ABSTRACT
Background
Controversial findings have been reported in human and animal studies regarding the influence of n–6 (ω-6) to n–3 (ω-3) fatty acid ratios on obesity and health. Two confounding factors may be related to interactions with other dietary lipid components or sex-specific differences in fatty acid metabolism.
Objective
This study investigated main and interactive effects of total dietary lipid, ratio of n–6 to n–3 fatty acids, and sex on growth, adiposity, and reproductive health in wild-type zebrafish.
Methods
Male and female zebrafish (3 wk old) were fed 9 diets consisting of 3 ratios of n–6 to n–3 fatty acids (1.4:1, 5:1, and 9.5:1) varied within 3 total lipid amounts (80, 110, and 140 g/kg) for 16 wk. Data were then collected on growth, body composition (determined by chemical carcass analysis), and female reproductive success (n = 32 breeding events/diet over 4 wk). Main and interactive effects of dietary lipid and sex were evaluated with regression methods. Significant differences within each dietary lipid component were relative to the intercept/reference group (80 g/kg and 1.4:1 ratio).
Results
Dietary lipid and sex interacted in their effects on body weight (P = 0.015), total body length (P = 0.003), and total lipid mass (P = 0.029); thus, these analyses were stratified by sex. Female spawning success decreased as dietary total lipid and fatty acid ratio increased (P = 0.030 and P = 0.026, respectively). While total egg production was not associated with either dietary lipid component, females fed the 5:1 ratio produced higher proportions of viable embryos compared with the 1.4:1 ratio [median (95% CI): 0.915 (0.863, 0.956) vs 0.819 (0.716, 0.876); P < 0.001].
Conclusions
Further characterization of dietary lipid requirements will help define healthy balances of dietary lipid, while the sex-specific responses to dietary lipid identified in this study may partially explain sex disparities in the development of obesity and its comorbidities.
Keywords: zebrafish, diet-induced obesity, dietary lipid composition, reproductive health, body composition
Introduction
Defining the effects of dietary lipid on adiposity and human health remains a fundamental challenge. Conflicting results from studies evaluating the effects of diets with high lipid content on weight gain and metabolism have led to multiple debates regarding the magnitude to which dietary lipid contributes to obesity. Recent publications have noted that different responses to dietary lipid may be attributed to variations in fatty acid composition profiles, suggesting that lipid quality may be equally important as lipid quantity in the influence of obesity development and metabolic syndrome (1–3). Individual fatty acids can vary significantly in oxidation and deposition rates, which may, in turn, influence outcomes in weight gain, adiposity, and health in both human and animal models (1, 2, 4–6).
In mammals and fish, n–6 and n–3 fatty acids are crucial for nutritional health, physiology, and reproduction (7). They are categorized as essential and must be obtained through diet (8). Several studies have proposed that imbalances in the ratio of n–6 to n–3 fatty acids can negatively impact health. Empirical and epidemiological data suggest that an excess intake of n–6 fatty acids increases inflammation in metabolic tissues (9–12); however, results from other studies indicate that arachidonic acid (ARA; 20:4n–6) also serves as a precursor for a group of potent anti-inflammatory molecules. Significant associations of increased dietary n–6 fatty acid content with both decreased inflammation and increases in lean tissue mass have also been observed (13–15). In addition to metabolic health, fertility and reproductive performance have also been linked to the ratio of n–6 to n–3 fatty acids in multiple species (16–20). As previous research has demonstrated that n–6 and n–3 fatty acid intake can significantly impact physiology and disease, a better understanding is needed regarding whether a specific ratio of n–6 to n–3 fatty acids is required for optimal health, and whether the responses of these ratios on health are modified by the total amount of dietary fat (21).
Sexual dimorphisms in the response to dietary manipulation must also be considered for obesity-related outcomes. Males and females differ in terms of how and where body fat is stored, hormone secretion in response to fat, and how the brain responds to signals that regulate body fat (22, 23). While sex-specific responses to dietary manipulation have been observed in multiple species, the influence of sex on the association between obesity-related phenotypes and dietary lipid composition has not been well defined (1, 24, 25). Therefore, when studying the impacts of dietary lipid manipulation on obesity, any potential sexually dimorphic responses should be considered (24).
To address gaps in our knowledge regarding the impacts of dietary lipid composition on obesity and metabolic health, many researchers have turned to animal models to answer these questions. In recent years, results from multiple studies have demonstrated that zebrafish are a powerful model for diet-induced obesity in humans and also offer multiple advantages over rodent models (26–31). Similar to humans, zebrafish exhibit increased weight gain, adiposity, and serum triglycerides (TGs) when fed a high-fat diet (HFD), as well as early evidence of metabolic diseases (26, 30). In this study, we examined both the individual and combined effects of total dietary lipid and dietary ratios of n–6 to n–3 fatty acids on weight gain, body composition, and reproductive success in juvenile male and female wild-type zebrafish. Results from this study will further define dietary lipid requirements for zebrafish in a research setting and translate this information to studies in human nutrition and health.
Methods
Diet preparation
Nine chemically defined experimental diets were formulated and produced using purified and semipurified ingredients (Supplemental Table 1). To ensure that all other macronutrients remained constant among diets, experimental diets were formulated with a single, common base mix. Total dietary lipid amounts were adjusted with Alpha-CelTM, a nonnutritive bulking agent (MP Biomedicals, LLC). Safflower oil (food grade; MP Biomedicals, LLC) served as the primary source of n–6 fatty acids, while menhaden fish oil (Virginia Prime® Gold Fish Oil; Omega Protein, Inc.) supplied the primary source of n–3 fatty acids. The amounts of each oil were adjusted to achieve the desired amount of total lipid and n–6 to n–3 fatty acid ratio for each diet. Diet analysis for crude fat and fatty acid composition was performed by Eurofins Scientific Laboratories, Inc. (Supplemental Table 2). Crude fat was determined by ethyl ether extraction, while fatty acid composition was determined by GC according to the American Oil Chemists' Society methods Ce 2–66 and Ce 1–62 (32, 33). Analyses were performed for the 4 diets considered the “extremes” as a cost-saving measure, and values for other diets were interpolated.
The ingredients for the base mix were combined first using a KitchenAid Professional 600 Series Orbital Mixer (Whirlpool, Inc.). Next, the safflower and menhaden oils were added to each diet using a Cuisinart Food Processor (Conair, Inc.). Diets were then extruded with a KitchenAid Extruder (KPEXTA; Whirlpool, Inc.) fitted with the pasta-maker attachment. Feed strands were air-dried on wire trays for 24 h and then stored at 4°C in air-tight storage bags until use. Prior to feeding, diets were ground to a powder (250–500 μm, sieved).
Experimental protocols
This study was conducted using recommendations of the Guide for the Care and Use of Laboratory Animals, NRC (34). All procedures abided by standard requirements for husbandry and euthanasia (34, 35). Protocols were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham (UAB).
Zebrafish were housed in 4 re-circulating systems with mechanical, chemical, and biological filtration and UV sterilization (Aquaneering, Inc.) on a 14-h light/10-h dark schedule. Tanks were siphoned weekly to remove uneaten food/debris, and flow rates were adjusted to provide a minimum of 2 water changes/h per tank. Sodium bicarbonate was added to adjust pH to the desired level as needed. At minimum, 20% of conditioned system water was exchanged once weekly from each system. Prior to being added to the re-circulating systems, municipal tap water was filtered through a reverse osmosis unit (Kent Marine) and conductivity was adjusted with synthetic sea salts (Instant Ocean). Water-quality parameters (given in Table 1 and Supplemental Table 3) were maintained at recommended levels (35) in all re-circulating systems throughout the experiment.
TABLE 1.
