Abstract
Context
Several studies have reported increased risk of fragility fractures in patients with mild autonomous cortisol secretion (MACS), discordant to the degree of bone density deterioration.
Objective
To evaluate the effect of MACS on bone metabolism in patients with adrenal adenomas.
Design
Cross-sectional study with prospective enrollment, 2014-2019
Setting
Referral center.
Patients
213 patients with adrenal adenomas: 22 Cushing syndrome (CS), 92 MACS and 99 nonfunctioning adrenal tumors (NFAT).
Main Outcome Measures
Osteocalcin, procollagen I intact N-terminal (PINP), C-terminal telopeptide (CTX), sclerostin.
Results
Patients with CS demonstrated lower markers of bone formation compared with patients with MACS and NFAT (CS vs MACS vs NFAT: mean osteocalcin 14.8 vs 20.1 vs 21.3 ng/mL [P < 0.0001]; mean PINP 34.8 vs 48.7 vs 48.5 µg/L [P = 0.003]). Severity of cortisol excess was inversely associated with sclerostin (CS vs MACS vs NFAT: mean sclerostin 419 vs 538 vs 624 ng/L, [P < 0.0001]). In a multivariable model of age, sex, body mass index, cortisol, and bone turnover markers, sclerostin was a significant predictor of low bone mass in patients with MACS (OR 0.63 [CI 95%, 0.40–0.98] for each 100 ng/L of sclerostin increase).
After adrenalectomy, osteocalcin, CTX, and sclerostin increased by a mean difference of 6.3 ng/mL, 0.12 ng/mL, and 171 pg/mL (P = 0.02 for all), respectively.
Conclusions
Lower sclerostin level in patients with MACS reflects a reduction in osteocyte function or number associated with exposure to chronic cortisol excess. Increase in bone turnover markers after adrenalectomy suggests restoration of favorable bone metabolism.
Keywords: MACS, Cushing syndrome, nonfunctioning adrenal tumors, sclerostin, osteopenia, osteoporosis, bone turnover markers
Incidentally discovered adrenal tumors are reported in approximately 5% of adults undergoing cross-sectional abdominal imaging, with roughly 95% representing benign adrenocortical adenomas (1). Overt adrenal hormonal excess is demonstrated in about 15% of patients with adenomas and includes mainly primary hyperaldosteronism and overt cortisol excess (Cushing syndrome, CS). Mild autonomous cortisol secretion (MACS), defined as an abnormal dexamethasone suppression test (cortisol > 1.8 µg/dL) (2) but without the classical external manifestations of overt CS, is more prevalent, affecting up to 48% of patients with adenomas (3–6).
Chronic exposure to subtle cortisol excess in patients with MACS has been reported to be detrimental to bone health (7). The prevalence of vertebral fractures in patients with MACS was reported to be 4 times higher compared with patients with nonfunctioning adrenal tumors (NFAT) (8–11). In a small retrospective comparative study of patients with MACS undergoing adrenalectomy versus those followed conservatively, the risk for new vertebral fractures was 5-fold lower in subjects who underwent adrenalectomy (12). Several studies have shown that the risk of new fractures in patients with MACS is discordant with the degree of bone mineral density (BMD) loss, suggesting that a reduction in bone quality rather than density contributes to the increased fracture risk (10, 11, 13). Patients with MACS were reported to have abnormal bone metabolism with low circulating concentrations of osteocalcin (14–17), consistent with reduced bone formation. In addition, while several studies demonstrated elevated levels of carboxyterminal telopeptide of type 1 collagen [CTX] (14–17) reflecting increased bone resorption, other studies did not (15, 18–20).
Abnormal bone metabolism may be a precursor to development of fragility fractures. However, small sample sizes, selective inclusion criteria, and heterogeneous definitions of MACS have collectively decreased the confidence and generalizability of reported results to date (15–17, 19–23). Our study aimed to prospectively assess the effect of MACS on bone metabolism by evaluating bone turnover markers in patients with MACS, CS, and NFAT. In a subset of patients undergoing adrenalectomy, we also aimed to evaluate the effect of remission of hypercortisolism on bone metabolism.
Participants and Methods
We prospectively enrolled patients evaluated for adrenal adenomas at Mayo Clinic in Rochester, Minnesota, from 2014 to 2019. The study protocol was approved by the Mayo Clinic Institutional Review Board and all subjects provided written, informed consent. Clinical, imaging, and biochemical data were prospectively collected at the time of enrollment and the medical record was again reviewed at the time of study conclusion. Fasting blood was collected in the morning from eligible participants and stored at −80°C. At the study conclusion, blood samples were analyzed for 2 markers of bone formation, osteocalcin (secreted by osteoblasts) and PINP (N-terminal propeptide of type 1 collagen, a cleaved byproduct of bone formation), as well as a marker of bone resorption, CTX (C-terminal telopeptide of type 1 collagen, cleaved byproduct of osteoclast activity), and sclerostin (produced by osteocytes).
