Abstract
Context
We previously reported the first female with a causative ESR1 gene variant, who exhibited absent puberty and high estrogens. At age 15 years, she presented with lower abdominal pain, absent breast development, primary amenorrhea, and multicystic ovaries. The natural history of complete estrogen insensitivity (CEI) in women is unknown.
Objective
The purpose of this report is to present the neuroendocrine phenotype of CEI, identify potential ligands, and determine the effect of targeted treatment.
Design
We have characterized gonadotropin pulsatility and followed this patient’s endocrine profile and bone density over 8 years. Seventy-five different compounds were tested for transactivation of the variant receptor. A personalized medicine approach was tailored to our patient.
Setting
Academic medical center.
Patient or Other Participants
A 24-year-old adopted white female with CEI.
Intervention(s)
The patient was treated with diethylstilbestrol (DES) for approximately 2.5 years.
Main Outcome Measure(s)
Induction of secondary sexual characteristics.
Results
Luteinizing hormone (LH) pulse studies demonstrated normal pulsatile LH secretion, elevated mean LH, and mildly elevated mean follicle-stimulating hormone (FSH) in the presence of markedly increased estrogens. DES transactivated the variant ESR1 in vitro. However, DES treatment did not induce secondary sexual characteristics in our patient.
Conclusions
Treatment with DES was not successful in our patient. She remains hypoestrogenic despite the presence of ovarian cysts with a hypoestrogenic vaginal smear, absent breast development, and low bone mineral mass. Findings suggest additional receptor mechanistic actions are required to elicit clinical hormone responses.
Keywords: ESR1, ERα, diethylstilbestrol, delayed puberty, complete estrogen insensitivity
Estrogenic compounds possess various important biological roles and affect multiple tissues, most notably the reproductive, neuroendocrine, and skeletal systems. Estrogens exert their effect on organs by binding and activating the estrogen receptors (ERs), ERα and ERβ, which are encoded by ESR1 and ESR2, respectively (1). Both ERα (2, 3) and ERβ (4–6) are members of the nuclear receptor superfamily (7) and were cloned and characterized as ligand-dependent transcription factors in 1985 and 1996, respectively. Subsequently, knockout mice for Esr1 (8) and Esr2 (9) were generated and characterized. Ligand binding to both receptors leads to a conformational change that regulates receptor interaction with proteins and deoxyribonucleic acid (DNA) in both the cytoplasm and the nucleus (10). Importantly, ERα activity is affected by transcriptional coactivators and corepressors that bind to the receptor to form the ERα activation complex (11). While the function of ERα and ERβ was initially thought to be limited to the nucleus (12), nonnuclear estrogen signaling via a G-protein–coupled estrogen receptor (GPER/GPR30) has now been proposed; and ERα has been shown to interact directly with G proteins (13–15).
Estrogen signaling is critical for normal physiology and development in both males and females and plays an important role in reproduction. The first report of a human ESR1 variant was described in a 28-year-old male with elevated serum estrogens and gonadotropins who failed to respond to estrogen therapy (16). His phenotypic presentation included genu valgum, tall stature, low bone mineral density, unfused epiphyses, hyperinsulinemia, and a mildly abnormal semen analysis (16), similar to the phenotypic findings in ERα knockout mice (8). DNA sequencing in this patient revealed a homozygous frameshift ESR1 variant creating a premature stop codon resulting in the loss of ERα protein expression. It had been hypothesized that ESR1 variants in females might be lethal. However, nearly 20 years after the report of the affected male (16), our group was the first to identify complete estrogen insensitivity (CEI) in a female patient with a sequence variation at Q375H (17). This homozygous loss-of-function ESR1 variant is in a completely conserved residue of the ligand-binding domain and impaired estrogen signaling (17). Our adolescent woman presented with pelvic pain due to multicystic ovaries. She was noted to have absent breast development with markedly elevated serum levels of estrogens and mildly elevated serum gonadotropins (17). This clinical phenotype is consistent with that of female ERα knockout mice (8). Subsequently, Bernard et al identified a loss of function ESR1 variant at R394H in the ligand-binding domain in a consanguineous family with CEI with 2 affected daughters and 1 affected son (18). All 3 siblings presented with delayed puberty associated with markedly elevated estradiol levels and elevated gonadotropins. More recently, ESR2 variants have been reported in patients with primary amenorrhea (19, 20) and in patients with 46,XY disorders of sex development (21).
The 3 reported families with CEI provide convincing evidence for causation of the ESR1 variants. The first ESR1 variant, p.Cys157Thr, found in the affected male, is located in the A/B domain upstream to both the DNA-binding domain and ligand-binding domain (16). Because it is predicted to introduce a premature stop codon, both the DNA- and ligand-binding domains would be absent, and the receptor would have no functional activity (16). The p.Glyn375His variant found in this patient (17), as well as the subsequently reported p.Arg394His variant (18), are both missense sequence variations in exon 5 involving the ligand-binding domain that impair function both in vitro and in vivo.
Here, we report the follow-up findings of the first described female with CEI up to 8 years after her initial presentation. We have characterized gonadotropin pulsatility and followed her endocrine profile and bone density over time. A personalized medicine approach was tailored to our patient; various compounds were used in transient transfection assays to determine if estrogen signaling could be induced. Diethylstilbestrol (DES) proved to successfully transactivate the variant ESR1 in vitro and was used as treatment. However, DES treatment did not induce any secondary sexual characteristics in our patient. These findings underscore the important function of transcriptional coregulators in mediating physiological and clinical responsiveness to DES in this patient but will require further study.
Materials and Methods
Clinical studies: frequent sampling study and ultrasound measurements
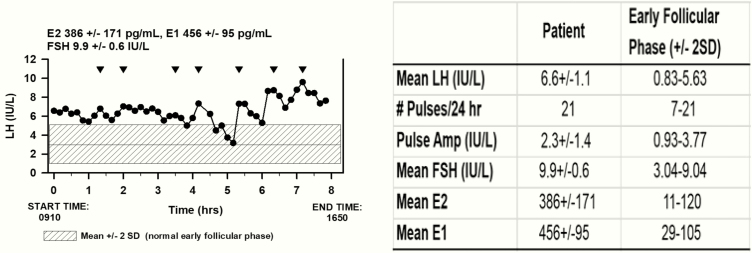
The patient signed an informed consent approved by the Institutional Review Board of the Medical College of Georgia at Augusta University for an 8-hour frequent sampling study. She presented to the Medical College of Georgia Research Center at 8:00 am, and a heparin lock was inserted into a peripheral vein 30 minutes before blood sampling began. Venous blood sampling was carried out every 10 minutes for a period of 8 hours. Two mL of blood was removed every 10 minutes. The blood samples remained at room temperature for 90 minutes before centrifugation at 2000 xg for 15 minutes after which serum was removed. Samples of 250 µL were sent to the University of Virginia Ligand Assay & Analysis Core for follicle-stimulating hormone (FSH) and luteinizing hormone (LH) analysis by an Immulite, automated chemiluminescence assay with intra-assay and inter-assay coefficient of variation (%) of 3.0 and 5.5 IU/L for FSH and 3.9 and 5.2 IU/L for LH, respectively. Two samples were pipetted (~400 µL) for E2 and E1 analysis at the same laboratory (Fig. 1) (22, 23). Serum E2 was analyzed by Calbiotech, ELISA, while E1 was analyzed by Beckman, RIA with intra-assay and inter-assay coefficient of variation (%) of 6.7 and 9.8 for E2 pg/mL and 5.0 and 6.7 pg/mL for E1, respectively. LH, measured at 10-minute intervals, was analyzed as previously described using a validated modification of the Santen and Bardin method (Fig. 1) (24). For ultrasound measurement, mean ovarian volume was calculated by the simplified formula 0.5 × length × width × thickness.
