Abstract
The viability of pathogenic fungi in the scale was investigated during topical administration of 1% luliconazole (LLCZ). Thirteen tinea pedis patients found to be positive on KOH examination were assessed by mycological examinations and quantitative real-time polymerase chain reaction (PCR) targeted internal transcribed spacer (ITS) in ribosomal RNA gene at the initial visit and after 2 and 4 weeks of treatment. Assays showed that the average copy number of ITS DNA had significantly decreased to 22.9% at 2 weeks and 4.8% at 4 weeks compared with the initial visit. LLCZ topical treatment could defeat almost pathogenic dermatophytes in the scales within 4 weeks.
Keywords: dermatophyte, quantitative real-time PCR, viability, tinea pedis, luliconazole
Luliconazole (LLCZ), (-)-(E)-[(4R)-4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene] (1H-imidazol-1-yl) acetonitrile), is an imidazole antifungal agent that interferes with ergosterol biosynthesis in Trichophyton species and Candida albicans by inhibition of sterol 14 alpha-demethylase.1,2 The in vitro antifungal activity of LLCZ against dermatophytes is reportedly superior to that of other topical antifungal agents.3–5 Previous clinical trials have reported that the topical application of 1% LLCZ to tinea pedis patients once a day for 14 days had improved skin lesions and mycological cure (negative result by microscopy), as assessed at week 4, at rates of 90.5% and 79.7%, respectively.6 The minimum fungicidal concentrations (MFC) of LLCZ are reportedly 0.0020–0.0160 μg/ml for Trichophyton rubrum and Trichophyton mentagrophytes isolates (n = 14, respectively),7 which are the most frequently isolated dermatophyte species, accounting for more than 90% of patients with tinea pedis.8–10 Pharmacokinetically, the concentration of LLCZ in the stratum corneum is estimated to be approximately 130 μg/cm3 (calculated at a stratum corneum thickness of 20 μm) at 24 hours after topical application.11 Because the concentration of LLCZ in the stratum corneum is 8000-fold greater than the MFC for dermatophyte species, pathogenic dermatophytes in the scales should be completely eradicated by topical LLCZ treatment. However, the viability of pathogenic fungi during LLCZ topical treatment remains unknown, as no appropriate method to evaluate the viability of fungi in scales has yet been developed. In our previous report, we developed a quantitative real-time polymerase chain reaction (qPCR) method targeting the internal transcribed spacer (ITS) of ribosomal RNA, and it could be in accordance with dermatophyte viability assay, colony forming unit.12 With this method, dermatophyte viability was evaluated in toenail lesions at 16 weeks after oral TBF treatment, which showed that one-third of dermatophytes in the nail specimens had survived, despite TBF concentration in the nails of 10-fold greater than the MFC.13 Hence, the aim of the present study was to assess the viability of pathogenic fungi during LLCZ topical treatment for tinea pedis.
The study cohort was limited to patients with tinea pedis, as confirmed by positive KOH examinations, who visited the Division of Dermatology, Kanazawa Medical University Hospital, from August 2012 to December 2015. The main exclusion criterion was the use of antimycotic therapy during the previous 12 months. After providing written informed consent, patients were treated with topical 1% LLCZ cream, solution, or ointment once daily for 4 weeks. Scales were collected from the affected sites at the initial visit and at 2 and 4 weeks after initiation of therapy. Each scale was subjected to KOH examination, fungal culture, and qPCR assays. If sufficient scales could not be obtained for these three assays by improvement of tinea pedis, the scales were used for KOH examinations and qPCR assays preferentially. The specimens of up to 10 mg were collected from the lesions for DNA extraction. Then ITS DNA was quantified by qPCR using the primers, ITS-forward (5′-AGCCCGGCTTGTGTGATG-3′) and ITS-reverse (5′-CATTCGCCTAGGAAGCCG-3′) and a 7900HT Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA) with a SYBR Green PCR Kit (Qiagen) as described previously.13 The copy number per 1 mg specimen was calculated using standard curves. Significant differences were determined using one-way analysis of variance (ANOVA) followed by Tukey–Kramer post hoc analysis. This study protocol was approved by the Ethics Committee of Kanazawa Medical University and performed in accordance with the guidelines of the Helsinki Declaration.
In total, 13 patients with tinea pedis (seven men, six women; median age, 66 years; age range, 48–81 years) were enrolled in this study and treated with 1% topical LLCZ. The eight patients were diagnosed as intertriginous type of tinea pedis, five were vesiculobullous type, and four patients also had tinea unguium. Because total two patients did not visit for following examinations, 12 and 11 patients evaluated at 2 and 4 weeks, respectively. At week 4, all evaluated patients improved clinically compared with the initial visit, and no scale was observed from one patient reached complete cure. At the start of the study (initial visit), 13 of 13 (100%) patients had positive results for the KOH examination, while 10 of 13 (77%) were positive for fungal culture, and 13 of 13 (100%) were positive by qPCR. After 2 weeks of treatment, none of the 10 evaluated samples was positive for fungal culture, while 10 of 12 (83%) were positive on KOH examination, and 11 of 12 (92%) were positive by qPCR. At 4 weeks, 3 of 10 (30%) patients were positive on KOH examination, 1 of 7 (14%) was positive for fungal culture, and 4 of 10 (40%) were positive by qPCR. Six patients were negative for mycological examinations and qPCR at week 4 (Table 1).