Experimental water quality conditions for each recirculating system1
| Recirculating system | |||||
|---|---|---|---|---|---|
| Parameter | Target range | 1 | 2 | 3 | 4 |
| pH | 6.80–8.50 | 7.45 ± 0.01 | 7.40 ± 0.01 | 7.39 ± 0.01 | 7.40 ± 0.01 |
| Conductivity, mS/cm | 1.40–1.50 | 1.42 ± 0.01 | 1.50 ± 0.01 | 1.52 ± 0.01 | 1.49 ± 0.01 |
| Salinity, ppt | 0.60–0.70 | 0.69 ± 0.003 | 0.70 ± 0.003 | 0.70 ± 0.004 | 0.70 ± 0.003 |
| Temperature,°C | 27–28 | 27.4 ± 0.02 | 27.2 ± 0.02 | 27.2 ± 0.02 | 27.9 ± 0.03 |
| TAN, mg/L | ∼ 0 | 0.01 ± 0.01 | 0.03 ± 0.03 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| Nitrite, mg/L | ∼ 0 | 0.19 ± 0.08 | 0.33 ± 0.09 | 0.36 ± 0.07 | 0.22 ± 0.08 |
| Nitrate, mg/L | < 200 | 101 ± 1.25 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Alkalinity, mg/L CaCO3 | 50–150 | 50–52 | 50–52 | 50–52 | 50–52 |
Values are mean ± SEM obtained over the course of the experiment. mS, milliSiemens; ppt, parts per thousand; TAN, total ammonia nitrogen.
Zebrafish embryos (AB strain) were acquired from the Zebrafish Research facility at UAB. Due to the large sample size required for the study, zebrafish were divided into 4 cohorts and stocked once weekly over a 4-wk period. Embryos were collected, maintained until 5 d postfertilization (dpf), and then polycultured with the rotifer Branchionus plicatilis in 6-L static tanks (240 larvae/tank), as previously described (36). Beginning at 11 dpf, each tank of fish was fed 25 mL of concentrated stage I Artemia nauplii (>500 nauplii per fish) 3 times daily. At 22 dpf, all fish were combined and 56 fish were randomly sampled to obtain initial wet weights. The remaining fish were randomly distributed into 2.8-L tanks at a density of 14 fish/tank (36 tanks/cohort, 144 tanks total for the study). After stocking, tanks of fish were randomly assigned to 1 of 9 diets (n = 16 tanks and 224 fish total per diet; within each cohort, n = 4 tanks and 56 fish per diet) and haphazardly, but evenly, distributed across rack positions on all 4 re-circulating systems. Each system held 4 tanks/diet, or 36 tanks/total.
During the 16-wk feeding trial (initiated at 23 dpf), each tank of fish was fed a daily ration of ∼5–7% of body weight, with half the ration fed at ∼0900 h and the other half at ∼1700 h. Fish weights from each diet were monitored weekly to maintain this ration throughout the feeding trial. Using Excel's RANDBETWEEN function, 25% of fish tanks from each diet were randomly selected each week to be weighed as a group. The daily ration was adjusted weekly from the average group wet weight calculated for each diet. Rations were measured with a powder measure (Lee Precision, Inc.) calibrated to dispense the ration for each diet. To minimize cross-contamination, each dietary lipid amount had its own powder measure.
Experiment termination
At the termination of the feeding trial, fish were 4.5 mo of age. Sex and terminal measures of body mass (BM) and total body length (TBL; measured from tip of the snout to tip of the top of the caudal fin) were determined individually for all fish in the study as previously described (36). After recording this information, fish were randomized to evaluation of body composition, reproductive success, or additional outcomes not discussed in the current paper. Zebrafish randomized to reproductive success were returned to the re-circulating system. Fish assigned to all other outcomes were killed by rapid submersion in ice water for a minimum of 10 min (after which time cessation of all opercular movement was observed) and then stored at −20°C until analysis.
Body-composition assessment
Twelve males and 12 females from each diet (3 males and 3 females per cohort) were analyzed for body composition (assessed gravimetrically as total body lipid and TG mass). Female zebrafish were ovariectomized prior to analysis. After removal from −20°C storage, total body lipid was extracted from whole-fish samples with chloroform and methanol (37) using a protocol modified for zebrafish that has been described elsewhere (38). TG mass was determined from each total lipid sample by solid-phase extraction with chloroform and methanol (39).
Evaluation of reproductive performance
From each diet, 40 females (n = 10/tank, 1 tank/cohort) were reserved for evaluation of reproductive success over a 4-wk breeding period. During the breeding period, females were maintained under the same feeding regime and husbandry conditions as described for the feeding trial. As we were primarily interested in the effects of dietary lipid composition on egg production and embryo quality, experimental males were not used in our evaluation of reproductive performance. Instead, randomly selected females from each diet were paired with Artemia-fed broodstock males (AB strain and 4–6 mo of age) from the Aquatic Animal Resource Core at UAB. Each breeding pair represented 1 breeding event, with n = 32 breeding events evaluated per diet. Protocols for breeding and embryo assessment have been described elsewhere (36). Females evaluated for reproduction were killed at the end of the breeding period as previously described.
Spawning success rate was defined as the proportion of successful breeding events (eggs released) to total breeding events. Total egg production represented the number of eggs produced from each individual clutch (breeding event). Embryo viability was determined from successful breeding events as the proportion of viable embryos to total eggs. Embryos exhibiting a stage of development consistent with the pharyngula period at 24–30 h postfertilization (hpf) were considered viable (40).
Statistical analysis
Sample sizes for growth and body composition parameters are given in Table 2. Lengths for 16 male and 27 female zebrafish were unable to be measured from photographs. Measures of total lipid mass (TLM) were missing from 9 samples (6 males and 3 females) due to loss in storage, while TG mass was unable to be measured in 26 samples (10 males and 16 females) due to malfunction of sample-processing equipment.
TABLE 2.
Sample sizes of male and female zebrafish evaluated for outcomes in growth and body composition, by dietary lipid component and individual diets1
| Wet body mass, n | Total body length, n | Total lipid mass, n | TG mass, n | |||||
|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | |
| TDL (g/kg) | ||||||||
| 80 | 239 | 370 | 234 | 353 | 35 | 36 | 32 | 28 |
| 110 | 238 | 369 | 232 | 363 | 32 | 33 | 32 | 33 |
| 140 | 222 | 356 | 217 | 352 | 35 | 36 | 28 | 28 |
| RFA | ||||||||
| 1.4:1 | 206 | 396 | 197 | 385 | 34 | 35 | 27 | 28 |
| 4:1 | 261 | 345 | 257 | 341 | 34 | 36 | 34 | 36 |
| 9.5:1 | 232 | 354 | 229 | 342 | 35 | 34 | 31 | 30 |
| Individual diet | ||||||||
| 80 g/kg TDL | ||||||||
| 1.4:1 | 69 | 126 | 66 | 119 | 12 | 12 | 9 | 9 |
| 5:1 | 92 | 117 | 91 | 116 | 11 | 12 | 11 | 12 |
| 9.5:1 | 78 | 127 | 77 | 118 | 12 | 12 | 12 | 12 |
| 110 g/kg TDL | ||||||||
| 1.4:1 | 70 | 138 | 66 | 137 | 10 | 11 | 10 | 11 |
| 5:1 | 89 | 120 | 87 | 118 | 12 | 12 | 12 | 12 |
| 9.5:1 | 79 | 111 | 79 | 108 | 10 | 10 | 10 | 10 |
| 140 g/kg TDL | ||||||||
| 1.4:1 | 67 | 132 | 65 | 129 | 12 | 12 | 8 | 8 |
| 5:1 | 80 | 108 | 79 | 107 | 11 | 12 | 11 | 12 |
| 9.5:1 | 75 | 116 | 73 | 116 | 12 | 12 | 9 | 8 |
RFA, dietary ratio of n–6 to n–3 fatty acids; TDL, total dietary lipid; TG, triglyceride.
All data analyses were performed with R Statistical Software (R Core Team, 2016, version 3.4.2) and were 2-tailed, with P < 0.05 considered statistically significant. Figures were produced with the “ggplot2,” “ggpubr,” and “metR” packages in R (41–43). All analyses evaluated main and interactive effects of total dietary lipid and ratio of n–6 to n–3 fatty acids. Diet-by-sex interactions were analyzed when applicable, and when significant, analyses for these outcomes were stratified by sex. All models controlled for cohort as either a random effect (growth outcomes) or fixed effect (body composition and reproduction outcomes). Unless otherwise indicated, outcomes calculated as percentages were log-transformed prior to analysis. For categorical variables (both dietary lipid components and week), differences were analyzed relative to the reference group (intercept).
