Abstract
A substantial body of evidence suggests the existence of MUC1-specific antibodies and cytotoxic T cell activities in pancreatic cancer patients. However, tumor-induced immunosuppression renders these responses ineffective. The current study explores a novel therapeutic combination wherein tumor-bearing hosts can be immunologically primed with their own antigen, through opsonization with a tumor antigen-targeted antibody, mAb-AR20.5. We evaluated the efficacy of immunization with this antibody in combination with PolyICLC and anti-PD-L1. The therapeutic combination of mAb-AR20.5 + anti-PD-L1 + PolyICLC induced rejection of human MUC1 expressing tumors and provided a long-lasting, MUC1-specific cellular immune response, which could be adoptively transferred and shown to provide protection against tumor challenge in human MUC1 transgenic (MUC.Tg) mice. Furthermore, antibody depletion studies revealed that CD8 cells were effectors for the MUC1-specific immune response generated by the mAb-AR20.5 + anti-PD-L1 + PolyICLC combination. Multichromatic flow cytometry data analysis demonstrated a significant increase over time in circulating, activated CD8 T cells, CD3+CD4−CD8−(DN) T cells, and mature dendritic cells in mAb-AR20.5 + anti-PD-L1 + PolyICLC combination-treated, tumor-bearing mice, as compared to saline-treated control counterparts. Our study provides a proof of principle that an effective and long-lasting anti-tumor cellular immunity can be achieved in pancreatic tumor-bearing hosts against their own antigen (MUC1), which can be further potentiated using a vaccine adjuvant and an immune checkpoint inhibitor.
Electronic supplementary material
The online version of this article (10.1007/s00262-017-2095-7) contains supplementary material, which is available to authorized users.
Keywords: mAb-AR20.5 antibody, PolyICLC, Anti-PD-L1, CD8 T cells, MUC1, Pancreatic cancer
Introduction
Recent investigations have provided evidence for immune activity against pancreatic cancer, including the presence of specific protective antibodies and cytotoxic T cell activities in pancreatic cancer patients [1, 2], and some responses have been observed that are associated with survival benefits in patients [3]. However, pancreatic tumors subvert these responses through several immunosuppressive pathways. A number of strategies that tackle tumor-derived immunosuppression have shown promise, including the use of anti-CD40 and GVAX vaccines [4, 5]. Though successful in establishing initial responses, the longer-term results of these studies demonstrate the need for additional approaches to invoke and sustain productive T cell and complementary anti-tumor immunity. Studies to block the effects of T cell checkpoint and inhibitory molecules have failed as single agents in treating pancreatic cancer, in part because of the lack of pre-existing intra/peri-tumoral T cells in these patients [6, 7]. Hence, there is an urgent need to identify and develop therapeutic modalities that can induce and sustain efficient tumor-targeted cytotoxic T cell responses in pancreatic cancer patients.
Murine monoclonal antibody mAb-AR20.5 (MsIgG1), which recognizes the DTRPAP sequence in human MUC1, has shown moderate activity in a small clinical trial for patients with advanced adenocarcinoma [8]. The therapeutic efficacy of mAb-AR20.5 is mediated in part by the formation of immune complexes with circulating or tumor-associated MUC1 antigen, which facilitates effective processing and cross-presentation of the MUC1 antigen to T cells. Though specific anti-MUC1 responses were noted in mAb-AR20.5 treated cancer patients, sustained anti-tumor activity was not observed. This led us to explore the capacity of additional immune-modulators to amplify and sustain MUC1-specific immune responses produced by administration of mAb-AR20.5. We investigated the anti-tumor efficacy of administering mAb-AR20.5 in combination with anti-PD-L1 and PolyICLC, and observed rejection of human MUC1 expressing Panc02 (Panc02.MUC1) tumor cells in a significant fraction of mice treated with mAb-AR20.5 + anti-PD-L1 + PolyICLC. There was evidence of a sustained immune response in a second round of tumor challenge that was mediated by CD8 T cells. This is the first report demonstrating that mAb-AR20.5 in combination with anti-PD-L1 and PolyICLC reduces tumor-associated immune suppression and promotes sustained MUC1-specific cellular immune responses.
