Abstract
Context
The reproductive axis is controlled by a network of gonadotropin-releasing hormone (GnRH) neurons born in the primitive nose that migrate to the hypothalamus alongside axons of the olfactory system. The observation that congenital anosmia (inability to smell) is often associated with GnRH deficiency in humans led to the prevailing view that GnRH neurons depend on olfactory structures to reach the brain, but this hypothesis has not been confirmed.
Objective
The objective of this work is to determine the potential for normal reproductive function in the setting of completely absent internal and external olfactory structures.
Methods
We conducted comprehensive phenotyping studies in 11 patients with congenital arhinia. These studies were augmented by review of medical records and study questionnaires in another 40 international patients.
Results
All male patients demonstrated clinical and/or biochemical signs of GnRH deficiency, and the 5 men studied in person had no luteinizing hormone (LH) pulses, suggesting absent GnRH activity. The 6 women studied in person also had apulsatile LH profiles, yet 3 had spontaneous breast development and 2 women (studied from afar) had normal breast development and menstrual cycles, suggesting a fully intact reproductive axis. Administration of pulsatile GnRH to 2 GnRH-deficient patients revealed normal pituitary responsiveness but gonadal failure in the male patient.
Conclusions
Patients with arhinia teach us that the GnRH neuron, a key gatekeeper of the reproductive axis, is associated with but may not depend on olfactory structures for normal migration and function, and more broadly, illustrate the power of extreme human phenotypes in answering fundamental questions about human embryology.
Keywords: arhinia, GnRH, Kallmann
Reproductive function in humans rests entirely in the hands of a network of about 2000 gonadotropin-releasing hormone (GnRH) (1) neurons in the hypothalamus. Pulsatile release of GnRH stimulates pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which act on the gonads to induce gametogenesis and sex steroid synthesis. The GnRH neurons have a unique birth history: They arise completely outside the confines of the brain, in the primitive nose. During the first trimester, neurons of the olfactory system and GnRH neurons are intimately associated as they make the long, tortuous journey from the nose through the nasal mesenchyme, across the skull base, and into the forebrain (2). At this early stage of development, the olfactory structures are divided into 2 systems: 1) the main olfactory system of olfactory nerves and bulbs, and 2) the accessory olfactory system, which includes the vomeronasal nerve (VN) and its target, the accessory olfactory bulb, as well as the terminal nerve (TN), which penetrates the brain and extends toward the septal, limbic, and hypothalamic areas (2, 3). Whereas the main olfactory bulbs persist into adulthood, the accessory olfactory bulbs, VN, and TN regress late in fetal life (4) and have thus been regarded as vestigial structures in humans.
Indeed, the hypothesis that GnRH neuronal migration might be entirely dependent on the integrity of the main olfactory nerve/bulbs provided an attractive explanation for the etiology of Kallmann syndrome (KS), or congenital anosmia with GnRH deficiency (5-7). Modern imaging studies have in fact shown that most, but not all (8, 9), patients with KS have no olfactory bulbs, tracts, and sulci (10, 11). In addition, some patients with mutations in KS-associated genes (12) (eg, ANOS1, PROK2, PROKR2, SEMA3A, and CHD7), including family members of KS patients (13), have complete olfactory agenesis yet display apparently normal reproductive function. Such patients have not been interpreted as evidence against this hypothesis; rather, it has been proposed that they must lack key genetic modifiers responsible for KS (12). Studies in a number of other species, however, also suggest that GnRH migration is not inexorably tied to the integrity of the main olfactory system. Arx-1null (Aristaless-related homeobox) mice show defective routing of olfactory and VN axons and severe olfactory bulb aplasia, yet GnRH neurons reach the forebrain via the TN (14). The unique development of the olfacto-terminalis systems in toothed whales provides additional support for this alternative hypothesis: These mammals have no VN and only a rudimentary main olfactory structure that disintegrates in late fetal life (15), leaving only a GnRH-immunoreactive TN system postnatally. In the chick embryo, GnRH neurons are not entirely faithful to the olfactory system because they have also been identified along the ophthalmic division of the trigeminal nerve (16). Taken together, these findings suggest that GnRH neurons may demonstrate greater flexibility in their migratory route to the brain than has been previously appreciated.
To investigate the dependency of GnRH neuronal ontogeny on the main olfactory system in humans, we performed a comprehensive phenotyping study of a cohort of patients with congenital arhinia. Arhinia, the complete absence of an external nose and internal olfactory structures, is arguably the most severe nasal malformation compatible with life in humans. It is an extremely rare condition (< 100 case reports in the past century) with no sex or ethnic bias (17) and has recently been linked to mutations in SMCHD1 (17, 18), which encodes an epigenetic repressor. Although a subset of arhinia patients have ocular and reproductive defects (19-23), a triad called Bosma arhinia microphthalmia syndrome (BAMS) (20, 24), the underlying etiology and spectrum of reproductive defects, including the potential for normal reproductive function, has not been determined in these patients.
Methods
Study patients
Twenty-seven male and 24 female patients, age 1 to 75 years, with either arhinia (n = 47) or arhinia subphenotypes (nasal hypoplasia, n = 2; absent nasal spine [Binder maxillonasal dysplasia], n = 1; hemiarhinia, n = 1) in the absence of holoprosencephaly participated in studies at the National Institutes of Health (NIH) or Massachusetts General Hospital (MGH) between 1996 and 2019 (Tables 1A and 1B and Table S1) (27). Basic clinical information and results of genetic studies were previously reported in a subset of patients (17, 28, 29); 7 male patients and 9 female patients are new (including patients 2, 3, 7, and 8 studied at NIH). All patients are known to harbor rare (minor allele frequency < 0.1%), heterozygous missense mutations in SMCHD1 except for 1 male patient who did not undergo genetic testing but who is part of a multiplex family (family AB, Table S1) with a known mutation (27). Variants were identified by either whole-exome sequencing (WES; n = 29) or targeted sequencing. No additional rare sequence variants in known KS genes were identified in the patients who underwent WES. The study was approved by the hospital human research committees and signed informed consent (or parental assent, where appropriate) was obtained before participation.
Table 1.