Study participants
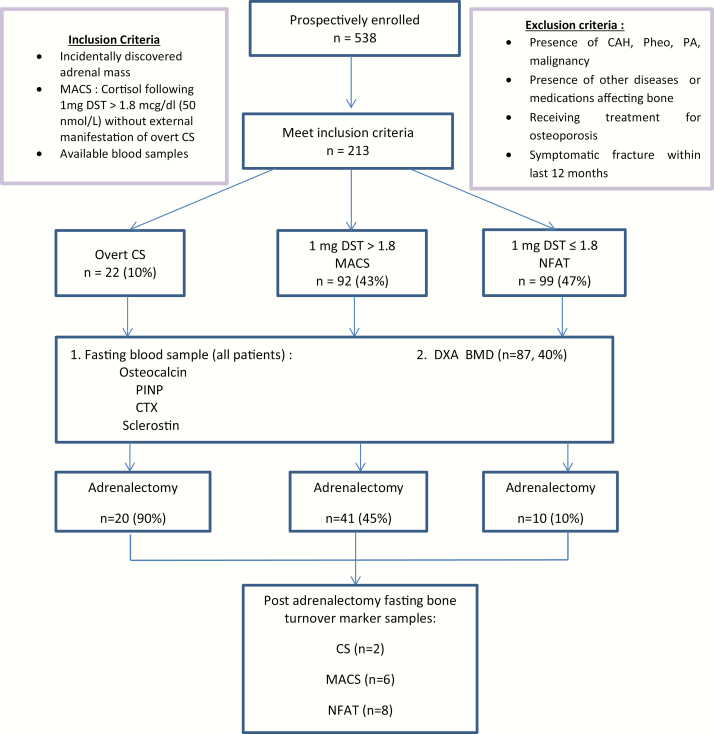
Patients were included in the study based on the following inclusion criteria: (1) adults with nonfunctioning adrenal adenoma, MACS, or CS; (2) available blood samples at the time of diagnosis (for the baseline cohort); (3) available blood samples at the time of diagnosis and also postadrenalectomy (at least 6 weeks after adrenalectomy and/or at least 6 weeks without the provision of exogenous glucocorticoid therapy, when recovery of the hypothalamic-pituitary-adrenal (HPA) axis had been documented with 8 am serum cortisol of ≥ 10 µg/dL [≥ 276 nmol/L] when assessed 24 hours after the last administered dose of glucocorticoid); (4) absence of symptomatic fracture within the last 12 months; (5) absence of drugs affecting bone metabolism, dexamethasone metabolism, or cortisol secretion; (6) absence of active extra-adrenal malignancy; and (7) absence of other known diseases that may affect bone metabolism (such as hypogonadism, thyrotoxicosis, hyper/hypoparathyroidism, malabsorptive disorders, chronic renal failure (eGFR < 30 mL/min), chronic hepatic disease, depression, alcoholism, eating disorders, or rheumatologic or hematologic diseases) (Fig. 1). MACS was diagnosed when cortisol levels following 1 mg dexamethasone overnight suppression was >1.8 µg/dL (>50 nmol/L) (2) without external manifestations of overt CS. CS was diagnosed based on the most recently published clinical practice guidelines (24). The diagnosis of osteopenia (−2.5 < T-score ≤ −1) at the hip and/or spine) or osteoporosis (T-score ≤ −2.5 at the hip and/or spine, or osteopenia with associated fragility fractures), presence of cardiovascular risk factors such as the presence of hypertension, dyslipidemia, prediabetes, and type 2 diabetes mellitus (DM2) were collected for all patients in the cohort. Hypertension was defined as a documented diagnosis of hypertension treated with at least 1 antihypertensive medication. Composite diabetes included patients with a documented diagnosis of either DM2 (HbA1c ≥ 6.4%) or prediabetes (5.7% < HbA1c < 6.4%) and therapy with at least 1 glucose‐lowering medication. Dyslipidemia was defined as documented diagnosis and therapy with at least 1 lipid‐lowering medication.
Figure 1.
Flow chart of study process. Abbreviations: MACS, mild autonomous cortisol secretion; DST, dexamethasone suppression test; CAH, congenital adrenal hyperplasia; PA, primary aldosteronism; CS, Cushing syndrome; NFAT, nonfunctioning adrenal tumor; PINP, N-terminal propeptide of type 1 collagen; CTX, C-terminal telopeptide of Type 1 collagen; DXA BMD, dual-energy X-ray absorptiometry.
Measurements
Biochemical analysis.
All patients were evaluated by a combination of various endocrine studies that included 1 and/or 8 mg overnight dexamethasone suppression test (DST), morning serum adrenocorticotropic hormone (ACTH) concentration, dehydroepiandrosterone sulphate (DHEA‐S), 24‐hour urinary free cortisol (UFC), and midnight salivary cortisol concentrations. Laboratory analyses were performed at Mayo Clinic Laboratories (Rochester, MN). Serum cortisol and DHEA‐S were measured on an immunoenzymatic assay (Beckman Coulter UniCel DxI 800), plasma ACTH was measured on an automated electrochemiluminescent immunoassay (Roche Cobas 8000), and 24‐hour UFC and midnight salivary cortisol were measured by liquid chromatography‐tandem mass spectrometry (LC‐MS/MS).