Materials and methods
Mice, cell lines and reagents
MUC1.Tg mice, immunologically tolerant to human MUC1, used for these studies were obtained from the breeding colony at the University of Nebraska Medical Center [9]. All animal studies were performed in accordance with the Institutional Animal Care and Use Committee guidelines (IACUC). MAb-AR20.5 and PolyICLC were supplied by Oncoquest Inc, and anti-PD-L1 (Clone, 10F.9G2) was purchased from Bio X cell (New Hampshire, USA). Panc02.MUC1, Panc02.Neo, KPC.MUC1 cells were prepared and maintained as described [10].
Tumor challenge and antibody treatment
MUC1.Tg mice were challenged subcutaneously on the hind flank with 1 × 106 Panc02.MUC1 tumor cells. Gemcitabine (30, 60 and 100 mg/kg) was administered either alone (twice a week with one week rest) or in combination with mAb-AR20.5 (50 μg, treatment on 0, 5 and 7 days after the second gemcitabine dose) through intraperitoneal injections (i.p). The anti-tumor efficacy of mAb-AR20.5 (50 μg, at days 7, 17, 27 and 37) in combination with anti-PD-L1 (200 μg, every 1 and 3 days after PolyICLC injection) and PolyICLC (50 μg, at days 8, 13, 18, 23, 28, 33, 38, 43) was evaluated in MUC.Tg mice challenged subcutaneously or orthotopically with Panc02.MUC1 (1 × 106) or KPC.MUC1 (1 × 104) cells. Subsequently, mice were divided into 8 experimental and control groups (n = 8/gp) (saline control; anti-PD-L1; PolyICLC; mAb-AR20.5; anti-PD-L1 + PolyICLC; mAb-AR20.5 + anti-PD-L1; mAb-AR20.5 + PolyICLC; and mAb-AR20.5 + PolyICLC + anti-PD-L1). Tumor growth was monitored for 65 days. Tumor-free mice (post 65 days) underwent a second round of challenge on opposite flanks with control (Panc02.Neo) and MUC1-expressing (Panc02.MUC1) tumor cells. Post-treatment, mice were monitored for time-to-tumor progression (TTP) every 2–3 days. Tumor diameters (3/tumor) were measured every 4 days for calculation of tumor volume (V = 4/3 × π × r 3). Mice were euthanized when tumors reached 1.2 cm diameter in accordance with IACUC requirements.
Adoptive transfer and depletion studies
Immune cell depletion studies were performed using appropriate antibodies (anti-CD8, anti-CD4, anti-NK) as described previously [1]. Additionally, splenocytes from tumor-free MUC1.Tg mice were harvested for post-rechallenge and processed for adoptive transfer as previously described [11].
Enzyme-linked immunosorbent assay for MUC1
Serum levels of MUC1 were determined by sandwich ELISA, where mAb-AR20.5 (recognizing DTRPAP epitope) was used for capture and detection [12]. 96 well plates were coated with mAb-AR20.5 (2.5 μg/ml) overnight at 4 °C, then blocked with 3% BSA in PBS/0.06% Thimoseral, followed by incubation with MUC1 standards or serum samples for 1 h at RT. A 23-mer MUC1 peptide, E23 [synthesized by Biotools Inc. (Edmonton Canada)] was used as MUC1 standard (units/ml) for ELISA. Post-incubation; plates were washed and treated with mAb-AR20.5-biotin and detected by horseradish peroxidase (HRP)-conjugated to streptavidin.
Immunofluorescence staining and flow cytometry
Spleen and tumor tissue were freshly harvested from tumor-bearing mice and processed for histology and flow cytometry as described previously [13]. mAb-AR20.5-FITC was used for detection of MUC1 in subcutaneous tumors. For flow cytometry staining of immune cells, blood was collected through the submandibular vein in accordance with IACUC requirements, processed for BD LSRII flow cytometer and analyzed by FlowJo software TreeStar Version 8.8.7.
Proliferation and antibody-dependent cell-mediated cytotoxicity assay
Functional status of spleen- and blood-derived T cells was assessed from tumor free and tumor-bearing mice by CFSE-based proliferation assays as described previously [14]. ADCC activity of mAb-AR20.5 was performed using murine splenic NK cells and analyzed as previously described [12, 15].