Clinical characteristics of the A, 5 male and B, 6 female patients with arhinia or absent nasal spine (patient No. 8) who underwent detailed phenotyping studies
| A, Evaluation of Males at Initial Diagnosis (Patient No.) | No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | |
|---|---|---|---|---|---|---|
| Age, y | 14.0 | 12.5 | 15.8 | 14.0 | 13.8 | |
| Physical exam | ||||||
| Micropenis | Yes | Yes | Yes | Yes | Yes | |
| Cryptorchidism | No | Yes, b/l | Yes, R | No | Yes, L | |
| Testicular volume, cc | 3 | [Removed at age 4 y] | 2–3 | 1 | 2 | |
| Tanner stage pubic hair | I | – | II | II | II | |
| Endocrine studies | ||||||
| LH, IU/L | < 0.1 | – | <0.1 | <0.15 | <0.1 | |
| FSH, IU/L | < 0.3 | – | <0.3 | <0.5 | <0.3 | |
| Total testosterone, ng/dL | 5.6 | 3.0 | <10.0 | 29.0 | 31.0 | |
| Peak LH (IU/L), GnRH stimulation testa | 1.6 | – | – | 1.5 | – | |
| Peak testosterone (ng/dL), hCG stimulation testb | – | 19 | – | 249 | – | |
| Bone age, y | 12.5 | 12.5 | 13.5 | 13.0 | 14.0 | |
| Hormone therapy (age at initiation, y) | Testosterone (14 y) | Testosterone (12 y) | Testosterone (16 y) | HMG/hCG (16-18); testosterone (14-16; 18) | Testosterone (14 y) | |
| Evaluation as Study Participant | ||||||
| Clinical parameters | ||||||
| Age, y | 25 | 36 | 19 | 28 | 23 | |
| Height, cm | 177.7 | 174.0 | 171.1 | 179.8 | 177.0 | |
| Weight, kg | 90.1 | 92.1 | 97.0 | 114.9 | 91.8 | |
| BMI, kg/m2 | 28.5 | 30.2 | 33.1 | 35.5 | 29.5 | |
| Biochemical studies | ||||||
| LH, IU/L | 0.1 | <0.1 | 0.1 | <0.3 | 0.1 | |
| FSH, IU/L | 0.3 | 0.1 | 0.1 | <0.7 | 0.3 | |
| Total testosterone, ng/dL | 13.0 | 12.0 | 34.6 | 25.0 | 44.0 | |
| Inhibin B, pg/mL | <10.0 | <10.0 | <10.0 | 22.2 | <10.0 | |
| Imaging studies | ||||||
| Brain MRI | ||||||
| Pituitary and hypothalamus | Normal | Normal | Normal | Normal | Pituitary hypoplasia | |
| Olfactory structures | No nerves, bulbs, sulci | No bulbs | No bulbs | No sulcid | No bulbs, tracts | |
| Ophthalmic structures | Hypoplastic | Hypoplastic | Hypoplastic | – | Normal | |
| Internal auditory structures | Normal | Normal | Normal | – | Normal | |
| Testicular volume on ultrasound, R, L, cc | 0.6, 0.7 | c | L 1.1c | – | R 0.4c | |
| Nonreproductive phenotypes | ||||||
| Mirror movements | No | Yes | No | Yes | Yes | |
| Renal agenesis | No | No | No | No | No | |
| Skeletal abnormalities | Hypoplastic ribs (#12) | Hypoplastic ribs (#5, 6, 12) | No | – | No | |
| Dental abnormalities (No. of missing teeth) | Agenesis (2) | 1 ectopic tooth | Agenesis (1) | Normal | Agenesis (3) | |
| Hearing | Normal | Normal | Losse | Losse | Normal | |
| External ears | Small; low-set; no upper pinna | Low-set; protruding; infolded cartilage | Normal | Normal | Normal | |
| Ocular | Normal | Microphthalmia | Anophthalmia | Normal | Normal | |
| Anosmia (UPSIT score) | Yes (8/40) | Yes (6/40) | Yes (12/40) | Yes (–) | Yes (11/40) | |
| Cleft lip/palate (CL/CP) | No | CP | No | CP | CL and CP | |
| SMCHD1 mutation | p.S135N | p.E473G | p.M129R | p.N524S | p.L141F | |
| B, Evaluation of Females at Initial Diagnosis (Patient No.) | No. 6 | No. 7 | No. 8 | No. 9 | No. 10 | No. 11 |
| Age, y | 17.5 | 16.0 | 15.0 | 14.7 | 19.0 | 15.9 |
| Physical exam | ||||||
| Tanner stage, breast | II | II | II | I | I | I |
| Tanner stage, pubic hair | IV | II | II | IV | I | V |
| Endocrine studies | ||||||
| LH (IU/L) | <0.2 | 0.3 | <0.02 | 0.05 | <1.0 | |
| FSH (IU/L) | 0.8 | 1 | <0.09 | 0.7 | <2.0 | |
| Estradiol, pg/mL | 9.7 | 15 | 5 | <1.0 | – | |
| Bone age, y | – | – | 13.5 | 10.0 | – | 13.0 |
| Estrogen replacement, age at initiation, y | None | Yes, 15 | Yes, 15 | Yes, 15 | Yes, 21 | Yes, 16 |
| Evaluation as Study Participant | ||||||
| Clinical parameters | ||||||
| Age, y | 21 | 29 | 19 | 18 | 53 | 23 |
| Height, cm | 161.4 | 169.0 | 182.9 | 169.2 | 165.6 | 165.2 |
| Arm span, cm | 171.8 | 169.0 | 182.0 | 170.0 | 166.3 | – |
| Midparental height, cm | 168.9 | 163.8 | 174.0 | 170.0 | 157.5 | 160.0 |
| Weight, kg | 49.4 | 79.7 | 70.1 | 72.7 | 75.8 | 53.9 |
| BMI, kg/m2 | 19.0 | 27.7 | 21.0 | 25.4 | 27.6 | 21.4 |
| Biochemical studies | ||||||
| LH (IU/L) | <0.1 | <0.1 | 0.1 | <0.1 | 0.2 | <0.3 |
| FSH (IU/L) | 0.4 | 0.3 | 0.1 | <0.1 | 0.5 | <0.7 |
| Estradiol (pg/mL) | <10.0 | <10.0 | <10.0 | <10.0 | <10.0 | – |
| Imaging studies | ||||||
| Brain MRI | ||||||
| Pituitary and hypothalamus | Normal | Normal | Normal | Normal | Normal | Normal |
| Olfactory structures | Hypoplastic sulci, no bulbs | No bulbs | No bulbs | No bulbs | Hypoplastic sulci, no bulbs | d |
| Ophthalmic structures | Hypoplastic orbits | Normal | Thin L optic n. | Normal | Normal | d |
| Internal auditory structures | Normal | Normal | Normal | Normal | Normal | Bulbous deformity R semicircular canal |
| Evaluation as Study Participant | ||||||
| Pelvic ultrasound | ||||||
| R and L ovarian volumes, cc | Not visible | 0.2, 0.4 | 5.5, 3.3 | Not visible | Not visible | – |
| Uterine volume, cc | 1.4 | 3.6 | 21.0 | 12.3 | 15.7 | – |
| Nonreproductive phenotypes | ||||||
| Mirror movements | Yes | No | No | Yes | No | – |
| Renal agenesis | No | No | No | No | No | No |
| Skeletal abnormalities | Clinodactyly, short 4th L MC | Hypoplastic ribs (R; #6, 7) | Hypermobility, arachnodactyly | No | No | Short 4th MCs, hypermobility, leg- and arm-length discrepancy |
| Dental abnormalities (No. of missing teeth) | Agenesis (7) | Normal | Normal | Agenesis (2) | Agenesis (–) | Normal |
| External ears | Normal | Low-set | Normal | Normal | R pinna cupped | Normal |
| Ocular | Anophthalmia | Normal | Normal | Normal | Colobomatous microphthalmia, cataract | Colobomatous microphthalmia, cataract |
| Anosmia (UPSIT score) | Yes (12/40) | Yes (10/40) | Yes (11/40) | Yes (8/40) | Yes (14/30) | Yes (–) |
| Cleft lip/palate | No | No | No | CP | No | CP |
| SMCHD1 mutation | p.N139H | p.E136D | p.M129R | p.G137E | p.H348R | p.N139H |
Although not specifically noted in B, all 6 female patients had normal hearing based on a formal audiologic evaluation. SMCHD1 variants identified by whole-exome sequencing are in bold.
Abbreviations: –, information unavailable; b/l, bilateral; BMI, body mass index; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; R, right; L, left; LH, luteinizing hormone; HMG, human menopausal gonadotropins; hCG, human chorionic gonadotropin; MC, metacarpal; MRI, magnetic resonance imaging; UPSIT, University of Pennsylvania Smell Identification Test.
aGnRH-stimulated LH levels greater than 5 IU/L indicate activation of the neuroreproductive axis (25).
bA peak testosterone level greater than 90 ng/dL after hCG stimulation suggests normal testicular Leydig cell function (26).
cOne or both testicles had been surgically removed.
dRadiologist was unable to comment on olfactory structures because of resolution and motion artifact.
eHistory of recurrent ear infections and myringotomy tubes.
Protocol
Eleven patients (5 male, 6 female) were evaluated in person at NIH or MGH. The remaining patients provided DNA samples and primary medical records and completed detailed questionnaires and standardized smell tests with the assistance of the referring physician.
Phenotyping.