Differences in BM and length were evaluated with linear mixed-effects regression analysis [“lme4” and “lmerTest” packages in R (44, 45)]. Analyses of both outcomes controlled for tank as a random effect. Body-composition differences were assessed with additive effects regression analysis [“gamlss” package in R (46)], with the most parsimonious model selected for each outcome.
Differences in total egg production were evaluated with a zero-inflated negative binomial regression model (46, 47), while embryo viability was assessed with a zero-inflated B regression analysis (46). The zero-inflated B regression model used the parameters μ (location) and ε (scale) to compare expected proportions of viable embryos to the reference group. The zero-inflated components used logistic regression to compare probability of a successful spawn with the reference group. Models for reproduction outcomes also controlled for week as a fixed effect.
Results
Survivorship surpassed 95% for all diets (data not shown), which was comparable to what has been observed in other zebrafish nutrition studies (14, 36). All diets promoted growth and weight gain over the course of the study, with no apparent limitations in palatability observed.
Both wet BM (Figure 1 and Supplemental Table 4) and TBL were significantly higher in female zebrafish (BM: males = 331 ± 3.94 mg and females = 509 ± 5.65 mg; TBL: males = 32.6 ± 0.120 mm and females = 34.8 ± 0.123 mm; P < 0.001 for both outcomes). Sex-by-diet interactions were observed for both outcomes (BM: P-interaction = 0.015; TBL, P-interaction = 0.003). In males, BM was negatively associated with total dietary lipid and ratio of n–6 to n–3 fatty acids; additionally, these dietary lipid components significantly interacted in their effects on BM. Female BM was not significantly associated with either dietary lipid component, although a trend for a negative association with total dietary lipid was observed (P-trend = 0.060). In both sexes, total dietary lipid was negatively associated with TBL and a significant interaction with the ratio of n–6 to n–3 fatty acids was observed (males, P-interaction = 0.003; females, P-interaction = 0.020) (Supplemental Table 4).
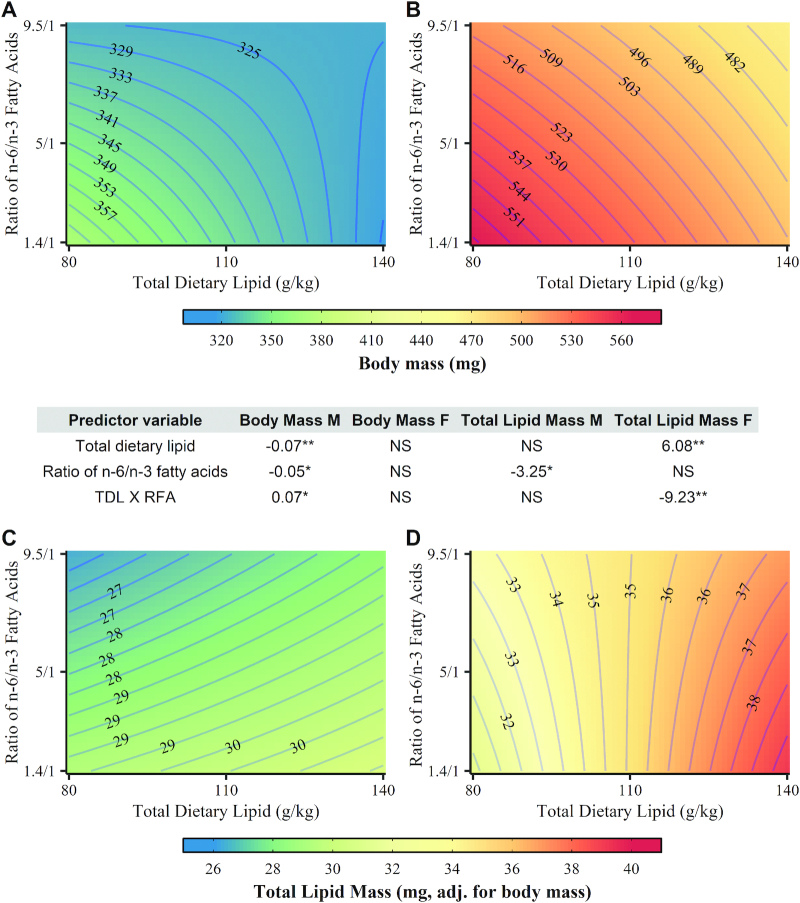
FIGURE 1.
Main and interactive effects of TDL and RFA on terminal body mass and body composition in male and female zebrafish. Contour plots: (A) male body mass; (B) female body mass; (C) male total lipid mass; and (D) female total lipid mass. Table values represent parameter estimates from regression analysis. *,**Significant change from reference group (80 g/kg TDL or 1.4/1 RFA): *P < 0.05, **P < 0.01. NS, P > 0.05. adj., adjusted; RFA, ratio of n–6 to n–3 fatty acids; TDL, total dietary lipid.
Body composition
Diet and sex significantly interacted in their effects on TLM (P-interaction = 0.029). TLM was negatively associated with the ratio of n–6 to n–3 fatty acids in male zebrafish and positively associated with total dietary lipid in female zebrafish (Figure 1 and Supplemental Table 5). A significant total dietary lipid by ratio of n–6 to n–3 fatty acids was also observed in females. Similar to TLM, TG mass in males was inversely associated with the ratio of n–6 to n–3 fatty acids (Table 3 and Supplemental Table 6). In contrast to TLM, TG mass in females was not significantly associated with either dietary lipid component, and no evidence of a significant total dietary lipid by ratio of n–6 to n–3 fatty acid interaction was observed in either sex (males, P-interaction = 0.15; females, P-interaction = 0.06).
TABLE 3.
Terminal triglyceride mass content of male and female zebrafish by dietary lipid component1
| Percentage of dry body mass | ||
|---|---|---|
| Male | Female | |
| Total dietary lipid (g/kg) | ||
| 80 (reference) | 9.92 ± 0.625 | 9.87 ± 0.856 |
| 110 | 11.2 ± 0.869 | 12.11 ± 0.876 |
| 140 | 9.87 ± 0.608 | 11.7 ± 0.741 |
| Dietary ratio of n–6 to n–3 fatty acids | ||
| 1.4:1 (reference) | 11.9 ± 0.804 | 12.3 ± 0.971 |
| 5:1 | 10.8 ± 0.660 | 11.5 ± 0.852 |
| 9.5:1 | 8.51 ± 0.596** | 9.75 ± 0.645 |
Values are percentage mean ± SEM and represent measures obtained at termination of the 16-wk feeding trial. Differences were evaluated with additive effects regression and analyzed separately in males and females. **Different from the reference group (P < 0.01) within each dietary lipid component. NS, P > 0.05.
Reproduction
Both total dietary lipid and ratio of n–6 to n–3 fatty acids significantly predicted spawning probability (Table 4). In contrast, egg production was not significantly associated with either dietary lipid component, while embryo viability was only significantly associated with the ratio of n–6 to n–3 fatty acids (Figure 2 and Supplemental Table 7). Females fed diets with an n–6 to n–3 ratio of 5:1 produced the highest proportion of viable embryos. An interaction between the 5:1 ratio of n–6:n–3 ratio and 80 g/kg of total dietary lipid was also observed, as females fed this diet produced the highest proportion of viable embryos (Supplemental Table 8).
TABLE 4.