Bone turnover markers.
Eligible patients were requested to provide fasting blood samples for baseline assessment of markers of bone formation, consisting of serum osteocalcin measured by an electrochemiluminescence immunoassay assay (Roche Cobas e411; intra- and interassay coefficients of variance [CV] were 1.7% and 3.9%, respectively) and serum amino-terminal propeptide of type I collagen (PINP) measured by radioimmunoassay (Orion Diagnostica; intra-and interassay CVs were 2.3% and 3.8%, respectively); as well as resorption, consisting of serum CTX measured by an electrochemiluminescence immunoassay assay (Roche Cobas e411; intra- and interassay CVs were 1.9% and 7.8%, respectively). Sclerostin (heparinized plasma sample) was measured by a quantitative, solid-phase, sandwich enzyme immunoassay, (R&D Systems; intra- and interassay CVs were 1.2% and 9.6%, respectively). CV values were obtained from kit inserts.
Assessment of bone health.
Dual-energy x-ray absorptiometry (DXA) imaging was assessed at the lumbar spine, total hip, and femoral neck using a Lunar Prodigy scanner (General Electric Healthcare, Waukesha, WI).
Statistics
Descriptive statistics were used to determine mean and standard deviation (SD) or median and ranges depending on data distribution, while categorical data are shown as a number (%). Associations between variables were assessed using 1‐way ANOVA for continuous variables. P values less than 0.05 were considered significant. Data were analyzed using JMP SOFTWARE, VERSION 14 (SAS, Carey, NC). We performed multivariate regression analyses to investigate the relationship between bone turnover markers and morning cortisol concentrations after overnight DST in predicting bone disease (osteopenia/osteoporosis), while controlling for confounding factors including age, sex and body mass index (BMI).
Results
Demographic and endocrine data for patients with adrenal adenomas
A total of 213 patients (143 [67%] women), median age 58 years (range, 18-93) with adrenal adenomas (median tumor size 2.4 cm [range, 0.3-14.4]) were included (Table 1). Of the 213 patients, 22 (10%) were diagnosed with overt CS, 92 (43%) with MACS, and 99 (47%) with NFAT. Morning serum cortisol concentrations following dexamethasone administration (DST cortisol) were higher in patients with overt CS and MACS compared with patients with NFAT (median 8.5 vs 3 vs 1.2 µg/dL, respectively). ACTH concentrations and DHEA-S index (DHEA-S level divided by the lower limit of the sex- and age-based reference range) were lower in patients with overt CS when compared to patients with MACS and NFAT (ACTH: median 5 vs 8.95 vs 15.5 pg/mL [P < 0.0001] and DHEA-S index: median 0.65 vs 2.64 vs 4.62 [P < 0.0001], respectively). UFC concentrations were highest in patients with CS, but were similar in other groups (CS vs MACS vs NFAT: median 108 vs 26 vs 22 µg/24h, normal < 45 µg/24h; P < 0.0001), Table 1.
Table 1.
Demographic Data and Bone Turnover Marker Levels of Patients With Adrenal Adenomas
| CS (n = 22) | MACS (n = 92) | NFAT (n = 99) | P Value (CS vs MACS vs NFAT) | P Value (MACS vs NFAT) | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, median (range), years | 41.5 (18-61) | 59.5 (28-82) | 59 (28-93) | <0.0001 | 0.664 |
| Female, n (%) | 18 (86%) | 57 (62%) | 67 (68%) | 0.456 | 0.41 |
| BMI median (range), kg/m2 | 30.1 (21.9 – 54.6) | 31 (17 – 53) | 31 (15 – 51) | 0.06 | 0.525 |
| Weight, median (range), kg | 84 (62-113) | 89 (62-128) | 90 (63-126) | 0.29 | 0.94 |
| Computed tomography imaging | |||||
| Tumor size, median (range), cm | 3 (0.5-6.1) | 3.1 (0.5-14.4) | 1.7 (3-4.5) | <0.0001 | <0.001 |
| Unenhanced CT attenuation, n, median (range) Hounsfield units, n = 151 | n = 13 | n = 68 | n = 70 | 0.416 | 0.32 |
| 10 (-55 to 36) | 8.25 (-20 to 36) | 7 (-27 to 41) | |||
| Endocrine work up | |||||
| Cortisol after overnight 1 mg dexamethasone, median, nmol/L (range), µg/dL (range) | 235 (61-689) | 83 (52-441) | 33 (25-49) | <0.0001 | <0.001 |