Statistical analyses
Time-to-tumor progression (TTP) was assessed using Kaplan–-Meier plots and analyzed by the log-rank test (125 mm3 tumor volume was defined as a “detectable tumor” and an end point for TTP curves). The difference in tumor volume and tumor growth between mice groups was calculated using the two-tailed Student’s t test and mixed model with random animal effect test, respectively. Flow cytometry data for immune subsets were analyzed using two-way ANOVA (Bonferroni post-test adjustment for multiple measurements) in Prism 6 software (GraphPad). All the p values ≤ 0.05 were considered significant.
Results
The mAb-AR20.5 antibody in combination with gemcitabine prolongs survival of Panc02.MUC1 tumor-bearing MUC1.Tg mice
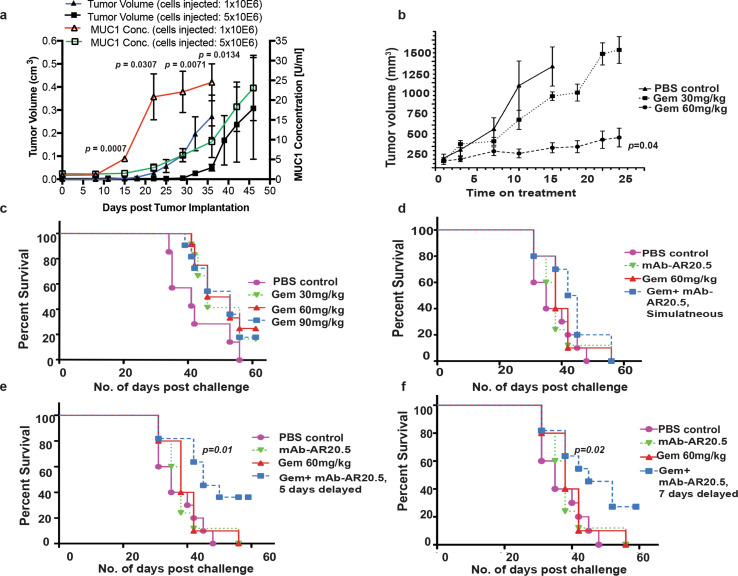
A phase I evaluation of mAb-AR20.5 antibody has shown promising results in an early clinical trial of adenocarcinomas [8]; however, this antibody has not been evaluated for treatment of pancreatic cancer. Thus, we sought to determine therapeutic efficacy of mAb-AR20.5 alone or in combination with gemcitabine in MUC1.Tg mice, which are immunologically tolerant to human MUC1, while otherwise having a fully competent immune system. ELISA experiments revealed low levels of circulating MUC1 in naïve MUC1.Tg mice, which increased significantly with progressive tumor burden (Fig. 1a). Circulating MUC1 levels above those found in normal control mice were detected as early as 15–21 days after tumor cell implantation. Additionally, gemcitabine at a 60 mg/kg dose significantly reduced MUC1-expressing tumor growth (Fig. 1b). In parallel, 60 and 90 mg/kg doses were found to prolong the overall survival of Panc02.MUC1 orthotopic tumor-bearing mice as compared to other groups (Fig. 1c). However, at these doses gemcitabine did not eliminate pancreatic tumors. Furthermore, we noted that administration of mAb-AR20.5, 5 or 7 days after gemcitabine treatment resulted in a significant increase in survival compared to other treatment groups (Fig. 1d–f). Our data suggest that combination of mAb-AR.20.5 + gemcitabine delivers a protective anti-tumor response and prolongs survival of tumor-bearing MUC1.Tg mice.