Spawning success of female zebrafish by dietary lipid component and week1
| Successful/total breeding events | Proportion successful | Estimate2 (SE) | P | |
|---|---|---|---|---|
| Total dietary lipid (g/kg) | ||||
| 80 (reference) | 72/96 | 0.75 | ||
| 110 | 67/96 | 0.70 | −0.22 (0.33) | 0.51 |
| 140 | 57/96 | 0.59 | −0.70 (0.32) | 0.030 |
| Dietary ratio of n–6 to n–3 fatty acids | ||||
| 1.4:1 (reference) | 75/96 | 0.78 | ||
| 5:1 | 61/96 | 0.64 | −0.69 (0.33) | 0.038 |
| 9.5:1 | 60/96 | 0.63 | −0.74 (0.33) | 0.026 |
| Week | ||||
| 1 (reference) | 39/72 | 0.54 | ||
| 2 | 51/72 | 0.71 | 0.82 (0.36) | 0.024 |
| 3 | 50/72 | 0.69 | 0.68 (0.36) | 0.06 |
| 4 | 53/72 | 0.74 | 0.97 (0.37) | 0.009 |
Assessed over a 4-wk breeding period following termination of the 16-wk feeding trial. Successful events = female released eggs; n = 32 total breeding events per diet.
Estimates are from the logistic component of zero-inflated negative binomial regression analysis and represent probability of a successful spawn relative to the reference group within each predictor variable. Significant P values (P < 0.05) represent differences from the reference group.
FIGURE 2.
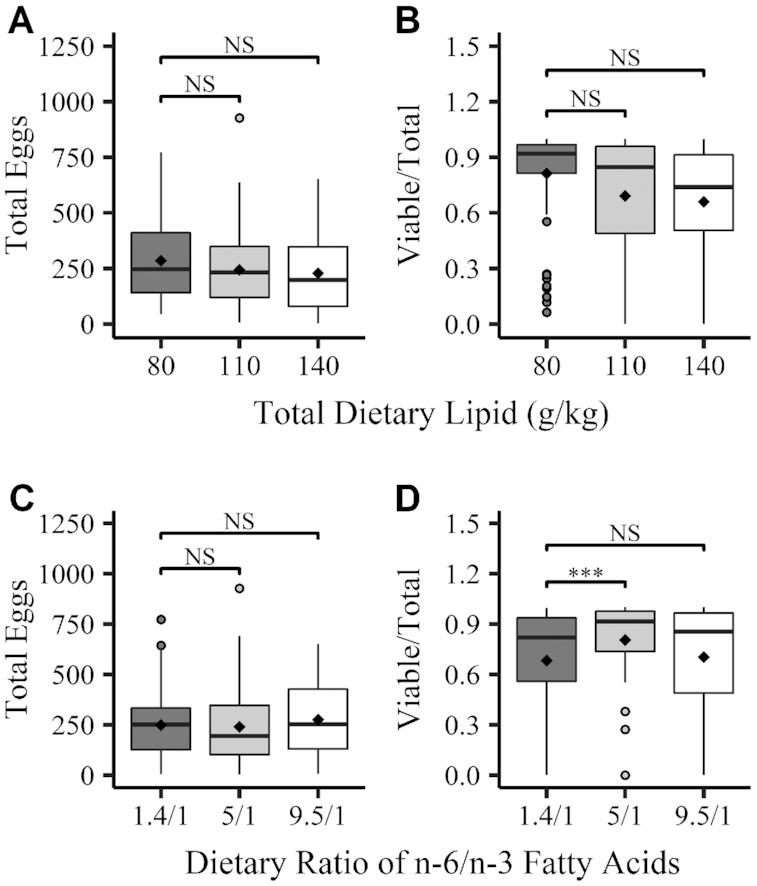
Effects of total dietary lipid and ratio of n–6 to n–3 fatty acids on reproductive success in female zebrafish. (A, C) Boxplots for total egg production. (B, D) Boxplots for embryo viability (ratio of viable eggs/total eggs produced). Data are from successful breeding events and represent counts from individual events. Boxes represent the IQR of values (bottom, lower quartile/25th percentile; center bar, median value/50th percentile; top, upper quartile/75th percentile). Upper/lower whiskers signify values within 1.5 IQR of the upper/lower quartiles. Outliers (closed circles) and mean values (diamond markers) are also shown on plots. Differences from the reference group within each dietary lipid component were evaluated with zero-inflated regression analysis. ***Different from 1.4:1 ratio, P < 0.001. NS, P > 0.05.
It was also noted that breeding trial week was significantly associated with egg production and spawning success. Compared with week 1, egg production was higher in weeks 3 and 4 (P < 0.001; data not shown), while spawning success increased in weeks 2, 3, and 4 (Table 4).
Discussion
Our results demonstrate that both total dietary lipid and ratio of n–6 to n–3 fatty acids significantly impact growth, body composition, and reproductive success. We report interactions of these 2 dietary components for multiple outcomes. Additionally, our findings reveal sexual dimorphisms in response to dietary lipid intake, emphasizing the need to include the influence of sex in evaluations of future nutrition studies.
Both increasing total dietary lipid and ratio of n–6 to n–3 fatty acids were independently associated with reduced terminal BM in male, but not female, zebrafish. In males, the negative association of dietary n–6 fatty acids with BM conflicts with results from previous studies, which found either positive or no associations between these variables (14, 48, 49). One potential explanation is that only 1 amount of total dietary lipid was utilized for the diets in previous studies, while results from the current study reflect a different effect for the ratio of n–6 to n–3 fatty acids when evaluated across multiple dietary lipid amounts. The negative association between BM and total dietary lipid in male zebrafish also conflicted with previous studies in adult zebrafish (26, 27, 29, 50). However, a similar negative association between growth performance and amount of total dietary lipid was also observed in juvenile turbot, halibut, and Sengalese sole, indicating that life stage may significantly influence the response to dietary lipid (51–53).
Variations in nutrient requirements, utilization, and partitioning among life stages may influence these differential responses. It has been suggested that diets composed of 32% protein are sufficient to meet the growth requirements of older zebrafish, but for juveniles, the requirement is higher (40%, or 14 mg ·g BM−1 · d−1 for maximal growth) (54). While the diets in our study were isonitrogenous, the protein-to-energy balance differed, which can significantly impact efficiency of protein retention. Previous research has suggested that there is an optimal ratio for zebrafish (51, 54, 55). Fish consuming the lowest amount of dietary lipid had the highest growth performance, suggesting that these diets promoted the best feed conversion and protein efficiency ratios. Alternatively, an excess of energy intake and a diet with an improper protein-to-energy balance may decrease protein gain and retention with deleterious effects on growth performance, which would provide an explanation for declining growth as dietary lipid amounts increased in the present study (54). Juvenile zebrafish consuming diets with a higher lipid content may not have been able to consume enough protein to meet requirements, and consequently were smaller than those fed the low-lipid-content diets. Results from our study and previous studies indicate that both life stage and nutrient ratios should be carefully considered when designing translational nutrition studies in zebrafish.
Sexual dimorphisms in lipid metabolism and adipose tissue deposition have been observed in both mammals and fish, and are believed to be primarily regulated by sex hormones (1, 23, 56). Estrogens promote the allocation of body fat to subcutaneous depots in females, while testosterone shifts body fat to abdominal and visceral depots in males. Estrogens have also been observed to protect against weight gain and increases in adiposity by increasing energy expenditure rates in humans and mice (4, 50, 56–58). These differences in body fat deposition and metabolism can potentially contribute to variations between men and women in the processing and allocation of dietary lipid (59, 60).
In our study, the ratio of n–6 to n–3 fatty acids was only associated with total lipid mass in male zebrafish. Consistent with our results, sex-specific responses to dietary fatty acid composition have also been described in humans. Dietary fatty acid content has been observed to have a larger effect on the postprandial lipemia response in men compared with women (57, 59). As visceral fat is associated with an increased risk of cardiovascular disease and metabolic dysfunction in humans and zebrafish (26, 61, 62), the higher sensitivity to metabolic disturbances in response to alterations in dietary fatty acid content could be influenced by the larger proportion of visceral fat in males. While dietary fatty acid content was not observed to significantly affect body composition in these short-term studies, another study reported a positive association between visceral adiposity and the postprandial TG response in both men and women (60). Therefore, it could be speculated that, in longer-term studies, acute variations in the postprandial TG response to dietary manipulation could translate to changes in adiposity over time (60).
