Abstract
Feeling emotionally close to others during social interactions is a ubiquitous and meaningful experience that can elicit positive affect. The present study integrates functional magnetic resonance imaging (fMRI) and ecological momentary assessment (EMA) to investigate whether neural response to social reward (1) is related to the experience of emotional closeness and (2) moderates the association between emotional closeness and positive affect during and following social interactions. In this study, 34 typically developing adolescents (ages 14–18) completed a social reward fMRI task, a monetary reward fMRI task, and a two-week EMA protocol regarding their social and affective experiences. Adolescents with greater right posterior superior temporal sulcus/temporoparietal junction (pSTS/TPJ) response to social reward reported greater mean momentary emotional closeness. Neural response to social reward in the right pSTS/TPJ moderated how strongly momentary emotional closeness was associated with both concurrent positive affect and future peak happiness, but in different ways. Although emotional closeness had a significant positive association with concurrent positive affect among adolescents at both high and low right pSTS/TPJ response based on a follow-up simple slopes test, this association was stronger for adolescents with low right pSTS/TPJ response. In contrast, emotional closeness had a significant positive association with future peak happiness among adolescents with high right pSTS/TPJ response but not among those with low right pSTS/TPJ response. These findings demonstrate the importance of neural response to social reward in key social processing regions for everyday experiences of emotional closeness and positive affect in the context of social interactions.
Keywords: social reward, positive affect, social interaction, emotional closeness, temporoparietal junction, posterior superior temporal sulcus
Positive affect promotes well-being and protects against maladaptive outcomes, such as depression (Lyubomirsky et al., 2005). Social interactions are meaningful and ubiquitous experiences that can elicit positive affect and engage reward circuitry (Kahneman et al., 2004); however, people differ in how they perceive, respond to, and benefit from rewarding social interactions (Healey et al., 2014). For instance, some may respond to pleasant interactions (e.g., receiving social approval) with heightened cognitive engagement and greater neural response in associated social circuitry, which may contribute to a greater positive affective response. Others might find the same experience much less pleasant or even aversive, which may not elicit significant positive affect. Thus, examining individual differences in neural response to social rewards may help elucidate how people differ in their experience of positive affect during everyday pleasant interactions.
Social Reward in Adolescence
Adolescence is a developmental period during which reward systems integrating behavior, brain function, and experience undergo dramatic changes in the service of developmental tasks such as exploration, learning about rewards, and individuation (Crone & Dahl, 2012). For example, reward pursuit (Somerville et al., 2010) and responsivity (Ernst et al., 2005; Steinberg, 2008) peak in adolescence. Adolescents’ activation in neural reward circuitry (e.g., increased ventral striatum, decreased medial prefrontal cortex [mPFC]) differs from that in children and adults (e.g., Bjork et al., 2004; Ernst et al., 2005; Forbes et al., 2010). Alterations in neural reward circuitry in adolescence are related to potentially maladaptive outcomes such as the development of substance use (Plichta & Scheres, 2014), other impulsive behaviors (Hyman et al., 2006), and depression (Forbes & Dahl, 2012). Differential neural and behavioral response to reward in adolescence also has a positive side, however, and is not necessarily maladaptive. Depending on the nature of the reward and the social context, such as maternal presence, greater ventral striatum reactivity is related to prosocial and adaptive outcomes (e.g., family orientedness, less risk taking; Telzer, 2016). For instance, heightened ventral striatum reactivity to donating money to family is related to decreases in depression, whereas heightened ventral striatum reactivity to risky rewards is related to increases in depression (Telzer, Fuligni, Lieberman, & Galván, 2014).
Most research on reward in adolescence has focused on non-social rewards (e.g., monetary reward). However, social contexts have important and potentially specific influences on brain and behavior. For instance, adolescents—compared with children and adults—are more likely to engage in risky behaviors (Steinberg, 2008) and exhibit greater ventral striatum activation in response to reward (Smith et al., 2015; Somerville et al., 2010) when in the presence of peers than when alone. In addition, adolescents have greater sensitivity and altered neural response to acceptance and rejection by peers compared to children and adults (Pfeifer & Blakemore, 2012). Thus, social reward is an essential component of reward processing in adolescence. In fact, social rewards are arguably the most important class of reward during adolescence given that adolescence is characterized by a preoccupation with attaining social status, seeking friendships, and fostering intimacy with peers (Davey et al., 2008). Alterations in response to social reward are associated with maladaptive outcomes, such as depression vulnerability (Monk et al., 2008; Olino et al., 2015), social anhedonia (Healey et al., 2014), and social anxiety disorder (Richey et al., 2014). Social reward has been investigated by examining neural response to various stimuli, including standardized positive peer feedback (Davey et al., 2010; Guyer et al., 2012; Silk et al., 2012), presentation of happy and/or familiar faces (Lin et al., 2012), provision of praise (Izuma et al., 2008), and simulated increases in social status (Zink et al., 2008).
Social Brain Network in Adolescence
Given the importance of social rewards in adolescence, the social brain network—which is a collection of brain regions that are involved in understanding others and social interactions (Blakemore, 2008; Burnett et al., 2011)—may play an important role in adolescents’ positive affect and reward processing. Processes associated with the social brain network include mentalizing, which refers to understanding the mental states of others and is often used synonymously with theory of mind and perspective-taking, and emotion regulation during social interactions. Key brain regions of the social brain network that are associated with mentalizing include the temporoparietal junction (TPJ), posterior superior temporal sulcus (pSTS), temporal poles, and medial prefrontal cortex (mPFC); those associated with emotion regulation in social interactions include the mPFC and ventrolateral prefrontal cortex (VLPFC; Burnett et al., 2011; Frith & Frith, 2003). Changes in neural activation to mentalizing (Gunther Moor et al., 2012) and peer acceptance and rejection (Pfeifer & Blakemore, 2012) occur over the course of adolescence. For instance, in the transition from adolescence to adulthood, there tends to be a linear decrease in mPFC activity and increase in pSTS/TPJ activity during mentalizing and social decision-making tasks, as well as a linear increase in VLPFC activity in response to social rejection (Burnett et al., 2011; Crone & Dahl, 2012). These changes may reflect an increase in the use and ability to consider the thoughts of others in social situations and an increase in regulating negative emotion in response to social rejection as adolescents get older and transition into adulthood. Importantly, effective mentalizing and emotion regulation during social interactions improve social likeability (Gross & John, 2003; Lopes et al., 2005), which is a highly salient social reward in adolescence (Davey et al., 2008).
Emotional Closeness as a Naturalistic Social-Affective Experience
Emotional closeness refers to feeling close and connected to others (i.e., the perception of emotional closeness). There have also been conceptualizations of emotional closeness that include objective components, such as engagement in emotionally intimate behaviors (e.g., disclosure of intimate thoughts, receipt of verbal and physical affection, the provision/receipt of emotional support; Flores & Berenbaum, 2014). Importantly, emotional closeness is a key component of intimate relationships that helps fulfill the basic human need of belongingness (Baumeister & Leary, 1995). During social interactions, feelings of closeness are associated with having heightened positive affect during the past hour’s most positive event (i.e., peak positive affect; Morgan et al., 2016). Following pleasurable events, describing the emotional experience of those events to others also enhances positive affect (Rimé, 2009). Notably, positive affect enhancement most strongly occurs during social sharing when close others engage constructively to further discuss the meaning or details of the events (Gable et al., 2004). Emotional closeness also reduces the distressing experiences of daily worry and depressive symptoms among people who highly desire emotional closeness (Flores & Berenbaum, 2014). Altogether, emotional closeness is an important social-affective experience that enhances social relationship quality, promotes well-being, and elicits positive affect.
The Role of Social Brain Regions in Social Reward, Emotional Closeness, and Positive Affect during Social Interactions
Social brain regions may play a role in how adolescents’ social interactions elicit positive affect. There is evidence that perceptions of emotional closeness may be associated with response in social brain regions. For example, emotional closeness modulates response in social brain regions implicated in empathy (e.g., temporal poles) when viewing a friend experiencing social rejection (Beeney et al., 2011). Although this finding was in the context of a negative peer event, it is plausible that emotional closeness may also be related to neural response to social rewards (e.g., positive peer feedback). Also, this finding is an example of emotional closeness having an impact on social brain functioning. It is worth noting though that the association between emotional closeness and social brain regions may be bidirectional, such that function in neural social circuitry may also facilitate the experience of emotional closeness (e.g., recruiting brain regions key to empathy may contribute to someone feeling emotionally close to another person). Also, neural response in social brain regions may reflect interpersonally relevant personality traits (e.g., sociability, agreeableness) and trait-like tendencies in how an individual processes socially rewarding contexts (e.g., engaging in greater social processing), which may alter an affective response to a positive social interaction. Thus, individual differences in neural response in social brain regions to objective social rewards may moderate the positive association between emotional closeness and positive affect in adolescents’ everyday lives.