Figure 1.
Luteinizing hormone (LH) in samples drawn every 10 minutes revealed pulsatile secretion (inverted triangles). The shaded area represents the range for LH in healthy normal women in the early follicular phase of the menstrual cycle (22, 23). (Mean ± SD for E2, E1, and FSH are indicated at the top of the graph.) Abbreviations: E1, estrone; E2, estradiol; FSH, follicle-stimulating hormone; hrs, hours, SD, standard deviation.
Plasmid construction
The plasmid pcDNA3-DEST-hERα (595 aa) was generated by Celplor Inc (Cary, NC) by cloning the complementary DNA (cDNA) into pENTR-3C (Invitrogen) using BamHI/EcoRI [GGATCCATG TGAGAATTC]). The plasmid pcDNA3-DEST-hERα-Q375H (595 aa) was generated by Celplor Inc (Cary, NC) via polymerase chain reaction-based site-directed mutagenesis [CAG > CAT]. These plasmids (pENTR-3C-hERa and pENTR-3C-hERa-Q375H) were then separately cloned into pcDNA3-DEST by gateway cloning (attL and attR reaction) at attR1 and attR2. pcDNA3-DEST was made by the McDonnell lab by removing the V5 tag ([TAC CAT GGG TAA GCC TAT CCC TAA CCC TCT CCT CGG TCT CGA TTC TAC GGA AAA CCT GTA TTT TCA GGG CCC GGA TCC GTC GAC GAA TTC TGC AGA T]; 97 nucleotides) from pcDNA3.1-nV5-DEST (Invitrogen). The pGL3–3xERE-TATA-Int-Luc (3xERE-Luc) plasmid contains 3 repeats of vitellogenin estrogen-responsive element (ERE). The pRL-TK renilla luciferase expression plasmid (Promega) was used as an internal control for transfection efficiency.
Cell culture
The human hepatocellular carcinoma cell line (HepG2) was purchased from the American Type Culture Collection. HepG2 cells were cultured in minimum essential media without phenol red (Life Technologies) supplemented with 10% fetal bovine serum (Gemini-Bio), 4 mM L-glutamine (Invitrogen), and 1% penicillin-streptomycin (Sigma).
Transient transfection and luciferase reporter assay
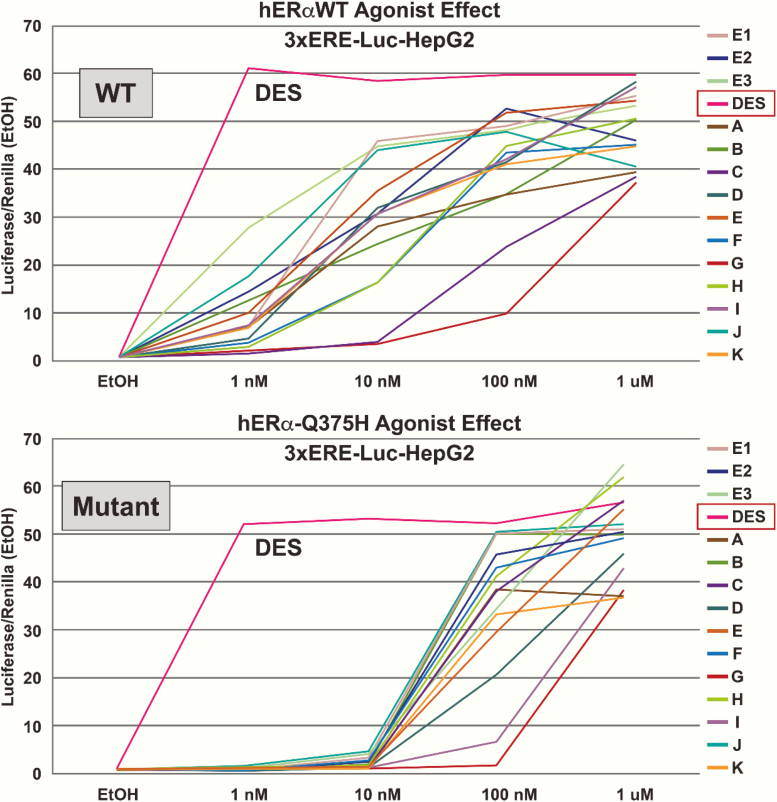
A total of 75 different compounds were screened to determine if transactivation of the variant receptor (hERα-Q375H) was possible via 3xERE reporter assay. Screened ligands were selected from a variety of chemically diverse classes that included 17 DES/hexestrol/bibenzyl/AZA analogs; 8 steroids; 14 heterocyclic core compounds; 3 imine core compounds; 5 cyclofenil and related 1,1 diarylethylenes; and 28 others. All screened ligands are listed in a supplementary table located in a digital research material repository (25). A representative dose response curve of some compounds is shown in Fig. 2. For transient transfections, the cells were cultured in media without phenol red and supplemented with 10% charcoal/dextran-stripped fetal bovine serum (Gemini-Bio) and seeded overnight in 24-well plates at a density of 1.2 × 105 cells/well. The cells were transfected with the following DNA mixture for 6 hours using lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. A DNA mixture containing 50 ng of expression plasmids of WT hERα or hERαQ375H, 100 ng of reporter plasmids of 3xERE-Luc, and 100 ng of renilla luciferase expression plasmid pRL-TK was transfected in each well. The cells were cultured in fresh medium supplemented with vehicle (ethanol, final concentration < 0.01%), or ligands (1 nM-1 µM) 6 hours after transfections. Luciferase and renilla luciferase activities were assayed 18 hours after treatments using the Dual Luciferase Reporter Assay System (Promega). Luciferase activity was normalized for transfection efficiency using renilla luciferase as an internal control. Fold change was calculated relative to vehicle control (ethanol) or pcDNA-3-DEST (Vector) in Figure 2 (26). All experiments were repeated at least 3 times (Fig. 2).