| 8.5 (2.2-25) | 3 (1.9-16) | 1.2 (0.9-1.8) | |||
| ACTH, n, median, pmol/L (range), pg/mL (range n = 162 | n = 18 | n = 78 | n = 66 | <0.0001 | <0.0001 |
| 1.1 (1.09-2.94) | 1.97(1.1-6.2) | 3.41 (2.0-6.16) | |||
| 5 (4.99-13.4) | 8.95 (5-28.2) | 15.5 (9.27- 28) | |||
| Urine cortisol, n, median | n = 21 | n = 74 | n = 61 | <0.0001 | 0.049 |
| µg/dL/24h (range) | 108 (25-875) | 26(2.6-84) | 22(3.4-71) | ||
| nmol/24h (range) n = 156 | 298 (69-2415) | 72(7.2-232) | 61(9.4-196) | ||
| DHEA-S index, n, median (range), n = 146 | n = 19 | n = 71 | n = 56 | <0.0001 | 0.007 |
| 0.65 (0.18-7.03) | 2.63 (0.11-17.2) | 4.63 (0.58-16.41) | |||
| Cardiovascular Risk Factors | |||||
| Hypertension, n (%) | 16(73%) | 66(73%) | 63(64%) | 0.36 | 0.17 |
| Dyslipidemia, n (%) | 7(32%) | 57(63%) | 53(54%) | 0.03 | 0.18 |
| Diabetes Mellitus/IFG, n (%) | 9(41%) | 38(41%) | 40(41%) | 0.99 | 0.98 |
| Bone Disease | |||||
| DXA Bone density scans performed, n (%) | 8 (37%) | 48 (52%) | 31 (31%) | ||
| Osteopenia, n (%) | 5 (62%) | 27 (56%) | 16 (52%) | 0.84 | 0.69 |
| Osteoporosis, n (%) | 3 (38%) | 10 (21%) | 6 (19%) | 0.56 | 0.87 |
| Osteopenia and osteoporosis, n (%) | 8 (100%) | 37 (77%) | 22 (71%) | 0.09 | 0.54 |
| Bone Turnover Markers | |||||
| Osteocalcin, mean (SD), (range), ng/mL | 14.8 (16.3) (2.2-79.6) | 20.1 (10.2) (5.4-53.7) | 21.3 (12.1) (2.2-78.8) | <0.0001 | 0.39 |
| PINP, mean (SD), (range), µg/L | 34.8 (23.6) (12.3-91.6) | 48.7 (23.5) (13.3-123) | 48.5 (22) (3-152) | 0.003 | 0.84 |
| CTX, mean (SD), (range), ng/mL | 0.3 (0.2) (0.09-0.93) | 0.4 (0.3) (0.05-1.28) | 0.4 (0.2) (0.10-1.16) | 0.15 | 0.5 |
| Sclerostin, mean (SD), (range), pg/mL | 419 (199) (73.2-683) | 538 (296) (111-873) | 624 (218) (202-1273) | <0.0001 | 0.005 |
All P values < 0.05 were considered statistically significant.
Abbreviations: ACTH, Adrenocorticotrophic hormone; BMI, body mass index; CS, Cushing syndrome; CT, computed tomography; CTX, C-terminal telopeptide of Type 1 collagen; DHEA-S, dehydroepiandrosterone sulfate; DXA, dual-energy x-ray absorptiometry; IFG, impaired fasting glucose; MACS, mild autonomous cortisol secretion; NFAT, nonfunctioning adrenal tumors; PINP, N-terminal propeptide of type 1 collagen; SD, standard deviation.
No significant differences in the prevalence of composite diabetes and hypertension were noted between patients with overt CS, MACS, and NFAT, while dyslipidemia was most frequently detected in patients with MACS, followed by NFAT, and was lowest in CS (MACS vs NFAT vs CS: 63% vs 54% vs 32%), respectively, P = 0.03), reflecting demographic differences, Table 1.
Of the 87 patients (40%) with BMD assessed at the time of diagnosis (8 with CS, 48 with MACS, and 31 with NFAT), all patients with CS had low bone mass (5 with osteopenia and 3 with osteoporosis), 37 (77%) of patients with MACS had low bone mass (27 with osteopenia and 10 with osteoporosis), and 22 (71%) of patients with NFAT had low bone mass (16 with osteopenia and 6 with osteoporosis), Table 1.
Bone turnover markers
Unadjusted for confounding factors, in patients with overt CS, values for markers of bone formation (osteocalcin and PINP) were significantly lower when compared to patients with MACS and NFAT (CS vs MACS vs NFAT: mean osteocalcin 14.8 vs 20.1 vs 21.3 ng/mL [P < 0.0001]; mean PINP 34.8 vs 48.7 vs 48.5 µg/L [P = 0.003]) (Table 1). No significant differences were noted in CTX measurements between the 3 groups (Table 1). Severity of cortisol excess was inversely associated with sclerostin measurements (CS vs MACS vs NFAT: mean sclerostin 419 vs 538 vs 624 ng/L; P < 0.0001) (Table 1). In patients with MACS, sclerostin was a significant predictor of bone disease (osteopenia and/or osteoporosis) in a multivariable model which included age, sex, BMI, DST cortisol, and bone turnover markers (OR 0.63 [95% CI, 0.40-0.98] for each 100 ng/L of sclerostin increase; P = 0.04) (Table 2).
