Abstract
High resolution mass spectrometry (HR-MS) offers the opportunity to track large numbers of non-target analytes through water treatment processes, providing a more comprehensive view of reactor performance than targeted evaluation. Both approaches were used to evaluate the performance of a pilot scale advanced oxidation process (AOP) employing ultraviolet light and hydrogen peroxide (UV/H2O2) to treat municipal wastewater effluent. Twelve pharmaceuticals and personal care products were selected as target compounds and added to reactor influent. Target compound removal over a range of flow rates and hydrogen peroxide addition levels was assessed using a liquid chromatograph combined with a quadrupole time-of-flight mass spectrometer (LC-qTOF-MS). Target compound removals were used to determine hydroxyl radical concentrations and UV fluence under pilot scale conditions. The experiments were also analyzed using a nontarget approach, which identified “molecular features” in either reactor influent or effluent. Strong correlation (r=0.94) was observed between target compound removals calculated using the targeted and non-targeted approaches across the range of reactor conditions tested. The two approaches also produced consistent rankings of the performance of the various reactor operating conditions, although the distribution of compound removal efficiencies was usually less favorable with the broader, nontarget approach. For example, in the UV only treatment 8.3% of target compounds and 2.2% of non-target compounds exhibited removals above 50%, while 100% of target compounds and 74% of non-target compounds exhibited removals above 50% in the best condition tested. These results suggest that HR-MS methods can provide more holistic evaluation of reactor performance, and may reduce biases caused by selection of a limited number of target compounds. HR-MS methods also offer insights into the composition of poorly removed compounds and the formation of transformation products, which were widely detected.
Keywords: Non-target analysis, advanced oxidation, wastewater, pharmaceuticals and personal care products, LC-QTOF-MS
Graphical Abstract
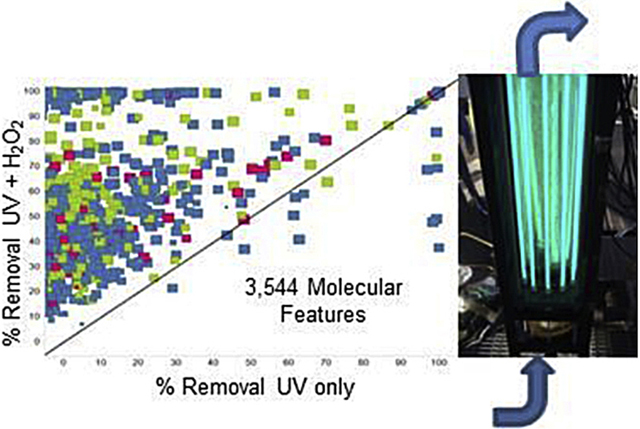
1.0. Introduction
Pharmaceuticals and personal care products (PPCP) have been found in wastewater effluents (Miège et al., 2009). While their environmental effects are largely unknown, there are concerns regarding growing antibacterial resistance (Goñi-urriza et al., 2000; Iwane et al., 2001; Watkinson et al., 2007; Y. Zhang et al., 2009) and endocrine disruption in aquatic systems (Roefer et al., 2014). Advanced oxidation processes (AOPs) have been shown to effectively degrade these compounds in wastewater (Rosario-Ortiz et al., 2010; Yuan et al., 2009). AOP reactors are typically optimized for PPCP removal by adding a suite of target chemicals and quantitating their removal while changing reactor operating conditions (Dickenson et al., 2011). With the advent of more widely available high resolution mass spectrometry (HR-MS) techniques another more complete approach for reactor evaluation may be possible.
HR-MS data can collect a full scan of extracted and ionisable microconstituents and can identify them by database matches, molecular formula, or simply by accurate mass and retention time. Collectively, the identified components are referred to as molecular features and comparing their behavior across different AOP reactor conditions can provide insight into the impact of changing reactor operating parameters.
Targeted evaluation of reactor performance relies on well validated techniques for accurately quantifying PPCPs with standards; while HR-MS may provide an alternative method, there are associated pitfalls. As a technique HR-MS is data intensive and demands the development and application of careful and systematic approaches to data processing. Software tools available from instrument vendors or as freeware (e.g., non-target R package) need to be applied systematically to provide meaningful results. Several studies have presented methodologies for screening of water samples for organic contaminants (Hug et al., 2014; Moschet et al., 2013; Nurmi et al., 2012). They involve algorithms for feature detection, curation (i.e., peak height requirements, blank subtraction, etc.), evaluation of false negative and false positive rates (Hug et al., 2014; Moschet et al., 2013; Nurmi et al., 2012) and sometimes establishment of screening limits of detection (Diaz et al., 2013). These techniques allow the creation of a complete dataset of the ionized and detected chemicals not limited by pre-selection of target compounds.
The objective of this study was to evaluate an innovative AOP reactor with both target and non-target techniques in tandem, to fully evaluate the potential of HR-MS as a tool for reactor assessment. To our knowledge, no comparison of the two analyses applied to treatment reactors has been performed. Our goal was to fully evaluate the reactor system based on target compound data, assess whether non-target data was comparable with non-target removals, and glean additional information from the advantages of the non-targeted technique, such as a more complete characterization of microconstituents in reactor effluent and byproduct formation.
2.0. Materials and Methods
2.1. Chemicals
The PPCPs used were carbamazepine (CBZ), diclofenac (DCF), ibuprofen (IBF), N,N-diethyl-m-toluamide (DEET), phenytoin (PYT), trimethoprim (TMP), tetracycline (TTC), triclosan (TCS), sulfamethoxazole (SFX), metoprolol (MTP), gemfibrozil (GFB), and fluoxetine (FXT). Reasons for their selection and key parameters from the literature related to their degradation by direct photolysis (molar absorption coefficient, ε, quantum yield, ϕ) and reactivity toward hydroxyl radical (second order rate constant, k.OH) are provided in Table S1, Appendix A. All standards were purchased at Sigma Aldrich with the exception of TCS (Accustandard). Labeled TMP (13C3) was added at 100 ng/L to monitor for matrix effects (Cambridge Isotope Laboratories). Ethylenediamine tetraacetic acid, tetrasodium salt (EDTA) (CAS #10378-23-1, Sigma-Aldrich) had a purity of 99% by titration. Methanol and acetonitrile (Burdick and Jackson) were LC-MS grade. Hydrogen peroxide (CAS# 7722-84-1, Ricca Chemical Company, 30% (w/w)) was ACS reagent grade.
2.2. Test System Setup
Effluent from the UC Davis tertiary wastewater treatment plant was used as reactor “influent”. A test tank was filled with 1517 L and gravity fed to the reactor pump. Targeted compounds were added in amounts to achieve final concentrations between 200 – 400 ng/L when the tank contained approximately 500 L. The remaining water was added rapidly ensuring adequate mixing. Flow rate to the reactor was controlled by a constrictor between the tank and the pump. Hydrogen peroxide was introduced immediately before the reactor pump using a peristaltic pump. Reactor effluent was periodically tested with peroxide strips (WaterWorks) to ensure a consistent addition; nominal peroxide concentration was calculated by flow rates. To quantify water loss and estimate reactor retention time, the flow rate was measured in two ways: via the drawdown rate of the water tank (water in) and the discharge rate of the reactor (water out). Peroxide concentration was calculated using the flow rate of incoming water, while the reactor “flow rate” is taken to be the effluent flow rate. A diagram of the sampling setup is provided in Fig. S1.