Conversely, a significant association between total dietary lipid and TLM was only observed in female zebrafish in our study. In mice, previous studies have demonstrated that males consuming an HFD are more susceptible to metabolic dysregulation, inflammation, and glucose intolerance (63–65). It could be speculated that this increased sensitivity in males could result in a stronger drive to adjust feed intake relative to energy density of the diet, and explain why total dietary lipid was positively associated with TLM only in females (56).
In vertebrates, TGs are predominantly associated with fat mass storage, with main storage sites including visceral, intramuscular, and subcutaneous depots (28). Analogous to total body lipid, body TG mass in males was significantly associated with the ratio of n–6 to n–3 fatty acids. In contrast, body TG mass in females was not influenced by either dietary lipid component. We are unsure as to why no significant effects of diet were observed for TG mass in females, indicating that additional investigations are needed to further explore these outcomes. It is also unclear whether the amounts of stored TG mass observed in our study represent positive or negative effects on health. To answer this question, healthy levels of adiposity will need to be defined for male and female zebrafish by evaluating additional markers for health and fitness.
Reproductive performance is influenced by both dietary lipid quantity and quality in fish and mammals (17, 66–69). In our study, the total amount of dietary lipid was significantly associated with spawning rate, but not total egg production or embryo viability. Spawning rate was observed to be lower in females consuming higher amounts of dietary lipid. This observation conflicts with studies in channel catfish and the snakehead murrel, where spawning rate and dietary lipid amount were positively associated (70, 71). However, the diets of catfish and murrels differ from zebrafish, resulting in diverse requirements for total dietary lipid intake. Zebrafish are a predominantly low-trophic-level species with a lower requirement, and consequently tolerance, for total dietary lipid intake (72). This lower tolerance may translate to a reduced ability to effectively utilize large amounts of dietary lipid for energy, resulting in increased ectopic fat storage (71–73). Previous research in mice has demonstrated that ectopic fat deposition in ovaries and other tissues resulting from diet-induced obesity initiates a cascade of lipid-induced programmed cell death known as lipotoxicity (74, 75). Consequently, female mice fed HFDs exhibit impaired oocyte release and fecundity. Analogous to mammals, zebrafish express markers associated with lipotoxicity in response to HFDs; therefore, higher amounts of dietary lipid could negatively impact spawning success in female zebrafish through a similar mechanism (26, 76). The total dietary lipid amounts selected for this study were presumed to be within a healthy range, which may provide an explanation for why neither total egg production nor embryo viability were affected. If diets with total lipid amounts outside this range were included in the study, larger differences in reproductive success among diets may have been observed (72).
It has been well established that mechanisms affecting egg release are influenced by dietary n–6 and n–3 fatty acid intake (67, 77, 78). These fatty acids regulate and serve as precursors to a group of physiologically active lipid compounds known as eicosanoids. ARA is a precursor for proinflammatory series-2 prostaglandins [prostaglandin E2 (PGE2) and prostaglandin F2α (PGF2a)], while EPA (20:5n–3) and DHA (22:6n–3) are precursors for the less inflammatory series-3 prostaglandins (PGE3) (77, 79). Ovaries have a high capacity to generate these prostaglandins, which affect gonadal steroidogenesis and ovulation in both mammals and fish (77, 80, 81). The negative association between spawning rate and ratio of n–6 to n–3 fatty acids observed in our study is supported by previous studies that evaluated ovulation and egg release in humans and animal models (6, 66, 67, 73, 78, 79). While production of PGF2a is necessary for ovulation and spawning behavior in mammals and fish, administration of PGE2 has been shown to inhibit ovulation (78, 82). Increasing dietary intake of n–3 fatty acids may enhance ova release by reducing production of PGE2, potentially through reduced ARA availability and elevated PGE3 synthesis (79).
Similar to total dietary lipid, the ratio of n–6 to n–3 fatty acids was not associated with total egg production. However, the significant association between embryo viability and dietary ratio of n–6 to n–3 fatty acids observed in our study reflects a hormetic dose–response relation. While the proportions of viable embryos produced by females fed diets with 1.4:1 and 9.5:1 ratios were similar (76% and 75%, respectively), embryo viability was significantly higher for the 5:1 ratio (87%). Hormetic relations have been described in variable intakes of other macronutrients or micronutrients and are believed to occur through a phenomenon known as “Bertrand's rule” (83–85). With Bertrand's rule, an increase in the intake of a specific nutrient is associated with increasing health benefits until reaching an optimal intake; beyond this optimal intake, further increases in intake may lead to adverse health consequences (83, 84). In zebrafish, the 5:1 ratio may represent an optimal intake ratio of n–6 to n–3 fatty acids for embryo viability, whereas the 1.4:1 and 9.5:1 ratios may represent imbalances in the ratio of n–6 to n–3 fatty acids.
The dietary ratio of n–6 to n–3 fatty acids may have also influenced embryo viability through spawning interactions. In teleosts, prostaglandins also act as pheromones to induce male spawning behavior and fertilization (86). The effects of dietary n–6 and n–3 fatty acids on prostaglandin production and release in females could influence signaling mechanisms for sperm release, ultimately affecting whether eggs are fertilized. In addition to their effects on female fish, another study found that ARA and EPA also modulate steroidogenesis in goldfish testis (87). However, caution should be exercised in extrapolating results from these previous studies to effects of dietary n–6 and n–3 fatty acids on production and release of prostaglandins and steroidogenesis in males or females, as influences of diet were not considered in either of these previous studies. Rather, findings from these studies merit further investigation.
A second factor that may have impacted embryo viability in our study is the effect of dietary long-chain (LC) PUFAs on oocyte development and quality (69). In both mammals and fish, the fatty acid composition of the ovaries is significantly influenced by maternal dietary LC-PUFA intake and is known to influence egg quality (17, 88). In particular, both n–6 and n–3 fatty acids are required for normal oocyte development, and an optimal ratio would improve both egg morphology and hatching rates (48, 77). In marine species, higher egg quality was positively associated with increased ARA and DHA/EPA content (89). However, optimal intakes of dietary n–6 fatty acids and ARA:EPA ratios are likely to be species dependent and influenced by the geography and ecosystem of the species’ food sources (90). This may explain the discrepancy between our results and those from previous studies in marine species. Whether the higher proportion of viable embryos observed from females fed the 5:1 ratio in our study is attributed to spawning behavior, egg quality, or a combination of both is a topic that should be explored in future studies.
Strengths of our study reside in the use of chemically defined diets with purified ingredients, the administration of daily rations during the feeding trial, and the evaluation of sex-specific responses to dietary lipid composition on obesity-related phenotypes. The duration of the feeding trial allowed us to examine the long-term effects of dietary lipid on our outcomes of interest. However, our study also had some intrinsic limitations. We had a female-biased sex ratio of zebrafish in our study sample, which may lead to aggressive interactions and the production of dominant individuals that control access to food resources (91). These behaviors could potentially influence our outcomes evaluated, and in future studies may be attenuated with an even distribution of males and females in each tank (91, 92). The time period of our study included the juvenile life stage of the zebrafish. Inclusion of this life stage may have potentially confounded effects of the diets on weight gain, as nutrient requirements and energy allocation differ from the adult life stage (72, 93). Thus, adult zebrafish may be a better choice for translational applications relating to diet-induced obesity in future studies. Given that vitamin E content is significantly associated with gonad development, fertility, and larval survival rate in fish (77), an additional limitation is that we did not control for variations in vitamin E content between the safflower and menhaden oils. Finally, a major challenge in all zebrafish nutritional studies is accurate measurement of feed intake due to the potential leaching of nutrients in the water (94). While we attempted to address this issue with administration of a daily ration, the development of direct methods for measuring feed intake in zebrafish should continue to be explored (94).
In summary, while requirements for total intake of dietary lipid and ratio of n–6 to n–3 fatty acids in both human and animal diets need to be defined, our results demonstrate that the balance of macronutrients may be as important as their individual intake. Dietary lipid quality and quantity exhibited independent and interactive effects on weight gain and reproductive success, suggesting that maximum benefits from their intake may be reached only when they are in the appropriate proportions (14). Optimal intakes of dietary lipid may also vary by sex. Our observations for body composition suggest that processes mediating the partitioning and utilization of dietary lipid are sexually dimorphic. Identification of sex-specific physiologic responses to dietary manipulation could lead to improved treatment and prevention strategies for obesity. Findings from this study and other studies continue to validate the zebrafish as a high-throughput model to identify underlying mechanisms that contribute to the development of diet-induced obesity, which will ultimately contribute to the development of sex-specific therapies (24).