The Present Study
The present study examined the association of adolescents’ neural response to social reward with their experience of emotional closeness and positive affect in natural settings. The primary hypothesis was that adolescents who demonstrate greater response in social reward circuitry will also experience greater emotional closeness in their everyday lives. Although the tendency to experience greater emotional closeness during social interactions may include factors that are not socially specific (e.g., high trait positive affect), they may also include socially specific factors (e.g., high valuing of and experience/comfort with emotionally intimate interactions). Thus, individual differences in emotional closeness may be distinctly related to social reward despite significant overlap in the circuitry processing social and monetary reward (Izuma et al., 2008). Although the focus of the present study is on social reward, we tested an exploratory hypothesis that emotional closeness would be specifically associated with and neural response to social but not monetary reward. The present study was not designed to test this exploratory hypothesis but provided a preliminary means to address it, and the intention of including analyses with monetary reward task was to help inform future studies testing this hypothesis. The secondary hypothesis of the present study was that function in regions identified in the initial hypothesis—that is, regions showing an association between emotional closeness and neural response to social reward—will moderate the association between naturalistic emotional closeness and positive affect (both concurrent positive affect and future peak positive affect), such that the associations between emotional closeness and positive affect will be stronger among those with greater neural response than those with lower neural response to social reward. This moderation is expected, as greater recruitment of social brain regions during social rewards may facilitate greater affective responses to emotionally close interactions.
Method
Participants included in the analyses were 34 typically developing adolescents, ages 14–18 (M= 16.3±1.5yrs; 65% female; 3% Hispanic/Latino; 79% White, 15% Black, and 6% other or multiracial), with no history of psychiatric or serious medical problems, as confirmed by brief interview. Additional participants were excluded due to not participating in the ecological momentary assessment (EMA; n = 8), completing fewer than 50% of the EMA prompts (n = 12), not completing the fMRI tasks (due to technical difficulties, claustrophobia, or experiencing a concussion between visits; n = 5), or having fMRI data with insufficient coverage (n = 2). The demographics of the excluded participants were not significantly different than included participants (Age: M= 16.1±1.2yrs, Mdiff = 0.21, t(59) = 0.60, p = .552; Gender: 43% female, χ2 (1) = 3.48, p = .062; Ethnicity: 0% Hispanic/Latino, we were unable to run a chi-square test due to there being one included participant and zero excluded participants identifying as Hispanic/Latino; Race: 63% White, 26% Black, and 11% other or multiracial, χ2 (2) = 2.03, p = .362). Exclusion was also unrelated to EMA variables (Mean Closeness, Mdiff = −0.27, t(53) = −1.46, p = .150; Mean Positive Affect, Mdiff = −0.04, t(54) = −0.22, p = .828; Mean Peak Happiness, Mdiff = −0.14, t(54) = −1.09, p = .280; Percent of Time with Someone, Mdiff = 8.2%, t(54) = 1.68, p = .099). One participant was excluded from any analyses that included the social reward fMRI task due to being an outlier (i.e., ±2 standard deviations from the mean). One participant whose neural response was an outlier on the monetary reward fMRI task and four participants who indicated that they suspected that the monetary reward fMRI task had predetermined outcomes were excluded from analyses that included that task. They were not excluded from other analyses. Thus, 33 participants were included in the primary social reward analyses and 29 participants were included in the exploratory monetary reward analyses. Given that there is not a widely accepted gold standard to conduct a power analysis for multilevel models, we conducted a power analysis for a related statistical approach (i.e., repeated measures ANOVA). Using G*Power Version 3.1.9.2 (Faul, Erdfelder, Lang, & Buchner, 2007), we found that a sample size of 32 with at least 14 observations for each participant provides 80% power to detect a medium-sized effect of a within-between interaction at α < .05. Participants completed an EMA protocol, a social reward fMRI task, and a monetary reward fMRI task. The University of Pittsburgh IRB approved all research procedures, and written informed consent was obtained from each participant and a parent/guardian.
Measures
Ecological momentary assessment (EMA).
Participants received 28 phone calls to cellular phones over a two-week period (two calls on Thursdays, Fridays, and Mondays after school hours; and four calls throughout the day on Saturdays and Sundays, at semi-random times). They responded to a variety of questions related to their affect, behavior, and social context. The included participants (who all completed at least 50% of the prompts) completed an average of 72.7% of the EMA prompts (M = 20.35, SD = 3.66, range = 14–27).
Positive affect.
Current Positive Affect:
In each phone call, participants responded to four questions from the Positive and Negative Affective Schedule for Children (PANAS-C; Laurent, 1999) that assessed positive affect at the time the phone rang (i.e., “How would you rate how [happy/cheerful/interested/excited] you were?”) using a 5-point Likert scale (1 = Not at all; 5 = Extremely; two-week mean positive affect: M = 2.82, SD = 0.70). Peak Happiness: Participants were also asked to “Think about the most enjoyable or happy time in the past hour.” They then responded to the question of “At the best point, how happy did you feel?” on a 5-point Likert scale (1 = Not at all; 5 = Extremely; two-week mean peak happiness: M = 3.89, SD = 0.46).
Emotional closeness.
In each phone call, participants were asked whether they were interacting with someone when they received the phone call and with whom. Participants reported interacting with someone on an average of 40.5% (SD = 16.2; range = 11.1%−87.5%) of their completed prompts. If they were interacting with someone, they were also asked to rate “How close or connected did you feel to [person they were interacting with]?” on a 5-point Likert scale (1 = Not at all; 5 = Extremely; two-week mean emotional closeness: M = 3.84, SD = 0.65). Who they were interacting with included friends (37.7% of the prompts they endorsed interacting with someone; Emotional Closeness: M = 4.09, SD = 0.86), child/adolescent family members (20.6%; M = 3.89, SD = 0.94), adult family members (30.3%; M = 3.59, SD = 0.98), family in general (7.2%; M = 4.13, SD = 0.99), and others (4.2%; M = 3.37, SD = 1.21).
Social reward fMRI task.
Participants completed an adapted version of the likeability task developed by Davey and colleagues (2010); see Healey and colleagues (2014) for further details. In this block-design task, participants received positive social feedback in the form of being liked by other adolescents. In the first visit, participants rated thirty-two (50% female) peer photographs based on how much they thought they would like the person depicted on a 9-point Likert scale (1 = not at all; 9 = very much). They had their own picture taken and were told that these peers would rate their picture. At the neuroimaging assessment (M = 5.88 weeks later, SD = 9.13 weeks, Median = 3.79 weeks; M = 3.94 weeks later, SD = 2.80 weeks, when excluding two outliers; number of weeks between visits was not associated with neural response to social reward, ρ = −.15, p = .418), they viewed the same peer photographs in the scanner with feedback that the peer rated them highly (positive feedback) or did not rate them yet (neutral feedback). Photographs were ranked ordered within each gender for each participant based on the ratings they made in the first visit. The top and bottom four photographs in each gender were categorized as “highest rated” and “lowest rated,” respectively. The remaining photographs were rated in the middle. Pseudo-feedback was presented, such that participants received positive feedback from photographs that they rated highest (i.e., highly rewarding feedback) and lowest (i.e., less rewarding feedback) within each gender. Participants received neutral feedback from photographs that they rated in the middle. Each photograph had a green (positive) or white (neutral) background to indicate feedback. Each photograph was presented 3 times within 8 blocks of 12 stimuli each. There were 2 primarily high positive blocks, 2 primarily low positive blocks, and 4 primarily neutral blocks. Blocks included 10 stimuli with feedback of their respective valence (i.e., high positive, low positive, or neutral) and 2 stimuli of the opposite type (i.e., neutral feedback in positive blocks, high or low positive feedback in neutral blocks) to reduce predictability and habituation. Participants were unaware that that the feedback would be presented in blocks. Each photograph was presented for 3s with a jittered inter-trial crosshair display between stimuli (1, 3, 5, or 7s). The inter-block interval of a “please take a break” message was 8s. Task duration was 12.8 minutes. After the scan, participants completed ratings in which they recalled how good they felt when they saw each of the stimulus photographs on a 9-point Likert scale (1 = not good at all; 9 = very good). When presented for these ratings, stimulus photographs were explicitly grouped by feedback (i.e., positive and neutral) with feedback group order counterbalanced. We grouped stimulus photographs by feedback to orient them to the feedback they received to prevent them from misremembering and providing inaccurate ratings. As expected, participants rated feeling better when getting positive feedback from peers they rated highest (M = 6.00, SD = 0.97) than peers they rated lowest (M = 5.47, SD = 0.85; Mdiff = 0.53, t(35) = 3.53, p = .001) or when getting neutral feedback(M = 4.97, SD = 1.03; Mdiff = 1.03, t(35) = 6.10, p < .001). Participants did not report significant differences in feeling better from getting feedback from either gender.
Monetary reward fMRI task.
The fMRI monetary reward task used was a card-guessing paradigm adapted from Delgado and colleagues (2000) to include both anticipation and outcome conditions (Nusslock et al., 2012). In this event-related paradigm, each trial included both an anticipation and an outcome period, and participants received win, loss, or no-change feedback for each trial. Participants were told that they would receive $1 for each win, lose 50 cents for each loss, and experience no earnings change for neutral outcomes. Participants were unaware of the fixed outcomes.