The vortex reactor consists of a central quartz tube surrounded by 12 low pressure UV lamps (each with 28 W output at 253.7 nm). Placing lamps outside the water flow reduces chemical/biological fouling and reduces friction losses. Water is introduced at the base of the reactor through several nozzles, rising to form a vortex, confining the water between an air column and the inner wall of the tube, exiting through a weir on the top of the reactor (Fig. S2). The vortex serves to maximize mixing and to keep the water close to the lamps on the outside of the quartz tube. Hydraulic retention time (HRT, θ) was estimated as the volume of the reactor (23 L) divided by the measured discharge rate. Unless a plug is installed at the bottom, the reactor has a stabilized air core through the center. Only one set of conditions was run without the air core (125 L/min, 6 mM H2O2) to test its effect; all others were run with it. While the HRT overestimates the true fluid residence time when the air core is present because the liquid volume is less than the total reactor volume, HRT is assumed to be comparable among conditions with the air core present. Before samples were collected the system was allowed to run for five times the estimated retention time to reach steady state. All test parameters are available in Table S2.
The primary purpose of this research was to compare target and non-target analytical approaches for assessing the performance of unit operations for microconstituent removal in wastewater treatment. The experiments were therefore designed to achieve a range of target compound removals. Preliminary tests with the vortex reactor at a flow rate of 125 L/min and peroxide dosages of 0.45 mM and 6 mM indicated substantially higher, but still incomplete removal at 6 mM. These results supported the selection of 3 and 6 mM as target peroxide dosages for subsequent experiments. Although these peroxide doses are higher than those employed in most AOP research, and are beyond the range expected to be economically viable in practice, they provided the desired distribution of removal efficiencies. The overall experimental design involved tests at three different flow rates: 23, 45 and 125 L/min, a range chosen to include the lowest and highest flow rates for the vortex reactor and one intermediate value. At the 45 L/min flow rate three peroxide concentrations were tested 0, 3 mM, and 6 mM. At the 125 L/min condition 6 mM peroxide was added and the reactor was tested with and without the stabilized air core. At the slowest flow rate, 23 L/min, 5 mM peroxide was added.
The fluence of the UV reactor (H’) was estimated from the removal of the target compounds with the greatest susceptibility to direct photolysis, as determined from the product of their quantum yield and molar absorption coefficient (ϕε) at 254 nm. Four target compounds had ϕε values above 50 L/ein·cm (PYT, SFX, DCF, and TCS; Table S1). Despite its potentially significant transformation by direct photolysis, TCS was not used as an actinometer because the pH dependence of its quantum yield would introduce additional uncertainty into the fluence estimate (Latch et al., 2005). The other three compounds were used as chemical actinometers following the approach outlined in (Jin et al., 2006):
| (1) |
where C0/C is the ratio of actinometer concentration in the influent to that in the effluent, U= photon energy at wavelength of the lamp (4.713×108 mJ/ein at 254 nm), ϕ254=quantum yield at 254 nm (mol/ein), ε=molar absorption coefficient at 254 nm (M−1cm−1), and the factor of 2303 converts among logarithmic bases and embeds the conversion from L to cm3. Fluence values determined using this approach have the advantage of being measured directly in wastewater under the conditions being tested for removal of the nontarget features (in this case, 45 L/min, 0 mM peroxide addition).
The steady state hydroxyl radical concentration ([·OH]) under each test condition was determined using CBZ, MTP, TMP, GFL and DEET as chemical actinometers. Values of the second order rate constant of each of these compounds with hydroxyl radical (k.OH) are available in the literature, and in most cases multiple independent values were found (4 for CBZ, 3 for MTP, 2 for TMP and GFB) resulting in a total of 12 estimates of [·OH] per condition using eq. 2.
| (2) |
2.3. Sampling, target PPCP extraction and analysis
At each test condition, 4 one-liter replicates of both influent and effluent were collected in pre-cleaned amber glass bottles. They were stored on ice and transported back to the lab where they were stored a 4 °C until extraction. All samples were filtered through glass fiber filters (GF/B, Whatman), 1 g of EDTA was added increase recovery of some compounds, and the pH was adjusted to 4 with 6 M HCl. Samples were concentrated by solid phase extraction with HLB (200 mg/6 mL, Sigma) cartridges preconditioned with 2 volumes (~ 12 mL) of methanol, 1 volume (~ 6 mL) of de-ionized (MilliQ) water and 1 volume of pH 4 water (prepared with 6 M HCl). After water extraction, cartridges were dried for at least 2 hours under high vacuum before being eluted with 14 mL of 1:1 acetonitrile: methanol. Samples were evaporated to 1 mL at 40 °C under nitrogen with an automated evaporation system (turbovap, Biotage).
Samples were analyzed with an Agilent 1260 high pressure liquid chromatography (HPLC) system attached to an Agilent 6530 quadrupole-time of flight (QTOF) instrument. Collision induced dissocation (CID) experiments allowed analysis of feature fragmentation patterns. Detailed instrument parameters are described in Appendix A.
Extraction recovery and precision was evaluated by spiking target compounds at 50 ng/L into 7 de-ionized water samples. Recovery and precision of target compounds in wastewater was evaluated by wastewater samples discussed in section 2.5. Results are reported in Table S3. Target compounds were quantified using external standards. Concentrations should be considered semi-quantitative given the low recovery of some compounds. Our goal was internal comparison between treatments and no labeled surrogates for each compound were used so results were not recovery corrected.
Water quality parameters (e.g., total suspended solids (TSS), turbidity, and UV transmittance (UVT)) were measured in accordance with standard methods (APHA, 1998) and are available in Table S4.
2.4. Feature filtering and evaluation
Feature extraction and identification were performed using Agilent MassHunter Qualitative analysis software (version B.06.00), which was used to isolate peaks as potential compounds, referred to as “features”. Complete extraction parameters are available in Table S5. Features were then imported into Mass Profiler Professional, MPP, (version 12.05) a statistical package used to align, filter and analyze data across conditions. Alignment of features across samples was based on a matching retention time (RT) (±0.15 min) and mass to ±15 ppm. All components of the method blank and field blank were removed. Any features not appearing in all replicates from at least one condition were eliminated. Remaining features, aligned across all samples, created a recursive filter that was used to rescan data files The reprocessed data set was re-filtered as described above. After the complete process the data set contained 3,544 potential compounds (ESI+ and ESI− combined) and was screened with an exact mass in-house database containing 1167 compounds including those previously detected in wastewater (Kolpin et al., 2002; Miège et al., 2009; Wols and Hofman-Caris, 2012) or predicted by production volume and physical-chemical properties to be potential contaminants of concern (Howard and Muir, 2011, 2013). If a feature was not matched with the database it was assigned a molecular formula based on user selected parameters (Table S6) or simply defined as its RT and mass.
2.5. Evaluation of nontarget screening
To evaluate the nontarget filtering procedure in the context of the target compounds, they were added into four wastewater effluent replicates at four different concentrations (100, 200, 500, and 1000 ng/L) to estimate the concentration required for them to pass the screening process. Unspiked wastewater was also analyzed to account for any PPCP in the native wastewater.
2.6. Data and statistical processing
Analysis of variance and Tukey’s honest significance difference test was conducted with RStudio (Version 0.98.501). T-test and probability values were calculated with Microsoft Excel (2010). Log Kow values were generated with the US Environmental Protection Agency EPISuite ™.
3.0. Results and discussion
3.1. Reactor performance based on target compound removal
Target compound removals under all reactor operating conditions are summarized in Fig. 1. Statistcal differences between average fractional removals for the specified compound across treatment conditions is available in Figure S5. Removals under UV only test conditions were modest, with only SFX, PYT, TCS, and DCF exhibiting removals above 15%. The average fluence for the reactor during the 45 L/min UV only test condition was 116 mJ/cm2 with a standard deviation of 6.7 mJ/cm2 across the three target actinometers (SFX, PYT, DCF). The calculated UV fluence is similar to the value of 145 mJ/cm2 previously determined for this reactor at a flow rate of 125 L/min using an MS2 phage decay test (Younis, 2014). These fluence values are at the upper end of the range of typical disinfection dosages (40–140 mJ/cm2) and lower than those typically required for PPCP removal in AOP systems (Kim et al., 2009; Rosario-Ortiz et al., 2010; Yuan et al., 2009), accounting for the need to add relatively high peroxide doses during the testing. There was a good correlation (ρ=0.90, ρ=0.97 excluding TCS) between the removal under UV only conditions (Fig. 1) and the product of the quantum yield and the molar absorption coefficient (Table S1 and Figure S3), for the compounds for which these parameters were available, as would be expected from eq 1.