Table 2.
Predictors of Osteopenia and Osteoporosis in Patients With MACS
| Predictor | Multivariable Analysis | ||
|---|---|---|---|
| Odds Ratio | 95% CI | P Value | |
| Age (for every 10 yrs increase) | 1.20 | 0.57-2.53 | 0.83 |
| Female sex | 1.35 | 0.18-9.87 | 0.77 |
| BMI (for every 5 kg/m2 increase) | 0.94 | 0.49-1.80 | 0.85 |
| Osteocalcin (for every ng/mL increase) | 1.10 | 0.92-1.32 | 0.26 |
| PINP (for every 1µg/L increase) | 0.97 | 0.90-1.07 | 0.62 |
| CTX (for every ng/mL increase) | 2.44 | 0.0-3189 | 0.8 |
| Sclerostin (for every 100 pg/mL increase) | 0.63 | 0.40-0.98 | *0.04 |
| Cortisol after 1mg DST (for every 1 µg/dL increase) | 0.92 | 0.74-1.16 | 0.51 |
All P values < 0.05 were considered statistically significant.
BMI, Body mass index; PINP, N-terminal propeptide of type 1 collagen; CTX, C-terminal telopeptide of Type 1 collagen; DST, dexamethasone suppression test
Postadrenalectomy changes in bone metabolism
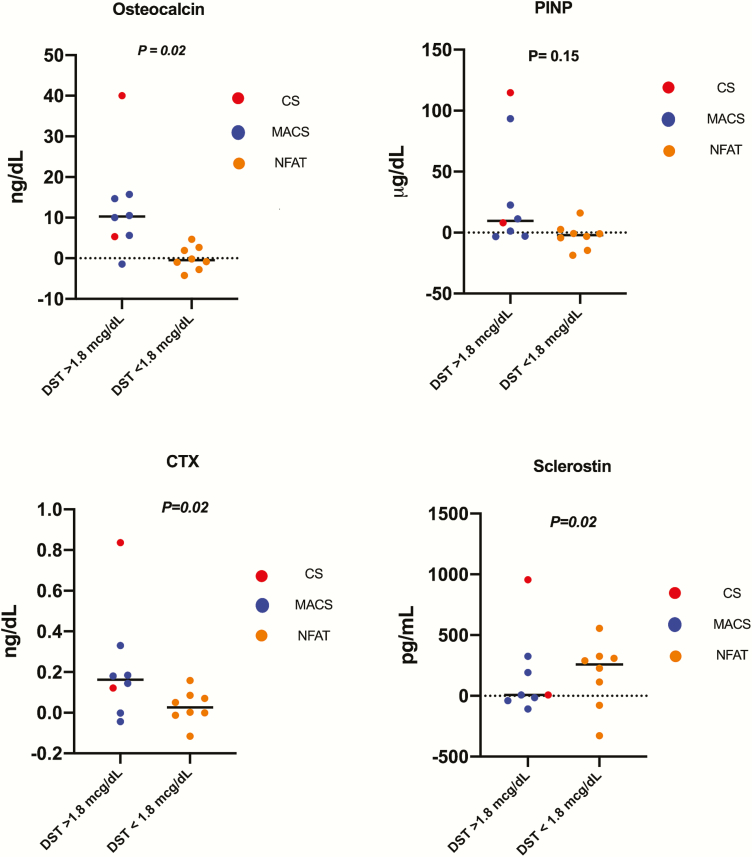
In 16 patients (2 patients with overt CS, 6 patients with MACS and 8 patients with NFAT), serum bone turnover markers were measured following adrenalectomy at a median of 37 weeks (range 6-92). Circulating levels of osteocalcin, CTX, and sclerostin increased significantly by a mean difference of 6.3 ng/mL (SD = 10.8; P = 0.02), 0.12 ng/mL (SD = 0.2; P = 0.02), and 171 pg/mL (SD = 302.4; P = 0.02), respectively. PINP concentrations demonstrated a nonsignificant increase after adrenalectomy (mean difference of 8.52 µg/L (SD = 32.24; P = 0.15) (Fig. 2). In patients with MACS only (n = 8), levels of osteocalcin and CTX increased significantly by a mean difference of 8.18 ng/mL (SD = 6.74; P = 0.04) and 0.14 ng/mL (SD = 0.12; P = 0.05), respectively. Sclerostin and PINP levels increased post adrenalectomy, thought nonsignificantly, by a mean difference of 107 pg/mL (SD = 181; P = 0.24) and 1.78 µg/L (SD = 35.4; P = 0.75), respectively.
Figure 2.
Mean difference of bone turnover markers pre- and post-adrenalectomy (remission). Abbreviations: CS, Cushing syndrome; MACS, mild autonomous cortisol secretion; NFAT, nonfunctioning adrenal tumor; DST, dexamethasone suppression test; PINP, N-terminal propeptide of type 1 collagen; CTX, C-terminal telopeptide of Type 1 collagen. All P values < 0.05 were considered statistically significant.