Trials were presented in pseudorandom order with predetermined outcomes. During each trial, participants had four seconds to guess, through button press, whether the value of a visually presented card with a possible value of 1–9 was higher or lower than 5 (index and middle finger, respectively). Afterward, the trial type (reward or loss) was presented visually for 6s (anticipation). This was followed by the “actual” numerical value of the card (500ms); outcome feedback (a green upward-facing arrow for win, a red downward-facing arrow for loss, or a yellow circle for neutral feedback; 500ms); and a crosshair presented for 9s (outcome period included the presentation of value of card, outcome feedback, and the first 6 seconds of the crosshair; baseline consisted of the last 3 seconds of the crosshair presentation). There were 6 trials of each outcome (i.e., “win,” “loss,” “no-win,” “no-loss”). Response time and percentage of responses were not significantly associated with demographic or EMA variables. During debriefing, all but four participants stated that they understood the task, thought that outcomes were due to chance, and found the task engaging.
fMRI Acquisition and Preprocessing
Participants were scanned using a Siemens 3T Trio scanner at the University of Pittsburgh Magnetic Resonance Research Center (MRRC). MPRAGE structural images were acquired with high-resolution T1-weighted images with 1 mm isometric voxels (TR/TE/flip angle = 2300 ms/2.98 ms/9; FOV = 256×240; 1.2 mm slice; 160 slices; 256×240 matrix; 1 Nex). Functional blood oxygen level dependent (BOLD) images were acquired using gradient echo planar imaging (EPI) sequences: 39 oblique axial slices (3.1 mm thick, 0 mm gap) oriented to the AC-PC line (TR/TE = 2000 ms/30 ms, FOV = 205×205, matrix = 64 ×64). A reference EPI scan acquired prior to fMRI data collection was visually inspected for artifacts and signal quality.
Statistical Parametric Mapping software, version 8 (SPM8; http://www.fil.ion.ucl.ac.uk/spm) was used to perform fMRI analyses. Images for each subject were realigned, motion-corrected, and high-pass temporally filtered with a cutoff of 128s. High-motion volumes (≥2mm) were adjusted using ART (http://gablab.mit.edu/index.php/software). The means (SD) for motion of this sample for the social reward task were x = 0.17 mm (0.12), y = 0.38 mm (0.51), z = 0.74 mm (0.90), roll = 0.58⁰ (0.57), pitch = 0.31⁰ (0.24), and yaw = 0.20⁰ (0.17). The means (SD) for motion of this sample for the monetary reward task were x = 0.16 mm (0.14), y = 0.28 mm (0.29), z = 0.54 mm (0.54), roll = 0.53⁰ (0.52), pitch = 0.22⁰ (0.19), and yaw = 0.23⁰ (0.27). The mean functional image was coregistered with the high-resolution 3D anatomic image, normalized to MNI space, and spatially smoothed (Gaussian kernel 6.0 mm FWHM).
Data Analytic Strategy
Neural response to social and monetary reward and mean emotional closeness.
Neural response to social reward was determined by individual level analyses contrasting brain activity during receipt of high positive feedback (i.e., mutual liking) compared with blocks of neutral feedback (high positive > neutral feedback), as this contrast reflects high social reward. Block periods included both feedback stimuli and crosshair interstimulus intervals. Neural response to monetary reward focused on consummatory reward (“win” outcome > “no win” outcome contrast) and did not include anticipatory reward given that the social reward task did not have an equivalent anticipatory period. Outcome periods included presentation of value of card, outcome feedback, and the first 6 seconds of the crosshair; baseline periods consisted of 3 seconds of the crosshair following the outcome period. These individual level analyses included non-interest crosshair displays (i.e., “interblock interval” for social reward, “baseline” for monetary reward) and did not include any other regressors of non-interest. The social and monetary reward tasks were designed separately and selected for their ability to engage reward circuitry (and social circuitry, in the case of the social reward task). Although the present study focused on social reward, including the monetary reward task provided the opportunity to test the primary hypothesis in a monetary reward task to help inform future studies focused on comparing these two types of reward. We conducted similar but separate regression analyses in SPM8 for each fMRI task with mean EMA-measured emotional closeness as an independent variable and neural response to social or monetary reward as the dependent variable. Age and gender were included as covariates in all analyses considering developmental changes in the social brain network (Burnett et al., 2011) and gender differences in social rewards (Spreckelmeyer et al., 2009). To focus on brain regions related to social processing in general without focusing on a particular type of social process, we masked results based on meta-analytic findings for studies examining the construct “social” in Neurosynth (www.neurosynth.org), a repository of functional neuroimaging masks based on findings across thousands of articles. We used a single forward-inference mask that was generated from 1,000 articles and included brain regions (e.g., mPFC, pSTS, TPJ, VLPFC) that are consistently reported to be activated in articles with frequent use of the term “social.” Given the high overlap between social and reward circuitry, it is not surprising that this mask also included key regions of reward circuitry (e.g., mPFC, striatum, insula, amygdala). We included clusters of at least 50 voxels in the cerebrum. This mask was used to constrain second-level regression analyses to social and reward brain regions. To avoid Type I error, we conducted Monte Carlo simulations using 3dClustSim in AFNI (https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html) with the auto-correlation function (ACF) estimated by 3dFWHMx, which helps address concerns of previous cluster thresholding techniques (Eklund et al., 2016). This procedure estimated that the minimum number of contiguous voxels per cluster (activated at punc < .005) required for a corrected p < .05 in a mask of 23,917 voxels was 150 voxels. Principal eigenvariates for clusters reaching significance in second-level analyses were extracted for use in multilevel modeling.
Neural response to social reward as moderator of within-person association between emotional closeness and concurrent positive affect.
We used multilevel modeling to examine whether emotional closeness was associated with concurrent positive affect and whether brain regions identified in the first set of analyses described above moderated the within-person association between emotional closeness and concurrent positive affect. Multilevel modeling is a ubiquitous technique to analyze nested data such as EMA data, in which repeated measurements of participant responses are nested within the participants. Multilevel modeling is useful to analyze EMA data because it does not assume that data points are independent and can handle missing data points (Snijders & Bosker, 2011). In addition, it allows the ability to examine whether the association between two within-person variables (Level 1; e.g., momentary emotional closeness and positive affect) is moderated by a between-person variable (Level 2; e.g, neural response to social reward measured at one timepoint). For each of the multilevel modeling analyses, we used the MIXED procedure of the SAS 9.4 software. We report parameter estimates with standard errors. We included random intercepts in each model (which means that intercepts are allowed to be different for each participant) and used unstructured covariance matrices (which means that each variance and covariance in the model is estimated from the data without assuming that variances or covariances are equal). Random slopes (which means that slopes are allowed to be different for each participant) were not included in the models due to final Hessian and estimated G matrix not being positive definite when they were included. We participant-centered within-person predictor variables and group-centered between-person predictor variables. Gender (male = 0; female = 1) was dummy-coded. A representative model is shown below (γ represents coefficient estimates; γ30 represents the coefficient estimate for the main effect of concurrent closeness; γ31 represents the coefficient estimate for the brain region × concurrent closeness interaction; Rij and U0j are error terms). Time refers to time of day in hours and PA refers to concurrent positive affect.
Neural response to social reward as moderator of within-person association between emotional closeness and future peak happiness.
Prospective analyses were conducted to test whether previous emotional closeness predicted peak happiness reported at the next call a few hours later and whether brain regions identified in the first set of analyses moderated this association. A representative model is shown below (γ30 represents the coefficient estimate for the main effect of previous closeness; γ31 represents the coefficient estimate for the brain region × previous closeness interaction).
Results
Between-Person Associations between Neural Response to Social and Monetary Reward and Mean Emotional Closeness
We conducted similar but separate regression analyses for each fMRI reward task with mean EMA-measured emotional closeness as an independent variable and neural response to social or monetary reward as the dependent variable. Age and gender were included as covariates. To focus on brain regions related to social processing, we masked results based on meta-analytic findings for studies examining the construct “social.” There were positive associations between mean emotional closeness and neural response to social reward in the right pSTS/TPJ region (see Table I and Figure 3). No brain regions were found to have a significant negative association. There were no significant age or gender differences in neural response to social reward. As anticipated, there were no significant associations between neural response in any brain region to consummatory monetary reward and mean emotional closeness. Furthermore, to test whether there was a significant difference between social and monetary reward in the correlations between mean emotional closeness and right pSTS/TPJ neural response, we extracted principal eigenvariates from both tasks for the significant cluster found in the right pSTS/TPJ region (see Table I and Figure 3).1 Although the correlation with response to the social reward task was significant (r = .48, p = .009) and the correlation with response to the monetary reward task was not (r = .31, p = .105), the two correlations were not significantly different when using Fisher r-to-z transformations (z = 0.73, p = .463).
Table I.
Regions with between-person positive associations between mean EMA emotional closeness and social reward BOLD contrast response (high positive > neutral feedback)
| Brain Region | Number of Voxels in Cluster | Max T-score at Peak Voxel | MNI Coordinates of Peak Voxel (x, y, z) | ||
|---|---|---|---|---|---|
| Right Posterior Superior Temporal Sulcus/ Temporoparietal Junction | 153 | 3.83 | 50 | −54 | 6 |
Note. Voxels were thresholded at p < .005. The findings reported here are significant at corrected p < .05 using Monte Carlo simulations.
Figure 3.