Fig. 1:
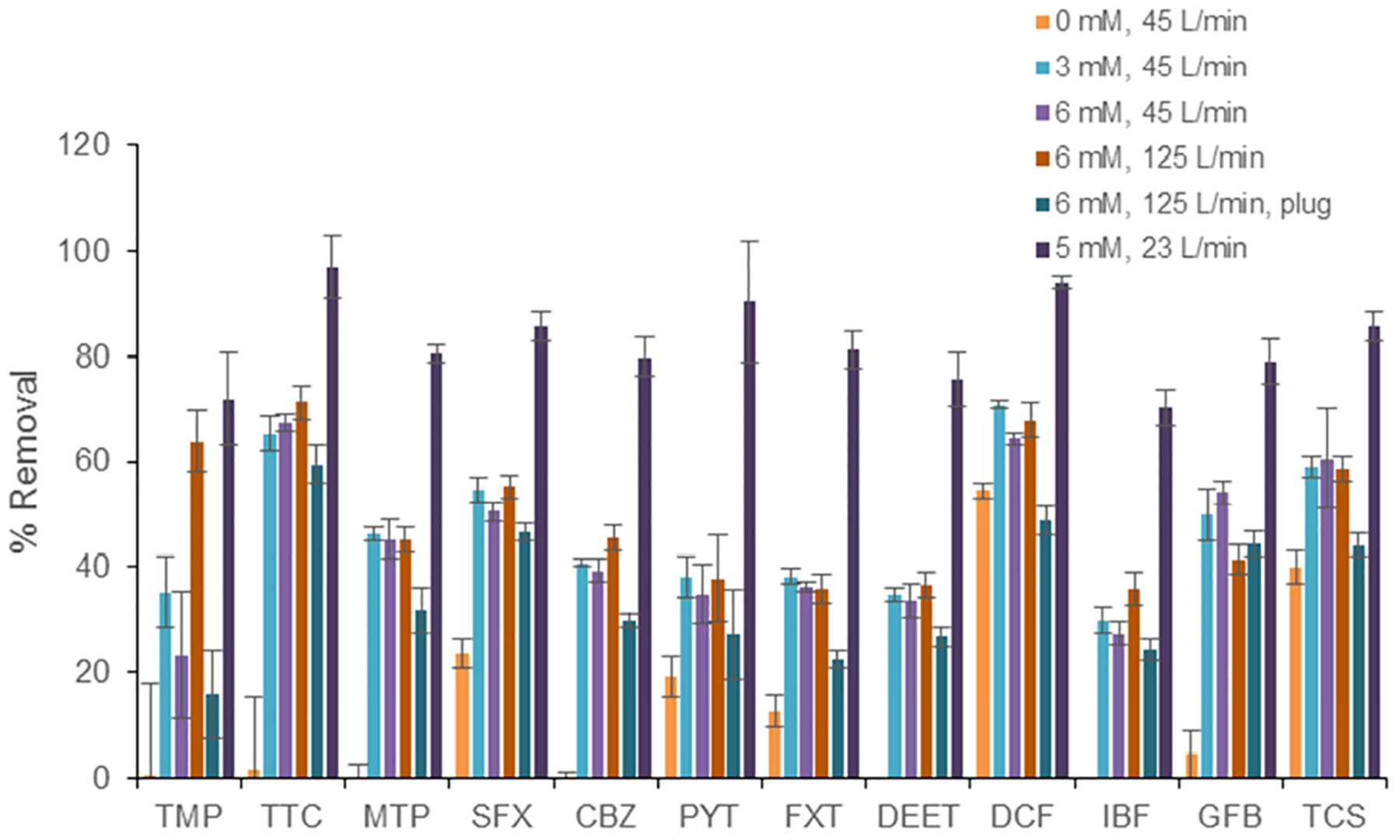
Removals of target compounds. All bars represent the average of 4 samples and error bars indicate standard deviation.
Addition of peroxide increased the removal of all target compounds at each test condition compared to the UV only case with the exception of DCF at the 125 L/min condition with the plug installed (no air core present). Degradation of compounds that experience significant direct photolysis, like DCF, would be expected to be impacted the most significantly by the absence of the air core because the same incident UV light must penetrate a thicker water column. All target compounds exhibited the highest removal efficiencies, ranging from 70% (IBF) to 94% (DCF), at the 23 L/min, 5 mM peroxide condition, which had the highest estimated detention time (60.9 s HRT). All of the target compounds had the worst removals (16 to 71%) under the highest flow rate conditions (125 L/min, 10.4 s HRT), and all but one minimum removal (for GFB) came under the condition with the plug inserted (no air core present).
The removals of the hydroxyl radical actinometers were used to determine the approximate steady state [·OH] concentration under each reactor operating condition (Table S2) and the CT value ([·OH]·θ). The highest value of [·OH] was obtained for the 125 L/min, 6 mM peroxide condition, while addition of the plug significantly reduced [·OH] despite the similar flow rate and peroxide dose, again confirming the importance of the stabilized air core for reactor performance. There was little difference in [·OH] between the 3 mM and 6 mM peroxide doses at 45 L/min. Increased peroxide doses do not necessarily lead to linear increases in hydroxyl radical concentration, for example because of reactions between the hydroxyl radicals and the hydrogen peroxide. At high doses ·OH radicals can self scavenge (Buxton et al., 1988), but self-scavenging has not been reported for removal of PPCPs or herbicides in wastewater at dosages of 5 mM (Yuan et al., 2009). A study examining treatment of reactive dye wastewater did not observe self –scavenging until peroxide doses reached 50–100 mM (Arslan et al., 2000).
Removal of all target compounds across reactor conditions was strongly related to the CT values, with linear correlation coefficients between Ln(C0/C) for each compound and CT ranging from 0.898 to 0.998 (Table S1). This linearity is expected from eq. 2, and all of the values have α<0.02 for being drawn from a random sample with 6 observations. This calculation is useful for subsequent comparison with the non-target results.
3.2. Comparison of performance estimated using target and nontarget approaches
Applying the data filtration method described in the methods section and in the supplementary information yielded a consensus list of 3544 molecular features. Because this feature list was aligned across all reactor conditions, and it required that features be reliably observed under just one test condition, some of these features were only observed under one or two reactor conditions and may have rarely or never been present in the influent samples. Therefore, depending on the purpose of the analysis, the number of features may be less than 3544 in the subsequent discussion. A key goal of the research was to compare reactor performance metrics obtained from automated extraction of nontarget “features” with performance estimates from the more conventional targeted approach discussed thus far. Of course, these nontarget features will not typically have pure compound standards available, so performance needs to be based on instrument counts rather than on concentrations. Using area counts in influent and effluent samples as surrogates for C0 and C, respectively, poses significant risks. In particular, ion suppression in electrospray ionization might be expected to yield variations in area counts for identical concentrations in different background matrices (e.g., reactor influent and effluent). Similarly, the presence of the complex mixture of a wastewater effluent may cause problems for both chromatographic and mass spectrometric performance compared to clean laboratory samples.
As an initial effort to draw this comparison, the target compounds in the test samples were treated as unknowns. Eight of the twelve target compounds (CBZ, DCF, DEET, FXT, GFB, SFX, TCS, and TMP) were successfully matched to the database using the filtering procedure described above. The four target compounds that were not successfully identified by the nontarget filtering procedure (IBF, PYT, MTP, TTC) were not successfully detected (TTC), required a high concentration to pass filtering procedures (IBF, PYT), and/or had low extraction recoveries (MTP) (Table S3). The false negative rate of 33% for target compounds is similar to that observed in previous suspect screening studies (Moschet et al., 2013; Vergeynst et al., 2015). Nontarget removals for the eight successfully detected target compounds, calculated as 100*(A/A0−1), where A represents ion counts in the effluent and A0 is ion counts in the influent, are compared to target removals calculated using quantified concentrations across all reactor operating conditions in Fig. 2. A strong correlation is observed between the two performance metrics across the entire range of performance and reactor operating conditions (ρ=0.939 including TCS; ρ=0.950 excluding TCS).