Participants discontinued hormone replacement at least 1 month before participation. A complete physical exam, including Tanner staging and determination of testicular volumes by Prader orchidometer in male patients, was performed. Frequent blood sampling studies were conducted overnight (every 10 minutes for 8-12 hours) to assess endogenous GnRH secretion as manifested by GnRH-induced LH pulsatility. All blood samples were analyzed for LH, and pulsatile LH secretion was determined using a validated modification of the Santen and Bardin method of pulse detection (30, 31). FSH was assayed from pools of the frequent sampling studies. Estradiol, total testosterone, and inhibin B (INHB) were measured in assay pools or at 3 time points (t = 0, 6 hours, 12 hours) and the average was reported. Anterior pituitary function was evaluated by measuring serum free thyroxine, dehydroepiandrosterone sulfate (DHEAS), insulin-like growth factor 1, and prolactin.
All participants underwent a pelvic or testicular ultrasound to calculate ovarian volumes (L × W × H × 0.52) or testicular volumes (L × W × H × 0.71) (32), respectively; a renal ultrasound; skeletal survey, and brain magnetic resonance imaging with fine cuts through the hypothalamic-pituitary unit and olfactory structures. Eight participants underwent 3-dimensional (3D) cone beam computed tomography (CBCT) of the head (Planmeca ProMax 3D Max; Planmeca, USA, Inc). Olfactory function was determined using the University of Pennsylvania Smell Identification Test (UPSIT) (33), in which complete anosmia is defined as a score less than the fifth percentile based on age- and sex-adjusted norms. Participants also underwent a standard ophthalmic exam, craniofacial and dental exam, and audiologic testing.
Genotyping.
Rare sequence variants in SMCHD1 were identified by WES in 29 participants and by targeted (Sanger) sequencing in the remainder, as previously described (17).
Hormone assays.
Serum LH and FSH were measured using a 2-site monoclonal nonisotopic system (Abbott Laboratories) and are expressed in international units per liter (IU/L) of the Pituitary 2nd International Standard 80/552. Serum testosterone and estradiol levels were measured by liquid chromatography–mass spectrometry with limits of detection of 8 ng/dL (0.28 nmol/L) and 10 pg/mL (36.71 pmol/L), respectively. INHB was measured using an enzyme-linked immunosorbent assay (Beckman Coulter, Inc) with a limit of quantification of 10 pg/mL. In patient 4, testosterone was measured using the DPC Coat-A-Count radioimmunoassay kit (Diagnostics Products Corp), which has intra-assay and interassay coefficients of variation (CVs) of less than 10%, and INHB was measured using a double-antibody enzyme-linked immunosorbent assay (Oxford Bio-Innovation, Ltd [formerly Serotec]) with an intra-assay CV of 4% to 6%, interassay CV of 15% to 18%, and a limit of quantification of 15.6 pg/mL. Because hormone levels reported for patients’ initial endocrine evaluations were abstracted from hospital medical records, details specific to each assay platform were not available.
Results
Clinical characteristics of arhinia patients
The 5 male patients had clinical signs of severe congenital GnRH deficiency, including a history of micropenis and/or cryptorchidism, prepubertal testicular volumes (1-3 cc), and minimal pubic hair (Table 1A). The remaining male patients (studied from afar) had similar clinical histories: A total of 82% (18 of 22) had micropenis, 73% (16 of 22) had cryptorchidism, and all 9 adult male patients demonstrated absent puberty and infertility (Table S1) (27).
Whereas the 6 female arhinia patients all had primary amenorrhea, 3 patients reported spontaneous breast development during early adolescence that failed to progress beyond the breast bud stage (Tanner stage II) (Table 1B). Ten of the 18 female patients studied from afar were of reproductive age (Table S1) (27). Seven had absent or arrested breast development and primary amenorrhea, 1 patient had arrested breast development and a single spontaneous menstrual period at age 14 years, and 2 sisters with arhinia had completely normal breast development (Table S1; patients R1 and R4) (27). The younger sister underwent menarche at the late age of 16.5 years; she was immediately placed on birth control pills because of intellectual disability and concerns about self-care/menstrual hygiene. The older sister had menarche at age 14 years and had regular menstrual cycles (ie, every 25-35 days, associated with typical moliminal symptoms such as mood swings, breast tenderness, and bloating) until menopause at age 50 years. She never attempted to conceive. Of note, preserved reproductive function could not be explained by a less deleterious SMCHD1 variant unique to these patients (p.S135N is recurrent) nor by a milder overall phenotype; rather, both patients had severe craniofacial dysmorphia of the eyes, external ears, palate, choanae, and dentition, and the cribriform plate was reported to be imperforate based on skull x-ray in the older sister (34).
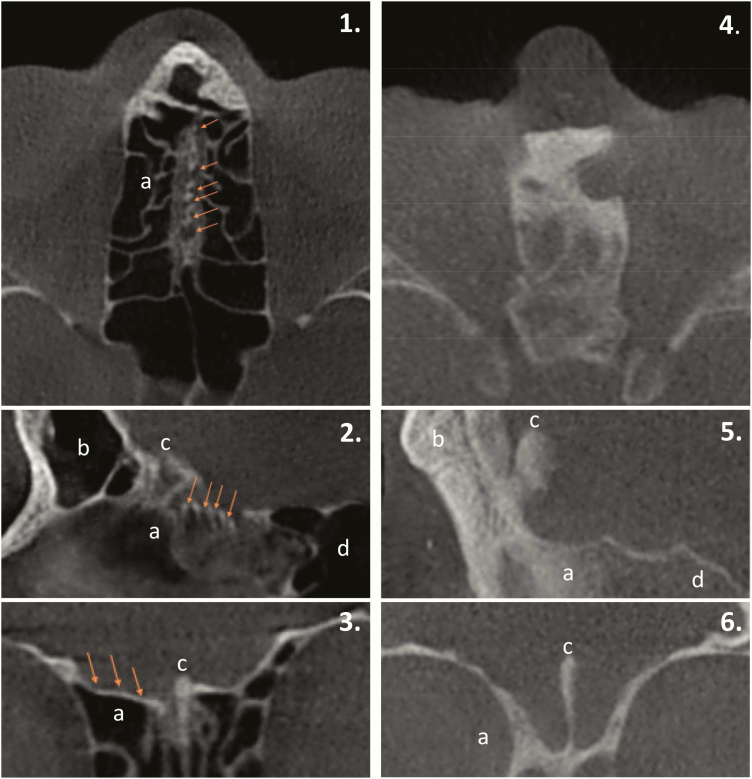
Serum LH, FSH, sex steroids, and INHB levels (in male patients) were uniformly low in all patients with available data (n = 27), and no LH pulses were identified in the 11 patients who underwent frequent blood sampling studies (Fig. 1, Fig S1) (27). Anterior pituitary function was otherwise normal (Fig. 1). Brain magnetic resonance imaging (n = 27) revealed a normal hypothalamic-pituitary unit with the exception of one male patient with pituitary hypoplasia. Olfactory bulbs and/or sulci were evaluated in 19 patients and revealed absent structures in all cases. In 8 patients, visualization of the skull base by 3D CBCT revealed an absence of perforations in the cribriform plate (Fig. 2) consistent with previous studies in arhinia patients (35-37).