Discussion
In our study, we demonstrated that sclerostin was the only biomarker that distinguished patients with MACS from patients with NFAT. We also showed that sclerostin concentrations were inversely proportional to the degree of hypercortisolism. Concentrations of osteocalcin, PINP and CTX were similar in patients with MACS and NFAT. In a multivariable analysis, sclerostin was a significant predictor of low bone mass in patients with MACS. Sclerostin has been recognized as a key negative regulator of bone formation, and glucocorticoid therapy has been reported to stimulate the expression of the SOST gene, which encodes sclerostin (25). Contrary to this observation, our study showed that plasma sclerostin levels are significantly decreased in patients with endogenous hypercortisolism. This could be explained by the differences of chronic endogenous cortisol excess versus transient/acute effect of exogenous glucocorticoid therapy on bone metabolism. In bone, sclerostin is exclusively produced by osteocytes (26) and chronic glucocorticoid exposure can induce autophagy in osteocytes (27) and increase oxidative stress (28). Decreased sclerostin concentrations in patients with MACS may be explained by the decrease in osteocyte function and/or number rather than by a direct, inhibitory effect on sclerostin production. Similar observation of low sclerostin was reported in one other study which included 21 patients with endogenous overt cortisol excess (29) , though no other studies have explored the role of sclerostin in MACS.
Our study confirmed previous reports that osteocalcin concentrations inversely correlate with the degree of cortisol excess in overt CS (30, 31). This is consistent with existing knowledge that cortisol excess inhibits the differentiation of osteoblasts from precursor mesenchymal cells and increases osteoblast apoptosis (32). In our study, we did not identify differences in concentrations of osteocalcin, PINP, and CTX in patients with MACS versus patients with NFAT. Several studies have reported low osteocalcin concentrations in patients with MACS (14–17, 20, 22, 23). However, other studies did not confirm a reduction in bone formation as measured by osteocalcin (18, 19, 21) and PINP (14, 15). Similar discrepancies have been reported in assessment of bone resorption, with CTX concentrations being increased (14, 17, 23) , normal (15, 19) or reduced (20) , and urinary deoxypyridinoline concentrations reported within the normal range in patients with MACS (15, 16, 19, 21, 22). These conflicting results may be attributed to small sample sizes, selection criteria based on age and gonadal status, as well as differences in definition of MACS.
In a small cohort of patients undergoing adrenalectomy, we observed an increase in bone turnover markers after surgery. The increase in osteocalcin and PINP concentrations are consistent with improvement in osteoblast activity. The noted rise in CTX levels is consistent with increased turnover with bone remodeling. The increase in sclerostin concentrations noted after adrenalectomy likely reflects recovery of osteocytic function after the cessation of metabolic stress, or to an increase in the number of osteocytes after the removal of the apoptotic effect of cortisol excess. This is further supported by the reported improvement in FGF23 levels in patients with treated endogenous hypercortisolism. FGF23 is also produced by osteocytes, and its rise after cure from hypercortisolism reflects restoration of osteocyte numbers and/or function (25).
Strengths and Limitations
We acknowledge that despite the prospective enrollment, evaluation of patients did not follow a prespecified protocol, which explains that not all patients had a uniform approach to testing. Our study lacks a control group without adrenal tumors, which would have allowed for a comparative assessment of patients with NFAT who may still have subtle cortisol secretion not detected by current standard of care testing (33). The length of time patients had hypercortisolism prior to diagnosis is also unknown and could potentially affect the degree of disruption to bone metabolism. In addition, although all patients were recruited from within the Mayo Clinic, only one of the clinics routinely obtained DXA imaging in all patients with MACS. Strengths of this study are consecutive enrollment, relatively large cohort size, uniform diagnosis based on the established 2016 ESE/ENSAT guidelines, and the same assays used for laboratory measurements. While BMD assessments were obtained in only in 40% of patients, all DXA imaging was performed using the same approach at a single institution. Finally, inclusion of a small cohort of patients with measurements pre- and post-adrenalectomy patients further supports the effect of cortisol secretion on bone metabolism.
Clinical Implications
MACS has been reported to have a detrimental effect on bone health, with a higher prevalence of vertebral fractures when compared to NFAT. Several studies have shown that the risk of new fractures in patients with MACS is discordant to BMD, suggesting that a reduction in bone quality rather than only BMD contributes to the increased fracture risk (10, 11, 13). Hence, existing screening tools to evaluate for osteopenia and osteoporosis via DXA may not be able to detect patients at risk of developing fragility fractures early enough to intervene. Whilst adrenalectomy has been reported to decrease the risk of new fractures, the appropriate selection of patients with MACS who would benefit most from adrenalectomy remains challenging.