Fig. 2:
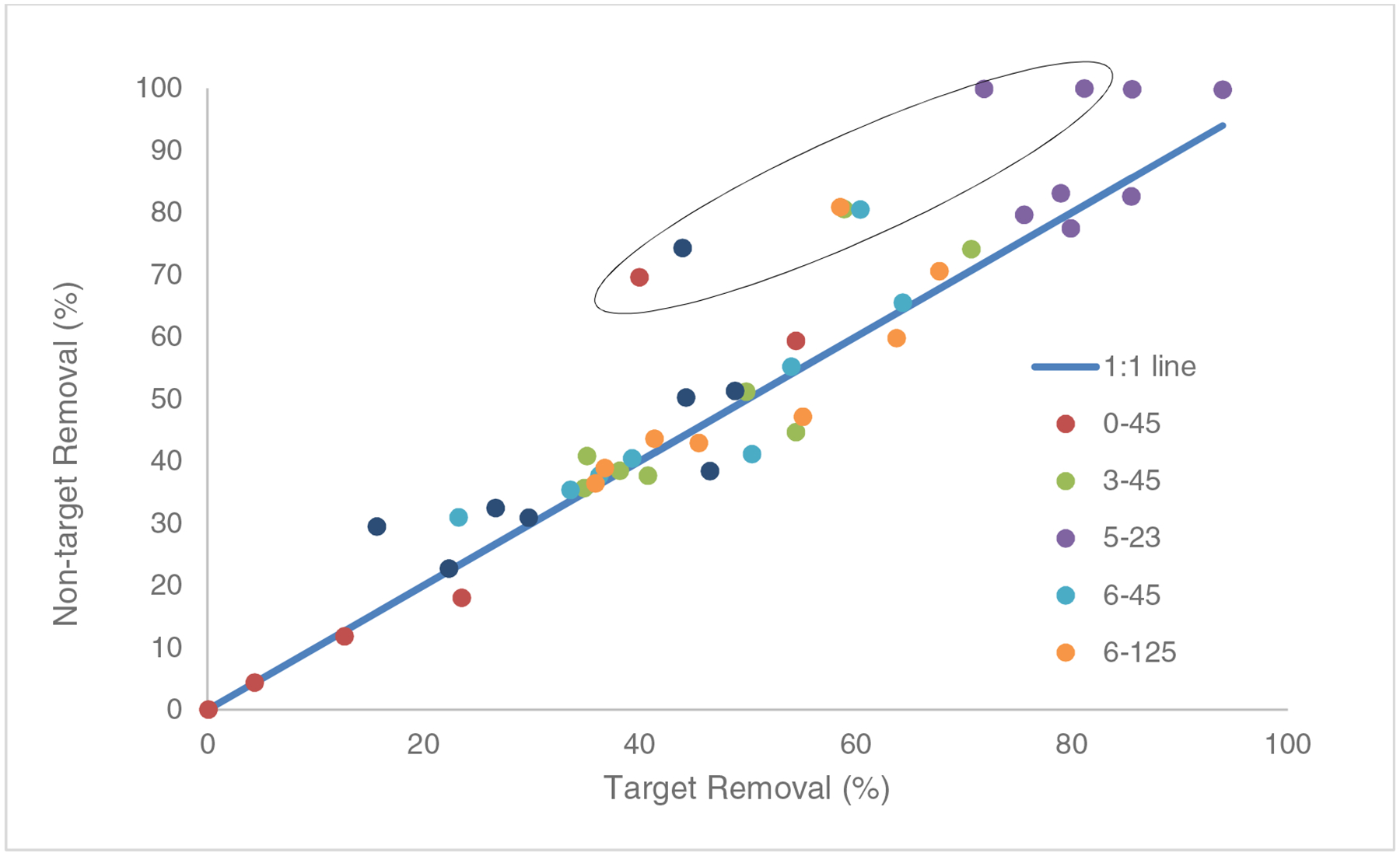
Comparison of the removal of 8 target compounds calculated using the average measured concentrations (Target removal, n= 4) and removal of target compounds calculated using averaged area counts (n=4) for molecular features matching the target compounds in the database (non-target removal). Symbol colors represent the reactor conditions as H2O2 concentration (mM) - flow rate (L/min). Triclosan data points are enclosed in the ellipse. The Pearson’s linear correlation coefficient of the data is ρ=0.939 including the TCS data and ρ=0.950 not including TCS data points).
All of the points representing TCS (identified by the ellipse in Fig. 2) plot significantly above the 1:1 line, and these comprise the majority of points with substantial deviation from the line. The reasons that TCS removal estimates are consistently higher using the nontarget approach are not completely clear, but they appear to relate to higher than expected ion counts in nontarget influent samples for TCS, biasing nontarget removal estimates upwards. The ratios of ion counts for TCS in nontarget mode to those in targeted mode averaged 2.2 (standard deviation 0.12) across the 20 replicates with successfully identified TCS in effluent samples (detections in each of 4 replicates at 5 treatment conditions). TCS was not detectable in any of the four nontarget replicates at the best performing condition of 23 L/min and 5mM added peroxide. The nontarget to target response ratio in the influent samples averaged 4.2 (standard deviation 0.37). We suspect that the nontarget counts for TCS include one or more contaminant ions incorrectly counted as TCS. Unfortunately, a software problem at the time of data collection prevented collection of the backup data that would allow for further investigation on this point.
Even with the problems associated with TCS, nontarget removal estimates are expected to serve as reasonable surrogates for target removals based on the overall results in Fig. 2, and removal estimates were calculated for all nontarget features that passed the filtering procedures. To limit analysis of nontarget removals to features reliably found in the influent, only features that were present in at least 3 of the 4 replicates in both batches of influent were considered (N=2,521). For these features, raw ion counts were averaged across replicates at each effluent condition and compared to the respective average influent ion count. A t-test was conducted between normalized averages of condition pairs to identify features that were significantly reduced in effluent samples compared to influent samples (α < 0.05). We considered two main methods of visualizing the removal data: comparisons of removal estimates across pairs of reactor operating conditions, and histograms displaying the distribution of removals at a single condition.
Two examples of the pairwise comparisons are shown in Fig. 3 (additional pairwise comparisons are provided in Figs. S4a–c). In the charts, the shading represents whether the feature was matched with the custom database (database), a molecular formula was generated for the feature (formula), or the feature could not be assigned a formula within the given parameters (“MFE” or molecular feature extractor, the algorithm used to identify potential compounds). The size of each data point is scaled to the statistical significance of non-zero fractional removal for the condition on the y-axis, which was selected to be the condition with the greatest removal for most features. Some features display negative removals because abundance was higher in the effluent than in the influent. This could be due to either experimental variability or production in the reactor. Points above the line indicate features more effectively removed under the operating condition on the y-axis, while points below the line represent features with greater removal under the condition on the x-axis. An even distribution of points across the 1:1 line indicates equivalent overall performance under the two conditions. Fig. 3a compares performance at two conditions at 45 L/min, one exposed to UV only and the other with 3 mM peroxide addition. Peroxide addition improves treatment effectiveness for the vast majority of features, as expected. A further increase from 3 mM to 6 mM of peroxide at 45 L/min provided little discernible improvement in performance (Fig. S4a). More subtle differences in performance can also be detected with the nontarget approach. Fig. 3b compares performance at the two 125 L/min conditions, both with 6 mM peroxide additions. Removal of the air core by installing the plug decreased reactor performance even though the HRT is slightly longer, presumably because of reduced UV penetration. This finding is consistent with targeted removal comparisons shown in Fig. 2. Overall, the pairwise non-target comparisons consistently showed that the condition with the longest HRT (23 L/min, 5 mM) had the best performance (Fig. S4c).