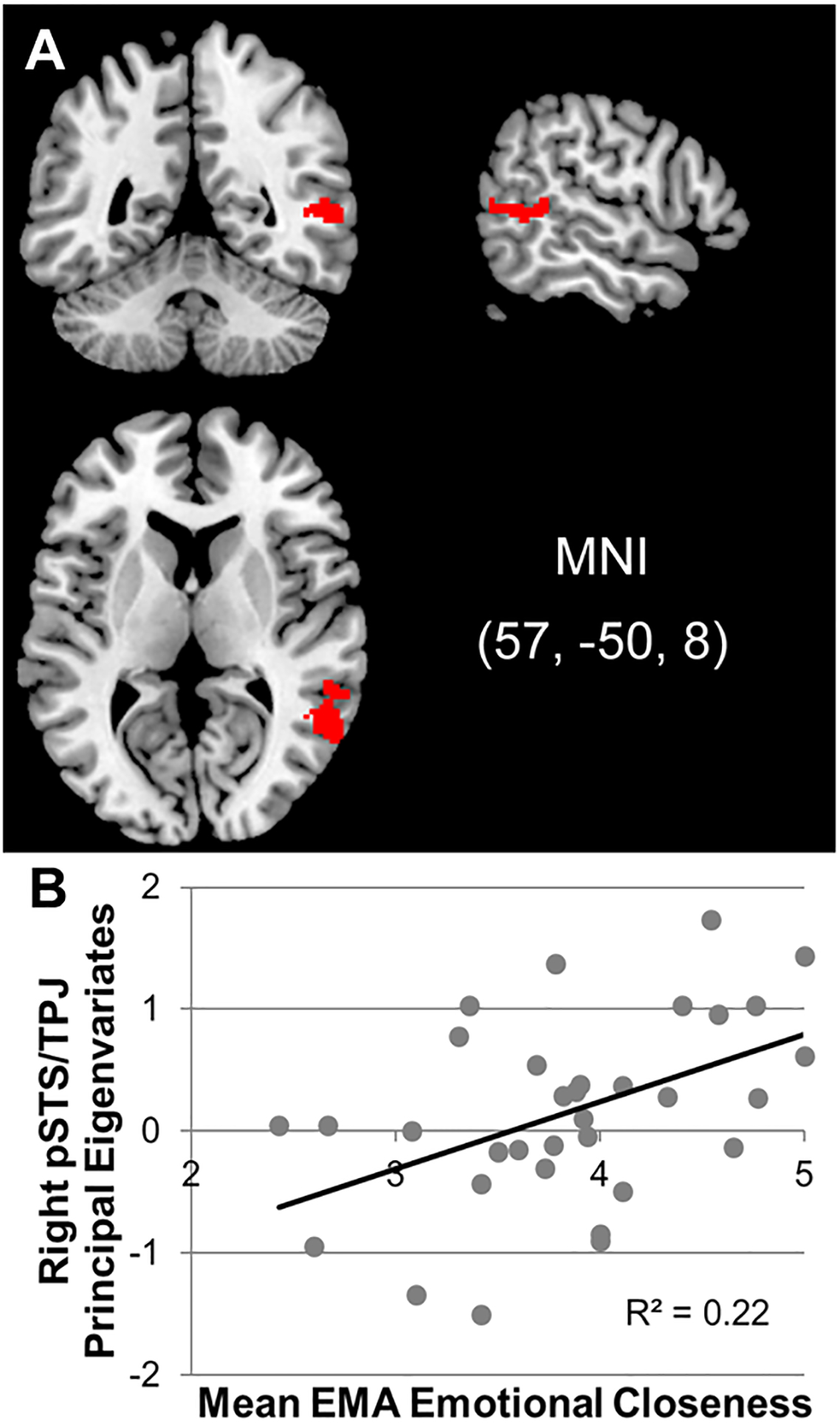
A. Brain region (right pSTS/TPJ) with a positive association between mean EMA emotional closeness and neural response to social reward (high positive > neutral feedback). B. Scatterplots of between-person associations between EMA emotional closeness and neural response to social reward.
Neural Response to Social Reward as Moderator of Concurrent Within-Person Association between Emotional Closeness and Positive Affect
Using the right pSTS/TPJ cluster identified in the previous analysis, we extracted principal eigenvariates from the social and monetary reward tasks to examine whether neural response to social or monetary reward moderates the within-person relation between emotional closeness and concurrent positive affect.2 As expected, emotional closeness was positively associated with concurrent positive affect (see Table II). In the social reward and concurrent positive affect model, neural response to social reward moderated the concurrent association between emotional closeness and positive affect.3 The positive association between emotional closeness and concurrent positive affect was unexpectedly greater among individuals who demonstrated lower right pSTS/TPJ response to social reward than those who demonstrated higher right pSTS/TPJ response (see Figure 4). A follow-up simple slopes test demonstrated that the positive association between emotional closeness and concurrent positive affect was significant when the principal eigenvariate of right pSTS/TPJ was less than 1.17 (i.e., 1.52 standard deviations above the mean in the present sample). Thus, the association was significant at both one standard deviation above, t(194) = 3.44, p = .001, and one standard deviation below, t(194) = 5.82, p < .001, the mean of right pSTS/TPJ response. It is worth noting that this simple slopes test does not require the sample to be divided into groups (see Preacher et al., 2006; http://www.quantpsy.org/interact/hlm2.htm).
Table II.
Unstandardized coefficient estimates (and standard errors) for multilevel models examining the moderating roles of right pSTS/TPJ response to social and monetary reward in the associations between emotional closeness and both concurrent positive affect and prospective peak happiness
| Social Reward | Monetary Reward | |||
|---|---|---|---|---|
| Concurrent Positive Affect | Prospective Peak Happiness | Concurrent Positive Affect | Prospective Peak Happiness | |
| Level 1 Variables | ||||
| Time, γ10 | 0.01 (0.02) | 0.03 (0.02) | 0.00 (0.02) | 0.04(0.02)† |
| Previous Positive Affect, γ20 | 0.08 (0.07) | 0.06 (0.08) | ||
| Proximal Positive Affect, γ20 | 0.48 (0.07)*** | 0.45 (0.08)*** | ||
| Concurrent Closeness, γ30 | 0.37 (0.05)*** | 0.22 (0.06)*** | ||
| Previous Closeness, γ30 | 0.20 (0.06)*** | 0.24 (0.06)*** | ||
| Level 2 Variables | ||||
| Intercept, γ00 | 3.08 (0.20)*** | 3.87 (0.17)*** | 3.20 (0.20)*** | 3.89 (0.18)*** |
| Gender, γ01 | −0.26 (0.23) | −0.11 (0.19) | −0.37 (0.25) | −0.17 (0.20) |
| Age, γ02 | 0.17 (0.08) | 0.04 (0.07) | 0.17 (0.08)* | 0.04 (0.07) |
| Right pSTS/TPJ, γ04 | 0.09 (0.15) | 0.20 (0.13) | 0.60 (0.39) | 0.31 (0.34) |
| Right pSTS/TPJ × Closeness, γ31 | −0.15 (0.08)* | 0.20 (0.09)* | −0.19 (0.22) | 0.05 (0.21) |
Note: Gray cells indicate that the predictor variable was not included in the model.
p < .05;
p < .001;
p <.10
Figure 4.
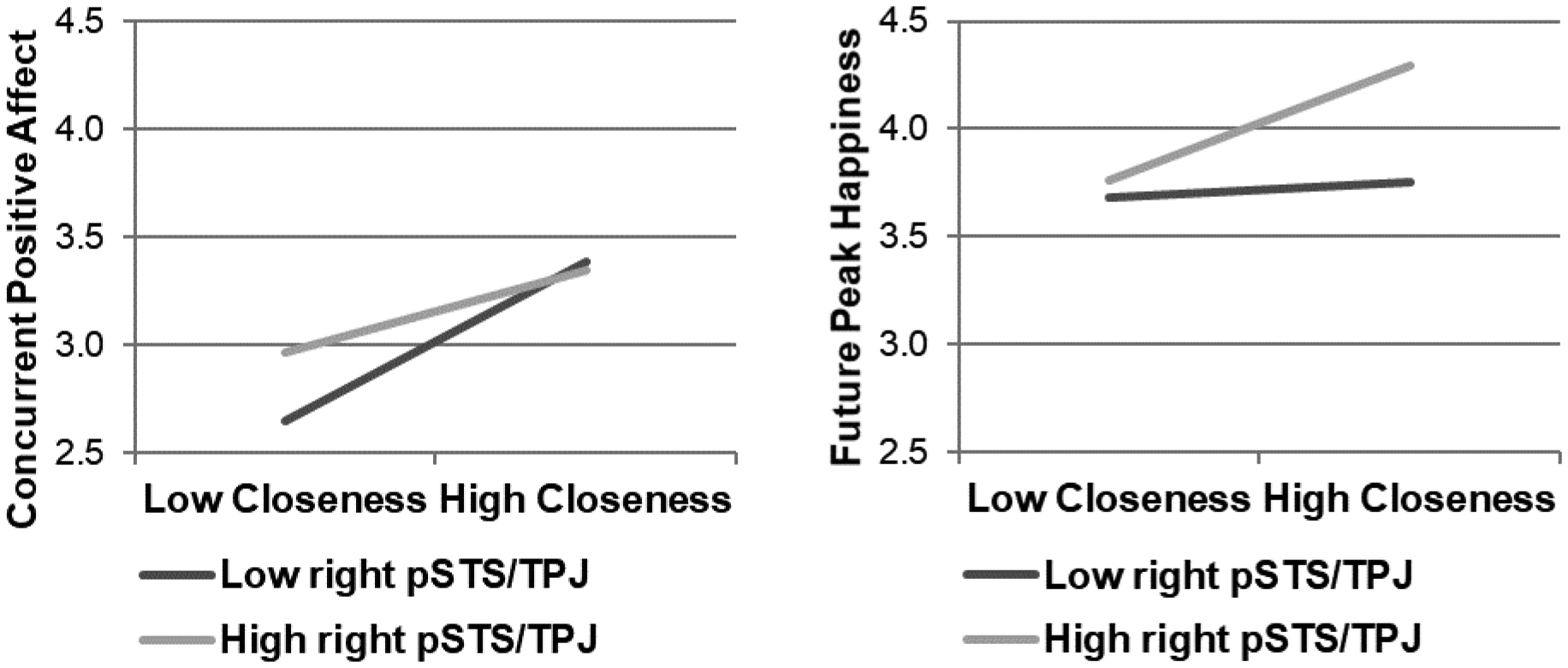
Graphs based on multilevel models demonstrating the EMA within-person association between emotional closeness and concurrent positive affect or future peak happiness at low and high right pSTS/TPJ contrast BOLD response (high positive > neutral feedback), with “low” and “high” defined as one standard deviation below and above the mean principal eigenvariate, respectively.