Figure 2.
Representative dose response reporter gene screen of a selection of compounds for estrogenic activity of the wild-type and Q-variant estrogen receptor (ER) in hepatocellular carcinoma cell line (HepG2) cells. DES was the most active compound tested compared with E2 and has a higher ligand-binding affinity to the wild-type ERα, surpassing the endogenous E2. Transactivation of the estrogen response element (ERE)-dependent–luciferase reporter gene in HepG2 cells resulted in a higher half maximal effective concentration (right-shifted), reflecting reduced estrogen signaling with the Q375H (variant). The data shown are the mean of the triplicate determinations in a representative experiment. (Values are presented as ± standard error of the mean [SEM]). Compounds tested: all 3 endogenous estrogen compounds: E1, estrone; E2, 17β estradiol; E3, estriol (not shown). Figure legend: [A] estrone; [B] D8,9-dehydroestrone; [C] equilin; [D] 17α-dihydroequilin; [E] 17β-dihydroequilin; [F] equilenin; [G] 17α-dihydroequilenin; [H] 17β-dihydroequilenin; [I] 17α-estradiol; [J] 17β-estradiol; [K] conjugated equine estrogen (mixed at the proprietary ratio for 10–2 M “estrogens”).
Results
Clinical report
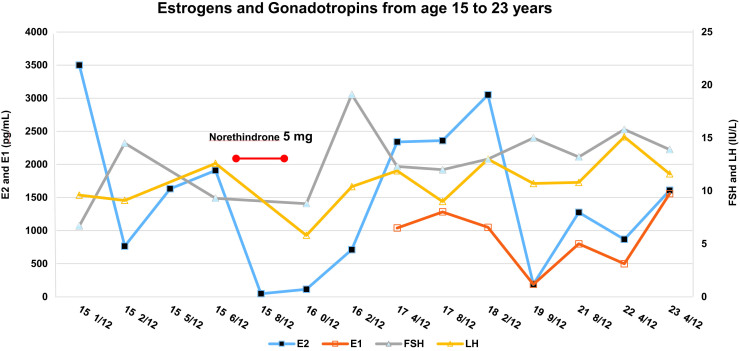
The patient is now a 24-year-old adopted white female who had initially presented at the age of 15 with lower abdominal pain secondary to multicystic ovaries and was found to have absent breast development and primary amenorrhea. Chromosomal microarray indicated that her parents were second-degree relatives supporting consanguinity (17). Her hormonal evaluation revealed persistently and markedly elevated serum estrogen levels, mildly elevated gonadotropins, and normal levels of sex hormone-binding globulin, thyroxine-binding globulin, prolactin, and triglycerides (Fig. 3, Table 1). More details of her history and diagnostic workup at initial presentation are described in our prior publication (17). Five months of oral estrogens failed to initiate breast development. DNA sequencing of ESR1 revealed a homozygous variant, c.112G->T (p.Gln375His) in the ligand-binding domain disrupting a highly conserved Gln residue, which was shown in vitro in COS-7 cells to significantly impair estrogen signaling by a factor of 240 (17).
Figure 3.
Measurement of FSH, LH, E2, and E1 for the patient from the initial evaluation at age 15 years and 1 month to the most recent follow-up visit at age 23 years and 4 months. Note the persistent elevation of both gonadotropins and estrogens. Note the decrease in E2 with norethindrone treatment (red arrow). E1 was first measured at age 17 years and 4 months. Abbreviations: FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Table 1.
Laboratory Values for the Patient With an ESR1 Variant
| Age (Years & Months) | 151/12 | 152/12 | 155/12 | 156/12 | 159/12 | 161/12 | 162/12 | 174/12 | 178/12 | 178/12 | 179/12 | 182/12 | 199/12 | 218/12 | 224/12 | 234/12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | 17 | 16.8 | 17.8 | 18.1 | 16.4 | 16.7 | 19.5 | |||||||||
| E2 (29-105 pg/mL) | 3,500 | 765 | 1632 | 1910 | 47 | 114 | 712 | 2340a | 2360a | 2,000 | 3,050a | 189 | 1275 | 868 | 1609 | |
| E1: (29-105 pg/mL) | 1040a | 1283a | 1,050a | 188 | 800 | 499 | 1559 | |||||||||
| Inhibin A b(pg/mL) | 319 | 184.4 | 21.6 | 58.3 | 85.5 | 109 | ||||||||||
| Inhibin Bc | 93.8 | 122.3 | 179.8 | 128.4 | 89.7 | |||||||||||
| AMH (0.30-11.21 ng/mL) | 2.8 | 8 | 5.2 | 3.7 | 3.15 | |||||||||||
| FSH (0.9-15 mIU/mL) | 6.7 | 14.5 | 9.3 | 8.8 | 19.1 | 12.3 | 12 | 13 | 15 | 13.2 | 15.8 | 13.9 | ||||
| LH (0.8-26 mIU/mL) | 9.6 | 9.1 | 12.6 | 5.8 | 10.4 | 11.9 | 9 | 13 | 10.7 | 10.8 | 15.1 | 11.6 | ||||
| TSH (0.4-5 uU/mL) | 5.2 | 4.4 | 1.9 | 3.1 | 3.1 | 4.1 | 4.3 | 9.3 | 0.3, 0.4 | 1.3 | 3.6 | 4.2 | ||||
| fT4 (0.93-1.60 ng/dL) | 0.9 | 1.02 | 0.79 | 1.1 | 1.1 | 1.24 | 1.0 | 2.3, 1.92 | 1.5d | 1.15 | ||||||
| T4 (5-12 ug/dL) | 6.4 | 7.1 | 7.7 | 6.9 | ||||||||||||
| T3 (71-180 ng/dL) | 136 | 127 | ||||||||||||||
| rT3 (13.5-34.2 ng/dL) | 43.6 | 23.8 | 14.9 | |||||||||||||
| TBG (15-30 ug/mL) | 24 | |||||||||||||||
| CBG (2.3-3.9 mg/dL) | 3.3 | |||||||||||||||
| SHBG (24.6-122 nmol/L) | 44 | 16 | 45.7 | 45 | 41.4 | 42.4 | ||||||||||
| PRL (3.6-12 ng/mL) | 6.8 | 18.5 | 10.7 | 9.9 | 16.3 | |||||||||||
| Insulin (2.6-24.9uU/mL) | 5.8 | 5.2 | ||||||||||||||
| IGF-1 (196-581 ng/mL) | 130 | 368 | ||||||||||||||
| DHEAS (37-307 ug/dL) | 114 | |||||||||||||||
| 2 AM Cortisol (6.2-19.4 ug/dL) | 23 | |||||||||||||||
| Total T (10-50 ng/dL) | 38 | 38 | 13 | 49.5 | 42 | 87.8a | 39 | 36 | 50 | 56 | ||||||
| Free T (0.5-3.9 pg/mL) | 0.6 | 4.5 | 3.4 | 5.6 | ||||||||||||
| P4 (<2 ng/mL) | 0.7 | 1.6 | 1.3 | 1.3 | 2 | 0.8 | 1.2 | 1.7 | ||||||||
| Cholesterol (100-169 mg/dL) | 149 | 149 | 164 | |||||||||||||
| Triglycerides (0-149 mg/dL) | 52 | 80 | 78 | |||||||||||||
| HDL (>39 mg/dL) | 64 | 52 | 78 | |||||||||||||
| LDL (0-99 mg/dL) | 75 | 81 | 70 | |||||||||||||
| VLDL 5-40 mg/dL) | 10 | 16 | 16 |
Thyroid peroxidase and antithyroglobulin antibodies were negative.