Fig. 3:
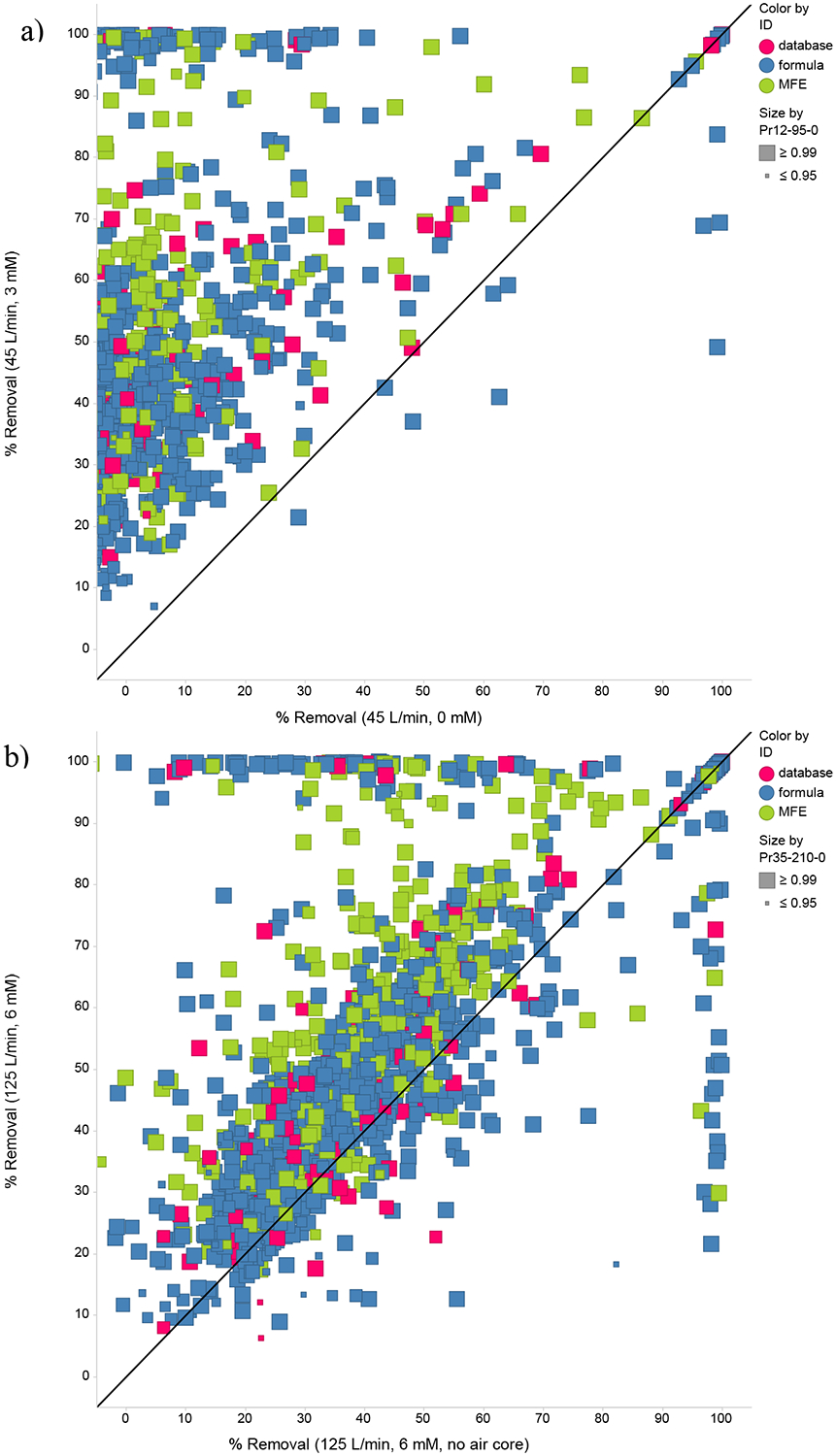
Pairwise comparisons of non-targeted removal at a) 45 L/min, 3 mM H2O2 versus 45 L/min, 0 mM H2O2 (air core present). b) 125 L/min, 6 mM H2O2 with and without the air core. Symbol color indicates whether the feature was a database match “database”, was assigned a molecular formula “formula”, or was unmatched “MFE”. Size indicates the probability of removal (1-α) of that feature under the reactor condition on the y-axis
A second method of analyzing performance of a treatment condition is to assess the statistical distribution of removal estimates. For these comparisons, the significance level was fixed at α = 0.05 and the maximum level of performance at this significance level was determined for each feature. Fig. 4 provides an example of this type of performance assessment for the 23 L/min, 5 mM peroxide case. Fig. 4a depicts the comparison for the nontarget features and Fig. 4b does the same for the target data. All of the target compounds displayed more than 50% removals under this condition. While most (85%) of the nontarget features had removals above 50%, the mode of nontarget removal estimates is still near zero. Among compounds with discernible nontarget removals, the distribution of removal efficiencies is very similar between the target and nontarget approaches. This similarity was observed across all operating conditions (Figs. S6a–e). The percentage of compounds (target) or features (nontarget) removed to specified performance levels (>0% or >50%) under each test condition is summarized in Table S7. These percentages are always lower for the nontarget approach.
Fig. 4:
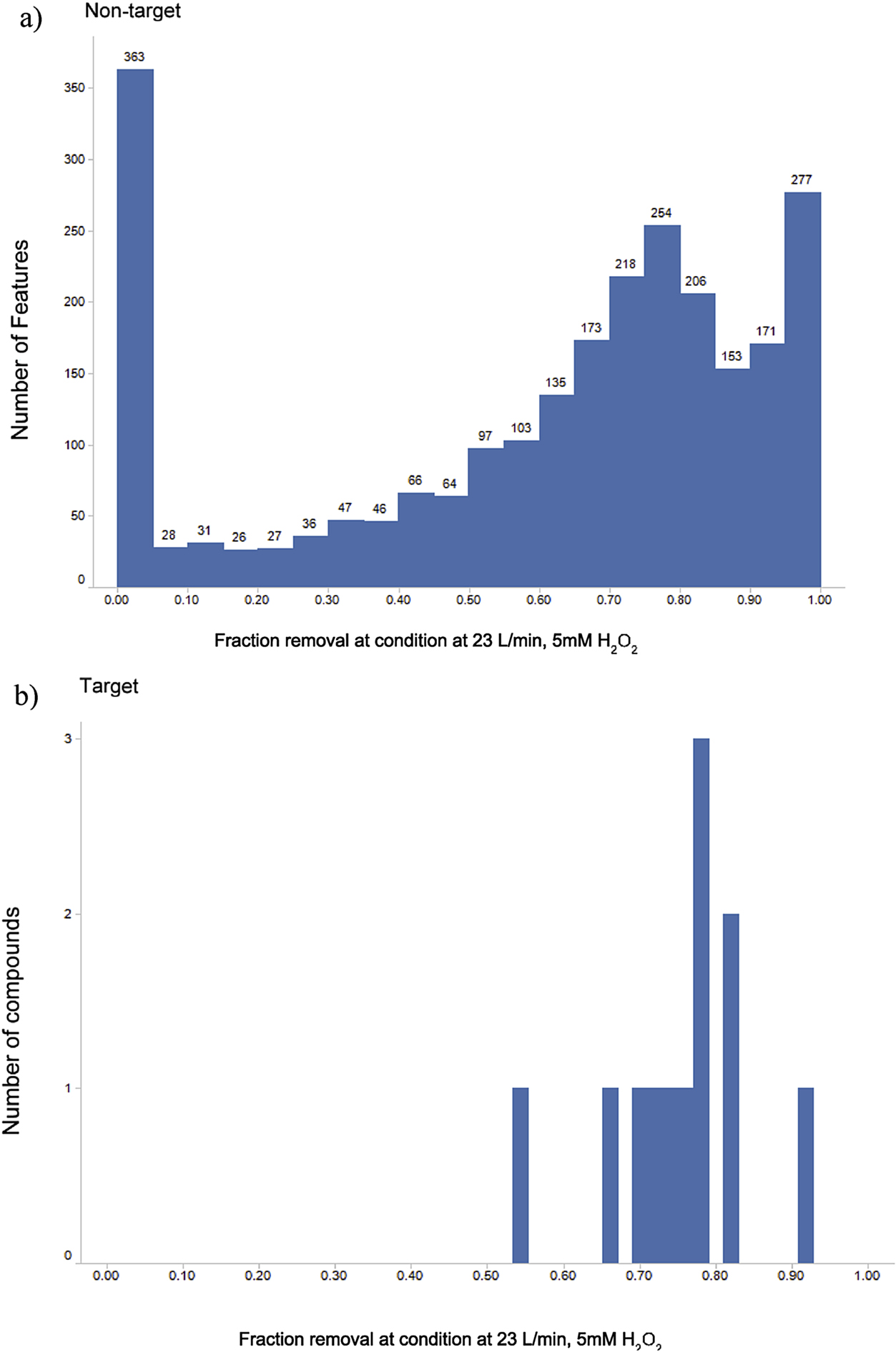
Fractional removal of features at α=0.05 significance in the 23 L/min, 5 mM H2O2 condition for (a) nontarget features and (b) target compounds.