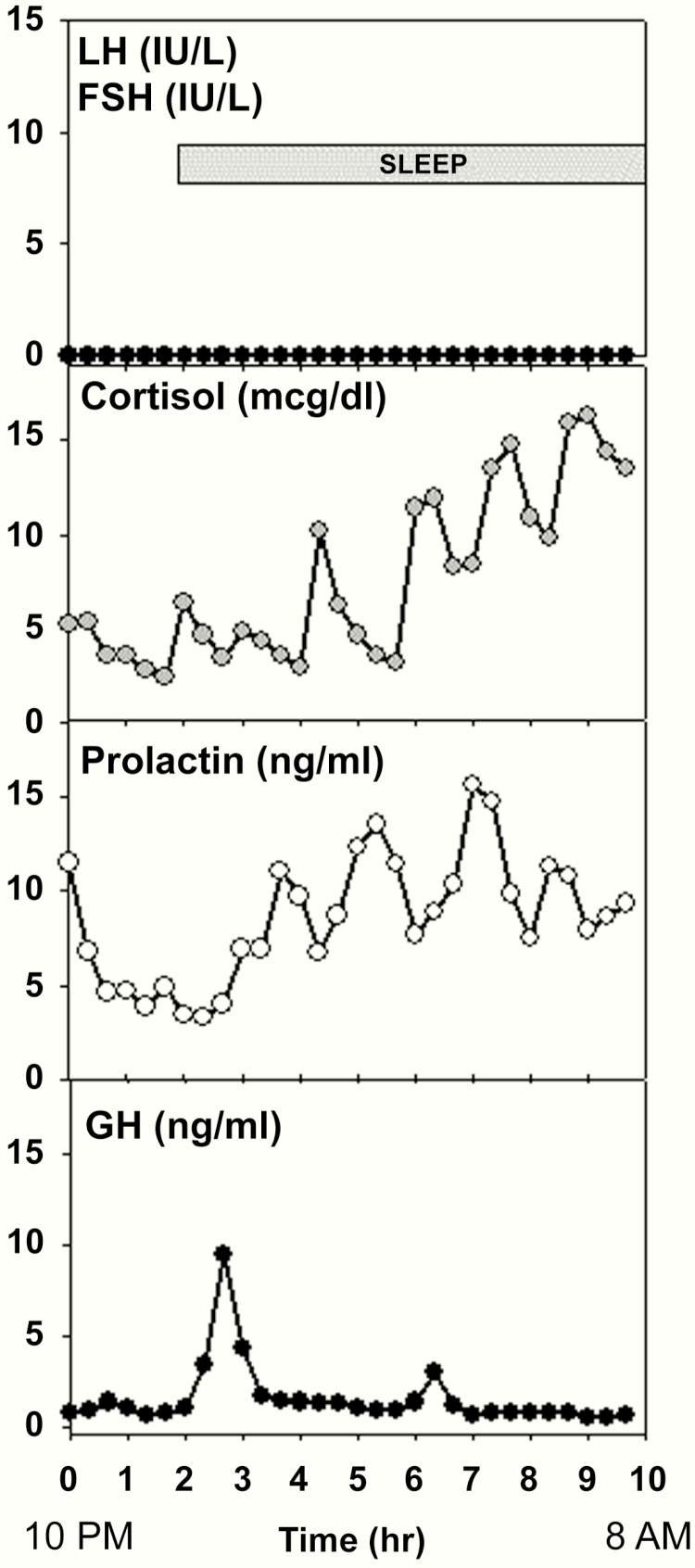
Figure 1.
Initial neuroendocrine evaluation in a female patient with arhinia (No. 11) at age 16 years (before hormone replacement therapy) demonstrating undetectable gonadotropins but normal cortisol, prolactin, and growth hormone (GH) secretion, including normal sleep augmentation of GH. Estradiol was undetectable (<20 pg/ml). Medical records indicated the patient was also clinically and biochemically euthyroid. A second frequent sampling study performed at age 23 years again showed apulsatile luteinizing hormone secretion (data not shown). To convert cortisol (μg/dL) to nmol/L, multiply by 27.59.
Figure 2.
Axial (1, 4), sagittal (2, 5), and coronal (3, 6) cone-beam computed tomography sections depicting the maxillofacial region in a, healthy control individual (left) and, patient with arhinia (right). Note the multiple olfactory foramina (arrows) on the surface of the cribriform plate in the control patient, whereas the cribriform plate, as well as the surrounding paranasal sinuses, are completely ossified in the patient with arhinia. a, ethmoid sinus; b, frontal sinus; c, crista galli; d, sphenoid sinus.
Response to pulsatile gonadotropin-releasing hormone or gonadotropins
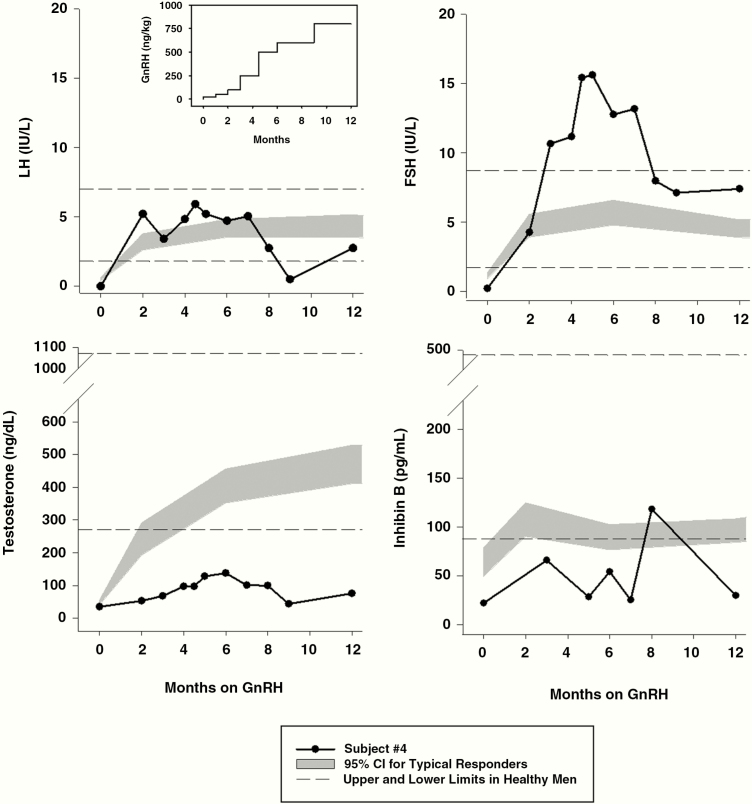
Two patients, 1 male and 1 female, who were treated with pulsatile GnRH using a portable infusion pump at a physiological dose and frequency (38-40), provide additional insight into pituitary and gonadal function in arhinia patients. The male patient (No. 4) was born with micropenis but did not have cryptorchidism. He showed no signs of spontaneous puberty by age 14 years and puberty was thus induced with exogenous testosterone (150 mg intramuscularly monthly). At age 16 years, he transitioned to exogenous combined gonadotropin therapy (75 U human menopausal gonadotropins and 1000 U human chorionic gonadotropin [hCG] 3×/week), and after 5 months of therapy, he showed a favorable response with an increase in testosterone (447 ng/dL [15.5 nmol/L]) and testicular growth (from 2 to 6 cc). Seminal fluid obtained via prostatic massage yielded no sperm, but a testicular biopsy revealed a normal complement of Leydig cells, active spermatogenesis (spermatid stage), occasional germ cells, and rare spermatozoa. He discontinued gonadotropins following a lapse in insurance coverage and returned to monthly testosterone injections. He remained on testosterone until age 42 years, when he began treatment with a GnRH pump. Despite incremental increases in his GnRH dose over the course of 1 year to a maximum of 800 ng/kg/bolus (vs physiological dose 25-50 ng/kg/bolus), his testicular volume never exceeded 3 to 4 cc and his testosterone peaked at 138 ng/dL (4.8 nmol/L). He also demonstrated GnRH-induced hypergonadotropism (maximum FSH 15.6 IU/L) and INHB levels remained low, consistent with the presence of testicular resistance in addition to a hypothalamic defect (Fig. 3). Medical records from several other male arhinia patients who had undergone hCG stimulation painted a similar picture of testicular resistance (Table S1; patients D1, E1, and F1) (27).
Figure 3.