atotal testosterone by liquid chromatography–mass spectrometry. bTanner stage I < 7.0 pg/mL. c(14-362 pg/mL for age 12-18 years, but < 100 pg/mL if Tanner I). dfree T4 by dialysis/mass spectrometry.
Abbreviations: AMH, anti-Müllerian hormone; BMI, body mass index; CBG, corticosteroid-binding globulin; DHEAS, dehydroepiandrosterone sulfate; E1, estrone; E2, estradiol; FSH, follicle-stimulating hormone; fT4, free T4; HDL, high-density lipoprotein; IGF-1, insulin-like growth factor-1; LDL, low density lipoprotein; LH, luteinizing hormone; P4, progesterone; PRL, prolactin; rT3, reverse T3; SHBG, sex hormone-binding globulin; T, testosterone; T3, tri-iodothyronine; T4, thyroxine; TBG, thyroxine-binding globulin; TSH, thyroid-stimulating hormone.
The affected patient with CEI was 15 years of age at presentation, 18 years of age at the time of initial publication, and is now 24 years of age. She has had no breast development, but she has had no more complaints of abdominal pain, although her ovaries have remained enlarged with cysts (Table 2). The mean ovarian volume in 6 of 7 ultrasounds spanning 8.5 years was consistently elevated, ranging from 38.2 to 123.7 cm3, with normal being < 20 cm3 (27). Uterine length ranged from 4 to 5.8 cm longitudinally, within the prepubertal age range, and the endometrium was thin, ranging from < 2 to 3.5 mm throughout the 8.5-year follow-up. A 1-month course of norethindrone resulted in a decrease in mean ovarian volume from 123.7 to 10.5 cm3, accompanied by a decrease in estradiol from 1910 to 47 pg/mL.
Table 2.
Pelvic Ultrasounds of the Patient With an ESR1 Variant
| Age (years & months) | 1411/12 | 150/12 | 151/12 | 1511/12 | 160/12 | 198/12 | 207/12 | 233/12 |
|---|---|---|---|---|---|---|---|---|
| Uterus (cm) | 4.8 | 4.9 × 2.1 × 2.5 | 5.2 × 1.2 × 1.9 | 5.5 | ___ | 4.91 × 2.04 × 2.0 | 4 × 3 × 3.3 | 5.25 × 1.35 × 2.86 |
| Uterine volume (cm3) | ___ | 13.5 | 6.2 | ___ | ___ | 10.5 | 20.7 | 10.6 |
| Endometrium (mm) | ___ | ___ | 2 | 4 | 2 | 4.21 | 3.5 | ___ |
| Mean ovarian volume (cm3) | 52.1 | 83.6 | 38.2 | 123.7 | 10.5a | 40.8 | ___ | 92 |
| Right ovarian volume (cm3) | 45.2 | 91.5 | 5.2 | 72.8 | 12.1 | 54.1 | 5.1 × 2.4b | 34 |
| Right ovarian volume (cm3) (Largest cyst with largest diameter) | ___ | 4.2 | 1.5 | 4.2 | 2.2 | 3.6 | 2.2 | 3.13 |
| Left ovarian volume (cm3) | 58.9 | 75.6 | 71.1 | 174.5 | 8.9 | 27.5 | 5.2 × 4.5b | 150 |
| Left ovarian cyst (cm) (largest cyst with largest diameter) | __ | 4.6 | 4.6 | 6.0 | 1.1 | 2.8 | 4.4 | 3.97 |
2-D ultrasounds of the patient from the initial evaluation until a recent follow-up visit at age 23 years and 3 months. Uterus and endometrium are included when measured. Bilateral ovarian volume and the largest ovarian cyst with the largest diameter from each ovary are included. aNote the decrease in ovarian volume at 16 years and zero months after treatment with progesterone (norethindrone). bAt age 20 years and 7 months, the 3 dimensions of the ovary were not measured, so the ovarian volume could not be calculated. Mean ovarian volume was calculated by the simplified formula 0.5 × length × width × thickness.
Additionally, the clinical evaluation consisted of follow-up laboratory studies (Table 1), a gonadotropin pulse study, and dual-energy x-ray absorptiometry (DXA). From age 15 years to the present, her laboratory studies have continued to demonstrate hyperestrogenemia with inhibin A and inhibin B levels within the normal postmenarchal and premenopausal range but elevated for prepubertal girls (22) and mildly elevated levels of FSH and LH. Serum progesterone has ranged from 0.7 to 2 ng/mL, while testosterone ranged from 13 to 87.8 ng/dL. She underwent 3 DXA scans, all revealing continued reduction in bone mineral density with lumbar Z-scores of –3.8 at age 15 years and 5 months and –4.3 at age 23 years (Table 3).
Table 3.
Dual-Energy X-ray Absorptiometry of the Patient With an ESR1 Variant
| Age (year & month) | Z-score (total) | Z-score (lumbar) | Z-score (femoral neck) | Z-score (total hip) | T-score (lumbar) | T-score (femoral neck) | T-score (total hip) |
|---|---|---|---|---|---|---|---|
| 155/12 | –2.3 | –3.8 | ___ | ___ | ___ | ___ | ___ |
| 174/12 | –2.4 | –4.4 | ___ | ___ | ___ | ___ | ___ |
| 233/12 | ___ | –4.3 | –3.2 | –2.9 | –4.5 | –3.2 | –2.9 |
Dual-energy x-ray absorptiometry (DXA) from the initial presentation at age 15 years and 5 months to the most recent follow-up visit at age 23 years and 3 months. Note the continued reduction in bone mineral density with the most recent T-score of –4.5 at the lumbar spine and –2.9 at the femoral neck.