The nontarget removal analysis can also provide some basic insight into the nature of the compounds that are easy or hard to remove, even without definitive identifications. For example, consider the 29 nontarget features with removals above 15% (α=0.05) and formula match scores above 90% under the UV only treatment condition. Two of these features were on our target list (DCF, SFX). The other 27 formulas were entered into Chemfinder to search for structures consistent with the formulas. Only 5 of the 27 formulas were present in Chemfinder, suggesting that the majority of these compounds are not industrially important, possibly because they are human or microbial metabolites of commercial chemicals. As an example of how this type of analysis can provide information not easily detected in other ways, one of the nontarget features with good UV removal, C13H9Cl3O2, was analyzed further. This feature exhibited >99% removal in the UV only condition and had an MS formula match score of 99.5. The eight Chemfinder matches for compound are shown in Fig. S7. It is immediately obvious that 6 of the 8 matches have structures similar to triclosan, one of our target chemicals. Although the TCS analogues in Fig. S7 involve the exchange of a carboxyl for a hydroxyl group (net addition of CO to TCS), which to our knowledge is not a byproduct of either biological or chemical degradation of TCS, various transformations of TCS or one of its metabolites could produce the observed empirical formula. In particular, biodegradation of the primary urinary metabolite of TCS, TCS-glucuronide, or one of its key wastewater byproducts, methyl-TCS, could lead to such structures. All of the structures in Fig. S7 involve chlorinated aromatic rings that would be expected to be subject to direct photolysis (1,2,3-trichlorobenzene has ϕε = 58.52 L/ein cm, and 1,4-dichlorobenzene has ϕε = 70.8 L/ein cm), consistent with the effective removal of the feature in the UV only condition. Overall, 20 of the 27 compounds that were removed significantly by UV, that were not present in the database, and that had confident formula matches contained at least one halogen atom (Cl and/or F). It is quite plausible that these halogenated species would be reistant to mineralization, might be subject to bioaccumulation, and might be responsible for a portion of effluent toxicity. Methods that can quantify the ability of treatment systems to remove such constituents, even in the absence of unequivocal identification of the chemical structure, will provide a more holistic view of treatment effectiveness.
Previous studies have suggested that performance of AOPs for the removal of trace organics can be evaluated using surrogates including DEET and PYT to represent alkyl aromatics (Dickenson et al., 2009). In our best performing condition, neither compound was removed to below our detection limit or by more than 90%. Reactor and experimental design need further optimization before any feasible large-scale implementation. Since the condition with the longest HRT (largest UV dose and highest OH radical contact time) had the best performance, and increases in the peroxide dosage beyond 3 mM had no observable effect, reactor design or operation should be modified to produce higher HRT, for example by running two reactors in series to increase UV exposure; HRT and peroxide dose would then need to be re-optimized at economically feasible concentrations.
3.3. Transformation product formation
Degradation of organics is the goal of AOPs. Concomitantly, transformation products are formed, in many cases transiently. Degradates of synthetic chemicals have become more common in some environmental systems than parent compounds (Boxall et al., 2004). Targeted analysis requires pre-selection of TPs for analysis based on prior laboratory degradation studies, which are not available for many compounds. HR-MS methods offer some additional approaches for detecting TPs, even if published degradation information is not available. In a non-targeted workflow, candidates (defined by mass and retention time) can be refined to those only present in effluent or those exhibiting a consistent increase in abundance. The reactor operating condition with the best overall removal had 88 features with higher abundance in the effluent, while the UV only treatment had 698 (p ≤ 0.05). The range of molecular masses of transformation product suspects was higher in UV only (1522 versus 1048 daltons). One potential explanation for this difference is that the addition of peroxide achieved greater degradation, rendering compounds nondetectable by our method. Non-target analysis can further enable structure elucidation through careful collision induced dissociation experiments.
3.3.1. Mass Defect filtering for transformation product identification
One method utilizing both knowledge of influent PPCPs and high mass resolution is mass defect filtering. This filtering strategy has been used to identify pharmaceutical metabolites by HR-MS during pharmacokinetic studies. The filter is based on the observation that the mass defect of metabolites rarely changes from that of the parent molecule by more than ±50 mDa (H. Zhang et al., 2009). Templates for common biological reactions, many oxidative, note the shift in nominal mass and the related mass defect shift (H. Zhang et al., 2009). Filters were calculated for the target compounds and applied to features in the effluent only and features with intensities that increased by at least 1.5x in any effluent condition. Ten possible byproducts were identified and CID experiments were performed. Two had promising collision spectra compared with proposed fragmentation patterns predicted using Molecular Structure Correlator (Agilent MSC). 4-nitro-sulfamethoxazole (Fig. 5b) is a known metabolite of SFX (Bonvin et al., 2013), and a commercial standard was available. Its identity was confirmed with a matching RT and MS/MS spectra Fig. S8. Although, as a pharmaceutical metabolite, it may be in raw wastewater, the feature was only present in the effluent conditions and for the conditions where it was present, it showed a negative spearman correlation with SFX abundance (r=−0.9, p=0.08) offering further evidence of its production via SFX degradation. It is possible that it was not detected in the influent and UV only treatments because of matrix suppression, but the correlation offers reasonable evidence of its formation by the degradation of SFX in the AOP reactor.