Hormonal responses to long-term gonadotropin-releasing hormone (GnRH) administration in a male arhinia patient (No. 4). GnRH was initially administered at a physiological dose (25 ng/kg/bolus subcutaneously) but was increased to pharmacologic doses as high as 800 ng/kg/bolus over the course of 1 year (see inset, upper left) in an attempt to overcome testicular resistance as manifested by hypergonadotropism with low testosterone and inhibin B levels. The patient’s data points are indicated by black circles and black lines. Horizontal dashed lines indicate the upper and lower limits of hormone levels in healthy adult men (41). The gray shaded area denotes the 95% CI of hormone levels in patients with congenital GnRH deficiency who responded in a typical fashion to GnRH with normalization of luteinizing hormone, follicle-stimulating hormone, and testosterone levels accompanied by mature sperm production (29). To convert testosterone (ng/dL) to nmol/L, multiply by 0.0347. To convert inhibin B (pg/mL) to ng/L, multiply by 1.0.
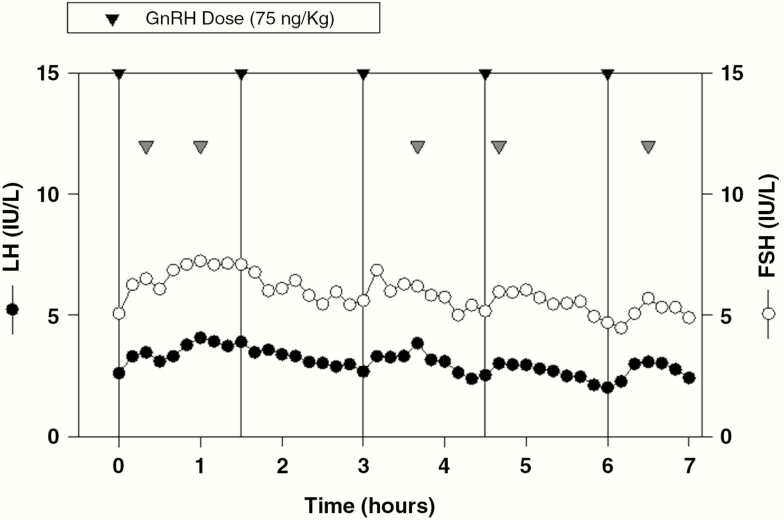
The female arhinia patient (No. 11) had also shown no signs of puberty and had started estrogen replacement at age 16 years. At age 23 years, she began treatment with pulsatile GnRH. A frequent sampling study performed during the course of 5 consecutive GnRH boluses on cycle day 6 revealed a normal pituitary response (Fig. 4). A dominant ovarian follicle was first visualized on ultrasound on cycle day 9 and ovulation was confirmed on cycle day 21 with detection of a corpus luteum.
Figure 4.
Frequent sampling study in patient No. 11 on day 6 of a physiological gonadotropin-releasing hormone (GnRH) regimen (75 ng/kg/bolus administered intravenously every 90 minutes for the first 7 days; then every 60 minutes until ovulation). Note normal luteinizing hormone and follicle-stimulating hormone levels and the presence of LH pulses (gray arrowheads) after 4 of 5 GnRH boluses (black arrowheads and vertical black lines). A pelvic ultrasound performed the same day demonstrated a maximum follicle diameter of 8 mm and an early proliferative (6 mm) endometrium.
Phenotypic overlap with syndromic forms of gonadotropin-releasing hormone deficiency
Arhinia patients demonstrated distinct nonreproductive phenotypes associated with other syndromic forms of congenital GnRH deficiency such as KS and CHARGE syndrome (coloboma, heart anomaly, choanal atresia, retardation, genital, and ear anomalies). The majority of patients, for example, had abnormalities of the palate, choanae, eyes, and external ears, 31% patients (4 of 13 with available data) had hypoplastic or absent ribs (but this may have been related to removal of rib cartilage for use in rhinoplasty (42) during childhood in at least 2 cases), and at least 29% (7 of 24 with data) had dental agenesis or ectopic tooth eruption. No patients had renal agenesis (confirmed by renal ultrasound in 14 patients), severe cardiac defects (confirmed by echocardiogram in 12 patients), congenital deafness, or hypopigmentation of the eyes, hair, or skin. Congenital mirror movements (CMMs), a common finding in patients with X-linked KS (43), were present in 5 (2 female, 3 male) of the 10 patients tested. Of note, 2 of the 3 male patients with CMMs had undergone WES that confirmed the absence of an ANOS1 mutation. The majority of adult patients were of normal intelligence, and at least 17 had matriculated in or completed college or a trade school (Table S1) (27).
Discussion
Sporadic patients (12) as well as KS family members (13) with pathogenic variants in KS-associated genes and congenital olfactory agenesis and anosmia have previously been found to have apparently normal reproductive function. We now report, through comprehensive reproductive phenotyping studies, that patients with complete absence of an external nose and all internal olfactory structures also have evidence of diminished or, in rare cases, normal reproductive function. Taken together, these findings suggest that, contrary to traditional views, the main olfactory system may not be required for normal GnRH ontogeny and reproductive competence in humans. These studies demonstrate further that individuals with extreme phenotypes represent a powerful research model to answer fundamental questions about human embryology.
Remarkably, 5 female arhinia patients demonstrated arrested pubertal development and 2 others reported mature reproductive function (though neither has attempted to conceive), suggesting preserved reproductive endocrine activity. Such strong clinical evidence for GnRH secretory activity was a surprise given the severity of the craniofacial and ocular defects in these female patients and the complete absence of perforations (a relic of olfactory nerve passage) in the anterior skull base in the 8 patients who underwent CBCT and in previously reported arhinia cases (35-37). In contrast to arhinia patients, a study of 35 adult KS patients found that they had a normal number of cribriform plate perforations as determined by CT of the ethmoid region (44). These imaging findings are in fact consistent with histopathological studies in fetuses with an ANOS1 deletion (2, 45) or CHARGE syndrome (45), demonstrating that olfactory structures crossed the cribriform plate, but instead of penetrating the forebrain, they formed an entangled mass (neuroma) on the dorsal surface of the cribriform plate. Nearly all GnRH neurons were found in the upper nasal region or within these neuromas rather than in the hypothalamus. These and the present studies would suggest that in at least some forms of KS, olfactory structures develop normally but terminate prematurely, whereas in arhinia, a more severe olfactory placode (OP) defect may entirely preclude olfactogenesis (see the following).
We were unable to measure reproductive hormones in the 2 women (R1 and R4) with apparently normal reproductive function. However, the only credible explanation for normal breast development and menstrual cycles is the existence of a functional GnRH neuronal network; an LH- and FSH-secreting pituitary adenoma or autonomous ovarian follicular cyst, for example, could not explain the regular episodes of vaginal bleeding that occurred over the course of 30 years in patient R1. Because we were also unable to visualize the skull base (cribriform plate) using contemporary imaging modalities, we cannot completely rule out the possibility that these 2 women have a rudimentary main olfactory system.
Female arhinia patients with partial or full reproductive function, who have no visible olfactory structures as adults nor evidence of olfactory nerve development in utero, therefore raise the intriguing question of how GnRH neurons successfully migrated from the primitive nose to the forebrain and finally to the hypothalamus. During normal human fetal development (~ weeks 5-9), the OP gives rise to the olfactory nerves and to the vomeronasal organ (VNO), a small, bean-shaped organ at the base of the nose that differentiates further into the vomeronasal nerve (VN), terminal nerve (TN), and GnRH neurons. The olfactory nerves, VN, and TN extend axons through the nasal mesenchyme and cross the primitive cribriform plate as they migrate toward the forebrain, where they then synapse on either the main olfactory bulb, the accessory olfactory bulb, or turn caudally toward the hypothalamus, respectively (2, 46, 47). GnRH neurons reach the forebrain by traveling along this “migratory mass” of bundled axons (48). The accessory olfactory bulb, VN, and TN are considered to be vestigial in humans and cannot be seen on neuroimaging. Thus, studies in KS patients have necessarily focused on the main olfactory system, where the gross structural defects provide a plausible explanation for the associated GnRH deficiency.