As expected, emotional closeness predicted future peak happiness (see Table II), and right pSTS/TPJ response to social reward moderated this association (see Figure 4). Follow-up simple slopes tests (see Preacher et al., 2006) demonstrated that there was a significant positive association between emotional closeness and future peak happiness when the principal eigenvariate of right pSTS/TPJ was at least −0.38 (i.e., 0.49 standard deviations below the mean in the present sample). Thus, this association was significant at one standard deviation above the mean of right pSTS/TPJ response to social reward, t(164) = 3.69, p < .001. However, the association was not significant at one standard deviation below the mean of right pSTS/TPJ response, t(164) = 0.51, p = .614. Right pSTS/TPJ response to monetary reward did not moderate either association (see Table II).
Discussion
To better understand adolescents’ individual differences in experiencing positive affect related to rewarding social interactions, the present study examined whether neural response to social reward is (1) associated with mean levels of real-world emotional closeness—an important social experience that can elicit positive affect in everyday life—and (2) a moderator of the within-person association between real-world emotional closeness and positive affect. Adolescents who exhibited greater neural response to social reward in a key social processing region (i.e., right pSTS/TPJ) generally experienced greater emotional closeness in their everyday lives; however, a similar association was not found in neural response to monetary reward. In addition, right pSTS/TPJ response to social reward moderated the within-person association between naturalistic emotional closeness and both concurrent positive affect and future peak happiness. The nature of the moderation was different, however, between concurrent and prospective associations. Surprisingly, adolescents with lower right pSTS/TPJ response demonstrated a stronger positive association between emotional closeness and concurrent positive affect than those with higher right pSTS/TPJ response. In contrast, emotional closeness was associated with future peak happiness among adolescents with higher right pSTS/TPJ response but not among those with lower right pSTS/TPJ response. Thus, adolescents with higher right pSTS/TPJ response to social reward seem to experience greater emotional closeness in their everyday life and have a sustained affective benefit from emotional closeness despite having a tempered immediate affective benefit (i.e., they have a relatively weak affective benefit in the short-term but a relatively strong affective benefit in the long-term).
Between-Person Association between Neural Response to Social Reward and Emotional Closeness
The lateralized findings for pSTS/TPJ response are consistent with previous research, where the right pSTS/TPJ is implicated in social processes, including mentalizing—a construct that generally includes theory of mind, perspective-taking, and cognitive empathy (Burnett et al., 2011; Krall et al., 2016). Right pSTS is more specifically implicated in face detection (Allison et al., 2000) and eye gaze (Puce et al., 1998), perception of biological motion (Saygin, 2007), and decoding social gestures to predict others’ action or intent (Haxby et al., 2000; Morris et al., 2005; Saxe et al., 2004). Right TPJ is also suggested to play a role in reorientation of attention to both social and nonsocial unexpected stimuli (Decety & Lamm, 2007). One possibility is that adolescents with greater emotional closeness may more readily engage social processing circuitries in social situations. Further, these adolescents may also typically engage in these processes in their everyday lives during positive social interactions. In fact, as adolescents develop and transition to adulthood, they tend to demonstrate greater consideration of others and shift from exhibiting greater mPFC activation to greater TPJ activation during social decision-making (Crone & Dahl, 2012). This could reflect greater other orientation, enhanced perspective taking, and stronger self-other identification. These social cognitive advances could provide a foundation for sophisticated social processing (and adaptive functioning) in adulthood. Future longitudinal studies examining the association between neural response to social reward and emotional closeness may find that emotional closeness may play an important role in the developmental change from greater mPFC to greater TPJ activation during social situations over the course of adolescence.
Potentially, engaging in social processing during socially rewarding situations may help facilitate experiencing greater emotional closeness. For example, greater engagement in social processing may facilitate conceptualizing the interaction as a shared experience. This may enhance the process of incorporating others as part of the self, which might contribute to feelings of emotional closeness (Aron et al., 1991). Although this possibility cannot be tested in the present study, future behavioral studies could help address this possibility by manipulating the use of social processes during a socially rewarding task and measuring emotional closeness and the incorporation of the other as part of the self during the task.
It is worth highlighting that research on reward does not commonly compare two classes of reward within the one study, despite the importance of understanding whether effects observed generalize across different classes of rewarding stimuli. Including both social reward and monetary reward, which is a more generic form of reward that is widely used in neuroimaging research, in the present study demonstrated that neural response to social—but not monetary—reward is related to experiencing greater naturalistic emotional closeness. However, the present study did not find a significant difference between social and monetary reward in terms of their correlations between emotional closeness and right pSTS/TPJ neural response. It is also worth noting though that there are notable differences between the social and monetary reward fMRI tasks used in the present study. For example, the social reward task used a block design and the monetary reward task used an event-related design. Thus, the finding that naturalistic emotional closeness is related to social but not monetary reward should be considered preliminary and suggests that it could beneficial for future studies to compare correlates of social and monetary reward using similarly designed tasks. Although there is overlap in reward circuitry activated by social and nonsocial rewards (Izuma et al., 2008), these preliminary findings suggest that there may be components of emotional closeness that are relevant to social but not nonsocial rewards. For example, valuing emotionally intimate experiences and having experience and comfort with emotional closeness may be related to neural response to social but not nonsocial rewards.
Right pSTS/TPJ Response to Social Reward Moderated Within-Person Associations between Emotional Closeness and Positive Affect
Whereas those with low right pSTS/TPJ response may be more emotionally reactive to the experience of emotional closeness in the immediate moment, those with high right pSTS/TPJ may build on the experience of emotional closeness to achieve a higher peak happiness hours later. Given that the right pSTS/TPJ is associated with social processing, the moderating role of right pSTS/TPJ suggests the potential importance of social processing in the affective benefits of emotional closeness. One possible explanation worthy of further investigation is that mentalizing may enhance the quality of an emotionally close interaction as it progresses by facilitating social competence (Davis, 1983) and prosocial behaviors (Eisenberg & Miller, 1987). Mentalizing may also increase the meaning of the interaction by enhancing the incorporation of the other person into one’s own self-concept (Galinsky et al., 2005). The combination of increased quality and meaning of an emotionally close social interaction may help explain how adolescents with higher right pSTS/TPJ response—but not adolescents with lower right pSTS/TPJ response—experienced sustained affective benefits from emotional closeness. Given the developmental shift from mPFC to TPJ activation over the course of adolescence and the transition into adulthood (Crone & Dahl, 2012), future studies may find that this shift may facilitate experiencing greater sustained affective benefits from emotional closeness over the course of adolescence.
Strengths, Limitations, and Future Directions
One notable strength of the present study is that it integrated fMRI and EMA methodologies to examine neural aspects of reward and naturalistic aspects of subjective emotional closeness and positive affect. Key advantages of incorporating the fMRI task include contributing a neural level of analysis and measuring social reward neural response to standardized stimuli. Advantages of EMA include its ecological validity, given that participants make ratings in the context of their everyday life, and its ability to capture both concurrent and prospective associations within a day, which helps demonstrate time-based characteristics of an association.
Although greater neural response to social reward in social brain regions suggest increased social processing during social reward, it is important to note that social processing was not measured directly in the present study. Future studies would benefit from measuring the spontaneous use of social processing during social reward (e.g., post-task self-report questionnaire) or manipulating the use of social-cognitive processes (e.g., instructing the use of mentalizing) during social reward. Given that there are developmental changes in both reward and social circuitries during adolescence, future studies should also include participants in other developmental periods (e.g., young adulthood) or a longitudinal design over several years. Incorporating participants with or at risk for psychopathology (e.g., depression) would also be beneficial to elucidate how the present findings may inform the development of psychopathology. Although the present study’s relatively modest sample size appears to be sufficient to detect a medium-sized interaction, future studies would be strengthened by having larger sample sizes to further improve the reliability of findings and to be able to detect smaller effect sizes.
Conclusion
Increased right pSTS/TPJ response to social reward was associated with mean emotional closeness and moderated the positive association between emotional closeness and positive affect. Study findings suggest that increased engagement of right pSTS/TPJ—a key brain region for social processing—during socially rewarding contexts may both facilitate the everyday experience of emotional closeness and support the sustained affective benefits of emotional closeness despite tempering the immediate affective benefits of emotional closeness. Thus, social brain regions (including right pSTS/TPJ) and social-affective experiences (e.g., emotional closeness) appear to play important roles in the experience of positive affect during positive social interactions.
Figure 1.
