Abstract
Background
Paclitaxel (PTX) is one of the widely used chemotherapy drugs in breast cancer (BC) treatment. Unfortunately, the survival rate of metastatic BC patients remains poor due to PTX resistance. Therefore, uncovering the underlying mechanism behind the PTX resistance of BC cells is crucial for BC therapy.
Methods
The enrichment of circular RNA ATP binding cassette subfamily B member 10 (circ-ABCB10), let-7a-5p and dual specificity phosphatase 7 (DUSP7) was measured by quantitative real time polymerase chain reaction (qRT-PCR) in PTX-resistant and PTX-sensitive BC tissues and cells. Chemoresistance, apoptosis, invasion and autophagy of BC cells were measured by 3-(4, 5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), flow cytometry, transwell invasion assay and Western blot assay, respectively. The binding sites between let-7a-5p and circ-ABCB10 or DUSP7 were predicted by Starbase bioinformatic software, and the combination was confirmed by dual-luciferase reporter assay. The protein expression of DUSP7 was examined by Western blot assay. Murine xenograft model was established to confirm the role of circ-ABCB10 in vivo.
Results
Circ-ABCB10 depletion promoted the PTX sensitivity and apoptosis while suppressed the invasion and autophagy of PTX-resistant BC cells. Circ-ABCB10 could bind to let-7a-5p in BC cells, and circ-ABCB10 contributed to PTX resistance of BC cells via let-7a-5p. DUSP7 is a direct target of let-7a-5p in BC cells, and the accumulation of DUSP7 reversed the promoting effects of let-7a-5p overexpression on the PTX sensitivity and apoptosis and the inhibitory impact on the invasion and autophagy of PTX-resistant BC cells. Circ-ABCB10 interference suppressed the growth of BC tumors in vivo.
Conclusion
Circ-ABCB10 mediated PTX resistance, apoptosis, invasion and autophagy of BC cells via let-7a-5p/DUSP7 axis.
Keywords: breast cancer, paclitaxel, chemoresistance, circ-ABCB10, let-7a-5p, DUSP7
Introduction
The prognosis of metastatic breast cancer (BC) patients remains dismal due to the chemoresistance. Paclitaxel (PTX) is a commonly used chemotherapy drug for BC treatment. Unfortunately, the BC patients with PTX treatment for 6 to 10 months developed resistance to PTX.1,2 Thus, it is urgent to investigate the mechanism of PTX resistance to prolong the period of survival of BC patients.
Circular RNAs (circRNAs) are non-coding RNAs (ncRNAs) and are characterized by covalently closed loop structure. CircRNAs are implicated in the modulation of many diseases, including cardiovascular disease and multiple cancers.3–6 CircRNAs have been reported to function as microRNA (miRNA) sponges and down-regulate the levels of target miRNAs.7 CircRNA ATP binding cassette subfamily B member 10 (circ-ABCB10) has been reported to be up-regulated in BC tissues, and loss-of-function experiments revealed that circ-ABCB10 accelerated the proliferation while restrained the apoptosis of BC cells through miR-1271.8 Nevertheless, the role of circ-ABCB10 in the PTX resistance of BC is barely known.
MiRNAs are another group of ncRNAs with 20–25 nucleotides in length. MiRNAs are implicated in the pathogenesis of multiple cancers.9–12 MiRNAs could modulate gene expression through reducing the abundance of target messenger RNAs (mRNAs) or restraining the translation of mRNAs via binding to the 3ʹ untranslated region (UTR) of mRNAs.12–16 Liu et al claimed that NEAT1 mediated the chemoresistance of nasopharyngeal carcinoma (NPC) through reducing the level of let-7a-5p.17 Herein, we concentrated on the role of let-7a-5p in the PTX resistance of BC cells.
Dual specificity phosphatase 7 (DUSP7) is a member of DUSPs, and it could dephosphorylate the extracellular signal-regulated kinases (ERK1/2), c-Jun N-terminal kinase (JNK) and p38 kinase.18,19 DUSP7 has been reported to be up-regulated in BC and myeloid leukemia.20,21 For instance, Luan et al reported that the level of DUSP7 was elevated in BC cells, and MIAT promoted the proliferation and metastasis and impeded the apoptosis of BC cells via miR-155-5p/DUSP7 axis.20 However, the biological significance of DUSP7 in the PTX resistance of BC is still undefined.
In this study, we first examined the abundance of circ-ABCB10 in PTX-resistant or sensitive BC tissues and cells. Loss-of-function experiments were conducted to assess the role of circ-ABCB10 on PTX-resistance, apoptosis, invasion and autophagy of BC cells. In addition, we investigated the downstream gene of circ-ABCB10 to further clarify the mechanism by which circ-ABCB10 contributing to the PTX resistance of BC cells.
Materials and Methods
Patients
PTX-sensitive (n=30) or PTX-resistant (n=30) BC patients were recruited in Qingdao Chengyang People’s Hospital. The abundance of circ-ABCB10, let-7a-5p and DUSP7 was detected in BC tissues of the above patients. This study was authorized by the Ethic Committee of Qingdao Chengyang People’s Hospital. All patients participated in this study had provided written informed consents.
Cell Culture and PTX Treatment
Human breast epithelial cell line MCF-10A and human BC cell lines (MDA-MB-468, MDA-MB-453, MCF-7 and MDA-MB-231) were obtained from BeNa Culture Collection (Beijing, China). Human BC cells were cultivated in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Carlsbad, CA, USA) added with 10% fetal bovine serum (FBS; Gibco), 100 units/mL penicillin and 100 μg/mL streptomycin. MCF-10A cells were cultured in Roswell Park Memorial Institute-1640 medium (RPMI-1640, Gibco) supplemented with 10% FBS (Gibco) and 10% penicillin (100 U/mL)-streptomycin (100 μg/mL). All cells were grown in a 37°C, 5% CO2 humidified incubator.