Supplementary Material
ACKNOWLEDGEMENTS
We thank the following individuals for their assistance: Erica Anderson, Johnathan Sahawneh, Jamiko Rose, and John Bradford. We also gratefully acknowledge Dr. David Allison for his support on this project. The authors’ responsibilities were as follows—MP and SW: formulated the research question and study conception; LAF, LND-C, JAD, RJB, JLD, MLP, MBW, LRD, and SAW: contributed to study design and implementation; LAF, RJB, and SAW: formulated and manufactured the experimental diets; LAF, LND-C, RJB, JLD, MLP, YY, and MBW: collected data; LAF, LND-C, YY, and MBW: processed samples for body-composition analysis; JAD and LAF: performed the statistical analysis; RM, JLD, and LAF: developed code for and designed figures produced in R; LAF, LRD, and SAW: interpreted the data; LAF: drafted the manuscript; LAD and SAW: provided critical revisions for the first draft of the manuscript; and all authors: read and approved the final manuscript.
Notes
The project described was supported by the American College of Laboratory Animal Medicine Foundation (2011905), award P30DK056336 from the National Institute of Diabetes and Digestive and Kidney Diseases, and NIH training grants T32HL105349 and T32HL072757.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–8 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: ARA, arachidonic acid; BM, body mass; dpf, days postfertilization; HFD, high-fat diet; hpf, hours postfertilization; LC, long-chain; PGE2, prostaglandin E2; PGE3, prostaglandin E3; PGF2α, prostaglandin F2α; TBL, total body length; TG, triglyceride; TLM, total lipid mass; UAB, University of Alabama at Birmingham.
References
- 1. Krishnan S, Cooper JA.. Effect of dietary fatty acid composition on substrate utilization and body weight maintenance in humans. Eur J Nutr. 2014;53(3):691–710. [DOI] [PubMed] [Google Scholar]
- 2. Yang JH, Chang JS, Chen CL, Yeh CL, Chien YW. Effects of different amounts and types of dietary fatty acids on the body weight, fat accumulation, and lipid metabolism in hamsters. Nutrition. 2016;32(5):601–8. [DOI] [PubMed] [Google Scholar]
- 3. Deckelbaum RJ. n-6 and n-3 Fatty acids and atherosclerosis: ratios or amounts?. Arterioscler Thromb Vasc Biol. 2010;30(12):2325–6. [DOI] [PubMed] [Google Scholar]
- 4. Clevenger HC, Kozimor AL, Paton CM, Cooper JA. Acute effect of dietary fatty acid composition on postprandial metabolism in women. Exp Physiol. 2014;99(9):1182–90. [DOI] [PubMed] [Google Scholar]
- 5. Kazemi A, Ramezanzadeh F, Nasr-Esfahani MH. Relationship between dietary fat intake, its major food sources and assisted reproduction parameters. J Reprod Infertil. 2014;15(4):214–21. [PMC free article] [PubMed] [Google Scholar]
- 6. Mumford SL, Chavarro JE, Zhang C, Perkins NJ, Sjaarda LA, Pollack AZ, Schliep KC, Michels KA, Zarek SM, Plowden TC et al.. Dietary fat intake and reproductive hormone concentrations and ovulation in regularly menstruating women. Am J Clin Nutr. 2016;103(3):868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agaba MK, Tocher DR, Zheng X, Dickson CA, Dick JR, Teale AJ. Cloning and functional characterisation of polyunsaturated fatty acid elongases of marine and freshwater teleost fish. Comp Biochem Physiol B Biochem Mol Biol. 2005;142(3):342–52. [DOI] [PubMed] [Google Scholar]
- 8. Bell MV, Tocher DR. Biosynthesis of polyunsaturated fatty acids in aquatic ecosystems: general pathways and new directions. In: Kainz M, Brett MT, Arts MT, editors. Lipids in aquatic ecosystems. New York: Springer-Verlag; 2009. pp. 211–36. [Google Scholar]
- 9. Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood). 2008; 233:674–88. [DOI] [PubMed] [Google Scholar]
- 10. Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56(8):365–79. [DOI] [PubMed] [Google Scholar]
- 11. Myles IA, Pincus NB, Fontecilla NM, Datta SK. Effects of parental omega-3 fatty acid intake on offspring microbiome and immunity. PLoS One. 2014;9:e87181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Allard JP, Aghdassi E, Mohammed S, Raman M, Avand G, Arendt BM, Jalali P, Kandasamy T, Prayitno N, Sherman M et al.. Nutritional assessment and hepatic fatty acid composition in non-alcoholic fatty liver disease (NAFLD): a cross-sectional study. J Hepatol. 2008;48(2):300–7. [DOI] [PubMed] [Google Scholar]
- 13. Fritsche KL. Too much linoleic acid promotes inflammation—doesn't it? Prostaglandins Leukotrienes Essent Fatty Acids. 2008;79(3–5):173–5. [DOI] [PubMed] [Google Scholar]
- 14. Powell ML, Pegues MA, Szalai AJ, Ghanta VK, D'Abramo LR, Watts SA. Effects of the dietary omega3:omega6 fatty acid ratio on body fat and inflammation in zebrafish (Danio rerio). Comp Med. 2015;65(4):289–94. [PMC free article] [PubMed] [Google Scholar]
- 15. Rosqvist F, Iggman D, Kullberg J, Jonathan Cedernaes J, Johansson H-E, Larsson A, Johansson L, Ahlström H, Arner P, Dahlman I et al.. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes. 2014;63(7):2356–68. [DOI] [PubMed] [Google Scholar]
- 16. Yan L, Bai X-L, Fang Z-F, Che L-Q, Xu S-Y, Wu D. Effect of different dietary omega-3/omega-6 fatty acid ratios on reproduction in male rats. Lipids Health Dis. 2013;12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meinelt BT, Schulz C, Wirth M, Kürzinger H, Steinberg C. Dietary fatty acid composition influences the fertilization rate of zebrafish (Danio rerio Hamilton-Buchanan). J Appl Ichthyol. 1999;15(1):19–23. [Google Scholar]
- 18. Butts IAE, Baeza R, Støttrup JG, Krüger-Johnsen M, Jacobsen C, Pérez L, Asturiano JF, Tomkiewicz J. Impact of dietary fatty acids on muscle composition, liver lipids, milt composition and sperm performance in European eel. Comp Biochem Physiol A Mol Integr Physiol. 2015;183:87–96. [DOI] [PubMed] [Google Scholar]
- 19. Feng Y, Ding Y, Liu J, Tian Y, Yang Y, Guan S, Zhang C. Effects of dietary omega-3/omega-6 fatty acid ratios on reproduction in the young breeder rooster. BMC Vet Res. 2015;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod. 2007;77(2):190–201. [DOI] [PubMed] [Google Scholar]
- 21. Arts MT, Kohler CC.. Health and condition in fish: the influence of lipids on membrane competency and immune response. In: Kainz M, Brett MT, Arts MT, editors. Lipids in aquatic ecosystems. New York: Springer-Verlag; 2009. pp. 237–56. [Google Scholar]
- 22. Shi H, Clegg DJ. Sex differences in the regulation of body weight. Physiol Behav. 2009;97(2):199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Magkos F, Mittendorfer B. Gender differences in lipid metabolism and the effect of obesity. Obstet Gynecol Clin North Am. 2009;36(2):245–65., vii. [DOI] [PubMed] [Google Scholar]
- 24. Robison BD, Drew RE, Murdoch GK, Powell M, Rodnick KJ, Settles M, Stone D, Churchill E, Hill RA, Papasani MR et al.. Sexual dimorphism in hepatic gene expression and the response to dietary carbohydrate manipulation in the zebrafish (Danio rerio). Comp Biochem Physiol D Genomics Proteomics. 2008;3(2):141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Westerterp-Plantenga MS, Lejeune MPGM, Smeets AJPG, Luscombe-Marsh ND. Sex differences in energy homeostatis following a diet relatively high in protein exchanged with carbohydrate, assessed in a respiration chamber in humans. Physiol Behav. 2009;97(3–4):414–9. [DOI] [PubMed] [Google Scholar]