Table 1.
Positive rate.
| Initial visit (13 patients) | At 2 weeks (12 patients) | At 4 weeks (11 patients) | |
|---|---|---|---|
| KOH | 100% (13/13) | 83% (10/12) | 30% (3/10) |
| Culture | 77% (10/13) | 0% (0/10) | 14% (1/7) |
| qPCR | 100% (13/13) | 92% (11/12) | 40% (4/10) |
qPCR, quantitative real-time PCR.
At the initial visit, the dermatophyte ITS DNA copy number/mg of the 13 scales ranged from 599 to 244,511, which significantly decreased to 22.9% and 4.8% after 2 and 4 weeks of treatment, respectively (P < .01, one-way ANOVA with Tukey–Kramer post hoc analysis) (Fig. 1).
Figure 1.
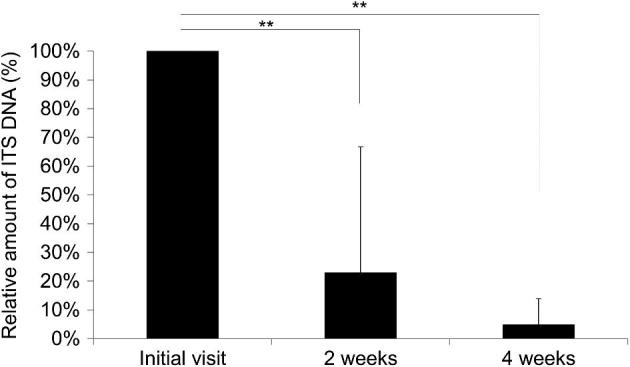
ITS DNA amount after 1% topical LLCZ treatment. Patients were treated with 1% topical LLCZ for 4 weeks. The amount of ITS DNA in scales was measured at 0, 2, and 4 weeks by qPCR, and calculated relative to the initial visit (100%). Each bar represents the mean ± standard deviation of duplicate samples with results analyzed statistically by one-way ANOVA with Tukey–Kramer post hoc analysis. **P < .01. n = 12 at initial visit and 2 weeks, and n = 10 at 4 weeks.
The results of the present study showed that the qPCR assay was more sensitive than KOH examination and fungal culture to assess the viability of pathogenic fungi during LLCZ topical treatment.14,15 Moreover, the wide range of ITS DNA copy numbers from the 13 patients at the initial visit were in agreement with the results of a previous study of 10 patients with tinea pedis.16 There was no significant difference in the ITS DNA copy number (599–244,511 copies/mg) in the infected specimens from tinea pedis patients at the initial visit as compared to that of tinea unguium.13
LLCZ eradiated almost pathogenic dermatophytes in the scales, and six patients had negative results for the mycological examinations and qPCR at week 4. There were no significant difference in sex, age, infection type, and combination of tinea unguium between patients with negative and positive results for mycological examinations and qPCR assays.
Approximately 5% of dermatophytes in the scales survived despite topical application of 1% LLCZ, which achieved a concentration of approximately 8000-fold greater than the MFC.7,11 A previous study found that 36% of dermatophytes in the nail specimens of onychomycosis patients had survived 16 weeks of oral TBF treatment.13 There are some supportive facts to better interpret these results. Dermatophytes produce arthroconidia from hyphae in infected scales.17 It is difficult to completely eradicate arthroconidia because these are resistant to antimycotics in the dormant stage.18–20 However, in response to antimycotic administration, arthroconidia remain dormant, as resistance is completely lost upon germination.18 Overall, these findings suggest that the application of antifungal agents can lead to complete cure by eliminating pathogenic fungi (assumed to be in hyphae form) in the lesion site, and the infected stratum corneum and nail are renewed if surviving pathogenic fungi (assumed to be arthroconidia) remain in a dormant state that is alive but not growing by antifungal agents. Topical or oral antifungal treatment should be continued until desquamation of the infected site because dermatophytosis can recur by germination of surviving arthroconidia.
In conclusion, the results of the present study revealed the cure mechanism of tinea pedis by LLCZ topical treatment. Approximately 95% of pathogenic dermatophytes in the scale were eliminated after 4 weeks of 1% LLCZ topical treatment, and LLCZ resulted in approximately 5% of surviving dermatophytes in the dormant state until desquamation of the infected scales.
Declaration of interest
T.I. is an employee in POLA CHEMICAL INDUSTRIES, INC. now, and was in POLA PHARMA, INC.