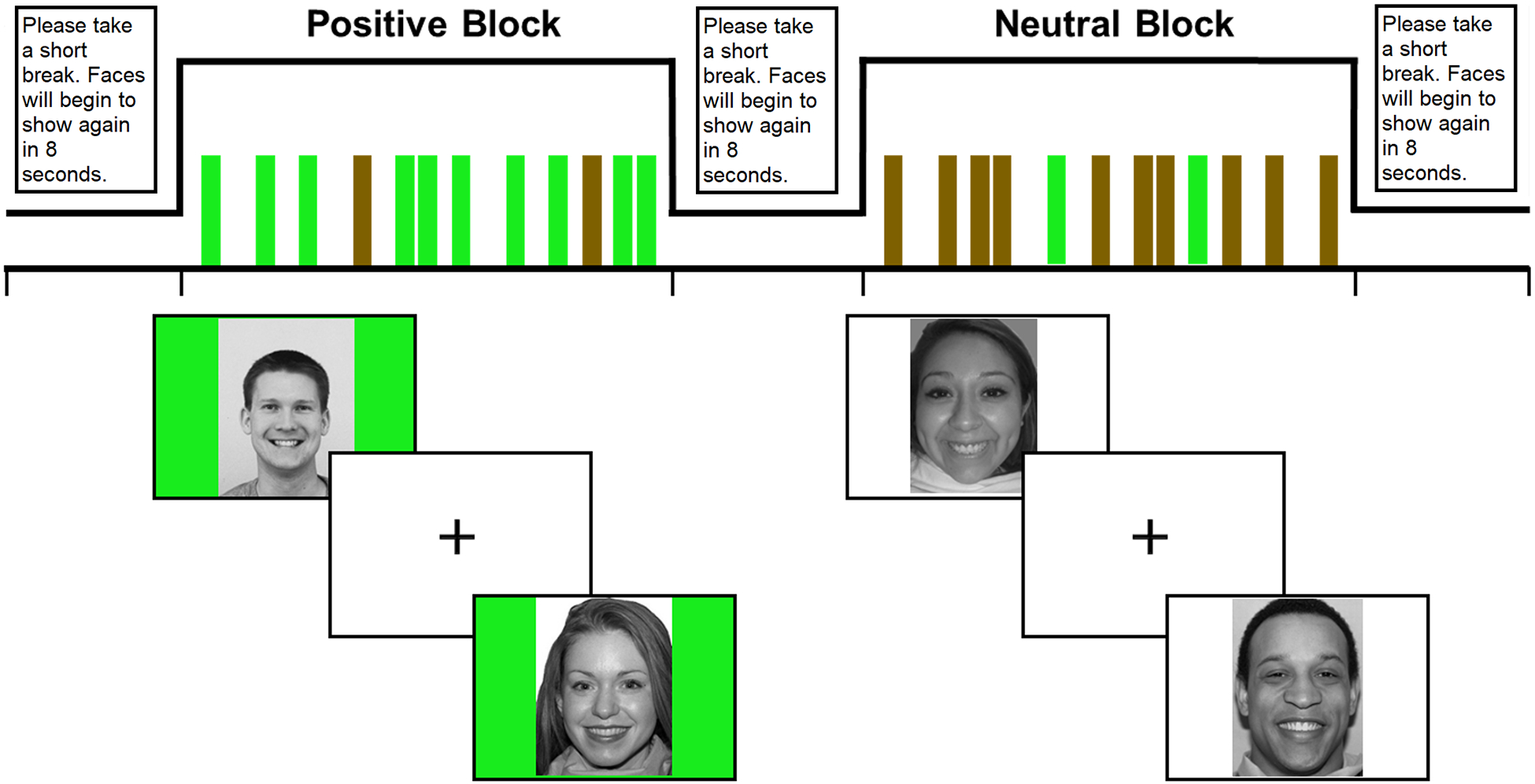
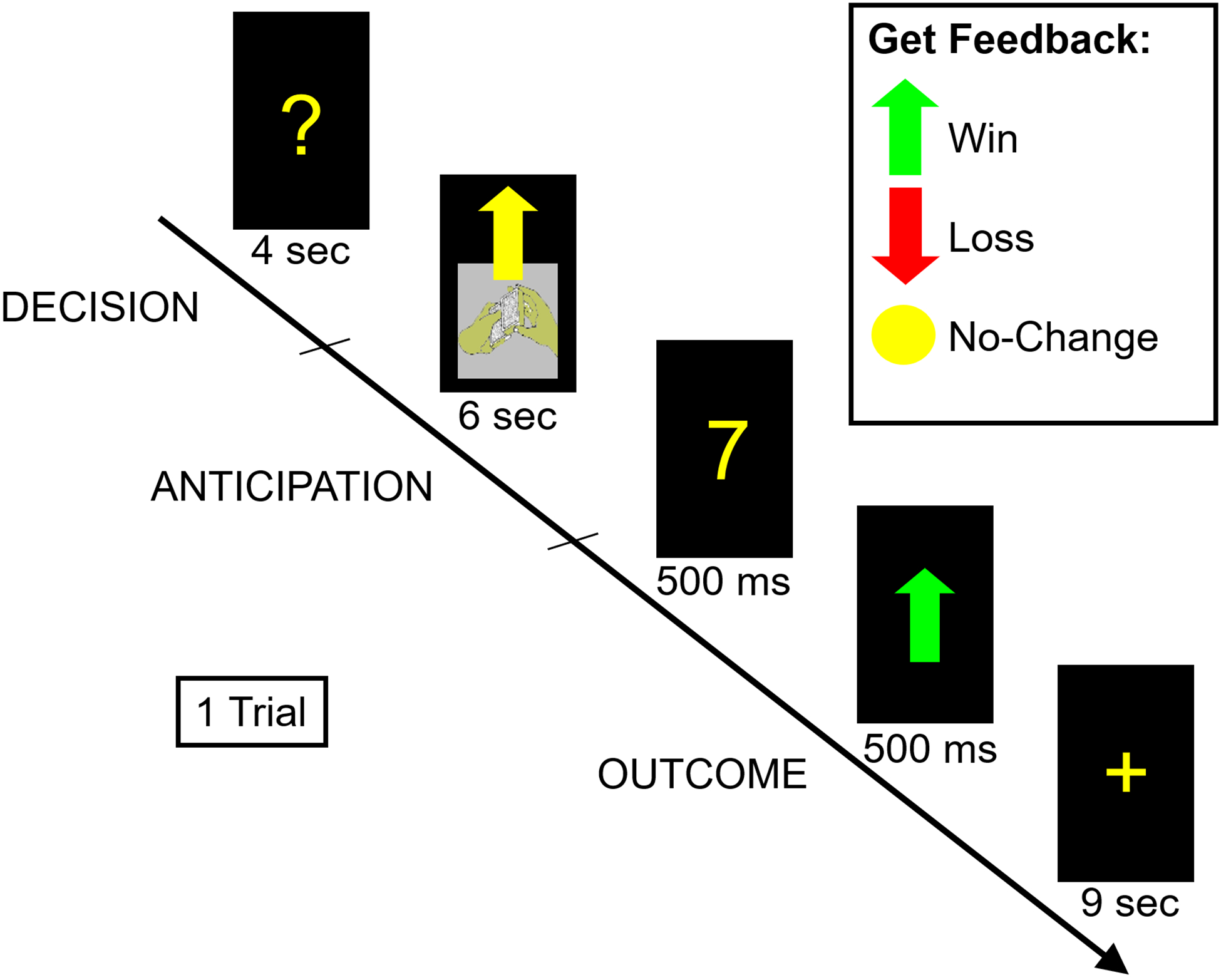
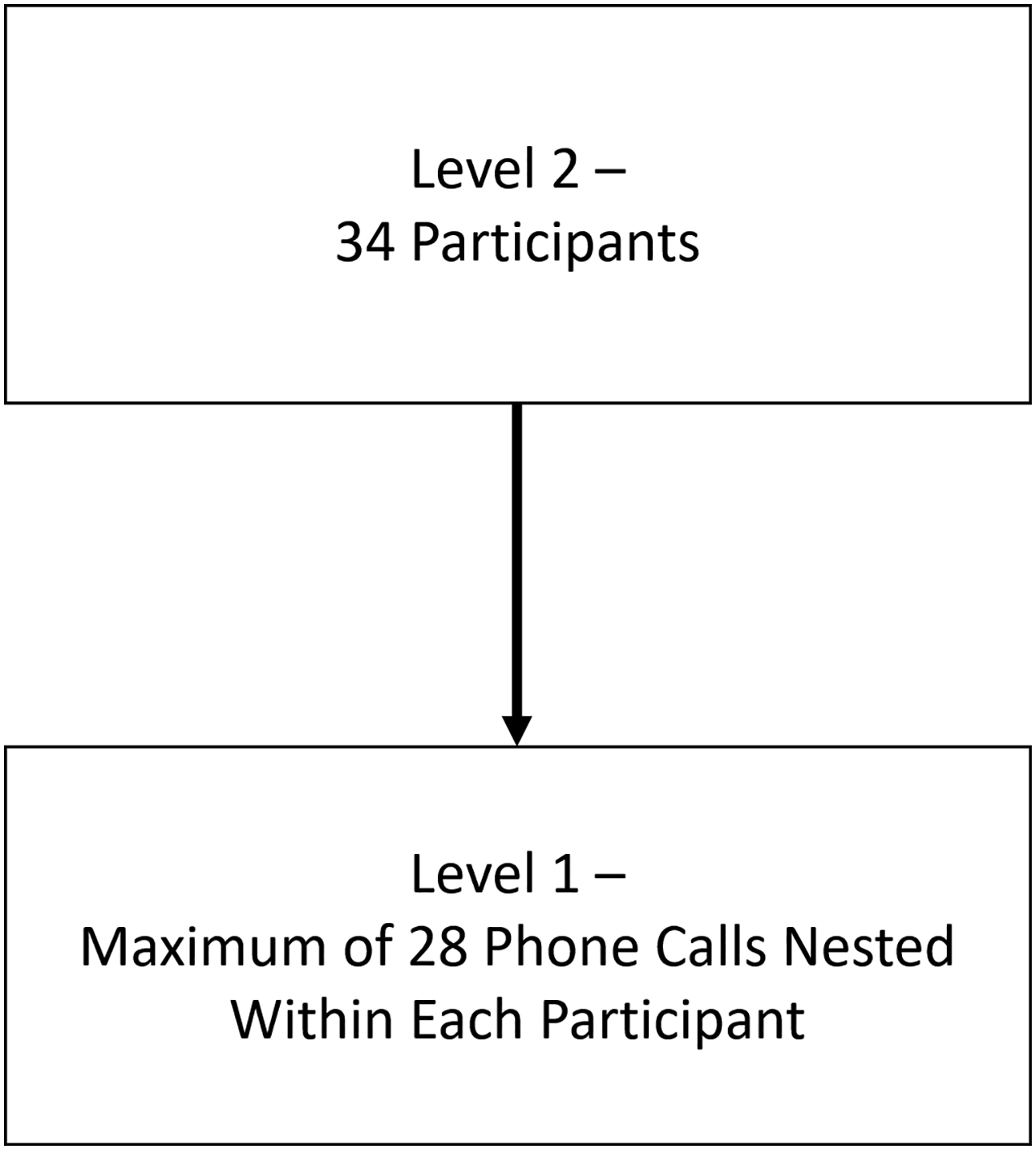
Illustrations of the block-design social reward task (modified from Healey et al., 2014), the event-related design monetary reward task (taken from Nusslock et al., 2012), and the nested nature of the ecological momentary assessment protocol.