PTX was obtained from Sigma (St. Louis, MO, USA) and dissolved in dimethyl sulfoxide (DMSO; Sigma). The PTX-resistant BC cell lines, MCF-7/PTX and MDA-MB-231/PTX, were established by treating parental MCF-7 and MDA-MB-231 cells with increased concentration of PTX.22
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
SYBR green were used in qRT-PCR. The levels of circ-ABCB10, let-7a-5p and DUSP7 were quantified using 2−ΔΔCt method.23 U6 or β-actin acted as the internal reference in this study. The primers used in this study were as follows: circ-ABCB10 (Forward, 5ʹ-CTAAGGAGTCACAGGAAGACATC-3ʹ; Reverse, 5ʹ-GTAGAATCTCTCAGACTCAAGGTTG-3ʹ), let-7a-5p (Forward, 5ʹ-CGATTCAGTGAGGTAGTAGGTTGT-3ʹ; Reverse, 5ʹ-TATGGTTGTTCTGCTCTCTGTCTC-3ʹ), DUSP7 (Forward, 5ʹ-CCAAGAAGTGTGGTGTCCTG-3ʹ; Reverse, 5ʹ-ACAAAGTCGTAGGCGTCGTT-3ʹ), U6 (Forward, 5ʹ-CTCGCTTCGGCAGCACA-3ʹ; Reverse, 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ), β-actin (Forward, 5ʹ-AGCCTCGCCTTTGCCGA-3ʹ; Reverse, 5ʹ-CTGGTGCCTGGGGCG-3ʹ).
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
MTT assay was used to detect the proliferation of BC cells. BC cells were seeded into 96-well plates at the density of 6000 cells per well and cultivated in the DMEM medium overnight. Cells were treated with 10 µL MTT (Invitrogen, Carlsbad, CA, USA) for 4 h and optical density was determined at 490 nm after adding 200 μL DMSO (Sigma).
Cell Transfection
Cell transfection was conducted by using Lipofectamine 2000 reagent (Invitrogen). Small interfering RNA negative control (si-NC), small interfering RNA against circ-ABCB10 (si-circ-ABCB10), pcDNA, circ-ABCB10 overexpression plasmid (circ-ABCB10) and DUSP7 overexpression plasmid (DUSP7) were purchased from Genepharma (Shanghai, China). Anti-miRNA-NC (anti-miR-NC), anti-let-7a-5p, miR-NC and let-7a-5p were purchased from Ribobio (Guangzhou, China).
Cell Apoptosis Analysis
BC cells were harvested and rinsed using cold phosphate buffer saline (PBS) buffer. Subsequently, cells were stained with Annexin V combined fluorescein isothiocyanate (FITC) and propidine iodide (PI; Solarbio, Beijing, China) mixture for 15 min in a dark room. Then the cells were tested by the flow cytometer (BD Biosciences, San Jose, CA, USA) and apoptotic cells were analyzed.
Transwell Invasion Assay
BD matrigel matrix (BD Biosciences) was coated on the polycarbonate membrane and BC cells were seeded into the pre-coated upper chambers. Medium added with 10% FBS was filled in the lower chambers. The invasive cells were dyed and calculated after 48-h incubation.
Western Blot Assay
BC cells were lysed on ice for 30 min using RIPA lysis solution (Beyotime, Shanghai, China). Samples of protein lysates were quantified, and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to the polyvinylidene fluoride (PVDF) membrane. The PVDF membrane was probed with primary and secondary antibody after blocking for 1 h with non-fat milk powder. The primary antibodies include anti-LC3 (ab51520), anti-P62 (ab155686), anti-DUSP7 (ab95960) and anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH; ab37168). The specific primary antibodies and the secondary antibody (ab205718) were obtained from Abcam (Cambridge, MA, USA). The protein signal level was determined via the enhanced chemiluminescent (ECL) system (Beyotime).
Immunofluorescence
Briefly, the coverslips were plated in 24-well plates, and BC cells after relevant treatment were seeded into the coverslips overnight. 4% paraformaldehyde was used to fix the BC cells for 10 min at room temperature, followed by blocking with 1% bull serum albumin (BSA) for 1 h at room temperature. The cells were incubated with anti-LC3B antibody (Abcam), and followed by the incubation with GFP-conjugated secondary antibody. The images were pictured with a LSM 700 confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany).
Dual-Luciferase Reporter Assay
Two reporter vectors named as WT-circ-ABCB10 and MUT-circ-ABCB10 were constructed which included wild-type or mutant type binding sites with let-7a-5p. Luciferase activity was determined in BC cells co-transfected with miR-NC or let-7a-5p and WT-circ-ABCB10 or MUT-circ-ABCB10 using dual-luciferase reporter assay kit (Promega, Madison, WI, USA). The confirmation of the binding relationship between let-7a-5p and DUSP7 was also carried out according to the same protocol.
Murine Xenograft Assay
Animal studies were performed in compliance with the ARRIVE guidelines and the Basel Declaration. Tumor formation assay was permitted by the Animal Research Committee of Qingdao Chengyang People’s Hospital. All animals received humane care according to the National Institutes of Health (USA) guidelines. MCF-7/PTX cells stably transfected with sh-NC or sh-circ-ABCB10 were harvested and re-suspended in PBS buffer. 200 μL cell suspension (6 × 106 cells) was injected to the BALB/c nude mice (Orient Bio Inc, Seongnam, South Korea). PBS or 3 mg/kg PTX was intraperitoneally injected into murine every 3 d following inoculation for 1 week. The tumor volume was measured using a caliper every 3 d using the formula of volume = π/6 × length (L) × width (W) × height (H). The mice were sacrificed and dissected after inoculation for 25 d. Tumor tissues were weighed and used to detect the level of circ-ABCB10.
Statistical Analysis
GraphPad Prism 7 was used for the statistical analysis. All data were expressed as mean±standard deviation (S.D.). Student’s t-test or one-way analysis of variance (ANOVA) was performed to calculate the P value. Spearman coefficient was used to analyze the liner relationship between levels of let-7a-5p and circ-ABCB10 or DUSP7 in PTX-resistant BC patients. P<0.05 was identified as statistically significant.