Fig. 1.
mAb-AR20.5 in combination with gemcitabine prolongs survival of Panc02.MUC1 tumor-bearing MUC1.Tg mice. a Representative plot showing circulating levels of human MUC1 and corresponding tumor volumes in MUC1.Tg mice post-orthotopic implantation of Panc02.MUC1 tumor cells. Circulating MUC1 levels above normal were detected as early as 15–21 days post-tumor cell implantation by ELISA (n = 3 for each group). The MUC1 levels were compared between the two groups by performing a two-sample t test for each time point. b Dose dependent effect of gemcitabine on the growth of Panc02.MUC1 tumor in MUC1.Tg mice. Gemcitabine at 60 mg/kg significantly reduced tumor growth over time, (n = 3/gp; p = 0.04). c Kaplan–Meier plots show dose-dependent effects of gemcitabine on overall survival of Panc02.MUC1 tumor-bearing MUC1.Tg mice. Statistically significant differences in survival were observed for tumor-bearing MUC1.Tg mice treated with 60 mg/kg gemcitabine compared to PBS treated mice (p = 0.05). d–f Kaplan–Meier plots showing survival curves for tumor-bearing MUC1.Tg mice post-treatment with PBS (i.p), mAb-AR20.5 (i.p) and gemcitabine (i.p) or combination of mAb-AR20.5 and gemcitabine using different schedules of treatment. For combination treatment, mAb-AR20.5 was injected on the same day (d), 5 days post (p = 0.01; combination vs. PBS) (e), or 7 days post (p = 0.02; combination vs. PBS, log-rank test) (f) second dose of gemcitabine injection. Representative of experiments repeated twice (n = 10/gp). The mixed effects model with random mice effects was used to compare the tumor growth over time between groups after accounting for the correlation among the measurements on the same mice
Combination of mAb-AR20.5 + anti-PD-L1 + PolyICLC induces tumor immunity and rejection of MUC1-expressing pancreatic tumors in MUC1.Tg mice
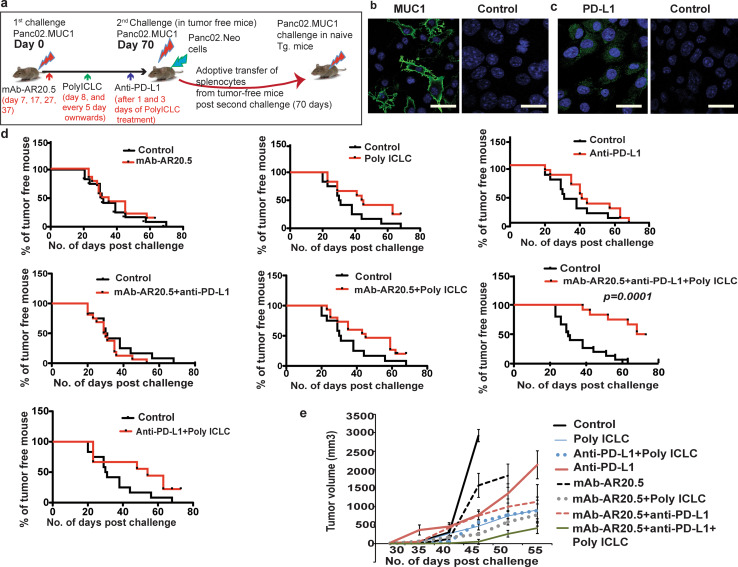
Though therapy with gemcitabine and mAb-AR20.5 showed promising initial results, we did not observe cures of tumors. Thus, we further explored whether the anti-tumor potential of mAb-AR20.5 could be improved by the incorporation of adjuvants and checkpoint inhibitors. The PD-L1-PD-1 axis regulates T-cell inhibitory responses and controls peripheral and central immune tolerance [16]. Blocking this interaction has led to better cytotoxic T-cell responses in several cancer models [17, 18]. PolyICLC promotes Type I IFNs and has been shown to produce effective antigen cross-presentation to cytotoxic T cells in several in vivo cancer models [19]. We explored whether MUC1-specific immune responses, achieved through administration of mAb-AR20.5, could be amplified and sustained by anti-PD-L1 and PolyICLC. (Fig. 2a). Panc02.MUC1 cells were found to express human MUC1 antigen and PD-L1 ligand on their surface (Fig. 2b, c). We assessed the efficacy of mAb-AR20.5 treatment alone or in combination with anti-PD-L1 and PolyICLC using a unique experimental design of tumor challenge and rechallenge with controls for antigen specificity (Fig. 2a). In three independent studies, we noted that 50% of mAb-AR20.5 + anti-PD-L1 + PolyICLC-treated mice were tumor free for 70 days, as compared to other treated groups (Fig. 2d). Animals that did not fully reject tumors showed significant delay in time-to-tumor progression and slower tumor growth in mAb-AR20.5 + anti-PD-L1 + PolyICLC-treated mice (Fig. 2d, e), supporting the hypothesis that this treatment produced immune responses capable of restraining tumor growth.