Frequent sampling study
Gonadotropin pulse studies have not been reported in patients with estrogen insensitivity. Since estradiol action is known to be involved in the negative feedback of gonadotropins on the hypothalamus and pituitary, the patient underwent a gonadotropin frequent sampling study. She had discontinued hormonal therapy for 3 years. Blood samples were drawn every 10 minutes, with measurement of LH and FSH in each sample and hourly measurement of estradiol (E2) and estrone (E1). LH and FSH levels [SD] were 6.6 [1.1] and 9.9 [0.6] IU/L, while E2 and E1 were 386 [171] and 456 [95] pg/mL, respectively (Fig. 1). LH and FSH were above the early follicular phase normal ranges of 0.83 to 5.63 and 3.04 to 9.04, respectively (Fig. 1). There were 7 pulses of LH detected within the 8-hour sampling period with an interpulse interval [SD] of 58.3 [19.4] minutes and a mean amplitude of 2.3 [1.4] IU/L. The interpulse interval was below the lower end of the range in women in the early follicular phase (94±11.3 minutes) (28), and well within the faster frequency range of young postmenopausal women (interpulse interval 65.4±17.9 minutes) (29), while the amplitude was within the early follicular phase range but considerably less than in young postmenopausal women (2.4±0.7 and 9.3±4.1 IU/L, respectively). While estrogen treatment had no clinical effect or effect on gonadotropin or estrogen secretion, a 1-month course of norethindrone resulted in a decrease in LH and FSH with a concomitant decrease in mean ovarian volume from 123.7 to 10.5 cm3 and a dramatic decrease in estradiol from 1910 to 47 pg/mL (Table 2, Fig. 3).
hERα-Q375: ligand screening
To determine if additional compounds other than the endogenous E2 and E1 were able to transactivate the variant ESR1, a construct of the variant receptor was transiently transfected into HepG2 cells along with the 3xERE luciferase reporter construct to assay activity. Various ER ligands were investigated as reported in Figure 2 and listed in Supplemental Table 1 (25) over a concentration range of 0.1 to 1000 nM, to identify potential pharmacological compounds that would activate the variant ERα. DES was shown to be the strongest transactivator of the mutated ERα (Fig. 2), and in fact, DES exhibited only slightly lower transactivation of Q375H compared with wild-type ERα. It is known that DES/hexestrol compounds have the highest ligand-binding affinities to the wild-type ERα, surpassing that of endogenous E2 (4, 30). This suggested that high-affinity ligands could present a potential therapy for hormone estrogen insensitivity in our patient.
Patient response to DES therapy
Since DES was found to transactivate the mutated ESR1 in an in vitro transient transfection reporter assay, we considered treating the patient with DES as she had no clinical or lab responses to high endogenous estrogen levels. We submitted these findings to the Ethics Board at Augusta University, and DES therapy was approved. At the age of 20 years and 1 month, the patient was treated with oral DES 1 mg/day for 8 months. She was observed for signs of estrogen responsiveness such as vaginal discharge and breast development. No signs of estrogen activity were noted after 8 months of therapy; DES was increased to 3 mg/day for 11 months, again with no response noted clinically. DES was then increased to 6 mg/day for 9 months. Serum DES levels were analyzed as the dose was adjusted and confirmed compliance with treatment.
After approximately 2.5 years of treatment with increasing doses of DES, there was no vaginal discharge and breasts remained at Tanner stage 1. At age 23 years and 4 months, there were no dramatic changes in our patient’s lab results (Table 1). Both FSH and LH remained mildly elevated and estradiol and estrone levels remained markedly elevated. Normal levels of sex hormone-binding globulin, thyroxine-binding globulin, prolactin, and triglycerides were noted. A vaginal maturation index showed a predominance of parabasal cells consistent with a hypoestrogenic state. Follow-up ultrasounds showed persistence of enlarged ovaries with bilateral ovarian cysts (Table 2). At chronologic age of 23 years and 4 months, her bone age was 17 years, and the ulnar epiphysis remained open. DXA imaging revealed a Z-score of –2.9 at the femoral neck and –4.5 at the lumbar spine (Table 3). In addition, both C-telopeptide and propetide type I collagen were elevated (798 pg/mL [normal, 34–635 pg/mL] and 124 μg/L [normal, 19–83 μg/L]), respectively, reflecting an ongoing increase in bone turnover.
Discussion
While ER variants are no longer believed to be lethal, as revealed by these several identified cases, the natural history and approach to the management of this rare condition remain unknown. To date, 3 different families have been reported with ESR1 variants showing impaired function in vitro and segregation with the phenotype. All 3 variants were inherited in an autosomal recessive fashion and identified in consanguineous families. The first characterized male presented because of a bone abnormality, genu valgum, and it was noticed that he had unfused epiphyses, which led to the evaluation and diagnosis of CEI. Pubertal development was normal. He was osteopenic; fertility was untested, but his sperm concentration was low normal, with significantly reduced motility (16). The first described female with CEI (our patient) presented with pelvic pain, multiple ovarian cysts, and absent breast development, which led to the correct diagnosis (17). In the third family, the 2 affected female siblings presented because of delayed puberty (18). The affected male within that family had a unilateral cryptorchid testis along with a very small descended testis. All patients had impaired hypothalamic-pituitary-gonadal function and impaired bone development, the phenotypes of which are similar to the Esr1 KO mouse (8, 31).
Our patient presented with pelvic pain due to multiple ovarian cysts. Although her pelvic pain is stable without medication, her ovarian cysts persist. Follow-up pelvic ultrasounds over 8 years revealed persistent multicystic ovaries with an abnormally large ovarian volume (27, 32, 33). These enlarged ovarian cysts are functional, producing elevated levels of E2, E1, inhibin A, and inhibin B and normal levels of testosterone and anti-Müllerian hormone and late follicular/early luteal levels of progesterone. This ovarian response is presumed to result from chronically elevated FSH due to a relative loss of ovarian negative feedback, similar to that seen in women with autoimmune oophoritis who lack estrogen but have intact inhibin feedback but with FSH levels that are incompletely suppressed by inhibin alone (34). It is of interest that the only normal ovarian volume and estradiol were observed in association with norethindrone. This finding is consistent with a marked suppressive effect of progesterone on gonadotropin-releasing hormone (GnRH) pulse frequency (35, 36) evident in this patient even in the absence of ERα. In addition, uterine size remained small with a thin to unmeasurable endometrium, which underscores the dramatic effect of estrogen on the endometrium acting via ESR1 signaling. Ovarian volume will need to be followed in our patient because it is known that increasing ovarian volume is associated with a greater risk for ovarian cancer (37, 38).