Fig. 5:
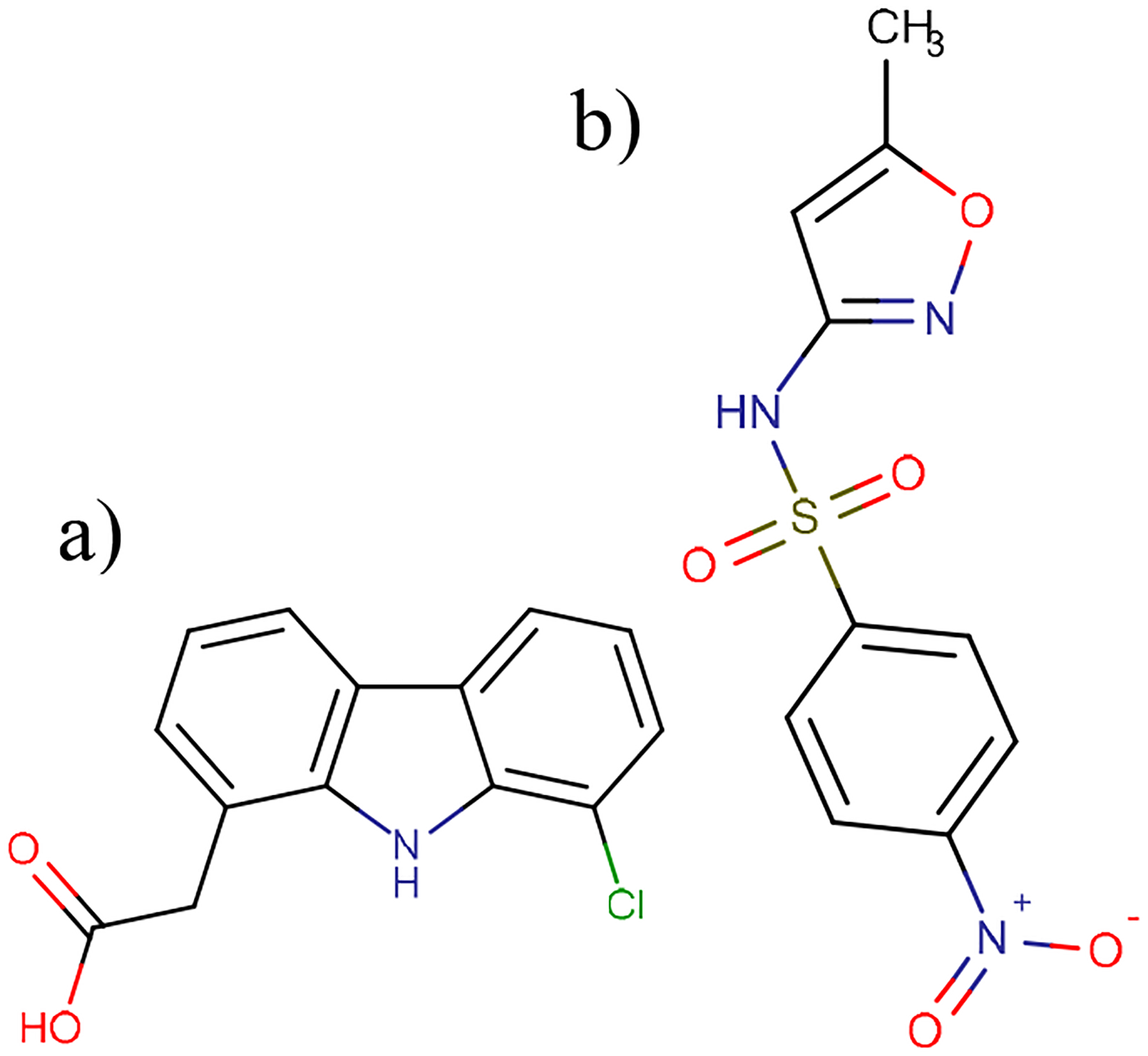
a)2-(8-chloro-9H-carbazol-1-yl)acetic acid and b) 4-nitro-sulfamethoxazole
The second compound, 2-(8-chloro-9H-carbazol-1-yl) acetic acid (D1) was not confirmed because an analytical standard was not commercially available. However, there is convincing evidence of its presence. Its accurate mass was consistent with the loss of a Cl and H from DCF, matching its proposed formula of C14H10ClNO2. A compound with this formula has been observed as a DCF photolysis product (Lekkerkerker-Teunissen et al., 2012; Salgado et al., 2013). Salgado et al., (2013) identified the structure seen in Fig. 5a. It was consistently present only in the UV only treatment and spottily in the other effluent conditions (peroxide dosed treatments). D1’s RT was slightly shifted forward (8.9 for DCF and 8.3 for D1) indicating an increase in polarity (log Kow change from 4.0 to 3.37) coinciding with the loss of a Cl.
While mass defect filtering is a promising tool for transformation product identification, it is mainly limited to degradation products that have undergone minimal transformation. To identify compounds further along the reaction pathways, in silico prediction of byproducts for known contaminants could be added to the screening process and putatively identified (Kern et al., 2009).
4.0. Conclusions
Non-targeted analysis can provide a more comprehensive view of reactor performance. Automated feature extraction algorithms successfully recovered 2/3 of the target compounds and allowed estimates of removal to be generated that were highly correlated with those based on target compound quantitation. This suggests that reactor performance estimates can be generated and analyzed for thousands of compounds, even though many of the compounds are not synthesized industrially or available commercially as standards. Because many of the compounds left out of targeted approaches have the potential to contribute to environmental persistence, bioaccumulation, and/or toxicity, this is a potentially significant innovation in reactor assessment.
In the case of the pilot scale reactor examined in this study, concurrently collected targeted and non-targeted data support the conclusion that the longest reactor retention time and a median peroxide dose (of those tested) provided the best removal of microconstituents. Analysis showed the best way to increase this AOP reactor’s performance was to increase the UV dose by increasing the reactor retention time. Transformation product evaluation can be refined to a list of compounds found only in the effluent.
Only 8% of target compounds exhibited removals above 50% in the UV only treatment. The best performing condition had 100% of target compounds with removals above 50%. The non-target results were qualitatively consistent with the targeted results, but indicate that target evaluation may overestimate reactor performance. UV only treatment removed 4% of constituents by more than 50%; removal increased to 85% in the best performing condition. Nontarget data suggests that a large number of organics are not effectively removed by AOP, but there is no data to indicate that these microconstituents would be a problem for human or environmental health. Ideally analysis would be conducted concurrently with some type of biological effects measure, such as in vitro bioassays to identify non-removed features with potential toxicological significance.
Supplementary Material
Highlights:
UV/H2O2 AOP process evaluated in tandem by targeted and non-targeted methods.
Strong correlation between removals calculated using non-target peak counts and traditional quantitation methods.
Both approaches gave consistent rankings of different reactor conditions.
The non-target method consistently gave broader and somewhat lower removal efficiency distributions than the target method.
Target results may overestimate reactor performance.
Acknowledgements
Support for this study was provided by in part by National Science Foundation Graduate Research Fellowship, Grant No. 1149997 and by the Superfund Research Program of the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number P42 ES004699. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. We would like to thank Professor Bassam Younis for allowing use of his reactor and Laura Mahoney for her reactor operating expertise and assistance in sampling. Daniel Cutherburtson (Agilent) provided invaluable assistance with Mass Profiler Professional. Jenny Mital supplied valuable statistical help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Suppementary materal
Supplementary material contains additional charts and methods.