- 26. Oka T, Nishimura Y, Zang L, Hirano M, Shimada Y, Wang Z, Umemoto N, Kuroyanagi J, Nishimura N, Tanaka1 T. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol. 2010;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seth A, Stemple DL, Barroso I. The emerging use of zebrafish to model metabolic disease. Dis Model Mech. 2013;6(5):1080–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Flynn E, Trent C, Rawls J. Ontogeny and nutritional control of adipogenesis in zebrafish (Danio rerio). J Lipid Res. 2009;50(8):1641–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hasumura T, Shimada Y, Kuroyanagi J, Nishimura Y, Meguro S, Takema Y, Tanaka T. Green tea extract suppresses adiposity and affects the expression of lipid metabolism genes in diet-induced obese zebrafish. Nutr Metab (Lond). 2012;9:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stoletov K, Fang L, Choi S-H, Hartvigsen K, Hansen LF, Hall C, Pattison J, Juliano J, Miller ER, Almazan F et al.. Vascular lipid accumulation, lipoprotein oxidation, and macrophage lipid uptake in hypercholesterolemic zebrafish. Circ Res. 2009;104(8):952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hölttä-Vuori M, Salo Veijo TV, Nyberg L, Brackmann C, Enejder A, Panula P, Ikonen E. Zebrafish: gaining popularity in lipid research. Biochem J. 2010;429(2):235–42. [DOI] [PubMed] [Google Scholar]
- 32. American Oil Chemists' Society. Preparation of methyl esters of fatty acids (method Ce 2-66)In: Official methods and recommended practices of the American Oil Chemists' Society. 4th ed Champaign (IL): American Oil Chemists' Society; 1989. [Google Scholar]
- 33. American Oil Chemists' Society. Fatty acid composition by gas chromatography (method Ce 1-62)In: Official methods and recommended practices of the American Oil Chemists' Society. 5th ed Champaign (IL): American Oil Chemists' Society; 1997. [Google Scholar]
- 34. National Research Council. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. 8th ed Washington (DC): National Academies Press; 2011. [PubMed] [Google Scholar]
- 35. Lawrence C, Mason T. Zebrafish housing systems: a review of basic operating principles and considerations for design and functionality. ILAR J. 2012;53(2):179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fowler LA, Williams MB, Dennis-Cornelius LN, Farmer S, Barry RJ, Powell ML, Watts SA. Influence of commercial and laboratory diets on growth, body composition, and reproduction in the zebrafish Danio rerio. Zebrafish. 2019;16(6):508–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Folch J, Lees M, Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 38. Fowler LA, Dennis LN, Barry RJ, Powell ML, Watts SA, Smith DL Jr.. In v ivo determination of body composition in zebrafish (Danio rerio) by quantitative magnetic resonance. Zebrafish. 2016;13(3):170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Juaneda P, Rocquelin G. Rapid and convenient separation of phospholipids and non phosphorus lipids from rat heart using silica cartridges. Lipids. 1985;20(1):40–1. [DOI] [PubMed] [Google Scholar]
- 40. Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203(3):253–310. [DOI] [PubMed] [Google Scholar]
- 41. Wickham H. ggplot2: elegant graphics for data analysis. 2nd ed New York: Springer-Verlag; 2016. [Google Scholar]
- 42. Kassambara A. ggpubr: ‘ggplot2’ based publication ready plots. R package version 0.2.2. [Internet] 2019, [cited 2019 Jun 16]. Available from: https://CRAN.R-project.org/package=ggpubr. [Google Scholar]
- 43. Campitelli E. metR: Tools for easier analysis of meteorological fields. R package version 0.3.0. [Internet].2019, [cited 2019 Oct 31]. Available from: https://CRAN.R-project.org/package=metR. [Google Scholar]
- 44. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Soft. 2015;67(1):1–48. [Google Scholar]
- 45. Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. J Stat Soft. 2017;82(13):1–26. [Google Scholar]
- 46. Rigby RA, Stasinopoulos DM. Generalized additive models for location, scale, and shape. Journal of the Royal Statistical Society: Series C (Applied Statistics). 2005;54:507–54. [Google Scholar]
- 47. Hu M-C, Pavlicova M, Nunes EV. Zero-inflated and hurdle models of count data with extra zeros: examples from an HIV-risk reduction intervention trial. Am J Drug Alcohol Abuse. 2011;37(5):367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jaya-Ram A, Kuah M-K, Lim P-S, Kolkovski S, Shu-Chien AC. Influence of dietary HUFA levels on reproductive performance, tissue fatty acid profile and desaturase and elongase mRNAs expression in female zebrafish Danio rerio. Aquaculture. 2008;277(3–4):275–81. [Google Scholar]
- 49. Meinelt T, Schulz C, Wirth M, Kürzinger H, Steinberg C. Correlation of diets high in n-6 polyunsaturated fatty acids with high growth rate in zebrafish (Danio rerio). Comp Med. 2000;50(1):43–5. [PubMed] [Google Scholar]
- 50. Meguro S, Hasumura T, Hase T. Body fat accumulation in zebrafish is induced by a diet rich in fat and reduced by supplementation with green tea extract. PLoS One. 2015;10:e0120142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Borges P, Oliveira B, Casal S, Dias J, Conceicao L, Valente LM. Dietary lipid level affects growth performance and nutrient utilisation of Senegalese sole (Solea senegalensis) juveniles. Br J Nutr. 2009;102(7):1007–14. [DOI] [PubMed] [Google Scholar]
- 52. Hamre K, Øfsti A, Næss T, Nortvedt R, Holm JC. Macronutrient composition of formulated diets for Atlantic halibut (Hippoglossus hippoglossus, L.) juveniles. Aquaculture. 2003;227(1–4):233–44. [Google Scholar]
- 53. Caceres-Martinez C, Cadena-Roa M, Métailler R. Nutritional requirements of turbot (Scophthalmus maximus): I. A preliminary study of protein and lipid utilization. J World Mariculture Soc. 2009;15(1–4):191–202. [Google Scholar]
- 54. Fernandes H, Peres H, Carvalho AP. Dietary protein requirement during juvenile growth of zebrafish (Danio rerio). Zebrafish. 2016;13(6):548–55. [DOI] [PubMed] [Google Scholar]
- 55. Conde-Sieira M, Soengas JL. Nutrient sensing systems in fish: impact on food intake regulation and energy homeostasis. Front Neurosci. 2017;10:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bosch TA, Steinberger J, Sinaiko AR, Moran A, Jacobs DR, Kelly AS, Dengel DR. Identification of sex-specific thresholds for accumulation of visceral adipose tissue in adults. Obesity. 2015;23(2):375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kien CL, Bunn JY. Gender alters the effects of palmitate and oleate on fat oxidation and energy expenditure. Obesity. 2008;16(1):29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Koutsari C, Zagana A, Tzoras I, Sidossis LS, Matalas AL. Gender influence on plasma triacylglycerol response to meals with different monounsaturated and saturated fatty acid content. Eur J Clin Nutr. 2004;58(3):495–502. [DOI] [PubMed] [Google Scholar]
- 60. Couillard C, Bergeron N, Prud'homme D, Bergeron J, Tremblay A, Bouchard C, Mauriege P, Despres JP. Gender difference in postprandial lipemia: importance of visceral adipose tissue accumulation. Arterioscler Thromb Vasc Biol. 1999;19(10):2448–55. [DOI] [PubMed] [Google Scholar]
- 61. Rasmussen OW, Lauszus FF. Six different fat tolerance tests in young, healthy subjects—gender dependent postprandial lipemia and glucose. Prensa Medica Argentina. 2016;102:(5):102–10. [Google Scholar]