Fig. 2.
mAb-AR20.5 in combination with anti-PD-L1 and PolyICLC induces rejection of Panc02.MUC1 tumors in MUC1.Tg mice. a Diagrammatic representation of in vivo experimental design for subcutaneous pancreatic tumor challenge in MUC1.Tg mice. b, c Representative images show immunofluorescence staining for human MUC1 (green), nucleus (blue) (b) and PD-L1 (green) (c) in Panc02.MUC1 tumor cells. d Time-to-tumor progression for different combination treatment groups receiving mAb-AR20.5, anti-PD-L1 and PolyICLC in MUC1.Tg mice. e Tumor growth curves for mice treated with different combinations of mAb-AR20.5, anti-PD-L1 and PolyICLC post Panc02.MUC1 tumor cell implantation in MUC1.Tg mice. The results shown are representative of three independent studies, p = 0.0001; mAb-AR20.5 based combination vs. saline control. Representative experiments were repeated thrice, (n = 6/gp), p = 0.0001; mAb-AR20.5 based combination vs. saline control, log-rank test (TTP curves). The mixed effects model with random mice effects was used to compare the tumor growth over time between groups after accounting for the correlation among the measurements on the same mice
mAb-AR20.5 + anti-PD-L1 + PolyICLC combination-treated mice display MUC1-specific immune responses
To examine the capacity of immunization with mAb-AR20.5 + anti-PD-L1 + PolyICLC treatment to induce antigen-specific responses including T-cell memory, we performed rechallenge experiment by implanting control (Panc02.Neo) and Panc02.MUC1 cells on opposite flanks of animals that rejected tumors following immunization with mAb-AR20.5 + anti-PD-L1 + PolyICLC. Previously unchallenged (control) MUC1.Tg animals served as controls. A significant proportion of the mAb-AR20.5 + PolyICLC + anti-PD-L1 treated mice exhibited antigen-specific rejection of Panc02.MUC1 upon rechallenge, but did not reject Panc02.Neo control tumor cells (Fig. 3a, b) demonstrating antigen specificity of the anti-tumor response. Moreover, mice that failed to reject a second round of tumor challenge with Panc02.MUC1 demonstrated significant delays in time-to-tumor progression and slower growth rates of Panc02.MUC1 compared to Panc02.Neo tumors (p = 0.0001, Fig. 3a, b). Panc02.Neo and Panc02.MUC1 cell lines exhibit indistinguishable growth rates in vitro (Supplementary Fig. 1) and hence delayed growth of Panc02.MUC1 tumor cells in treated mice supports our hypothesis that these animals produced MUC1 specific immune responses that restrained Panc02.MUC1 tumor growth. In functional studies, splenocytes from mAb-AR20.5 + anti-PD-L1 + PolyICLC-treated mice showed enhanced proliferative responses to general stimulation (PMA/ionomycin) compared to controls, as reflected by dilution of CFSE dye (Fig. 3c). To further validate the antigen specificity of these responses, we examined whether MUC1-specific cellular immune responses could be adoptively transferred into naïve mice. Splenocytes (2 × 106 cells/100 μl) from tumor immune mice were transferred through tail vein injection 2 days prior to tumor challenge (subcutaneous) in healthy naïve MUC1.Tg mice. We observed a significant delay and rejection of MUC1-expressing tumor cells in a significant fraction of recipient mice (p = 0.0143) (Fig. 3d). Tumor growth rates for mice that developed tumors were also decreased, though these differences among groups did not achieve statistical significance because of the relatively low numbers of animals examined in this study (data not shown).