REFERENCES
- APHA, 1998. Standard Methods for the Examination of Water and Wastewater, 20th ed. American Public Health Association, Washington DC. [Google Scholar]
- Arslan I, Balcioglu I, Tuhkanen T, Bahnemann D, 2000. H2O2/UV-C and Fe2+/H2O2/UV-C versus TiO2/UV-A treatment for reactive dye wastewater. J. Environ. Eng. Asce 126, 903–911. doi: 10.1061/(ASCE)0733-9372(2000)126:10(903), 903–911 [DOI] [Google Scholar]
- Bonvin F, Omlin J, Rutler R, Schweizer WB, Alaimo PJ, Strathmann TJ, McNeill K, Kohn T, 2013. Direct photolysis of human metabolites of the antibiotic sulfamethoxazole: Evidence for abiotic back-transformation. Environ. Sci. Technol 47, 6746–6755. doi: 10.1021/es303777k [DOI] [PubMed] [Google Scholar]
- Boxall AB, Sinclair CJ, Fenner K, Kolpin D, Maund SJ, 2004. When Synthetic Chemicals Degrade in the Environment. Environ. Sci. Technol 38, 368A–375A. doi: 10.1021/es040624v [DOI] [PubMed] [Google Scholar]
- Buxton GV, Greenstock CL, Helman WP, Ross AB, 1988. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (OH/O−) in Aqueous Solution. At. Energy 17, 513–886. doi: 10.1063/1.555805 [DOI] [Google Scholar]
- Diaz R, Ibáñez M, Sancho JV, Hernández F, 2013. Qualitative validation of a liquid chromatography-quadrupole-time of flight mass spectrometry screening method for organic pollutants in waters. J. Chromatogr. A 1276, 47–57. doi: 10.1016/j.chroma.2012.12.030 [DOI] [PubMed] [Google Scholar]
- Dickenson ERV, Drewes JE, Sedlak DL, Wert EC, Snyder SA, 2009. Applying surrogates and indicators to assess removal efficiency of trace organic chemicals during chemical oxidation of wastewaters. Environ. Sci. Technol 43, 6242–6247. doi: 10.1021/es803696y [DOI] [PubMed] [Google Scholar]
- Dickenson ERV, Snyder SA, Sedlak DL, Drewes JE, 2011. Indicator compounds for assessment of wastewater effluent contributions to flow and water quality. Water Res. 45, 1199–1212. doi: 10.1016/j.watres.2010.11.012 [DOI] [PubMed] [Google Scholar]
- Goñi-urriza M, Capdepuy M, Arpin C, Raymond N, Caumette P, Quentin C, 2000. Impact of an Urban Effluent on Antibiotic Resistance of Riverine Enterobacteriaceae Impact of an Urban Effluent on Antibiotic Resistance of Riverine Enterobacteriaceae and Aeromonas spp. Appl. Environ. Microbiol 66, 125–132. doi: 10.1128/AEM.66.1.125-132.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard PH, Muir DCG, 2011. Identifying New Persistent and Bioaccumulative Organics Among Chemicals in Commerce II: Pharmaceuticals. Environ. Sci. Technol 45, 6938. doi: 10.1021/es201196x [DOI] [PubMed] [Google Scholar]
- Howard PH, Muir DCG, 2013. Identifying new persistent and bioaccumulative organics among chemicals in commerce. III: Byproducts, impurities, and transformation products. Environ. Sci. Technol 47, 5259–5266. doi: 10.1021/es4004075 [DOI] [PubMed] [Google Scholar]
- Hug C, Ulrich N, Schulze T, Brack W, Krauss M, 2014. Identification of novel micropollutants in wastewater by a combination of suspect and nontarget screening. Environ. Pollut 184, 25–32. doi: 10.1016/j.envpol.2013.07.048 [DOI] [PubMed] [Google Scholar]
- Iwane T, Urase T, Yamamoto K, 2001. Possible impact of treated wastewater discharge on incidence of antibiotic resistant bacteria in river water. Water Sci. Technol 43, 91–99. [PubMed] [Google Scholar]
- Jin S, Mofidi A, Linden K, 2006. Polychromatic UV Fluence Measurement Using Chemical Actinometry, Biodosimetry, and Mathematical Techniques. J. Environ. Eng 132, 831–841. doi: 10.1061/(ASCE)0733-9372(2006)132:8(831) [DOI] [Google Scholar]
- Kern S, Fenner K, Singer HP, Schwarzenbach RP, Hollender J, 2009. Identification of transformation products of organic contaminants in natural waters by computer-aided prediction and high-resolution mass spectrometry. Environ. Sci. Technol 43, 7039–7046. doi: 10.1021/es901979h [DOI] [PubMed] [Google Scholar]
- Kim I, Yamashita N, Tanaka H, 2009. Photodegradation of pharmaceuticals and personal care products during UV and UV/H2O2 treatments. Chemosphere 77, 518–525. doi: 10.1016/j.chemosphere.2009.07.041 [DOI] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT, 2002. Pharmaceuticals, Hormones and Other Organic Wastewater Contaminants in U.S. Streams, 1999–2000: A National Reconnaissance. Environ. Sci. Technol 36, 1202–1211. doi: 10.1021/es011055j [DOI] [PubMed] [Google Scholar]
- Latch DE, Packer JL, Stender BL, VanOverbeke J, Arnold WA, McNeill K, 2005. Aqueous photochemistry of triclosan: formation of 2,4-dichlorophenol, 2,8-dichlorodibenzo-p-dioxin, and oligomerization products. Environ. Toxicol. Chem 24, 517–525. doi: 10.1897/04-243R.1 [DOI] [PubMed] [Google Scholar]
- Lekkerkerker-Teunissen K, Benotti MJ, Snyder SA, Van Dijk HC, 2012. Transformation of atrazine, carbamazepine, diclofenac and sulfamethoxazole by low and medium pressure UV and UV/H 2O 2 treatment. Sep. Purif. Technol 96, 33–43. doi: 10.1016/j.seppur.2012.05.005 [DOI] [Google Scholar]
- Miège C, Choubert JM, Ribeiro L, Eusèbe M, Coquery M, 2009. Fate of pharmaceuticals and personal care products in wastewater treatment plants Conception of a database and first results. Environ. Pollut 157, 1721–1726. doi: 10.1016/j.envpol.2008.11.045 [DOI] [PubMed] [Google Scholar]
- Moschet C, Piazzoli A, Singer H, Hollender J, 2013. Alleviating the reference standard dilemma using a systematic exact mass suspect screening approach with liquid chromatography-high resolution mass spectrometry. Anal. Chem 85, 10312–10320. doi: 10.1021/ac4021598 [DOI] [PubMed] [Google Scholar]
- Nurmi J, Pellinen J, Rantalainen AL, 2012. Critical evaluation of screening techniques for emerging environmental contaminants based on accurate mass measurements with time-of-flight mass spectrometry. J. Mass Spectrom 47, 303–312. doi: 10.1002/jms.2964 [DOI] [PubMed] [Google Scholar]
- Roefer P, Snyder S, Zegers R, Rexing DJ, Fronk JL, 2014. Endocrine-disrupting chemicals in a source water. Am. Water Work. Assoc 92, 52–58. [Google Scholar]
- Rosario-Ortiz FL, Wert EC, Snyder SA, 2010. Evaluation of UV/H2O2 treatment for the oxidation of pharmaceuticals in wastewater. Water Res 44, 1440–1448. doi: 10.1016/j.watres.2009.10.031 [DOI] [PubMed] [Google Scholar]
- Salgado R, Pereira V, Carvalho G, Soeiro R, Gaffney V, Alameida C, Cardoso V, Ferreira E, Benoliel M, Ternes T, Oehmen A, Reis M, Noronha J, 2013. Photodegradation kinetics and transformation products of ketoprofen, diclofenac, and atenolol in pure water and treated wastewater. J. Hazard. Mater 244, 516–527. doi: 10.1016/j.hazmat.2012.10.039 [DOI] [PubMed] [Google Scholar]
- Watkinson AJ, Micalizzi GB, Graham GM, Bates JB, Costanzo SD, 2007. Antibiotic-resistant Escherichia coli in wastewaters, surface waters, and oysters from an urban riverine system. Appl. Environ. Microbiol 73, 5667–5670. doi: 10.1128/AEM.00763-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wols BA, Hofman-Caris CHM, 2012. Review of photochemical reaction constants of organic micropollutants required for UV advanced oxidation processes in water. Water Res. 46, 2815–2827. doi: 10.1016/j.watres.2012.03.036 [DOI] [PubMed] [Google Scholar]
- Younis BA 2014. Demonstrating a vortex technology to disinfect wastewater with ultraviolet light, California Energy Commission, Publication Number CEC-500-2015-036. [Google Scholar]
- Yuan F, Hu C, Qu J, Yang M, 2009. Degradation of selected pharmaceuticals in aqueous solution with UV and UV/H2O2. Water Res. 43, 1766–1774. doi: 10.1016/j.watres.2009.01.008 [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang D, Ray K, Zhu M, 2009. Mass defect filter technique and its applications to drug metabolite identification by high-resolution mass spectrometry. J. Mass Spectrom 44, 999–1016. doi: 10.1002/jms.1610 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Marrs CF, Simon C, Xi C, 2009. Wastewater treatment contributes to selective increase of antibiotic resistance among Acinetobacter spp. Sci. Total Environ 407, 3702–3706. doi: 10.1016/j.scitotenv.2009.02.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