The ovarian phenotype of CEI differs significantly from polycystic ovary syndrome, isolated FSH deficiency, and hypogonadotropic hypogonadism. Polycystic-appearing ovaries demonstrate multiple small peripherally located follicles and a dense stroma (39), while the rare condition of isolated FSH deficiency due to FSHB variants is associated with small, multicystic follicles throughout the ovary (40). The ovaries in women with hypogonadotropic hypogonadism are typically very small but may display small cysts scattered throughout the stroma (41). Patients with aromatase deficiency, who have sequence variations in the CYP19A1 gene, also manifest with enlarged and multicystic ovaries similar to women with ESR1 variants (42, 43). However, the phenotype of the aromatase-deficient female differs from CEI with manifestations of sexual ambiguity at birth, elevated androgens, and unmeasurable estrogens. Women with aromatase deficiency, in contrast to our patient with estrogen insensitivity, will respond to exogenous estrogen therapy with breast development (44).
The pituitary secretion of gonadotropins is regulated by GnRH and ovarian negative feedback from estrogen, progesterone and the inhibins, and estradiol-positive feedback from ovarian steroids (45). In our patient with CEI, FSH and LH were chronically elevated but were far lower than in postmenopausal women who lack both ovarian steroid and peptide secretion (22). Inhibins A and B, which were elevated in conjunction with functional ovarian cysts, selectively inhibit FSH at the pituitary level, but there is no evidence of an inhibitory action of either inhibin on LH (46). As suggested by the effect of norethindrone in this patient, progestins have a profoundly suppressive effect on LH and FSH through slowing of pulsatile GnRH secretion (35, 36). However, LH pulse frequency is not suppressed in this patient, indicating that the low levels of circulating progesterone are insufficient to dramatically suppress GnRH and, thus, gonadotropin secretion. Taken together, these observations suggest that ERβ, which is present in both the hypothalamus and, to a lesser extent, in the pituitary, may play a role in suppressing FSH, and particularly LH, secretion in this patient. There is also a possible role for GPER suppression of FSH and LH in this context. Of interest, inhibin A is absent in males, which probably also contributes to the gonadotropin differences seen in affected males versus females. The findings from humans with ESR1 variants and ERα knockout mice indicate that reproductive steroid negative feedback of the hypothalamus and pituitary involves estrogen signaling in both men and women.
Estrogen insensitivity and lack of estrogenic regulation of bone metabolism leads to critical loss of bone mass. It is well established in the literature that 80% to 90% of peak bone mass is achieved by late adolescence (47, 48). Thus, a high degree and long duration of estrogen insensitivity likely has a significant negative impact on bone mineral density. Our patient continued to exhibit pronounced reduced bone mineral density with Z-scores of –2.4 at 15 age years and 5 months, –2.3 at age 17 years and 4 months, and –4.3 at age 23 years and 3 months. She also demonstrated increased bone turnover as indicated by elevated C-telopeptide and propeptide type I collagen. The first male described with an ESR1 variant also had decreased bone density (Z-score –3.85 at age 28.5 years) (16). Taken together, a complete lack of ERα function has a dramatic negative effect upon bone growth and mineral content (49). Bone density was not studied in the third family. Identification of a compound that activates the variant ERα receptor or signals via ERβ or GPR30 might provide a beneficial treatment modality for these patients.
Previously, in vitro analysis indicated that our variant ESR1 did not affect nuclear localization but rather markedly impaired estrogen signaling (17). To restore estrogen signaling, 75 different compounds were investigated to identify a pharmacological compound that would transactivate the variant ERα. DES was found to activate the transiently transfected construct (hERα-Q375) at a ligand concentration lower than estradiol, and LxxLL peptide recruitment also occurred at a lower ligand concentration with DES compared to estradiol. In addition, DES also activated the Q375 variant at a lower ligand concentration compared with estradiol. Therefore, we opted to treat this patient with DES following approval by the Ethics Board. Treatment was initiated with a low dose of 1 mg/day, a dose previously known to be beneficial for menopausal symptoms (50). The patient’s dose was subsequently increased to 3 mg/day and then to 6 mg/day, which are doses far less than those used in years past for prevention of pregnancy loss (51). Unfortunately, she had no breast development and no vaginal discharge. Her vaginal smear showed a complete absence of estrogen effect as indicated by the preponderance of parabasal cells.
Despite the in vitro reporter gene cell response to DES in transfected cells, DES did not improve the physiological response, which could explain why she failed to respond to treatment. Transient expression assays are often used as a convenient alternative to stable transformation because they enable rapid analysis (52). Yet, they are unable to emulate the cellular complex of the global changes in gene expression patterns and the influence of coactivators and corepressors and their posttranslational modifications on ERα activity. Future studies designed to investigate the discrepancy in cell-based and clinical responses to DES will require creation of a stable in vitro cell line of the variant receptor or a CRISPR/Cas9 KI mouse model. While indirect methods measure specific gene products resulting from nuclear receptor activation, these more direct approaches measure either the binding of ligands to the receptors or the transcriptional events produced by ligand binding (53).
The high-affinity ligand characteristic of DES and its activation of the transiently transfected construct presents an exciting potential therapeutic approach to hormone insensitivity; yet, its failure to restore estrogen sensitivity in this particular ESR1 variant receptor underscores the role of the ligand/receptor complex and other cellular components such as coactivators for the importance of their effectiveness in activation of ERα. Current breast cancer literature has focused on ligand-independent and estrogen-independent activation of ERα, which is proposed as the driving force of tamoxifen resistance (54–55). A potential therapeutic approach to activate this defective ERα receptor would be the use of transcriptional coactivator, small molecules that are reported to stabilize the ligand/receptor complex to allow restoration of the receptor-mediated signaling cascade in tumor cells (56). Whether their actions are sufficiently effective to stimulate normal physiological hormone responses is still unclear.
In summary, our patient with CEI demonstrates multiple ovarian cysts, a lack of observable estrogen effects, mildly impaired LH pulsatility with amenorrhea, and decreased bone density. Although DES was predicted to be beneficial in transient transfection assays, there was no effect clinically. Further studies to identify ligands that bind the variant receptor to stimulate coregulators, or to identify relevant signaling cascades to activate the ER could have future applications for treating patients with estrogen insensitivity. Whether additional patients with milder forms of estrogen insensitivity occur has yet to be determined.
Glossary
Abbreviations
- CEI
complete estrogen insensitivity
- DES
diethylstilbestrol
- DNA
deoxyribonucleic acid
- DXA
dual-energy x-ray absorptiometry
- E1
estrone
- E2
estradiol
- ER
estrogen receptor
- ERE
estrogen-responsive element
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- HepG2
hepatocellular carcinoma cell line
- LH
luteinizing hormone
Acknowledgments
Financial Support: This research is funded by the National Institute of Child Health and Human Development (NICHD) 5R01HD033004-15 to L.C.L., the National Institute of Environmental Health Sciences (NIEHS) Division of Intramural Research 1ZIAES070065 to K.S.K. and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) 5R01DK015556 and the Breast Cancer Research Foundation BCRF 18-04 to J.A.K. The authors would like to thank Dr Donald McDonnell (Duke University) for the generous supply of some of the compounds used in the screening assay of the variant receptor. We appreciate the comments and suggestions for the manuscript from Sylvia Hewitt and Dr Yin Li.