Figure 2.
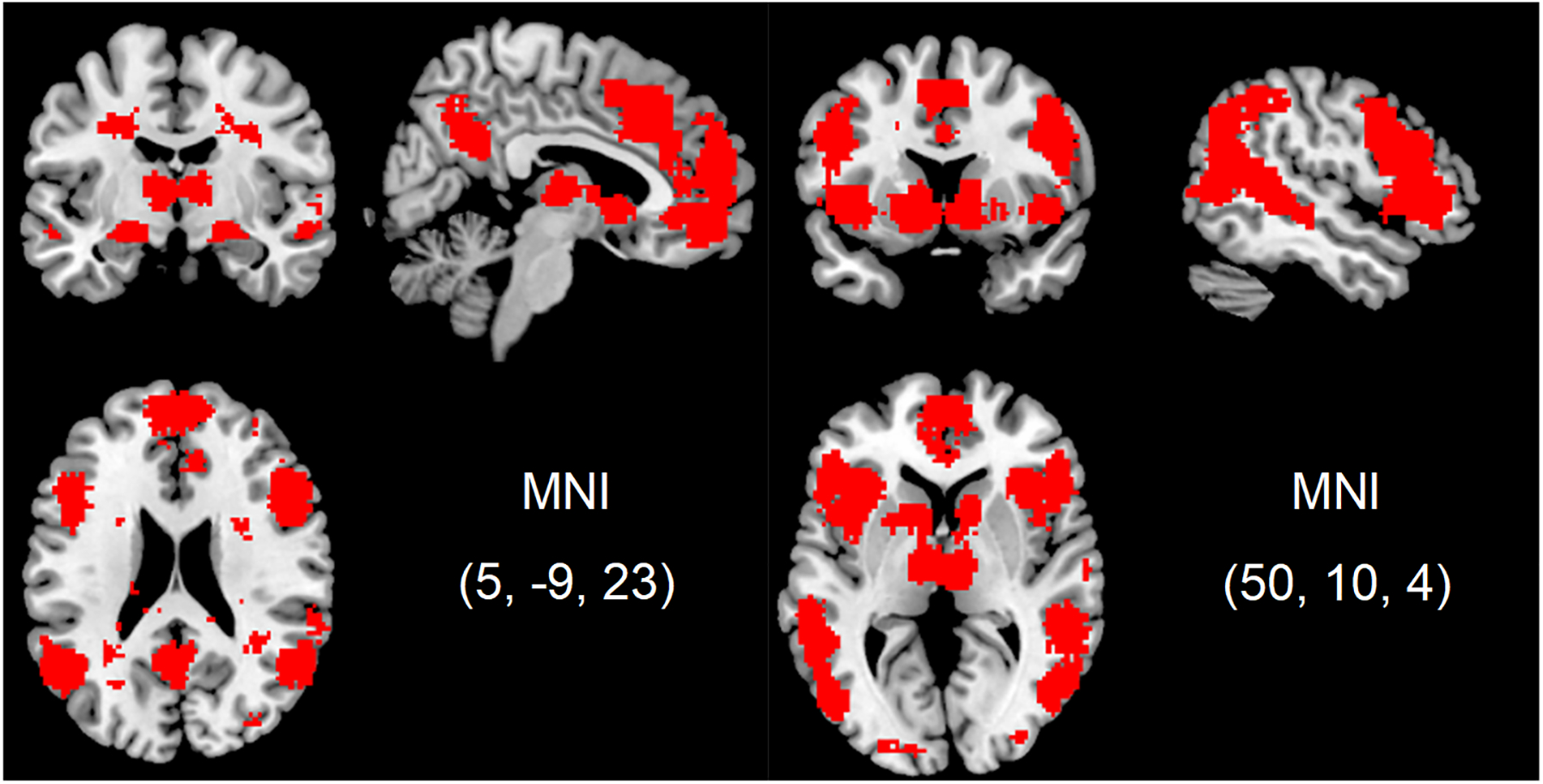
The Neurosynth-derived “social” mask used in analyses testing the association between momentary emotional closeness and neural response to social and monetary reward. Regions in this mask include the temporoparietal junction (TPJ), posterior superior temporal sulcus (pSTS), inferior parietal lobule (IPL), precuneus, posterior cingulate cortex (PCC), supramarginal gyrus, angular gyrus, parahippocampal gyrus, ventrolateral prefrontal cortex (VLPFC), dorsolateral prefrontal cortex (DLPFC), medial prefrontal cortex (mPFC), orbital frontal cortex (OFC), anterior cingulate cortex (ACC), insula, caudate, putamen, ventral striatum, amygdala, thalamus, globus pallidus, supplementary motor area, and portions of the occipital lobe.
Acknowledgements
We would like to thank the research assistants who contributed to this study and the families who participated. This research was supported by a grant from the National Institute on Drug Abuse (DA033612, PI: Forbes). Writing of this manuscript was supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the VA Pittsburgh Healthcare System, and the VISN 4 Mental Illness Research, Education and Clinical Center (MIRECC). Authors have no conflicts of interest to declare.
Footnotes
Publisher's Disclaimer: Disclaimer: The contents of this paper do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
As an alternative to extracting principal eigenvariates from the significant right pSTS/TPJ cluster found in the social reward analyses, we also tried using a meta-analytic functional mask (2,226 voxels) from Neurosynth.org by combining the primary right clusters from masks generated by using the terms “pSTS” (based on 73 articles) and “temporoparietal junction” (based on 130 articles). The results were similar when using the meta-analytic functional mask. Although the correlation with response to the social reward task was significant (r = .38, p = .046) and the correlation with response to the monetary reward task was not (r = .05, p = .788), the two correlations were not significantly different when using Fisher r-to-z transformations (z = 1.08, p = .282).
We also tried conducting the multilevel models with principal eigenvariates of right pSTS/TPJ response using the meta-analytic functional mask described in Footnote 1. Although right pSTS/TPJ response to social reward did not significantly moderate the association between emotional closeness and concurrent positive affect, γ31 = −0.13, SE = 0.09, t(194) = −1.34, p = .182, it did significantly moderate the association between emotional closeness and future peak happiness, γ31 = 0.20, SE = 0.09, t(161) = 2.15, p = .033. Right pSTS/TPJ response to monetary reward did not significantly moderate either association (concurrent positive affect: γ31 = −0.31, SE = 0.20, t(162) = −1.55, p = .123, future peak happiness: γ31 =0.15, SE = 0.23, t(140) = 0.63, p = .531).
We considered including mean level of closeness at Level 2 but decided to not include it given the focus of hypotheses on how neural response to social reward moderates the within-person association between emotional closeness and positive affect rather than the between-person association between in emotional closeness and positive affect. When including mean level of closeness at Level 2, mean closeness was significantly related to positive affect (Social Reward: γ04 = 0.64, SE = 0.18, t(32.6) = 3.47, p = .002; Monetary Reward: γ04 = 0.45, SE = 0.17, t(30.7) = 2.67, p = .012) and future peak happiness (Social Reward: γ04 = 0.59, SE = 0.15, t(34.3) = 3.87, p = .001; Monetary Reward: γ04 = 0.52, SE = 0.13, t(34.4) = 3.95, p < .001). The value of the right pSTS/TPJ × Level 1 emotional closeness interaction did not change in each model.