Fig. 3.
mAb-AR20.5 + anti-PD-L1 + PolyICLC combination-treated mice display MUC1 specific immunity and reject rechallenged Panc02.MUC1 tumors in MUC1.Tg mice. a Time-to-tumor progression curves following rechallenge with Panc02.Neo or Panc02.MUC1 tumors for control or mice that rejected Panc02.MUC1 tumors following treatment with mAb-AR20.5 + anti-PD-L1 + PolyICLC. b Tumor growth curves for Panc02.Neo and Panc02.MUC1 tumors for control or mAb-AR20.5 + anti-PD-L1 + PolyICLC-treated mice post second round of tumor cell challenge (p = 0.0001). c Representative plot showing proliferation of splenocytes as determined by CFSE-based proliferation assay of spleen cells from saline-treated control and combination-treated MUC1.Tg mice that had rejected tumors. The red peak shows mean fluorescence intensity (MFI) for CFSE-labeled splenocytes on day “0” while the blue peak shows MFI for CFSE-labeled splenocytes on day 4-post stimulation with PMA and ionomycin. d Representative plot showing time-to-tumor progression for Panc02.MUC1 tumors in control (no cells) and immune cell recipient mice, following adoptive transfer of immune cells from animals that previously rejected Panc02.MUC1 tumors. Rechallenge experiments were repeated thrice (n = 3–4/gp). Adoptive transfer experiments were repeated twice (3 recipient mice/1 donor mice splenocytes, n = 6/gp), log-rank test (TTP curves). The mixed effects model with random mice effects was used to compare the tumor growth over time between groups after accounting for the correlation among the measurements on the same mice
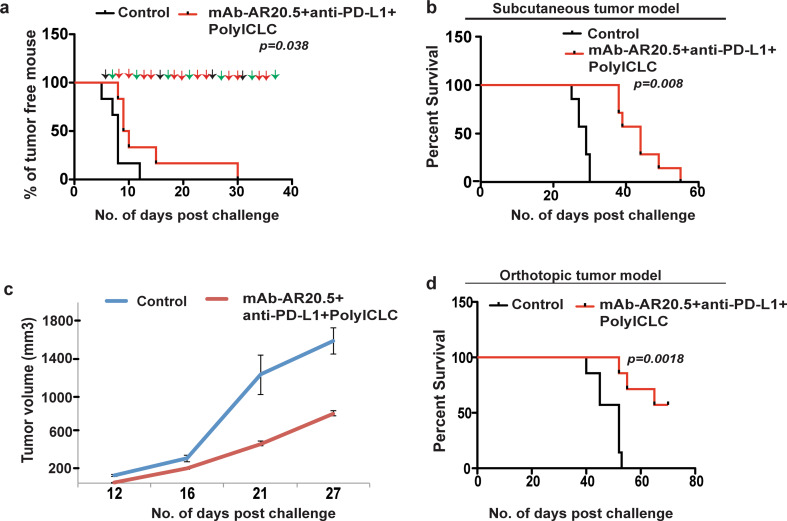
mAb-AR20.5 + anti-PD-L1 + PolyICLC combination prolongs overall survival of KPC.MUC1 tumor challenged mice
Our findings with Panc02.MUC1 were validated using a second more aggressive syngeneic MUC1 expressing pancreatic tumor cell line derived from KPC mice (KPC.MUC1 cells), which also expresses hMUC1 (data not shown) and PD-L1 (Supplementary Fig. 2). Immunization with mAb-AR20.5 + anti-PD-L1 + PolyICLC produced a significant delay in time-to-tumor progression, slower tumor growth and significantly prolonged survival in both subcutaneous and orthotopic tumor models as compared to control counterparts (Fig. 4a–d). These findings support the concept that the combination of mAb-AR20.5 + anti-PD-L1 + PolyICLC produces an immune response against aggressive pancreatic tumors that can restrain tumor growth and provide survival benefit.
