discontinued statin; the eruptions healed after four weeks; rechallenge with pravastatin led to identical plaque formation | [15] |
| Rosuvastatin, 10 mg/day | 65-year-old woman of unknown ethnicity | Trunk and extremities; onset three months after beginning statin treatment | Flat-topped and erythematous papules | A lymphocytic infiltrate was reported in the dermis with apoptotic keratinocytes and focal parakeratosis in the epidermis | Discontinued statin; treated with psoralen and ultraviolet A radiation therapy and with oral corticosteroid therapy. Remission in six months | [16] |
| Rosuvastatin, 10 mg/day and simvastatin, 10 mg/day | 55-year-old South Asian woman | Right thigh with onset one week after beginning rosuvastatin; eruptions on her right thigh, back, and oral mucosa were reported at one-month follow-up | An erythematous rash | Apoptotic keratinocytes, basal vacuolar changes, and focal parakeratosis were present | Discontinued rosuvastatin; treatment with clobetasol propionate 0.05% cream. Remission in two months | [17] |
| Simvastatin, 10 mg/day | 57-year-old woman of unknown ethnicity | Wrists, elbows, and buccal mucosa; onset after one month of statin use | Red papules and Wickham’s striae were noted | A lymphocytic infiltrate with eosinophils and histiocytes were reported. Compact orthokeratosis and focal parakeratosis in epidermis were found; Civatte bodies and vacuolar degeneration were also noted | Therapy with topical diflucortolone 0.1% cream did not resolve the eruption. Discontinued simvastatin and bezafibrate therapy; eruption began to resolve within four weeks, but the mucosal lesions persisted at the six-month follow- up | [18] |
Lichenoid drug eruptions have been reported in one patient taking pravastatin 10 mg/day, two patients taking rosuvastatin 10 mg/day, and two patients on simvastatin at 10 mg/day [15-18]. Our patient with the atorvastatin-induced lichenoid eruption was being treated at 40 mg/day. Another patient developed a lichenoid drug eruption with pravastatin; however, this patient’s dosage was not stated [14]. Another patient developed a lichenoid drug eruption on fluvastatin 20 mg daily; when she switched to lovastatin 20 mg daily, she redeveloped this drug-induced eruption [13].
To the best of our knowledge, lichenoid drug eruptions secondary to statin medications have been reported in two men and five women including our patient [13-18]. These individuals ranged in age from 55 to 75 years with a median onset age of 64 years [13-18]. The median onset age was 74 years for men and 59 years for women [13-18]. Four of the patients were of unknown ethnicity; however, a Black man, a Caucasian man, and a South Asian woman were described [15,17]. In the individuals who experienced a statin-induced lichenoid drug eruption, the onset of the eruption ranged from 2 to 12 weeks after starting the statin medication with a median of four weeks [13-18].
The cutaneous adverse event appeared on the trunk and extremities in six patients; one of the patients had skin lesions that developed on the face [13-18]. Six patients presented with lichen planus-like violaceous papules, and one patient demonstrated dense freckling on her face [13-18]. Oral involvement was reported in three of the individuals, and Wickham’s striae were observed in two patients [13,17-18].
Histologic evaluation of the statin-induced lichenoid drug eruptions demonstrated lymphocytic infiltration of the dermoepidermal junction similar to idiopathic lichen planus [13-18]. Focal parakeratosis was reported in three patients [16-18]. Eosinophils were noted in two patients, including ours [18]. Hyperkeratosis was also noted in two patients’ statin-induced lichenoid eruptions [13,15].
Management of statin-induced lichenoid drug eruptions includes discontinuation of the causative statin agent and treatment with topical and/or oral corticosteroids. Six of the seven patients’ skin lesions, including ours, resolved with cessation of the statin medication and additional therapy: an oral corticosteroid, a topical corticosteroid, or both [13,15-18]. In some instances, the eruption persisted for several months after discontinuing the instigating agent. Indeed, with or without additional treatment, the statin-induced drug eruptions resolved within three weeks to nine months after the causative drug was stopped [13-18].