References
- Allison T, Puce A, & McCarthy G (2000). Social perception from visual cues: Role of the STS region. Trends in Cognitive Sciences, 4(7), 267–278. [DOI] [PubMed] [Google Scholar]
- Aron A, Aron EN, Tudor M, & Nelson G (1991). Close relationships as including other in the self. Journal of Personality and Social Psychology, 60(2), 241. [Google Scholar]
- Baumeister RF, & Leary MR (1995). The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin, 117(3), 497–529. [PubMed] [Google Scholar]
- Beeney JE, Franklin RG Jr, Levy KN, & Adams RB Jr. (2011). I feel your pain: Emotional closeness modulates neural responses to empathically experienced rejection. Social Neuroscience, 6(4), 369–376. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, & Hommer DW (2004). Incentive-elicited brain activation in adolescents: similarities and differences from young adults. Journal of Neuroscience, 24(8), 1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ (2008). The social brain in adolescence. Nature Reviews Neuroscience, 9(4), 267–277. [DOI] [PubMed] [Google Scholar]
- Burnett S, Sebastian C, Kadosh KC, & Blakemore SJ (2011). The social brain in adolescence: Evidence from functional magnetic resonance imaging and behavioural studies. Neuroscience & Biobehavioral Reviews, 35(8), 1654–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, & Dahl RE (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13(9), 636–650. [DOI] [PubMed] [Google Scholar]
- Davey CG, Allen NB, Harrison BJ, Dwyer DB, & Yücel M (2010). Being liked activates primary reward and midline self‐related brain regions. Human Brain Mapping, 31(4), 660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Yücel M, & Allen NB (2008). The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neuroscience and Biobehavioral Reviews, 32(1), 1–19. [DOI] [PubMed] [Google Scholar]
- Davis MH (1983). Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of Personality and Social Psychology, 44(1), 113–126. [Google Scholar]
- Decety J, & Lamm C (2007). The role of the right temporoparietal junction in social interaction: How low-level computational processes contribute to meta-cognition. The Neuroscientist, 13(6), 580–593. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, & Fiez JA (2000). Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology, 84(6), 3072–3077. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, & Miller PA (1987). The relation of empathy to prosocial and related behaviors. Psychological Bulletin, 101(1), 91–119. [PubMed] [Google Scholar]
- Eklund A, Nichols TE, & Knutsson H (2016). Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences, 113(28),7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, … Pine DS (2005). Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage, 25(4), 1279–1291. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, & Buchner A (2007). G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–91. [DOI] [PubMed] [Google Scholar]
- Flores LE Jr., & Berenbaum H (2014). Desired emotional closeness moderates the prospective relations between levels of perceived emotional closeness and psychological distress. Journal of Social and Clinical Psychology, 33(8), 673–700. [Google Scholar]
- Frith U, & Frith CD (2003). Development and neurophysiology of mentalizing. Philosphical Transactions of the Royal Society of London B: Biological Sciences, 358(1431), 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, … & Dahl RE (2010). Healthy adolescents’ neural response to reward: Associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child & Adolescent Psychiatry, 49(2), 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, & Dahl RE (2012). Altered reward function in adolescent depression: What, when and how? Journal of Child Psychology and Psychiatry, 53(1), 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gable SL, Reis HT, Impett EA, & Asher ER (2004). What do you do when things go right? The intrapersonal and interpersonal benefits of sharing positive events. Journal of Personality and Social Psychology, 87(2), 228–245. [DOI] [PubMed] [Google Scholar]
- Galinsky AD, Ku G, & Wang CS (2005). Perspective-taking and self-other overlap: Fostering social bonds and facilitating social coordination. Group Processes and Intergroup Relations, 8(2), 109–124. [Google Scholar]
- Gross JJ, & John OP (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85(2), 348–362. [DOI] [PubMed] [Google Scholar]
- Gunther Moor B, Güroğlu B, Op de Macks ZA, Rombouts SA, Van der Molen MW, & Crone EA (2012). Social exclusion and punishment of excluders: neural correlates and developmental trajectories. NeuroImage, 59(1), 708–717. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Pine DS, & Nelson EE (2012). Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience, 7(1), 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, & Gobbini MI (2000). The distributed human neural system for face perception. Trends in Cognitive Sciences, 4(6), 223–233. [DOI] [PubMed] [Google Scholar]
- Healey KL, Morgan J, Musselman SC, Olino TM, & Forbes EE (2014). Social anhedonia and medial prefrontal response to mutual liking in late adolescents. Brain and Cognition, 89, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, & Nestler EJ (2006). Neural mechanisms of addiction: The role of reward-related learning and memory. Annual Review of Neuroscience, 29, 565–598. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, & Sadato N (2008). Processing of social and monetary rewards in the human striatum. Neuron, 58(2), 284–294. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Krueger AB, Schkade DA, Schwarz N, & Stone AA (2004). A survey method for characterizing daily life experience: The day reconstruction method. Science, 306(5702), 1776–1780. [DOI] [PubMed] [Google Scholar]
- Krall SC, Volz LJ, Oberwelland E, Grefkes C, Fink GR, & Konrad K (2016). The right temporoparietal junction in attention and social interaction: A transcranial magnetic stimulation study. Human Brain Mapping, 37(2), 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent J, Catanzaro SJ, Joiner TE Jr., Rudolph KD, Potter KI, Lambert S, … Gathright T (1999). A measure of positive and negative affect for children: Scale development and preliminary validation. Psychological Assessment, 11(3), 326–338. [Google Scholar]
- Lin A, Adolphs R, & Rangel A (2012). Social and monetary reward learning engage overlapping neural substrates. Social Cognitive and Affective Neuroscience, 7(3), 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes PN, Salovey P, Côté S, Beers M, & Petty RE (2005). Emotion regulation abilities and the quality of social interaction. Emotion, 5(1), 113–118. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, King L, & Diener E (2005). The benefits of frequent positive affect: Does happiness lead to success? Psychological Bulletin, 131(6), 803–855. [DOI] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton III JL, … Blair RJ (2008). Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. American Journal of Psychiatry, 165(1), 90–98. [DOI] [PubMed] [Google Scholar]
- Morgan JK, Lee GE, Wright AG, Gilchrist DE, Forbes EE, McMakin DL, … Silk JS (2016). Altered positive affect in clinically anxious youth: The role of social context and anxiety subtype. Journal of Abnormal Child Psychology, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JP, Pelphrey KA, & MCCarthy G (2005). Regional brain activation evoked when approaching a virtual human on a virtual walk. Journal of Cognitive Neuroscience, 17(11), 1744–1752. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Almeida JR, Forbes EE, Versace A, Frank E, LaBarbara EJ, … Phillips ML (2012). Waiting to win: Elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disorders, 14(3), 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, Silk JS, Osterritter C, & Forbes EE (2015). Social reward in youth at risk for depression: A preliminary investigation of subjective and neural differences. Journal of Child and Adolescent Psychopharmacology, 25(9), 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, & Blakemore SJ (2012). Adolescent social cognitive and affective neuroscience: Past, present, and future. Social, Cognitive, and Affective Neuroscience, 7(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, & Scheres A (2014). Ventral–striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: A meta-analytic review of the fMRI literature. Neuroscience and Biobehavioral Reviews, 38, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, & Bauer DJ (2006). Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics, 31, 437–448. [Google Scholar]
- Puce A, Allison T, Bentin S, Gore JC, & McCarthy G (1998). Temporal cortex activation in humans viewing eye and mouth movements. Journal of Neuroscience, 18(6), 2188–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey JA, Rittenberg A, Hughes L, Damiano CR, Sabatino A, Miller S, … Dichter GS (2014). Common and distinct neural features of social and non-social reward processing in autism and social anxiety disorder. Social Cognitive and Affective Neuroscience, 9(3), 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimé B (2009). Emotion elicits the social sharing of emotion: Theory and empirical review. Emotion Review, 1(1), 60–85. [Google Scholar]
- Saxe R, Xiao DK, Kovacs G, Perrett DI, & Kanwisher N (2004). A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia, 42(11), 1435–1446. [DOI] [PubMed] [Google Scholar]
- Saygin AP (2007). Superior temporal and premotor brain areas necessary for biological motion perception. Brain, 130(9), 2452–2461. [DOI] [PubMed] [Google Scholar]
- Silk JS, Stroud LR, Siegle GJ, Dahl RE, Lee KH, & Nelson EE (2012). Peer acceptance and rejection through the eyes of youth: pupillary, eyetracking and ecological data from the Chatroom Interact task. Social Cognitive and Affective Neuroscience, 7(1), 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AR, Steinberg L, Strang N, & Chein J (2015). Age differences in the impact of peers on adolescents’ and adults’ neural response to reward. Developmental Cognitive Neuroscience, 11, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snjiders TA, & Bosker RJ (1999). Multilevel analysis: An introduction to basic and advanced multilevel modeling. London: Sage. [Google Scholar]
- Somerville LH, Jones RM, & Casey BJ (2010). A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition, 72(1), 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, Rademacher L, Irmak A, Konrad K, … Gründer G (2009). Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience, 4(2), 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L (2008). A social neuroscience perspective on adolescent risk-taking. Developmental Review, 28(1), 78–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH (2016). Dopaminergic reward sensitivity can promote adolescent health: A new perspective on the mechanism of ventral striatum activation. Developmental Cognitive Neuroscience, 17, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, & Galván A (2014). Neural sensitivity to eudaimonic and hedonic rewards differentially predict adolescent depressive symptoms over time. Proceedings of the National Academy of Sciences, 111(18), 6600–6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, & Meyer-Lindenberg A (2008). Know your place: neural processing of social hierarchy in humans. Neuron, 58(2), 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]