Authorship Contributions: Each author has made substantial contributions to the conception, design, acquisition, analysis, or interpretation of the data. All authors have approved the submitted version.
Additional Information
Disclosure Summary: The authors have nothing to disclose. The authors have no competing financial interests as defined by the Endocrine Society or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Hewitt SC, Winuthayanon W, Korach KS. What’s new in estrogen receptor action in the female reproductive tract. J Mol Endocrinol. 2016;56(2):R55–R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walter P, Green S, Greene G, et al. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci U S A. 1985;82(23):7889–7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Green S, Walter P, Greene G, et al. Cloning of the human oestrogen receptor cDNA. J Steroid Biochem. 1986;24(1):77–83. [DOI] [PubMed] [Google Scholar]
- 4. Kuiper GG, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–870. [DOI] [PubMed] [Google Scholar]
- 5. Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392(1):49–53. [DOI] [PubMed] [Google Scholar]
- 6. Tremblay GB, Tremblay A, Copeland NG, et al. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol Endocrinol. 1997;11(3):353–365. [DOI] [PubMed] [Google Scholar]
- 7. Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90(23):11162–11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krege JH, Hodgin JB, Couse JF, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95(26):15677–15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levin ER, Hammes SR. Nuclear receptors outside the nucleus: extranuclear signalling by steroid receptors. Nat Rev Mol Cell Biol. 2016;17(12):783–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dasgupta S, Lonard DM, O’Malley BW. Nuclear receptor coactivators: master regulators of human health and disease. Annu Rev Med. 2014;65:279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. King WJ, Greene GL. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984;307(5953):745–747. [DOI] [PubMed] [Google Scholar]
- 13. Kumar P, Wu Q, Chambliss KL, et al. Direct interactions with G α i and G βγ mediate nongenomic signaling by estrogen receptor α. Mol Endocrinol. 2007;21(6):1370–1380. [DOI] [PubMed] [Google Scholar]
- 14. Navarro CE, Saeed SA, Murdock C, et al. Regulation of cyclic adenosine 3′,5′- monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotrophin-releasing hormone neurons. Mol Endocrinol. 2003;17(12):1792–1804. [PubMed] [Google Scholar]
- 15. Watson CS, Jeng YJ, Hu G, Wozniak A, Bulayeva N, Guptarak J. Estrogen- and xenoestrogen-induced ERK signaling in pituitary tumor cells involves estrogen receptor-α interactions with G protein-αi and caveolin I. Steroids. 2012;77(5):424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith EP, Boyd J, Frank GR, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331(16):1056–1061. [DOI] [PubMed] [Google Scholar]
- 17. Quaynor SD, Stradtman EW Jr, Kim HG, et al. Delayed puberty and estrogen resistance in a woman with estrogen receptor α variant. N Engl J Med. 2013;369(2):164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bernard V, Kherra S, Francou B, et al. Familial multiplicity of estrogen insensitivity associated with a loss-of-function ESR1 mutation. J Clin Endocrinol Metab. 2017;102(1):93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Asadi M, Ghafouri-Fard S, Zare-Abdollahi D, Ebrahim-Habibi A, Matin N. Estrogen receptor mutation in a girl with primary amenorrhea. Clin Genet. 2013;83(5):497–498. [DOI] [PubMed] [Google Scholar]
- 20. Lang-Muritano M, Sproll P, Wyss S, et al. Early-onset complete ovarian failure and lack of puberty in a woman with mutated estrogen receptor β (ESR2). J Clin Endocrinol Metab. 2018;103(10):3748–3756. [DOI] [PubMed] [Google Scholar]
- 21. Baetens D, Güran T, Mendonca BB, et al. ; ESR2 STUDY GROUP Biallelic and monoallelic ESR2 variants associated with 46,XY disorders of sex development. Genet Med. 2018;20(7):717–727. [DOI] [PubMed] [Google Scholar]
- 22. Sehested A, Juul AA, Andersson AM, et al. Serum inhibin A and inhibin B in healthy prepubertal, pubertal, and adolescent girls and adult women: relation to age, stage of puberty, menstrual cycle, follicle-stimulating hormone, luteinizing hormone, and estradiol levels. J Clin Endocrinol Metab. 2000;85(4):1634–1640. [DOI] [PubMed] [Google Scholar]
- 23. Hall JE, Schoenfeld DA, Martin KA, Crowley WF Jr. Hypothalamic gonadotropin-releasing hormone secretion and follicle-stimulating hormone dynamics during the luteal-follicular transition. J Clin Endocrinol Metab. 1992;74(3):600–607. [DOI] [PubMed] [Google Scholar]
- 24. Hayes FJ, McNicholl DJ, Schoenfeld D, Marsh EE, Hall JE. Free alpha-subunit is superior to luteinizing hormone as a marker of gonadotropin-releasing hormone despite desensitization at fast pulse frequencies. J Clin Endocrinol Metab. 1999;84(3):1028–1036. [DOI] [PubMed] [Google Scholar]
- 25. Brakta S, Chorich LP, Kim HG, et al. Supplemental Table 1.docx. figshare Figure. Deposited 4 February 2020. doi: 10.6084/m9.figshare.11800683.v2. [DOI]
- 26. Arao Y, Coons LA, Zuercher WJ, Korach KS. Transactivation function-2 of estrogen receptor α contains transactivation function-1-regulating element. J Biol Chem. 2015;290(28):17611–17627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pavlik EJ, DePriest PD, Gallion HH, et al. Ovarian volume related to age. Gynecol Oncol. 2000;77(3):410–412. [DOI] [PubMed] [Google Scholar]
- 28. Santoro N, Filicori M, Crowley WF Jr. Hypogonadotropic disorders in men and women: diagnosis and therapy with pulsatile gonadotropin-releasing hormone. Endocr Rev. 1986;7(1):11–23. [DOI] [PubMed] [Google Scholar]
- 29. Hall JE, Lavoie HB, Marsh EE, Martin KA. Decrease in gonadotropin-releasing hormone (GnRH) pulse frequency with aging in postmenopausal women. J Clin Endocrinol Metab. 2000;85(5):1794–1800. [DOI] [PubMed] [Google Scholar]
- 30. Katzenellenbogen BS, Iwamoto HS, Heiman DF, Lan NC, Katzenellenbogen JA. Stilbestrols and stilbestrol derivatives: estrogenic potency and temporal relationships between estrogen receptor binding and uterine growth. Mol Cell Endocrinol. 1978;10(1):103–113. [DOI] [PubMed] [Google Scholar]