Results
The Abundance of circ-ABCB10 and Let-7a-5p Is Associated with Sensitivity to PTX in BC Tissues and Cells
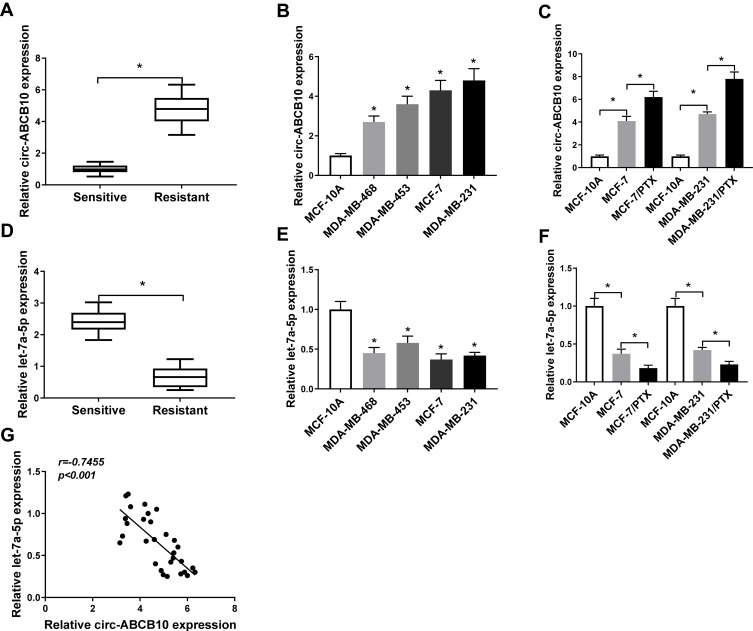
To assess whether the expression of circ-ABCB10 and let-7a-5p was related to PTX resistance of BC cells, we measured the abundance of circ-ABCB10 and let-7a-5p in PTX-sensitive or resistant BC tissues. As showed in Figure 1A and D, we found that circ-ABCB10 was up-regulated while the level of let-7a-5p was reduced in PTX-resistant BC tissues compared with that in PTX-sensitive tissues. As indicated in Figure 1B and E, the expression of circ-ABCB10 was higher while the enrichment of let-7a-5p was lower in BC cells than that in human breast epithelial cells MCF-10A. Besides, we examined the level of circ-ABCB10 and let-7a-5p in parental BC cells (MCF-7 and MDA-MB-231) and matching PTX-resistant cells (MCF-7/PTX and MDA-MB-231/PTX). The level of circ-ABCB10 was higher in MCF-7/PTX and MDA-MB-231/PTX than that in corresponding parental cells, while the alteration of let-7a-5p level revealed an inverse trend to circ-ABCB10 (Figure 1C and F). Based on the above results, we made the hypothesis that the level of circ-ABCB10 was negatively correlated with the expression of let-7a-5p in PTX-resistant BC tissues, and correlation analysis confirmed our hypothesis (Figure 1G).
Figure 1.
The abundance of circ-ABCB10 and let-7a-5p is associated with sensitivity to PTX in BC tissues and cells. (A) The expression of circ-ABCB10 was measured in PTX-sensitive (n=30) or resistant (n=30) BC tissues by qRT-PCR. (B) QRT-PCR was conducted to determine the level of circ-ABCB10 in human breast epithelial cells MCF-10A and BC cells (MDA-MB-468, MDA-MB-453, MCF-7 and MDA-MB-231). (C) The abundance of circ-ABCB10 was examined in MCF-10A, MCF-7, MDA-MB-231 and their matching PTX-resistant subclones (MCF-7/PTX and MDA-MB-231/PTX) by qRT-PCR. (D) The enrichment of let-7a-5p was determined in PTX-sensitive (n=30) or resistant (n=30) BC tissues by qRT-PCR. (E) QRT-PCR was performed to detect the level of let-7a-5p in MCF-10A cells and BC cells. (F) The level of let-7a-5p was examined in MCF-10A, MCF-7, MCF-7/PTX, MDA-MB-231 and MDA-MB-231/PTX by qRT-PCR. (G) Correlation analysis was carried out to analyze the expression of let-7a-5p and circ-ABCB10 in PTX-resistant BC tissues. *P<0.05.
Circ-ABCB10 Contributes to PTX Resistance of BC Cells
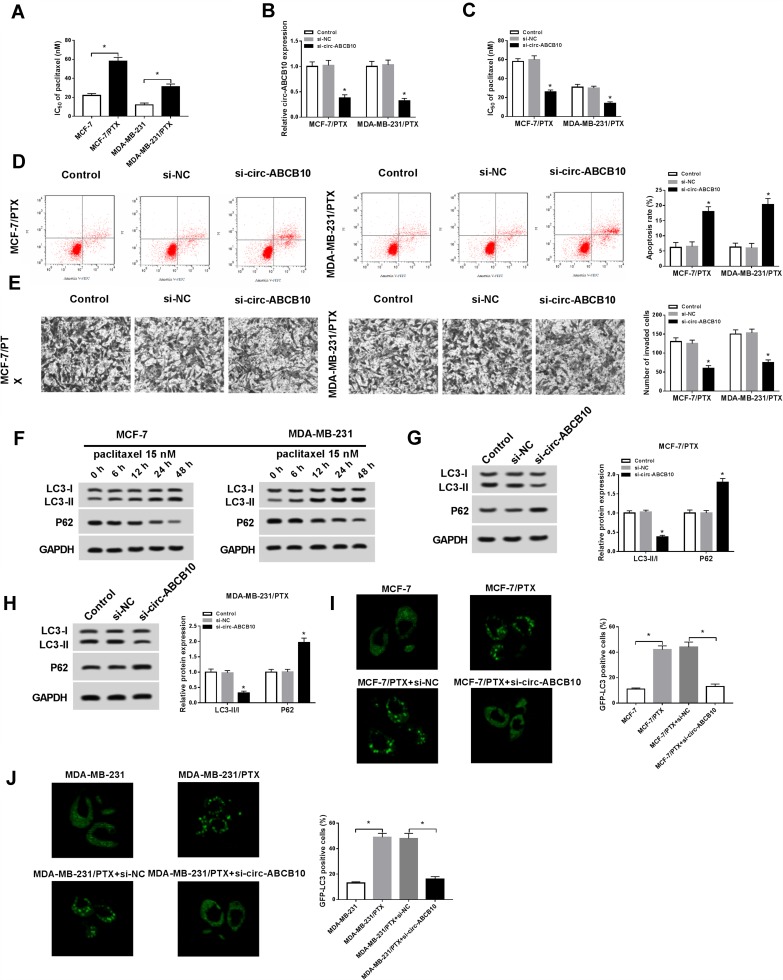
The expression of circ-ABCB10 was associated with chemoresistance of BC cells, suggesting that circ-ABCB10 might play an oncogenic role in BC cells. We first verified the PTX resistance of MCF-7 and MDA-MB-231 cells and their corresponding PTX-resistant subclones. As mentioned in Figure 2A, the IC50 value was higher in MCF-7/PTX and MDA-MB-231/PTX cells than that in the parental cells. We evaluated the knockdown efficiency of si-circ-ABCB10 in MCF-7/PTX and MDA-MB-231/PTX cells. As indicated in Figure 2B, the transfection of si-circ-ABCB10 prominently declined the level of circ-ABCB10 in the two PTX-resistant BC cells. MTT assay showed that circ-ABCB10 depletion decreased the IC50 value, suggesting that circ-ABCB10 knockdown sensitized MCF-7/PTX and MDA-MB-231/PTX to PTX (Figure 2C). As expected, the apoptosis of MCF-7/PTX and MDA-MB-231/PTX was notably elevated with circ-ABCB10 intervention (Figure 2D). Apart from this, we also found that circ-ABCB10 interference suppressed the invasion of the two PTX-resistant BC cells (Figure 2E).