- 62. Landgraf K, Schuster S, Meusel A, Garten A, Riemer T, Schleinitz D, Kiess W, Körner A. Short-term overfeeding of zebrafish with normal or high-fat diet as a model for the development of metabolically healthy versus unhealthy obesity. BMC Physiol. 2017;17(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, Garcia-Caceres C, Navas CR, Gordillo R, Neinast M, Kalainayakan SP et al.. Hypothalamic PGC-1alpha protects against high-fat diet exposure by regulating ERalpha. Cell Rep. 2014;9(2):633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stubbins RE, Holcomb VB, Hong J, Nunez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr. 2012;51(7):861–70. [DOI] [PubMed] [Google Scholar]
- 65. González-Granillo M, Helguero LA, Alves E, Archer A, Savva C, Pedrelli M, Ahmed O, Li X, Domingues MR, Parini P et al.. Sex-specific lipid molecular signatures in obesity-associated metabolic dysfunctions revealed by lipidomic characterization in ob/ob mouse. Biol Sex Differ. 2019;10(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Santiago CB, Reyes OS. Effects of dietary lipid source on reproductive performance and tissue lipid levels of Nile tilapia Oreochromis niloticus (Linnaeus) broodstock. J Appl Ichthyol. 1993;9(1):33–40. [Google Scholar]
- 67. Mazorra C, Bruce M, Bell JG, Davie A, Alorend E, Jordan N, Rees J, Papanikos N, Porter M, Bromage N. Dietary lipid enhancement of broodstock reproductive performance and egg and larval quality in Atlantic halibut (Hippoglossus hippoglossus). Aquaculture. 2003;227(1–4):21–33. [Google Scholar]
- 68. Rosero DS, Odle J, Mendoza SM, Boyd RD, Fellner V, van Heugten E. Impact of dietary lipids on sow milk composition and balance of essential fatty acids during lactation in prolific sows. J Anim Sci. 2015;93(6):2935–47. [DOI] [PubMed] [Google Scholar]
- 69. Wakefield SL, Lane M, Schulz SJ, Hebart ML, Thompson JG, Mitchell M. Maternal supply of omega-3 polyunsaturated fatty acids alter mechanisms involved in oocyte and early embryo development in the mouse. Am J Physiol Endocrinol Metab. 2008;294(2):E425–34. [DOI] [PubMed] [Google Scholar]
- 70. Bentley CD, Watanabe WO, Rezek TC, Seaton PJ. Preliminary investigations on the effects of dietary lipid on the spawning performance and egg quality of black sea bass Centropristis striata L. Aquaculture Res. 2009;40(16):1873–83. [Google Scholar]
- 71. Ghaedi A, Kabir MA, Hashim R. Effect of lipid levels on the reproductive performance of Snakehead murrel, Aquaculture Res. 2016;47(3):983–91. [Google Scholar]
- 72. National Research Council. Nutrient requirements of fish and shrimp. Washington (DC): National Academies Press; 2011. [Google Scholar]
- 73. Sink TD, Lochmann RT. Effects of dietary lipid source and concentration on channel catfish (Ictalurus punctatus) egg biochemical composition, egg and fry production, and egg and fry quality. Aquaculture. 2008;283(1–4):68–76. [Google Scholar]
- 74. Wu LL-Y, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, Robker RL. High-fat diet causes lipotoxicity responses in cumulus–oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151(11):5438–45. [DOI] [PubMed] [Google Scholar]
- 75. Grindler NM, Moley KH. Maternal obesity, infertility and mitochondrial dysfunction: potential mechanisms emerging from mouse model systems. Mol Hum Reprod. 2013;19(8):486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Faillaci F, Milosa F, Critelli RM, Turola E, Schepis F, Villa E. Obese zebrafish: a small fish for a major human health condition. Anim Models Exp Med. 2018;1(4):255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Izquierdo MS, Fernández-Palacios H, Tacon AGJ. Effect of broodstock nutrition on reproductive performance of fish. Aquaculture. 2001;197(1–4):25–42. [Google Scholar]
- 78. Abayasekara DRE, Wathes DC.. Effects of altering dietary fatty acid composition on prostaglandin synthesis and fertility. Prostaglandins Leukotrienes Essent Fatty Acids. 1999;61(5):275–87. [DOI] [PubMed] [Google Scholar]
- 79. Broughton KS, Bayes J, Culver B. High α-linolenic acid and fish oil ingestion promotes ovulation to the same extent in rats. Nutr Res. 2010;30(10):731–8. [DOI] [PubMed] [Google Scholar]
- 80. Knight OM, Van Der Kraak G. The role of eicosanoids in 17α, 20β-dihydroxy-4-pregnen-3-one-induced ovulation and spawning in Danio rerio. Gen Comp Endocrinol. 2015;213:50–8. [DOI] [PubMed] [Google Scholar]
- 81. Murdoch WJ, Hansen TR, McPherson LA. A review—role of eicosanoids in vertebrate ovulation. Prostaglandins. 1993;46(2):85–115. [DOI] [PubMed] [Google Scholar]
- 82. Lister AL, Van Der Kraak GJ. Regulation of prostaglandin synthesis in ovaries of sexually-mature zebrafish (Danio rerio). Mol Reprod Dev. 2009;76(11):1064–75. [DOI] [PubMed] [Google Scholar]
- 83. Hayes DP. Adverse effects of nutritional inadequacy and excess: a hormetic model. Am J Clin Nutr. 2008;88(Suppl):578S–81S. [DOI] [PubMed] [Google Scholar]
- 84. Simpson SJ, Raubenheimer D. The nature of nutrition: a unifying framework from animal adaptation to human obesity. Princeton (NJ): Princeton University Press; 2012. [Google Scholar]
- 85. Raubenheimer D, Lee KP, Simpson SJ. Does Bertrand's rule apply to macronutrients?. Proc R Soc B. 2005;272(1579):2429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kobayashi M, Sorensen PW, Stacey NE. Hormonal and pheromonal control of spawning behavior in the goldfish. Fish Physiol Biochem. 2002;26(1):71–84. [Google Scholar]
- 87. Wade MG, Van Der Kraak G, Gerrits M, Ballantyne J. Release and steroidogenic actions of polyunsaturated fatty acids in the goldfish testis. Biol Reprod. 1994;51(1):131–9. [DOI] [PubMed] [Google Scholar]
- 88. Bilby T, Block J, Do Amaral B, Sa Filho O, Silvestre F, Hansen P, Staples C, Thatcher W. Effects of dietary unsaturated fatty acids on oocyte quality and follicular development in lactating dairy cows in summer. J Dairy Sci. 2006;89(10):3891–903. [DOI] [PubMed] [Google Scholar]
- 89. Pickova J, Dutta PC, Larsson PO, Kiessling A. Early embryonic cleavage pattern, hatching success, and egg-lipid fatty acid composition: comparison between two cod (Gadus morhua) stocks. Can J Fish Aquat Sci. 1997;54(10):2410–6. [Google Scholar]
- 90. Bell JG, Sargent JR. Arachidonic acid in aquaculture feeds: current status and future opportunities. Aquaculture. 2003;218(1–4):491–9. [Google Scholar]
- 91. Spence R, Smith C. Male territoriality mediates density and sex ratio effects on oviposition in the zebrafish, Danio rerio. Anim Behav. 2005;69(6):1317–23. [Google Scholar]
- 92. Uusi-Heikkila S, Wolter C, Meinelt T, Arlinghaus R. Size-dependent reproductive success of wild zebrafish Danio rerio in the laboratory. J Fish Biol. 2010;77(3):552–69. [DOI] [PubMed] [Google Scholar]
- 93. Leibold S, Hammerschmidt M. Long-term hyperphagia and caloric restriction caused by low- or high-density husbandry have differential effects on zebrafish postembryonic development, somatic growth, fat accumulation and reproduction. PLoS One. 2015;10(3):e0120776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Watts SA, Powell M, D'Abramo LR. Fundamental approaches to the study of zebrafish nutrition. ILAR J. 2012;53(2):144–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.