Several mouse models have demonstrated an association between olfactory bulb aplasia or hypoplasia and disrupted GnRH neuron migration, as in KS. In Prok2 and Prokr2 null mice, for example, GnRH neurons become trapped in a tangled mass of olfactory nerves and the VN between the olfactory pit and forebrain (49, 50). Pitteloud et al (50), however, also showed that Prokr2 is highly expressed in the VNO, raising the possibility that Prokr2 deficiency causes GnRH deficiency not because of olfactory nerve defects but because of defects in the TN, a VNO derivative. Studies in the Arx-1 null mouse, which lacks olfactory nerves and bulbs yet has a normal complement of GnRH neurons, supports the idea that GnRH neurons may use the TN as a migratory scaffold (14). A mouse model of methotrexate teratogenicity also demonstrates the remarkable resiliency of GnRH neurons in the context of structural disarray akin to human arhinia: Affected embryos have a grossly abnormal nose and forebrain yet GnRH-immunoreactive neurons navigate to the forebrain (51). Thus, there is now a growing body of evidence suggesting that the association between olfactory bulb agenesis and GnRH deficiency in KS patients, and perhaps in arhinia patients, is just that—an association—and that the primary defect in KS may instead lie with the VNO and its derivatives, the VN, TN, and GnRH neurons. Such a model would predict that mutations that disrupt VNO development would consistently cause GnRH deficiency, but that the presence of an additional olfactory phenotype (eg, KS) would depend on whether the lesion extended to the lateral aspect of the OP, where the main olfactory system originates (52). This model is in fact consistent with clinical observations: For most KS-associated genes, patients who harbor identical mutations demonstrate a spectrum of olfactory phenotypes, including normosmia, hyposmia, or anosmia (53). In these patients, the size of the placode lesion and resulting olfactory function likely depend on genetic or environmental modifiers and/or stochastic processes during development (54). Detailed study of the human VNO, and in particular the TN, a “neglected cranial nerve” (55), will be necessary to confirm this hypothesis.
In contrast to the relatively broad spectrum of reproductive function in female arhinia patients, all male patients had signs of severe GnRH deficiency. In patient 4, long-term treatment with escalating doses of pulsatile GnRH uncovered testicular resistance as manifested biochemically by elevated LH and FSH levels and low testosterone and INHB levels, and clinically by minimal testicular growth. Several additional male patients demonstrated only a slight increase in testosterone in response to hCG stimulation, indicating Leydig cell dysfunction. Importantly, this testicular resistance could not be explained entirely by cryptorchidism, because 2 patients had descended testes. Such “dual defects” at the level of the hypothalamus and testis have previously been reported by our group in KS men and ascribed to coexpression of KS-associated genes in the hypothalamus and testes (29). During spermiogenesis, chromatin is extensively reorganized to repackage the genome within the small confines of the sperm head (56), and this process is regulated by chromodomain helicase DNA binding protein 5 (CHD5), a chromatin remodeler (57). Notably, SMCHD1, the gene implicated in arhinia, also encodes a chromatin remodeler, and single-cell RNA sequencing studies in human testes are consistent with low-level SMCHD1 expression in Leydig cells, although the highest expression was seen in primary spermatocytes (58). Thus, it is possible that aberrant SMCHD1 activity within the testis may contribute to the testicular resistance observed in some male arhinia patients, but this hypothesis awaits further study.
We observed a high prevalence of CMMs in arhinia patients, a phenotype that has not been reported in previous case series. CMMs are due to defective development of commissural (midline-crossing) axons in the brain and spinal cord; they are extremely rare (<1 in 1 million; (59)) but are a common feature of the Klippel-Feil sequence (KFS) and X-linked KS. The KFS is characterized by abnormal fusion of the cervical vertebrae, which manifests as a short neck with limited neck mobility; the phenotypic spectrum may include cleft palate, microphthalmia, and renal agenesis. The autosomal dominant forms of KFS are caused by mutations in growth differentiation factors (GDF)-6 (60, 61) or GDF3, which encode secretory peptides of the transforming growth factor β superfamily. It is of interest that SMCHD1, GDFs, and anosmin, the proteins implicated in BAMS, KFS, and X-linked KS, respectively, are all expressed at the neural plate border (NPB) (18, 62, 63). The NPB is a region in the primitive central nervous system that separates the surface ectoderm from the neural ectoderm and gives rise to neural crest cells, cranial placode cells, and the roof plate. Importantly, the roof plate is a critical signaling center in the dorsal neural tube (64) that controls the development of the dorsal spinal cord, including the commissural neurons—the very neurons implicated in CMMs (65, 66). Thus, the presence of CMMs in KFS and X-linked KS and now in BAMS suggests that these conditions are neither strictly neural crest defects nor cranial placodal defects; rather, an even earlier embryonic insult to the NPB (~ week 3 in humans) that affects a number of NPB derivatives may underlie these disorders.
The significant phenotypic variability in other nonreproductive phenotypes among arhinia patients with SMCHD1 mutations suggests an important role for genetic modifiers yet to be determined (oligogenicity). Of note, phenotypic differences cannot be explained by the SMCHD1 variants alone. For example, the 8 patients with the most common variant (p.His348Arg) demonstrate a range of ocular phenotypes from normal vision and ocular anatomy to bilateral anophthalmia. There is also no correlation between phenotypes and a given variant’s position in the 3D protein structure, effect on SMCHD1 ATPase activity, or effect on SMCHD1 N-terminal dimerization (67). We hypothesize, instead, that phenotypic differences arise from variation in prenatal exposures, SMCHD1 protein binding partners, and/or SMCHD1 downstream targets.
In conclusion, patients with arhinia raise the possibility that in humans, as in the mouse, a working hypothalamic-pituitary-gonadal axis may develop in the complete absence of internal and external olfactory structures. Studies in the mouse suggested that females require more GnRH neurons than males to achieve mature reproductive function (68), yet only female arhinia patients demonstrated signs of GnRH activity, suggesting that females may have unique developmental strategies to safeguard the reproductive axis. Taken together, patients with arhinia offer an unparalleled window into the integrated yet plastic development of NPB derivatives—the craniofacial skeleton, olfactory system, and the GnRH neuron—during human embryogenesis.
Acknowledgments
We thank and acknowledge the study staff of the Massachusetts General Hospital Clinical Research Center and the NIH Clinical Center for their support in conducting these studies, Dr Patrick Sluss and Ms Annette Rice for overseeing reproductive hormone assays, and Drs Paolo E. Forni, Kathleen K. Sulik, and Joseph R. Siebert for insightful discussions about these patients. We also pay our respects to and acknowledge the contribution of Dr Antonio Richieri-Costa, a distinguished geneticist, teacher, and colleague who died in August 2019.
Glossary
Abbreviations
- BAMS
Bosma arhinia microphthalmia syndrome
- CBCT
cone beam computed tomography
- CHARGE
coloboma, heart anomaly, choanal atresia, retardation, genital, and ear anomalies
- CMMs
congenital mirror movements
- CV
coefficients of variation
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- INHB
inhibin B
- KFS
Klippel-Feil sequence
- KS
Kallmann syndrome
- LH
luteinizing hormone
- MGH
Massachusetts General Hospital
- NIH
National Institutes of Health
- NPB
neural plate border
- OP
olfactory placode
- TN
terminal nerve
- VN
vomeronasal nerve
- VNO
vomeronasal organ
- WES
whole-exome sequencing
Financial Support: This work was supported in part by the Intramural Research Program of the National Institutes of Health, the National Institute of Environmental Health Sciences (Z01-ES103315), the National Institute of Child Health and Human Development (Z01-HD008919, P50 HD028138), by Grant 1UL1TR001102, and a Lasker Clinical Research Scholar grant (Grant 1SI2ES025429-01 to N.D.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the National Center for Advancing Translational Science or the National Institutes of Health.