Figure 2.
Circ-ABCB10 contributes to PTX resistance of BC cells. (A) The IC50 of PTX in MCF-7, MCF-7/PTX, MDA-MB-231 and MDA-MB-231/PTX was determined by MTT assay. (B–E) MCF-7/PTX and MDA-MB-231/PTX cells were transfected with si-NC or si-circ-ABCB10. (B) The level of circ-ABCB10 was detected in the above MCF-7/PTX and MDA-MB-231/PTX by qRT-PCR. (C) The IC50 of PTX in MCF-7/PTX and MDA-MB-231/PTX was measured by MTT assay. (D) Flow cytometry was performed to detect the apoptosis of the above MCF-7/PTX and MDA-MB-231/PTX cells. (E) Transwell invasion assay was carried out to detect the invasion of MCF-7/PTX and MDA-MB-231/PTX cells. (F) Western blot assay was performed to detect the abundance of autophagy-related proteins (LC3-I, LC3-II and P62) in MCF-7 and MDA-MB-231 cells treated with 15 nM PTX for 0 h, 6 h, 12 h, 24 h or 48 h. (G, H) The abundance of autophagy-related proteins was determined in si-NC or si-circ-ABCB10 transfected MCF-7/PTX and MDA-MB-231/PTX cells by Western blot assay. (I, J) Immunofluorescence was conducted to detect the number of GFP-LC3 positive cells in different groups. *P<0.05.
Autophagy has been reported to be associated with PTX resistance in BC cells.24 The autophagy was promoted in MCF-7 and MDA-MB-231 cells treated with 15 nM PTX for 0 h, 6 h, 12 h, 24 h or 48 h, and this promoting effect was PTX treatment time-dependent in BC cells (Figure 2F). As showed in Figure 2G and H, circ-ABCB10 interference suppressed the autophagy of MCF-7/PTX and MDA-MB-231/PTX cells. The number of GFP-LC3 positive cells was significantly elevated in MCF-7/PTX and MDA-MB-231/PTX cells compared with that in their parental cells, and the intervention of circ-ABCB10 decreased the number of GFP-LC3 positive cells in the two PTX-resistant BC cells (Figure 2I and J). Collectively, circ-ABCB10 intervention elevated the sensitivity of MCF-7/PTX and MDA-MB-231/PTX cells to PTX and promoted the apoptosis while inhibited the invasion and autophagy of the two cells.
Let-7a-5p Is a Direct Target of circ-ABCB10 in BC Cells
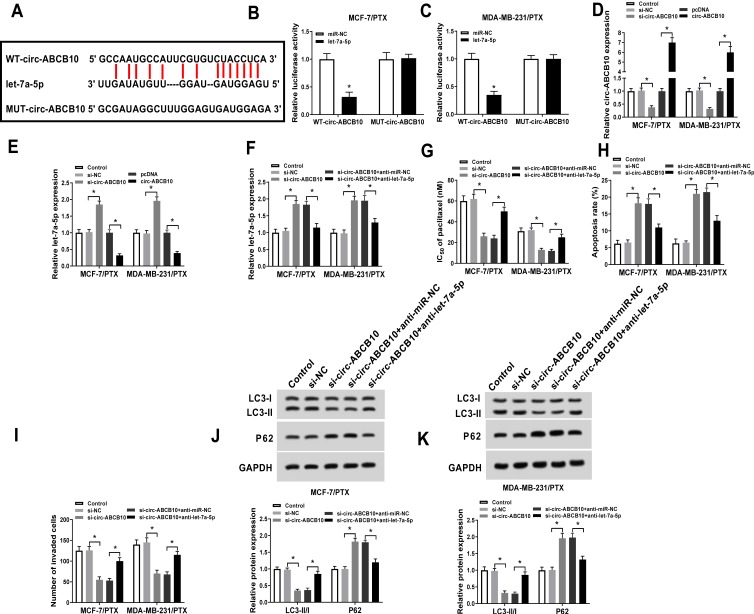
Let-7a-5p was predicted as a target of circ-ABCB10 by Starbase software (Figure 3A). Subsequently, we validated this combination by dual-luciferase reporter assay. As indicated in Figure 3B and C, luciferase activity was conspicuously declined in MCF-7/PTX and MDA-MB-231/PTX cells co-transfected with let-7a-5p and WT-circ-ABCB10, while it has little impact in let-7a-5p and MUT-circ-ABCB10 group, suggesting that let-7a-5p was a direct target of circ-ABCB10 in BC cells. To illuminate the modulatory relationship between circ-ABCB10 and let-7a-5p in PTX-resistant BC cells, we transfected si-NC, si-circ-ABCB10, pcDNA or circ-ABCB10 into MCF-7/PTX and MDA-MB-231/PTX cells. As showed in Figure 3D, the level of circ-ABCB10 was notably decreased by si-circ-ABCB10 transfection while the transfection of circ-ABCB10 prominently elevated the expression of circ-ABCB10 in MCF-7/PTX and MDA-MB-231/PTX cells. As mentioned in Figure 3E, let-7a-5p was negatively regulated by circ-ABCB10 in the two PTX-resistant BC cells.