Our study found sclerostin to be a predictor for osteopenia or osteoporosis in a multivariable model of age, sex, BMI, DST cortisol, and bone turnover markers. Measurements of sclerostin may help identify patients with MACS who are at risk of having osteopenia or osteoporosis; however, it is unclear whether sclerostin concentrations are predictive of future fragility fractures. Measurements of sclerostin concentrations during the evaluation of patients with MACS may be of value in selectively identifying those patients most likely to benefit from adrenalectomy. However, larger longitudinal studies are needed to validate sclerostin as a diagnostic and prognostic biomarker of skeletal health in patients with MACS.
Conclusions
In conclusion, patients with MACS demonstrate lower sclerostin concentrations reflecting a reduction in osteocyte function or number due to exposure to chronic cortisol excess. Overt CS is associated with reduced bone formation as demonstrated by lower osteocalcin and PINP concentrations. Increase in bone turnover markers after adrenalectomy suggests restoration of favorable bone metabolism.
Acknowledgments
The authors thank Jolaine M. Hines, Immunochemical Core Laboratory, Mayo Clinic.
Financial Support: This research was supported by the James A. Ruppe Career Development Award in Endocrinology (I.B.) and the Catalyst Award for Advancing in Academics from Mayo Clinic (I.B.).
This research was partly supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) USA under award K23DK121888 (to I.B). The views expressed are those of the author(s) and not necessarily those of the National Institutes of Health USA.
Glossary
Abbreviations
- ACTH
adrenocorticotropic hormone
- BMD
bone mineral density
- CS
Cushing syndrome
- CTX
C-terminal telopeptide of type 1 collagen
- DHEA-S
dehydroepiandrosterone sulfate
- DST
dexamethasone suppression test
- DXA
dual-energy x-ray absorptiometry
- MCAS
mild autonomous cortisol secretion
- NFAT
nonfunctioning adrenal tumors
- PINP
procollagen I intact N-terminal
- UFC
urinary free cortisol
Additional Information
Disclosure Summary: I.B. reports advisory board participation with Corcept and HRA Pharma outside the submitted work.
References
- 1. Bovio S, Cataldi A, Reimondo G, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006;29(4):298–302. [DOI] [PubMed] [Google Scholar]
- 2. Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1–G34. [DOI] [PubMed] [Google Scholar]
- 3. Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25(2):309–340. [DOI] [PubMed] [Google Scholar]
- 4. Barzon L, Sonino N, Fallo F, Palu G, Boscaro M. Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol. 2003;149(4):273–285. [DOI] [PubMed] [Google Scholar]
- 5. Bancos I, Chortis V, Lang K, et al. The natural history of adrenal incidentaloma - results from the international prospective multi-centre EURINE-ACT study. Endocrine Abstracts 201749:GP122 2017. [Google Scholar]
- 6. Prete A, Paragliola RM, Bottiglieri F, et al. Factors predicting the duration of adrenal insufficiency in patients successfully treated for Cushing disease and nonmalignant primary adrenal Cushing syndrome. Endocrine. 2017;55(3):969–980. [DOI] [PubMed] [Google Scholar]
- 7. Athimulam S, Bancos I. Evaluation of bone health in patients with adrenal tumors. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):125–132. [DOI] [PubMed] [Google Scholar]
- 8. Athimulam S, Delivanis D, Khosla S, Drake M, Bancos I.. SAT-366 The impact of mild autonomous cortisol secretion on bone metabolism. J Endocr Soc. 2019; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiodini I, Guglielmi G, Battista C, et al. Spinal volumetric bone mineral density and vertebral fractures in female patients with adrenal incidentalomas: the effects of subclinical hypercortisolism and gonadal status. J Clin Endocrinol Metab. 2004;89(5):2237–2241. [DOI] [PubMed] [Google Scholar]
- 10. Chiodini I, Morelli V, Masserini B, et al. Bone mineral density, prevalence of vertebral fractures, and bone quality in patients with adrenal incidentalomas with and without subclinical hypercortisolism: an Italian multicenter study. J Clin Endocrinol Metab. 2009;94(9):3207–3214. [DOI] [PubMed] [Google Scholar]
- 11. Chiodini I, Viti R, Coletti F, et al. Eugonadal male patients with adrenal incidentalomas and subclinical hypercortisolism have increased rate of vertebral fractures. Clin Endocrinol. 2009;70(2):208–213. [DOI] [PubMed] [Google Scholar]
- 12. Salcuni AS, Morelli V, Eller Vainicher C, et al. Adrenalectomy reduces the risk of vertebral fractures in patients with monolateral adrenal incidentalomas and subclinical hypercortisolism. Eur J Endocrinol. 2016;174(3):261–269. [DOI] [PubMed] [Google Scholar]
- 13. Morelli V, Eller-Vainicher C, Salcuni AS, et al. Risk of new vertebral fractures in patients with adrenal incidentaloma with and without subclinical hypercortisolism: a multicenter longitudinal study. J Bone Miner Res. 2011;26(8):1816–1821. [DOI] [PubMed] [Google Scholar]