Clinical Trials Information: The Clinicaltrials.gov registration numbers for this work are NCT01511588, NCT00383656, and NCT00392756.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability. The data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Casoni F, Malone SA, Belle M, et al. Development of the neurons controlling fertility in humans: new insights from 3D imaging and transparent fetal brains. Development. 2016;143(21):3969–3981. [DOI] [PubMed] [Google Scholar]
- 2. Schwanzel-Fukuda M, Bick D, Pfaff DW. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res Mol Brain Res. 1989;6(4):311–326. [DOI] [PubMed] [Google Scholar]
- 3. Pearson AA. XXX The development of the olfactory nerve, the nervus terminalis, and the vomeronasal nerve in man. Ann Otol Rhinol Laryngol. 1942;51(2):317–332. [Google Scholar]
- 4. Humphrey T. The development of the olfactory and the accessory olfactory formations in human embryos and fetuses. J Comp Neurol. 1940;73(3):431–468. [Google Scholar]
- 5. De Morsier G, Gauthier G. Olfacto-genital dysplasia [article in French]. Pathol Biol. 1963;11:1267–1272. [PubMed] [Google Scholar]
- 6. Kallmann FJ, Schoenfeld WA, Barrera SE. The genetic aspects of primary eunuchoidism. Am J Ment Defic. 1944;XLVIII:203–236. [Google Scholar]
- 7. Maestre de San Juan A. Falta total de nervios olfactorios con anosmia en un individuo en quien existía una atrofia congénita de los testículos y miembro viril. Siglo Médico. 1856;131:211–214. [Google Scholar]
- 8. Quinton R, Duke VM, de Zoysa PA, et al. The neuroradiology of Kallmann’s syndrome: a genotypic and phenotypic analysis. J Clin Endocrinol Metab. 1996;81(8):3010–3017. [DOI] [PubMed] [Google Scholar]
- 9. Vogl TJ, Stemmler J, Heye B, et al. Kallman syndrome versus idiopathic hypogonadotropic hypogonadism at MR imaging. Radiology. 1994;191(1):53–57. [DOI] [PubMed] [Google Scholar]
- 10. Knorr JR, Ragland RL, Brown RS, Gelber N. Kallmann syndrome: MR findings. AJNR Am J Neuroradiol. 1993;14(4):845–851. [PMC free article] [PubMed] [Google Scholar]
- 11. Truwit CL, Barkovich AJ, Grumbach MM, Martini JJ. MR imaging of Kallmann syndrome, a genetic disorder of neuronal migration affecting the olfactory and genital systems. AJNR Am J Neuroradiol. 1993;14(4):827–838. [PMC free article] [PubMed] [Google Scholar]
- 12. Alkelai A, Olender T, Dode C, et al. Next-generation sequencing of patients with congenital anosmia. Eur J Hum Genet. 2017;25(12):1377–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pitteloud N, Acierno JS Jr, Meysing A, et al. Mutations in fibroblast growth factor receptor 1 cause both Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci U S A. 2006;103(16):6281–6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taroc EZM, Prasad A, Lin JM, Forni PE. The terminal nerve plays a prominent role in GnRH-1 neuronal migration independent from proper olfactory and vomeronasal connections to the olfactory bulbs. Biol Open. 2017;6(10):1552–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oelschläger HA, Buhl EH, Dann JF. Development of the nervus terminalis in mammals including toothed whales and humans. Ann N Y Acad Sci. 1987;519:447–464. [DOI] [PubMed] [Google Scholar]
- 16. Murakami S, Seki T, Wakabayashi K, Arai Y. The ontogeny of luteinizing hormone-releasing hormone (LHRH) producing neurons in the chick embryo: possible evidence for migrating LHRH neurons from the olfactory epithelium expressing a highly polysialylated neural cell adhesion molecule. Neurosci Res. 1991;12(3):421–431. [DOI] [PubMed] [Google Scholar]
- 17. Shaw ND, Brand H, Kupchinsky ZA, et al. SMCHD1 mutations associated with a rare muscular dystrophy can also cause isolated arhinia and Bosma arhinia microphthalmia syndrome. Nat Genet. 2017;49(2):238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gordon CT, Xue S, Yigit G, et al. De novo mutations in SMCHD1 cause Bosma arhinia microphthalmia syndrome and abrogate nasal development. Nat Genet. 2017;49(2):249–255. [DOI] [PubMed] [Google Scholar]
- 19. Asirvatham AR, Mahadevan S, Balasubramanian S. Anosmia with hypogonadism: but NOT Kallmann syndrome. BMJ Case Rep. 2017;2017:bcr2017220045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bosma JF, Henkin RI, Christiansen RL, Herdt JR. Hypoplasia of the nose and eyes, hyposmia, hypogeusia, and hypogonadotrophic hypogonadism in two males. J Craniofac Genet Dev Biol. 1981;1(2):153–184. [PubMed] [Google Scholar]
- 21. Brasseur B, Martin CM, Cayci Z, Burmeister L, Schimmenti LA. Bosma arhina microphthalmia syndrome: Clinical report and review of the literature. Am J Med Genet A. 2016;170A(5):1302–1307. [DOI] [PubMed] [Google Scholar]
- 22. Hunter JD, Davis MA, Law JR. Hypogonadotropic hypogonadism in a female patient with congenital arhinia. J Pediatr Endocrinol Metab. 2017;30(1):101–104. [DOI] [PubMed] [Google Scholar]
- 23. Tryggestad JB, Li S, Chernausek SD. Hypogonadotropic hypogonadism presenting with arhinia: a case report. J Med Case Rep. 2013;7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Graham JM Jr, Lee J. Bosma arhinia microphthalmia syndrome. Am J Med Genet A. 2006;140(2):189–193. [DOI] [PubMed] [Google Scholar]
- 25. Neely EK, Wilson DM, Lee PA, Stene M, Hintz RL. Spontaneous serum gonadotropin concentrations in the evaluation of precocious puberty. J Pediatr. 1995;127(1):47–52. [DOI] [PubMed] [Google Scholar]
- 26. Knorr D, Beckmann D, Bidlingmaier F, Helmig FJ, Sippell WG. Plasma testosterone in male puberty. II. hCG stimulation test in boys with hypospadia. Acta Endocrinol (Copenh). 1979;90(2):365–71. [PubMed] [Google Scholar]
- 27.Supplementary material can be found at 10.5281/zenodo.3686220. [DOI] [Google Scholar]
- 28. Shaw ND, Seminara SB, Welt CK, et al. Expanding the phenotype and genotype of female GnRH deficiency. J Clin Endocrinol Metab. 2011;96(3):E566–E576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sykiotis GP, Hoang XH, Avbelj M, et al. Congenital idiopathic hypogonadotropic hypogonadism: evidence of defects in the hypothalamus, pituitary, and testes. J Clin Endocrinol Metab. 2010;95(6):3019–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hayes FJ, McNicholl DJ, Schoenfeld D, Marsh EE, Hall JE. Free alpha-subunit is superior to luteinizing hormone as a marker of gonadotropin-releasing hormone despite desensitization at fast pulse frequencies. J Clin Endocrinol Metab. 1999;84(3):1028–1036. [DOI] [PubMed] [Google Scholar]
- 31. Santen RJ, Bardin CW. Episodic luteinizing hormone secretion in man. Pulse analysis, clinical interpretation, physiologic mechanisms. J Clin Invest. 1973;52(10): 2617–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sakamoto H, Saito K, Oohta M, Inoue K, Ogawa Y, Yoshida H. Testicular volume measurement: comparison of ultrasonography, orchidometry, and water displacement. Urology. 2007;69(1):152–157. [DOI] [PubMed] [Google Scholar]