Figure 3.
Let-7a-5p is a direct target of circ-ABCB10 in BC cells. (A) Let-7a-5p was predicted as a target of circ-ABCB10 by Starbase. (B, C) Dual-luciferase reporter assay was conducted to verify the combination between circ-ABCB10 and let-7a-5p in MCF-7/PTX and MDA-MB-231/PTX cells. (D, E) The abundance of circ-ABCB10 and let-7a-5p was determined in MCF-7/PTX and MDA-MB-231/PTX cells transfected with si-NC, si-circ-ABCB10, pcDNA or circ-ABCB10 by qRT-PCR. MCF-7/PTX and MDA-MB-231/PTX cells were transfected with si-NC, si-circ-ABCB10, si-circ-ABCB10 + anti-miR-NC or si-circ-ABCB10 + anti-let-7a-5p. (F) QRT-PCR was conducted to measure the abundance of let-7a-5p in the above two cells. (G) MTT assay was applied to examine the IC50 of PTX in the above two cells. (H) Flow cytometry was applied to analyze the apoptosis rate of the above MCF-7/PTX and MDA-MB-231/PTX cells. (I) The invasion of the above two cells was determined by transwell invasion assay. (J, K) The abundance of LC3-I, LC3-II and P62 was measured in MCF-7/PTX and MDA-MB-231/PTX cells by Western blot assay. *P<0.05.
To clarify whether let-7a-5p was involved in circ-ABCB10-mediated chemoresistance of BC cells, MCF-7/PTX and MDA-MB-231/PTX cells were transfected with si-NC, si-circ-ABCB10, si-circ-ABCB10 + anti-miR-NC or si-circ-ABCB10 + anti-let-7a-5p. The transfection of anti-let-7a-5p reduced the level of let-7a-5p, which was up-regulated by circ-ABCB10 depletion in MCF-7/PTX and MDA-MB-231/PTX cells (Figure 3F). As showed in Figure 3G and H, the intervention of let-7a-5p reversed the promoting effects of circ-ABCB10 depletion on the PTX sensitivity and apoptosis of the two PTX-resistant BC cells. Moreover, circ-ABCB10 intervention repressed the invasion and autophagy of MCF-7/PTX and MDA-MB-231/PTX cells while the addition of anti-let-7a-5p abrogated these effects (Figure 3I–K). These findings suggested that circ-ABCB10 contributed to PTX resistance of BC cells through let-7a-5p.
Let-7a-5p Could Bind to DUSP7 in BC Cells
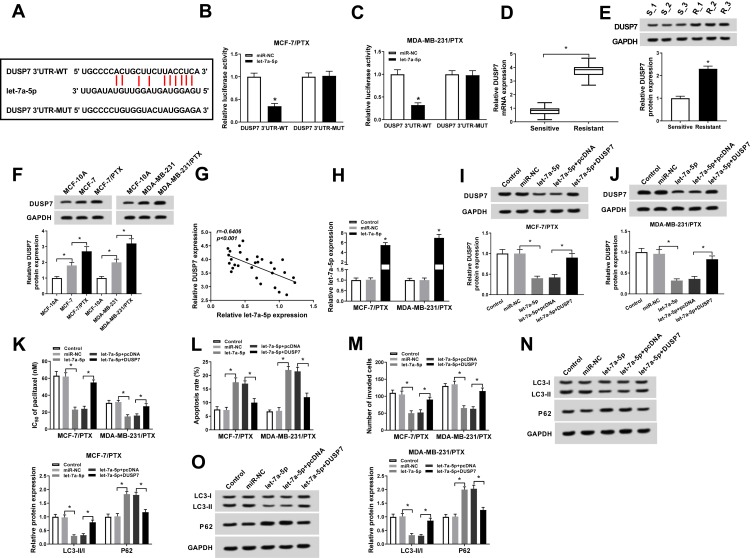
As mentioned above, circ-ABCB10 exerted its function at least partly through let-7a-5p in BC cells. But how does let-7a-5p affect the chemoresistance of BC cells? Starbase software predicted that DUSP7 could bind to let-7a-5p (Figure 4A). The combination was then verified by dual-luciferase reporter assay in MCF-7/PTX and MDA-MB-231/PTX cells co-transfected with miR-NC or let-7a-5p and DUSP7 3ʹ UTR-WT or DUSP7 3ʹ UTR-MUT. As showed in Figure 4B and C, the luciferase activity was significantly reduced with the co-transfection of DUSP7 3ʹ UTR-WT and let-7a-5p, suggesting that DUSP7 could bind to let-7a-5p in BC cells. To clarify the role of DUSP7 in BC, we first examined the mRNA and protein levels of DUSP7 in PTX-sensitive or resistant BC tissues. As mentioned in Figure 4D and E, the abundance of DUSP7 mRNA and protein was higher in PTX-resistant BC tissues than that in PTX-sensitive BC tissues. Meanwhile, DUSP7 was up-regulated in PTX-resistant BC cells compared with that in parental cells, suggesting its pivotal role in chemoresistance of BC cells (Figure 4F). Additionally, an obvious inverse correlation was found between the expression of DUSP7 and let-7a-5p in PTX-resistant BC tissues (Figure 4G).
Figure 4.