Conclusions
Lichenoid drug eruptions share several features with lichen planus. However, unique characteristics of these drug-induced eruptions (including delayed onset, absence of Wickham’s striae, and presence of eosinophils microscopically) can help distinguish lichenoid drug eruptions from idiopathic lichen planus. Statins are generally used in the management of hypercholesterolemia; however, several adverse cutaneous events have been observed in patients treated with statins. Lichenoid drug eruptions are an uncommon adverse cutaneous event associated with statin medications. The new onset of lichenoid dermatitis in an individual receiving statin therapy should raise the concern that this skin eruption may be associated with the medication.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
References
- 1.Statin adverse effects: a review of the literature and evidence for a mitochondrial mechanism. Golomb BA, Evans MA. Am J Cardiovasc Drugs. 2008;8:373–418. doi: 10.2165/0129784-200808060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alopecia associated with atorvastatin. Segal AS. Am J Med. 2002;113:171. doi: 10.1016/s0002-9343(02)01135-x. [DOI] [PubMed] [Google Scholar]
- 3.Linear IgA bullous dermatosis induced by atorvastatin. König C, Eickert A, Scharfetter-Kochanek K, Krieg T, Hunzelmann N. J Am Acad Dermatol. 2001;44:689–692. doi: 10.1067/mjd.2001.113462. [DOI] [PubMed] [Google Scholar]
- 4.Cheilitis due to treatment with simvastatin. Mehregan DR, Mehregan DA, Pakideh S. http://www.ncbi.nlm.nih.gov/pubmed/9798110. Cutis. 1998;62:197–198. [PubMed] [Google Scholar]
- 5.Drug-induced cutaneous lupus erythematosus: 88 new cases. Laurinaviciene R, Sandholdt LH, Bygum A. Eur J Dermatol. 2017;27:28–33. doi: 10.1684/ejd.2016.2912. [DOI] [PubMed] [Google Scholar]
- 6.Atorvastatin-induced dermatomyositis. Oztas M, Ugurlu S, Aydin O. Rheumatol Int. 2017;37:1217–1219. doi: 10.1007/s00296-017-3658-9. [DOI] [PubMed] [Google Scholar]
- 7.Current perspectives on erythema multiforme. Lerch M, Mainetti C, Terziroli Beretta-Piccoli B, Harr T. Clin Rev Allergy Immunol. 2018;54:177–184. doi: 10.1007/s12016-017-8667-7. [DOI] [PubMed] [Google Scholar]
- 8.Pityriasis lichenoides chronica associated with the use of HMG-CoA reductase inhibitors. Massay RJ, Maynard AA. http://www.ncbi.nlm.nih.gov/pubmed/23620974. West Indian Med J. 2012;61:743–745. [PubMed] [Google Scholar]
- 9.Drug-related pityriasis rubra pilaris with acantholysis. Gajinov ZT, Matić MB, Duran VD, Vucković N, Prcić ST, Vujanović LM. Vojnosanit Pregl. 2013;70:871–873. doi: 10.2298/vsp1309871g. [DOI] [PubMed] [Google Scholar]
- 10.Porphyria cutanea tarda induced by HMG CoA reductase inhibitors: simvastatin, pravastatin. (Article in French) Perrot JL, Guy C, Bour Guichenez G, Amigues O, Servoz J, Cambazard F. http://www.ncbi.nlm.nih.gov/pubmed/7631993. Ann Dermatol Venereol. 1994;121:817–819. [PubMed] [Google Scholar]
- 11.Pravastatin-induced rhabdomyolysis and purpura fulminans in a patient with chronic renal failure. [Mar;2020 ];Kato K, Onodera K, Iwasaki Y, et al. https://www.ncbi.nlm.nih.gov/pubmed/25644555/ Int J Surg Case Rep. 2015 8C:84–87. doi: 10.1016/j.ijscr.2015.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skin ulcers and myopathy associated with pravastatin therapy. Fernández-Torres R, del Pozo J, Almagro M, Yebra-Pimentel MT, Fernández-Jorge B, Mazaira M, Fonseca E. Clin Exp Dermatol. 2009;34:0–238. doi: 10.1111/j.1365-2230.2008.03098.x. [DOI] [PubMed] [Google Scholar]
- 13.Lichenoid drug eruption with HMG-CoA reductase inhibitors (fluvastatin and lovastatin) Sebök B, Tóth M, Anga B, Harangi F, Schneider I. Acta Derm Venereol. 2004;84:229–230. doi: 10.1080/00015550310006851. [DOI] [PubMed] [Google Scholar]
- 14.Pravastatin-induced lichenoid drug eruption. Pua VS, Scolyer RA, Barnetson RS. Australas J Dermatol. 2006;47:57–59. doi: 10.1111/j.1440-0960.2006.00225.x. [DOI] [PubMed] [Google Scholar]
- 15.Pravastatin-induced lichenoid drug eruption. Keough GC, Richardson TT, Grabski WJ. http://www.ncbi.nlm.nih.gov/pubmed/9515217. Cutis. 1998;61:98–100. [PubMed] [Google Scholar]
- 16.Statin-related lichenoid dermatosis: an uncommon adverse reaction to a common treatment. [Mar;2020 ];Vesza Z, Pires C, da Silva PM. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6346926/ Eur J Case Rep Intern Med. 2018 5:844. doi: 10.12890/2018_000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drug eruption to rosuvastatin with recurrence on simvastatin: a case report. Wong ITY, Huang Y, Zhou Y. J Cutan Med Surg. 2018;22:359–361. doi: 10.1177/1203475418756376. [DOI] [PubMed] [Google Scholar]
- 18.Simvastatin-induced lichenoid drug eruption. Roger D, Rolle F, Labrousse F, Brosset A, Bonnetblanc JM. Clin Exp Dermatol. 1994;19:88–89. doi: 10.1111/j.1365-2230.1994.tb01128.x. [DOI] [PubMed] [Google Scholar]
- 19.Lichenoid drug eruptions. Halevy S, Shai A. J Am Acad Dermatol. 1993;29:249–255. doi: 10.1016/0190-9622(93)70176-t. [DOI] [PubMed] [Google Scholar]
- 20.Lichenoid tissue reaction/interface dermatitis: recognition, classification, etiology, and clinicopathological overtones. Sehgal VN, Srivastava G, Sharma S, Sehgal S, Verma P. Indian J Dermatol Venereol Leprol. 2011;77:418–429. doi: 10.4103/0378-6323.82389. [DOI] [PubMed] [Google Scholar]