- 33. Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94(2 Pt 1):176–178. [DOI] [PubMed] [Google Scholar]
- 34. Ruprecht KW, Majewski F. Familiary arhinia combined with Peters’ anomaly and maxilliar deformities, a new malformation syndrome [(author’s transl) article in German]. Klin Monbl Augenheilkd. 1978;172(5):708–715. [PubMed] [Google Scholar]
- 35. Cusick W, Sullivan CA, Rojas B, Poole AE, Poole DA. Prenatal diagnosis of total arhinia. Ultrasound Obstet Gynecol. 2000;15(3):259–261. [DOI] [PubMed] [Google Scholar]
- 36. Mathur NN, Dubey NK, Kumar S, Bothra R, Chadha A. Arhinia. Int J Pediatr Otorhinolaryngol. 2005;69(1):97–99. [DOI] [PubMed] [Google Scholar]
- 37. Mondal U, Prasad R. Congenital arhinia: a rare case report and review of literature. Indian J Otolaryngol Head Neck Surg. 2016;68(4):537–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Filicori M, Santoro N, Merriam GR, Crowley WF Jr. Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J Clin Endocrinol Metab. 1986;62(6):1136–1144. [DOI] [PubMed] [Google Scholar]
- 39. Hoffman AR, Crowley WF Jr. Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med. 1982;307(20):1237–1241. [DOI] [PubMed] [Google Scholar]
- 40. Martin K, Santoro N, Hall J, Filicori M, Wierman M, Crowley WF Jr. Clinical review 15: Management of ovulatory disorders with pulsatile gonadotropin-releasing hormone. J Clin Endocrinol Metab. 1990;71(5):1081A–1081G. [DOI] [PubMed] [Google Scholar]
- 41. Spratt DI, O’Dea LS, Schoenfeld D, Butler J, Rao PN, Crowley WF Jr. Neuroendocrine-gonadal axis in men: frequent sampling of LH, FSH, and testosterone. Am J Physiol. 1988;254(5 Pt 1):E658–E666. [DOI] [PubMed] [Google Scholar]
- 42. Marin VP, Landecker A, Gunter JP. Harvesting rib cartilage grafts for secondary rhinoplasty. Plast Reconstr Surg. 2008;121(4):1442–1448. [DOI] [PubMed] [Google Scholar]
- 43. Quinton R, Duke VM, Robertson A, et al. Idiopathic gonadotrophin deficiency: genetic questions addressed through phenotypic characterization. Clin Endocrinol (Oxf). 2001;55(2):163–174. [DOI] [PubMed] [Google Scholar]
- 44. Maione L, Benadjaoud S, Eloit C, et al. Computed tomography of the anterior skull base in Kallmann syndrome reveals specific ethmoid bone abnormalities associated with olfactory bulb defects. J Clin Endocrinol Metab. 2013;98(3):E537–E546. [DOI] [PubMed] [Google Scholar]
- 45. Teixeira L, Guimiot F, Dodé C, et al. Defective migration of neuroendocrine GnRH cells in human arrhinencephalic conditions. J Clin Invest. 2010;120(10):3668–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schwanzel-Fukuda M, Pfaff DW. The migration of luteinizing hormone-releasing hormone (LHRH) neurons from the medial olfactory placode into the medial basal forebrain. Experientia. 1990;46(9):956–962. [DOI] [PubMed] [Google Scholar]
- 47. Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci U S A. 1989;86(20):8132–8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wray S, Key S, Qualls R, Fueshko SM. A subset of peripherin positive olfactory axons delineates the luteinizing hormone releasing hormone neuronal migratory pathway in developing mouse. Dev Biol. 1994;166(1):349–354. [DOI] [PubMed] [Google Scholar]
- 49. Matsumoto S, Yamazaki C, Masumoto KH, et al. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci U S A. 2006;103(11):4140–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pitteloud N, Zhang C, Pignatelli D, et al. Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci U S A. 2007;104(44):17447–17452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schwanzel-Fukuda M, Pfaff DW. Luteinizing hormone-releasing hormone (LHRH) and neural cell adhesion molecule (NCAM)-immunoreactivity in development of the forebrain and reproductive system. Ann Endocrinol (Paris). 1994;55(6):235–241. [PubMed] [Google Scholar]
- 52. Carstens MH. Development of the facial midline. J Craniofac Surg. 2002;13(1):129–1 87; discussion 188. [DOI] [PubMed] [Google Scholar]
- 53. Lewkowitz-Shpuntoff HM, Hughes VA, Plummer L, et al. Olfactory phenotypic spectrum in idiopathic hypogonadotropic hypogonadism: pathophysiological and genetic implications. J Clin Endocrinol Metab. 2012;97(1):E136–E144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fish JL. Developmental mechanisms underlying variation in craniofacial disease and evolution. Dev Biol. 2016;415(2):188–197. [DOI] [PubMed] [Google Scholar]
- 55. Vilensky JA. The neglected cranial nerve: nervus terminalis (cranial nerve N). Clin Anat. 2014;27(1):46–53. [DOI] [PubMed] [Google Scholar]
- 56. Ward WS, Coffey DS. DNA packaging and organization in mammalian spermatozoa: comparison with somatic cells. Biol Reprod. 1991;44(4):569–574. [DOI] [PubMed] [Google Scholar]
- 57. Li W, Wu J, Kim SY, et al. Chd5 orchestrates chromatin remodelling during sperm development. Nat Commun. 2014;5:3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Guo J, Grow EJ, Mlcochova H, et al. The adult human testis transcriptional cell atlas. Cell Res. 2018;28(12):1141–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Orphanet: an online database of rare diseases and orphan drugs. Copyright, INSERM 1997. Available at http://www.orpha.net Accessed February 24, 2020 [Google Scholar]
- 60. Tassabehji M, Fang ZM, Hilton EN, et al. Mutations in GDF6 are associated with vertebral segmentation defects in Klippel-Feil syndrome. Hum Mutat. 2008;29(8):1017–1027. [DOI] [PubMed] [Google Scholar]
- 61. Ye M, Berry-Wynne KM, Asai-Coakwell M, et al. Mutation of the bone morphogenetic protein GDF3 causes ocular and skeletal anomalies. Hum Mol Genet. 2010;19(2):287–298. [DOI] [PubMed] [Google Scholar]
- 62. Endo Y, Ishiwata-Endo H, Yamada KM. Extracellular matrix protein anosmin promotes neural crest formation and regulates FGF, BMP, and WNT activities. Dev Cell. 2012;23(2):305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schille C, Bayerlová M, Bleckmann A, Schambony A. Ror2 signaling is required for local upregulation of GDF6 and activation of BMP signaling at the neural plate border. Development. 2016;143(17):3182–3194. [DOI] [PubMed] [Google Scholar]
- 64. Lee KJ, Jessell TM. The specification of dorsal cell fates in the vertebrate central nervous system. Annu Rev Neurosci. 1999;22:261–294. [DOI] [PubMed] [Google Scholar]
- 65. Chizhikov VV, Millen KJ. Mechanisms of roof plate formation in the vertebrate CNS. Nat Rev Neurosci. 2004;5(10):808–812. [DOI] [PubMed] [Google Scholar]
- 66. Chizhikov VV, Millen KJ. Roof plate-dependent patterning of the vertebrate dorsal central nervous system. Dev Biol. 2005;277(2):287–295. [DOI] [PubMed] [Google Scholar]
- 67. Pedersen LC, Inoue K, Kim S, Perera L, Shaw ND. A ubiquitin-like domain is required for stabilizing the N-terminal ATPase module of human SMCHD1. Commun Biol. 2019;2(1):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev. 2008;57(2):277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]