Let-7a-5p could bind to DUSP7 in BC cells. (A) The binding sites between let-7a-5p and DUSP7 were predicted by Starbase. (B, C) Luciferase activity was determined in MCF-7/PTX and MDA-MB-231/PTX cells co-transfected with miR-NC or let-7a-5p and DUSP7 3ʹ UTR-WT or DUSP7 3ʹ UTR-MUT. (D, E) The abundance of DUSP7 mRNA and protein was measured in PTX-sensitive or resistant BC tissues by qRT-PCR and Western blot assay. (F) Western blot assay was conducted to measure the abundance of DUSP7 in MCF-10A, MCF-7, MCF-7/PTX, MDA-MB-231 and MDA-MB-231/PTX cells. (G) Correlation analysis was performed to analyze the abundance of let-7a-5p and DUSP7 in PTX-resistant BC tissues. (H) The overexpression efficiency of let-7a-5p was assessed in MCF-7/PTX and MDA-MB-231/PTX cells transfected with miR-NC or let-7a-5p by qRT-PCR. MCF-7/PTX and MDA-MB-231/PTX cells were transfected with miR-NC, let-7a-5p, let-7a-5p + pcDNA or let-7a-5p + DUSP7. (I, J) Western blot assay was applied to detect the enrichment of DUSP7 in the above two cells. (K) The IC50 of PTX in the above two cells was measured by MTT assay. (L) Flow cytometry was applied to examine the apoptosis of the above MCF-7/PTX and MDA-MB-231/PTX cells. (M) Transwell invasion assay was conducted to detect the invasion of MCF-7/PTX and MDA-MB-231/PTX cells. (N, O) The abundance of autophagy-related proteins was detected in MCF-7/PTX and MDA-MB-231/PTX cells by Western blot assay. *P<0.05.
The enrichment of let-7a-5p was notably enhanced in MCF-7/PTX and MDA-MB-231/PTX cells transfected with let-7a-5p (Figure 4H). To explore whether let-7a-5p exerted its function through DUSP7 in BC cells, we transfected miR-NC, let-7a-5p, let-7a-5p + pcDNA or let-7a-5p + DUSP7 into MCF-7/PTX and MDA-MB-231/PTX cells to detect the cell chemoresistance, apoptosis, invasion and autophagy. The expression of DUSP7 was declined by let-7a-5p transfection, and the addition of DUSP7 overexpression plasmid recovered the expression of DUSP7 in MCF-7/PTX and MDA-MB-231/PTX cells (Figure 4I and J). As showed in Figure 4K, let-7a-5p transfection reduced IC50 of MCF-7/PTX and MDA-MB-231/PTX cells to PTX, and the overexpression of DUSP7 abolished this effect. As mentioned in Figure 4L–O, DUSP7 accumulation suppressed let-7a-5p-mediated apoptosis and reversed the inhibitory effects of let-7a-5p accumulation on the invasion and autophagy of the two PTX-resistant BC cells. Taken together, let-7a-5p promoted the PTX sensitivity and apoptosis while suppressed the invasion and autophagy of BC cells through negatively regulating DUSP7.
The Enrichment of DUSP7 Is Modulated by circ-ABCB10/Let-7a-5p Axis in BC Cells
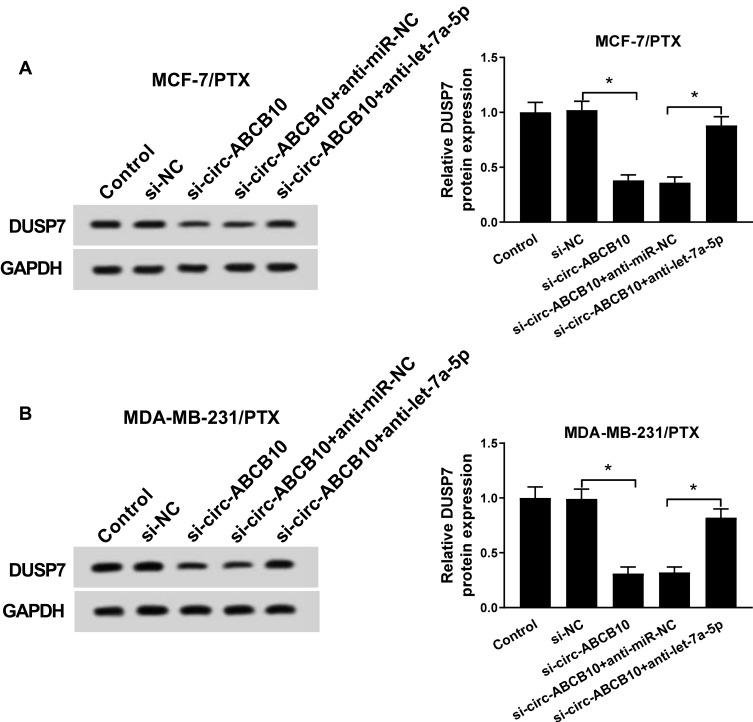
To further investigate the regulatory relationship of circ-ABCB10 and let-7a-5p on the level of DUSP7 in the two PTX-resistant BC cells, the enrichment of DUSP7 was detected in MCF-7/PTX and MDA-MB-231/PTX cells transfected with si-NC, si-circ-ABCB10, si-circ-ABCB10 + anti-miR-NC or si-circ-ABCB10 + anti-let-7a-5p by Western blot assay. As showed in Figure 5A and B, the abundance of DUSP7 was declined with circ-ABCB10 interference in MCF-7/PTX and MDA-MB-231/PTX cells, and the addition of anti-let-7a-5p recovered the expression of DUSP7. Taken together, DUSP7 was regulated by circ-ABCB10/let-7a-5p axis in BC cells.
Figure 5.
The enrichment of DUSP7 is modulated by circ-ABCB10/let-7a-5p axis in BC cells. (A, B) The protein expression of DUSP7 in MCF-7/PTX and MDA-MB-231/PTX cells transfected with si-NC, si-circ-ABCB10, si-circ-ABCB10 + anti-miR-NC or si-circ-ABCB10 + anti-let-7a-5p was determined by Western blot assay. *P<0.05.