References
- 1. Niwano Y, Kuzuhara N, Kodama H, Yoshida M, Miyazaki T, Yamaguchi H. In vitro and in vivo antidermatophyte activities of NND-502, a novel optically active imidazole antimycotic agent. Antimicrob Agents Chemother. 1998; 42: 967–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Niwano Y, Koga H, Kodama H, Kanai K, Miyazaki T, Yamaguchi H. Inhibition of sterol 14 alpha-demethylation of Candida albicans with NND-502, a novel optically active imidazole antimycotic agent. Med Mycol. 1999; 37: 351–355. [DOI] [PubMed] [Google Scholar]
- 3. Uchida K, Nishiyama Y, Yamaguchi H. In vitro antifungal activity of luliconazole (NND-502), a novel imidazole antifungal agent. J Infect Chemother. 2004; 10: 216–219. [DOI] [PubMed] [Google Scholar]
- 4. Koga H, Tsuji Y, Inoue K et al.. In vitro antifungal activity of luliconazole against clinical isolates from patients with dermatomycoses. J Infect Chemother. 2006; 12: 163–165. [DOI] [PubMed] [Google Scholar]
- 5. Koga H, Nanjoh Y, Makimura K, Tsuboi R. In vitro antifungal activities of luliconazole, a new topical imidazole. Med Mycol. 2009; 47: 640–647. [DOI] [PubMed] [Google Scholar]
- 6. Watanabe S, Takahashi H, Nishikawa T et al.. Dose-finding comparative study of 2 weeks of luliconazole cream treatment for tinea pedis: comparison between three groups (1%, 0.5%, 0.1%) by a multi-center randomised double-blind study. Mycoses. 2007; 50: 35–40. [DOI] [PubMed] [Google Scholar]
- 7. Maeda J, Nanjoh Y, Koga H, Toga T, Makimura K, Tsuboi R. [In vitro antifungal activity of luliconazole against Trichophyton spp]. Med Mycol J. 2016; 57: J1–6. [DOI] [PubMed] [Google Scholar]
- 8. Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008; 51: 2–15. [DOI] [PubMed] [Google Scholar]
- 9. Sei Y. [2011 Epidemiological survey of dermatomycoses in Japan]. Med Mycol J. 2015; 56: J129–135. [DOI] [PubMed] [Google Scholar]
- 10. Cai W, Lu C, Li X et al.. Epidemiology of superficial fungal infections in Guangdong, Southern China: a retrospective study from 2004 to 2014. Mycopathologia. 2016; 181: 387–395. [DOI] [PubMed] [Google Scholar]
- 11. Pharmaceuticals and Medical Devices Agency in Japan Drug Information of Lulicon. http://www.info.pmda.go.jp/go/pack/2655712M1025_1_03/. Accessed February 13, 2019. [Google Scholar]
- 12. Iwanaga T, Anzawa K, Mochizuki T. Quantification of dermatophyte viability for evaluation of antifungal effect by quantitative PCR. Mycopathologia. 2014; 177: 241–249. [DOI] [PubMed] [Google Scholar]
- 13. Iwanaga T, Ushigami T, Anzawa K, Mochizuki T. Pathogenic dermatophytes survive in nail lesions during oral terbinafine treatment for tinea unguium. Mycopathologia. 2017; 182: 673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luk NM, Hui M, Cheng TS, Tang LS, Ho KM. Evaluation of PCR for the diagnosis of dermatophytes in nail specimens from patients with suspected onychomycosis. Clin Exp Dermatol. 2012; 37: 230–234. [DOI] [PubMed] [Google Scholar]
- 15. Dhib I, Fathallah A, Yaacoub A, Hadj Slama F, Said MB, Zemni R. Multiplex PCR assay for the detection of common dermatophyte nail infections. Mycoses. 2014; 57: 19–26. [DOI] [PubMed] [Google Scholar]
- 16. Miyajima Y, Satoh K, Uchida T et al.. Rapid real-time diagnostic PCR for Trichophyton rubrum and Trichophyton mentagrophytes in patients with tinea unguium and tinea pedis using specific fluorescent probes. J Dermatol Sci. 2013; 69: 229–235. [DOI] [PubMed] [Google Scholar]
- 17. Tate P. The dermatophytes or ringworm fungi. Biol Rev. 1929; 4: 41–74. [Google Scholar]
- 18. Hashimoto T, Blumenthal HJ. Survival and resistance of Trichophyton mentagrophytes arthrospores. Appl Environ Microbiol. 1978; 35: 274–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seebacher C. Action mechanisms of modern antifungal agents and resulting problems in the management of onychomycosis. Mycoses. 2003; 46: 506–510. [DOI] [PubMed] [Google Scholar]
- 20. Fernandez-Torres B, Inza I, Guarro J. Comparison of in vitro antifungal susceptibilities of conidia and hyphae of dermatophytes with thick-wall macroconidia. Antimicrob Agents Chemother. 2003; 47: 3371–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]