- 31. Binder AK, Winuthayanon W, Hewitt SC, Couse JF, Korach KS. Steroid receptors in the uterus and ovary. In: Plant T, Zelesnick A, eds. Knobil and Neill: Physiology of Reproduction. New York: Academic Press; 2014:1099–1102. [Google Scholar]
- 32. Oppermann K, Fuchs SC, Spritzer PM. Ovarian volume in pre- and perimenopausal women: a population-based study. Menopause. 2003;10(3):209–213. [DOI] [PubMed] [Google Scholar]
- 33. Christensen JT, Boldsen J, Westergaard JG. Ovarian volume in gynecologically healthy women using no contraception, or using IUD or oral contraception. Acta Obstet Gynecol Scand. 1997;76(8):784–789. [DOI] [PubMed] [Google Scholar]
- 34. Welt CK, Falorni A, Taylor AE, Martin KA, Hall JE. Selective theca cell dysfunction in autoimmune oophoritis results in multifollicular development, decreased estradiol, and elevated inhibin B levels. J Clin Endocrinol Metab. 2005;90(5):3069–3076. [DOI] [PubMed] [Google Scholar]
- 35. Soules MR, Steiner RA, Clifton DK, Cohen NL, Aksel S, Bremner WJ. Progesterone modulation of pulsatile luteinizing hormone secretion in normal women. J Clin Endocrinol Metab. 1984;58(2):378–383. [DOI] [PubMed] [Google Scholar]
- 36. Gill S, Lavoie HB, Bo-Abbas Y, Hall JE. Negative feedback effects of gonadal steroids are preserved with aging in postmenopausal women. J Clin Endocrinol Metab. 2002;87(5):2297–2302. [DOI] [PubMed] [Google Scholar]
- 37. Lukanova A, Kaaks R. Endogenous hormones and ovarian cancer: epidemiology and current hypotheses. Cancer Epidemiol Biomarkers Prev. 2005;14(1):98–107. [PubMed] [Google Scholar]
- 38. Bodelon C, Pfeiffer RM, Buys SS, Black A, Sherman ME. Analysis of serial ovarian volume measurements and incidence of ovarian cancer: implications for pathogenesis. J Natl Cancer Inst. 2014; 106(10):dju262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adams J, Polson DW, Franks S. Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. Br Med J (Clin Res Ed). 1986;293(6543):355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barnes RB, Namnoum AB, Rosenfield RL, Layman LC. The role of LH and FSH in ovarian androgen secretion and ovarian follicular development: clinical studies in a patient with isolated FSH deficiency and multicystic ovaries. Hum Reprod. 2002;17(1):88–91. [DOI] [PubMed] [Google Scholar]
- 41. Bry-Gauillard H, Larrat-Ledoux F, Levaillant JM, et al. Anti-Mullerian hormone and ovarian morphology in women with isolated hypogonadotropic hypogonadism/kallmann syndrome: effects of recombinant human FSH. J Clin Endocrinol Metab. 2017;102(4):1102–1111. [DOI] [PubMed] [Google Scholar]
- 42. Conte FA, Grumbach MM, Ito Y, Fisher CR, Simpson ER. A syndrome of female pseudohermaphrodism, hypergonadotropic hypogonadism, and multicystic ovaries associated with missense mutations in the gene encoding aromatase (P450arom). J Clin Endocrinol Metab. 1994;78(6):1287–1292. [DOI] [PubMed] [Google Scholar]
- 43. Lin L, Ercan O, Raza J, et al. Variable phenotypes associated with aromatase (CYP19) insufficiency in humans. J Clin Endocrinol Metab. 2007;92(3):982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mullis PE, Yoshimura N, Kuhlmann B, Lippuner K, Jaeger P, Harada H. Aromatase deficiency in a female who is compound heterozygote for two new point mutations in the P450arom gene: impact of estrogens on hypergonadotropic hypogonadism, multicystic ovaries, and bone densitometry in childhood. J Clin Endocrinol Metab. 1997;82(6):1739–1745. [DOI] [PubMed] [Google Scholar]
- 45. Hall JE. Neuroendocrine control of menstrual cycle. In: Strauss JF, Barbieri RL, eds. Yen and Jaffe’s Reproductive Endocrinology. 7th ed Philadelphia, PA: Saunders/ Elsevier; 2014:141–156. [Google Scholar]
- 46. Gregory SJ, Kaiser UB. Regulation of gonadotropins by inhibin and activin. Semin Reprod Med. 2004;22(3):253–267. [DOI] [PubMed] [Google Scholar]
- 47. Henry YM, Fatayerji D, Eastell R. Attainment of peak bone mass at the lumbar spine, femoral neck and radius in men and women: relative contributions of bone size and volumetric bone mineral density. Osteoporos Int. 2004;15(4):263–273. [DOI] [PubMed] [Google Scholar]
- 48. Szeliga A, Maciejewska-Jeske M, Męczekalski B. Bone health and evaluation of bone mineral density in patients with premature ovarian insufficiency. Prz Menopauzalny. 2018;17(3):112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smith EP, Specker B, Bachrach BE, et al. Impact on bone of an estrogen receptor-alpha gene loss of function mutation. J Clin Endocrinol Metab. 2008;93(8):3088–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mashchak CA, Lobo RA, Dozono-Takano R, et al. Comparison of pharmacodynamic properties of various estrogen formulations. Am J Obstet Gynecol. 1982;144(5):511–518. [DOI] [PubMed] [Google Scholar]
- 51. Bamigboye AA, Morris J. Oestrogen supplementation, mainly diethylstilbestrol, for preventing miscarriages and other adverse pregnancy outcomes. Cochrane Database Syst Rev. 2003;( 3):CD004353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee MW, Yang Y. Transient expression assay by agroinfiltration of leaves. Methods Mol Biol. 2006;323:225–229. [DOI] [PubMed] [Google Scholar]
- 53. Raucy JL, Lasker JM. Current in vitro high throughput screening approaches to assess nuclear receptor activation. Curr Drug Metab. 2010;11(9):806–814. [DOI] [PubMed] [Google Scholar]
- 54. Bennesch MA, Segala G, Wider D, Picard D. LSD1 engages a corepressor complex for the activation of the estrogen receptor α by estrogen and cAMP. Nucleic Acids Res. 2016;44(18):8655–8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Song RX. Membrane-initiated steroid signaling action of estrogen and breast cancer. Semin Reprod Med. 2007;25(3):187–197. [DOI] [PubMed] [Google Scholar]
- 56. Wang L, Yu Y, Chow DC, et al. Characterization of a steroid receptor coactivator small molecule stimulator that overstimulates cancer cells and leads to cell stress and death. Cancer Cell. 2015;28(2):240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]