Circ-ABCB10 Knockdown Suppresses the Growth of BC Tumor in vivo
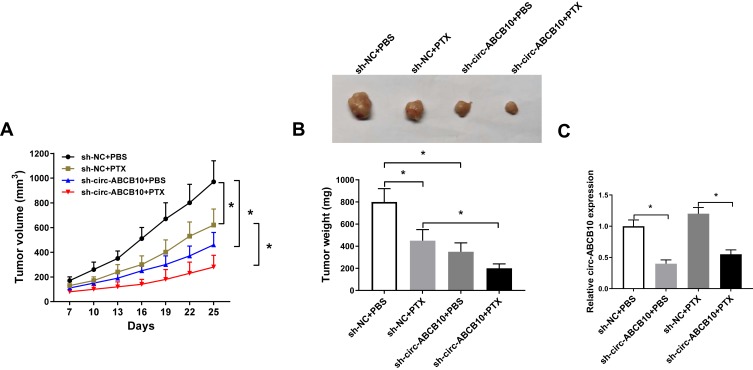
We built murine xenograft model using MCF-7/PTX cells stably transfected with sh-NC or sh-circ-ABCB10 to explore the effect of circ-ABCB10 in vivo. As showed in Figure 6A and B, PTX treatment or circ-ABCB10 depletion suppressed the BC tumor growth in vivo, especially in sh-circ-ABCB10 and PTX combined treatment group. The abundance of circ-ABCB10 was reduced in sh-circ-ABCB10 + PBS group and sh-circ-ABCB10 + PTX group compared with that in sh-NC + PBS group and sh-NC + PTX group (Figure 6C). Taken together, circ-ABCB10 knockdown inhibited the growth of BC tumor in vivo.
Figure 6.
Circ-ABCB10 knockdown suppresses the growth of BC tumor in vivo. MCF-7/PTX cells stably transfected with sh-NC or sh-circ-ABCB10 were subcutaneously injected into the back of murine. PBS or 3 mg/kg PTX was intraperitoneally injected into murine every 3 d following inoculation for 1 week. (A) Tumor volume was recorded every 3 d. (B) Tumor was weighed after inoculation for 25 d. (C) The expression of circ-ABCB10 was detected in resected tumors by qRT-PCR. *P<0.05.
Discussion
Exploring crucial molecules involved in chemoresistance is essential for BC treatment. Herein, we found that circ-ABCB10 was up-regulated in PTX-resistant BC tissues and cells compared with that in PTX-sensitive BC tissues and parental BC cells. Subsequently, we conducted loss-of-function experiments to assess the role of circ-ABCB10 in the chemoresistance of BC cells. Circ-ABCB10 depletion promoted the PTX sensitivity and apoptosis while impeded the invasion and autophagy of BC cells.
To illustrate the exact mechanism by which circ-ABCB10 contributing to PTX resistance of BC cells, we aimed to explore the downstream gene of circ-ABCB10 in BC cells. Let-7a-5p was predicted as a target of circ-ABCB10 by Starbase bioinformatic software, and this combination was verified by dual-luciferase reporter assay. Let-7a-5p played a suppressive role in colorectal cancer (CRC), lung cancer and BC. For example, Liu et al demonstrated that the expression of let-7a-5p was negatively associated with lymph node metastasis, tumor size and staging of CRC patients.25 Duan et al found that let-7a-5p restrained the proliferation and metastasis while facilitated the death of lung cancer cells via inversely modulating BCL2L1.26 Kim et al proved that the level let-7a-5p was reduced in BC tissues and cells, and it repressed the proliferation and metastasis of BC cells through CCR7.27 Herein, we focused on the role of let-7a-5p in the PTX resistance of BC cells. The level of let-7a-5p was lower in PTX-resistant BC tissues and cells than that in PTX-sensitive BC tissues and parental BC cells, and the level of let-7a-5p was negatively correlated with the abundance of circ-ABCB10 in PTX-resistant BC patients. The interference of let-7a-5p abolished the promoting effects of circ-ABCB10 depletion on the PTX sensitivity and apoptosis and the inhibitory impacts on the invasion and autophagy of PTX-resistant BC cells.
DUSP7 was predicted to be a target of let-7a-5p by Starbase software, and we validated the combination between DUSP7 and let-7a-5p in BC cells by dual-luciferase reporter assay. DUSP7 is a member of DUSP family which contains at least 11 members. DUSP family is a group of MAP kinase (MAPK) inhibitors, and they exerted their functions by dephosphorylating MAPK (ERK1/2, JNK and p38). DUSP7 has been identified as an oncogene in BC.20 Consistent with the above findings, we found that DUSP7 played an oncogenic role in BC. DUSP7 accumulation counteracted the promoting impacts of let-7a-5p overexpression on PTX sensitivity and apoptosis and the suppressive effects on the invasion and autophagy of PTX-resistant BC cells. Western blot assay revealed that let-7a-5p depletion reversed circ-ABCB10 intervention-mediated down-regulation on the protein level of DUSP7 in MCF-7/PTX and MDA-MB-231/PTX cells, suggesting that DUSP7 was regulated by circ-ABCB10/let-7a-5p axis in PTX-resistant BC cells.
We established a murine xenograft model to confirm the function of circ-ABCB10 in vivo. The BC tumors were significantly lesser in sh-circ-ABCB10 + PBS group and sh-circ-ABCB10 + PTX group than that in sh-NC + PBS group and sh-NC + PTX group, suggesting that circ-ABCB10 knockdown restrained the growth of BC tumors in vivo. Besides, BC tumors were the smallest in sh-circ-ABCB10 + PTX combined treatment group.
In summary, circ-ABCB10 contributed to PTX resistance of BC cells through up-regulating DUSP7 via sponging let-7a-5p. Circ-ABCB10/let-7a-5p/DUSP7 axis might provide new insight into developing an effective strategy for the treatment of PTX-resistant BC patients.
Highlights
Circ-ABCB10 contributes to PTX resistance of BC cells, and it promotes the invasion and autophagy while impedes the apoptosis of PTX-resistant BC cells.
Let-7a-5p could bind to circ-ABCB10 in BC cells.
DUSP7 is a direct target of let-7a-5p in BC cells.
Circ-ABCB10 contributes to PTX resistance of BC cells through let-7a-5p/DUSP7 axis.
Disclosure
The authors declare that they have no financial conflicts of interest in this work.