- 14. Osella G, Terzolo M, Reimondo G, et al. Serum markers of bone and collagen turnover in patients with Cushing’s syndrome and in subjects with adrenal incidentalomas. J Clin Endocrinol Metab. 1997;82(10):3303–3307. [DOI] [PubMed] [Google Scholar]
- 15. Torlontano M, Chiodini I, Pileri M, et al. Altered bone mass and turnover in female patients with adrenal incidentaloma: the effect of subclinical hypercortisolism. J Clin Endocrinol Metab. 1999;84(7):2381–2385. [DOI] [PubMed] [Google Scholar]
- 16. Chiodini I, Torlontano M, Carnevale V, et al. Bone loss rate in adrenal incidentalomas: a longitudinal study. J Clin Endocrinol Metab. 2001;86(11):5337–5341. [DOI] [PubMed] [Google Scholar]
- 17. Tauchmanovà L, Pivonello R, De Martino MC, et al. Effects of sex steroids on bone in women with subclinical or overt endogenous hypercortisolism. Eur J Endocrinol. 2007;157(3):359–366. [DOI] [PubMed] [Google Scholar]
- 18. Hadjidakis D, Tsagarakis S, Roboti C, et al. Does subclinical hypercortisolism adversely affect the bone mineral density of patients with adrenal incidentalomas? Clin Endocrinol. 2003;58(1):72–77. [DOI] [PubMed] [Google Scholar]
- 19. Bardet S, Rohmer V, Boux de Casson F, et al. [Bone mineral density and biological markers of bone repair in patients with adrenal incidentaloma: effect of subclinical hypercortisolism]. Rev Med Interne. 2002;23(6):508–517. [DOI] [PubMed] [Google Scholar]
- 20. Sartorio A, Conti A, Ferrero S, et al. Evaluation of markers of bone and collagen turnover in patients with active and preclinical Cushing’s syndrome and in patients with adrenal incidentaloma. Eur J Endocrinol. 1998;138(2):146–152. [DOI] [PubMed] [Google Scholar]
- 21. Chiodini I, Tauchmanovà L, Torlontano M, et al. Bone involvement in eugonadal male patients with adrenal incidentaloma and subclinical hypercortisolism. J Clin Endocrinol Metab. 2002;87(12):5491–5494. [DOI] [PubMed] [Google Scholar]
- 22. Francucci CM, Pantanetti P, Garrapa GG, Massi F, Arnaldi G, Mantero F. Bone metabolism and mass in women with Cushing’s syndrome and adrenal incidentaloma. Clin Endocrinol. 2002;57(5):587–593. [DOI] [PubMed] [Google Scholar]
- 23. Guo W, Li F, Zhu C, et al. Effect of hypercortisolism on bone mineral density and bone metabolism: A potential protective effect of adrenocorticotropic hormone in patients with Cushing’s disease. J Int Med Res. 2018;46(1):492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nieman LK, Biller BM, Findling JW, et al. ; Endocrine Society Treatment of Cushing’s syndrome: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yao W, Cheng Z, Busse C, Pham A, Nakamura MC, Lane NE. Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: a longitudinal study of gene expression in bone tissue from glucocorticoid-treated mice. Arthritis Rheum. 2008;58(6):1674–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Bezooijen RL, Roelen BA, Visser A, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199(6): 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xia X, Kar R, Gluhak-Heinrich J, et al. Glucocorticoid-induced autophagy in osteocytes. J Bone Miner Res. 2010;25(11):2479–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Almeida M, Han L, Ambrogini E, Weinstein RS, Manolagas SC. Glucocorticoids and tumor necrosis factor α increase oxidative stress and suppress Wnt protein signaling in osteoblasts. J Biol Chem. 2011;286(52):44326–44335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Lierop AH, van der Eerden AW, Hamdy NA, Hermus AR, den Heijer M, Papapoulos SE. Circulating sclerostin levels are decreased in patients with endogenous hypercortisolism and increase after treatment. J Clin Endocrinol Metab. 2012;97(10):E1953–E1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Szappanos A, Toke J, Lippai D, et al. Bone turnover in patients with endogenous Cushing’s syndrome before and after successful treatment. Osteoporos Int. 2010;21(4):637–645. [DOI] [PubMed] [Google Scholar]
- 31. Sereg M, Toke J, Patócs A, et al. Diagnostic performance of salivary cortisol and serum osteocalcin measurements in patients with overt and subclinical Cushing’s syndrome. Steroids. 2011;76(1-2):38–42. [DOI] [PubMed] [Google Scholar]
- 32. Chang JK, Li CJ, Liao HJ, Wang CK, Wang GJ, Ho ML. Anti-inflammatory drugs suppress proliferation and induce apoptosis through altering expressions of cell cycle regulators and pro-apoptotic factors in cultured human osteoblasts. Toxicology. 2009;258(2-3):148–156. [DOI] [PubMed] [Google Scholar]
- 33. Lopez D, Luque-Fernandez MA, Steele A, Adler GK, Turchin A, Vaidya A. “Nonfunctional” adrenal tumors and the risk for incident diabetes and cardiovascular outcomes: A cohort study. Ann Intern Med. 2016;165(8):533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]