References
- 1.Pallis AG, Boukovinas I, Ardavanis A, et al. A multicenter randomized Phase III trial of vinorelbine/gemcitabine doublet versus capecitabine monotherapy in anthracycline- and taxane-pretreated women with metastatic breast cancer. Ann Oncol. 2012;23(5):1164–1169. doi: 10.1093/annonc/mdr405 [DOI] [PubMed] [Google Scholar]
- 2.Seo JH, Oh SC, Choi CW, et al. Phase II study of a gemcitabine and cisplatin combination regimen in taxane resistant metastatic breast cancer. Cancer Chemother Pharmacol. 2007;59(2):269–274. doi: 10.1007/s00280-006-0266-x [DOI] [PubMed] [Google Scholar]
- 3.Qian L, Yu S, Chen Z, Meng Z, Huang S, Wang P. The emerging role of circRNAs and their clinical significance in human cancers. Biochim Biophys Acta Rev Cancer. 2018;1870(2):247–260. doi: 10.1016/j.bbcan.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 4.Chen LL, Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12(4):381–388. doi: 10.1080/15476286.2015.1020271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu L, Jiang Z, Li T, Hu Y, Guo J. Circular RNAs in hepatocellular carcinoma: functions and implications. Cancer Med. 2018;7:3101–3109. doi: 10.1002/cam4.1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou B, Yu JW. A novel identified circular RNA, circRNA_010567, promotes myocardial fibrosis via suppressing miR-141 by targeting TGF-beta1. Biochem Biophys Res Commun. 2017;487(4):769–775. doi: 10.1016/j.bbrc.2017.04.044 [DOI] [PubMed] [Google Scholar]
- 7.Chan JJ, Tay Y. Noncoding RNA: RNA regulatory networks in cancer. Int J Mol Sci. 2018;19(5):1310. doi: 10.3390/ijms19051310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang HF, Zhang XZ, Liu BG, Jia GT, Li WL. Circular RNA circ-ABCB10 promotes breast cancer proliferation and progression through sponging miR-1271. Am J Cancer Res. 2017;7(7):1566–1576. [PMC free article] [PubMed] [Google Scholar]
- 9.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10(10):704–714. doi: 10.1038/nrg2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arora A, Singh S, Bhatt AN, Pandey S, Sandhir R, Dwarakanath BS. Interplay between metabolism and oncogenic process: role of microRNAs. Transl Oncogenomics. 2015;7:11–27. doi: 10.4137/tog.s29652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122(1):6–7. doi: 10.1016/j.cell.2005.06.036 [DOI] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 13.Yu X, Li Z, Yu J, Chan MT, Wu WK. MicroRNAs predict and modulate responses to chemotherapy in colorectal cancer. Cell Prolif. 2015;48(5):503–510. doi: 10.1111/cpr.12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Lei H, Luo M, et al. DNA methylation downregulated mir-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer. 2015;18(1):43–54. doi: 10.1007/s10120-014-0340-8 [DOI] [PubMed] [Google Scholar]
- 15.Xiong X, Ren HZ, Li MH, Mei JH, Wen JF, Zheng CL. Down-regulated miRNA-214 induces a cell cycle G1 arrest in gastric cancer cells by up-regulating the PTEN protein. Pathol Oncol Res. 2011;17(4):931–937. doi: 10.1007/s12253-011-9406-7 [DOI] [PubMed] [Google Scholar]
- 16.He XP, Shao Y, Li XL, et al. Downregulation of miR-101 in gastric cancer correlates with cyclooxygenase-2 overexpression and tumor growth. FEBS J. 2012;279(22):4201–4212. doi: 10.1111/febs.12013 [DOI] [PubMed] [Google Scholar]
- 17.Liu F, Tai Y, Ma J. LncRNA NEAT1/let-7a-5p axis regulates the cisplatin resistance in nasopharyngeal carcinoma by targeting Rsf-1 and modulating the Ras-MAPK pathway. Cancer Biol Ther. 2018;19(6):534–542. doi: 10.1080/15384047.2018.1450119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chappell J, Sun Y, Singh A, Dalton S. MYC/MAX control ERK signaling and pluripotency by regulation of dual-specificity phosphatases 2 and 7. Genes Dev. 2013;27(7):725–733. doi: 10.1101/gad.211300.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orlev LN, Ehud B, Tamar BG, Orit SA, Yoel K, Witz IP. Does the dual-specificity MAPK phosphatase Pyst2-L lead a monogamous relationship with the Erk2 protein? Immunol Lett. 2004;92(1–2):149–156. doi: 10.1016/j.imlet.2003.11.024 [DOI] [PubMed] [Google Scholar]
- 20.Luan T, Zhang X, Wang S, et al. Long non-coding RNA MIAT promotes breast cancer progression and functions as ceRNA to regulate DUSP7 expression by sponging miR-155-5p. Oncotarget. 2017;8(44):76153–76164. doi: 10.18632/oncotarget.19190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy-Nissenbaum O, Sagi-Assif O, Raanani P, Avigdor A, Ben-Bassat I, Witz IP. cDNA microarray analysis reveals an overexpression of the dual-specificity MAPK phosphatase PYST2 in acute leukemia. Methods Enzymol. 2003;366:103–113. doi: 10.1016/s0076-6879(03)66009-x [DOI] [PubMed] [Google Scholar]
- 22.Fu Y, Ye D, Chen H, Lu W, Ye F, Xie X. Weakened spindle checkpoint with reduced BubR1 expression in paclitaxel-resistant ovarian carcinoma cell line SKOV3-TR30. Gynecol Oncol. 2007;105(1):66–73. doi: 10.1016/j.ygyno.2006.10.061 [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 24.Ajabnoor GM, Crook T, Coley HM. Paclitaxel resistance is associated with switch from apoptotic to autophagic cell death in MCF-7 breast cancer cells. Cell Death Dis. 2012;3:e260. doi: 10.1038/cddis.2011.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu TP, Huang CC, Yeh KT, et al. Down-regulation of let-7a-5p predicts lymph node metastasis and prognosis in colorectal cancer: implications for chemotherapy. Surg Oncol. 2016;25(4):429–434. doi: 10.1016/j.suronc.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 26.Duan S, Yu S, Yuan T, Yao S, Zhang L. Exogenous Let-7a-5p induces A549 lung cancer cell death through BCL2L1-mediated PI3Kgamma signaling pathway. Front Oncol. 2019;9:808. doi: 10.3389/fonc.2019.00808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SJ, Shin JY, Lee KD, et al. MicroRNA let-7a suppresses breast cancer cell migration and invasion through downregulation of C-C chemokine receptor type 7. Breast Cancer Res. 2012;14(1):R14. doi: 10.1186/bcr3098 [DOI] [PMC free article] [PubMed] [Google Scholar]