Fig. 4.
mAb-AR20.5 + anti-PD-L1 + PolyICLC combination treatment restrains tumor growth in KPC.MUCI tumor-bearing Tg mice. a Time-to-tumor progression curve for saline control and mAb-AR20.5 + Anti-PD-L1 + PolyICLC treated KPC.MUC1 tumor-bearing MUC1.Tg mice. Black, green and red arrows represent time of therapeutic intervention with mAb-AR20.5, PolyICLC and anti-PD-L1, respectively. b, c Kaplan–Meier survival curves (b), tumor growth curves (c) for saline and mAb-AR20.5 + anti-PD-L1 + PolyICLC treated MUC1.Tg mice (subcutaneous tumor challenge, p value was not statistically significant for tumor growth curves). The results shown are representative of 2 independent experiments (n = 6/gp). d Kaplan–Meier survival curves for different treatment post-orthotopic tumor (KPC.MUC1) implantation in MUC1.Tg mice (n = 8/gp, log-rank test for TTP and survival curves)
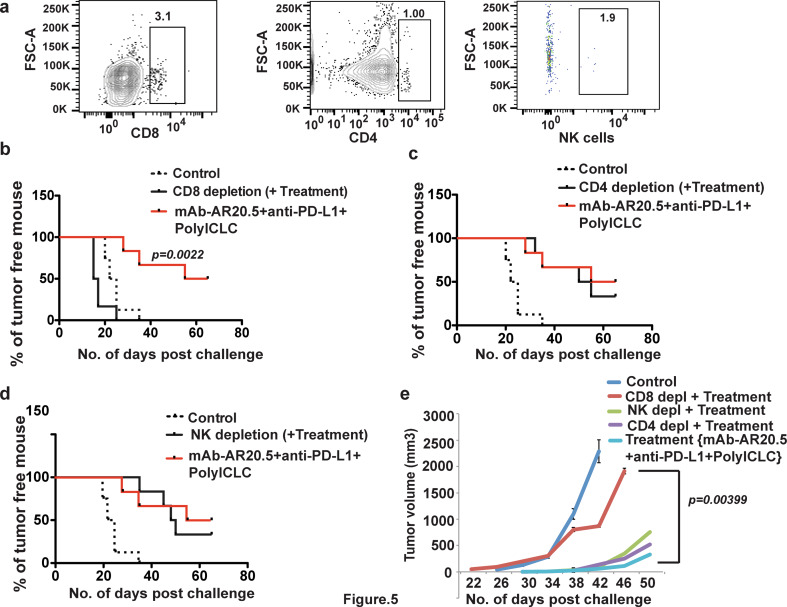
Depletion of CD8 T cells abrogates anti-tumor effects of mAb-AR20.5 + anti-PD-L1 + PolyICLC treatment in MUC1.Tg mice
To define the key immune cells that mediate immune rejection of Panc02.MUC1 tumors in this model system, we evaluated anti-tumor responses of mAb-AR20.5 + anti-PD-L1 + PolyICLC following depletion of CD8, CD4, or NK cells (Fig. 5a). Depletion of CD8 T cells reduced tumor immune responses induced by this combination therapy, as all mice failed to reject Panc02.MUC1 tumors. CD8-depleted mice showed early and rapidly progressing tumors as compared to other treatment counterparts (Fig. 5b, e). However, time-to-tumor progression and tumor growth were comparable between CD4-, NK-depleted and un-manipulated combination-treated MUC1.Tg mice (p values were not statistically significant) (Fig. 5c–e).
Fig. 5.
CD8 T cells are effectors of anti-tumor responses following mAb-AR20.5 + anti-PD-L1 + PolyICLC combination in MUC1 tumor-bearing transgenic mice. a Representative FACS plots showing CD8, CD4 and NK cell depletion in MUC1.Tg mice spleen. b–d Time-to-tumor progression (Panc02.MUC1 tumor challenge) curves for animals treated with PBS alone (control), or mAb-AR20.5 + anti-PD-L1 + PolyICLC-treated with or without CD8-depletion (b), CD4-depletion (c), or NK cells-depletion (d). e Tumor growth curves for mice treated with saline, mAb-AR20.5, anti-PD-L1 and PolyICLC or the indicated groups of immune cell-depleted MUC1.Tg mice. Depletion experiments were performed once, (n = 6/gp), log-rank test (for TTP curves). The mixed effects model with random mice effects was used to compare the tumor growth over time between groups after accounting for the correlation among the measurements on the same mice
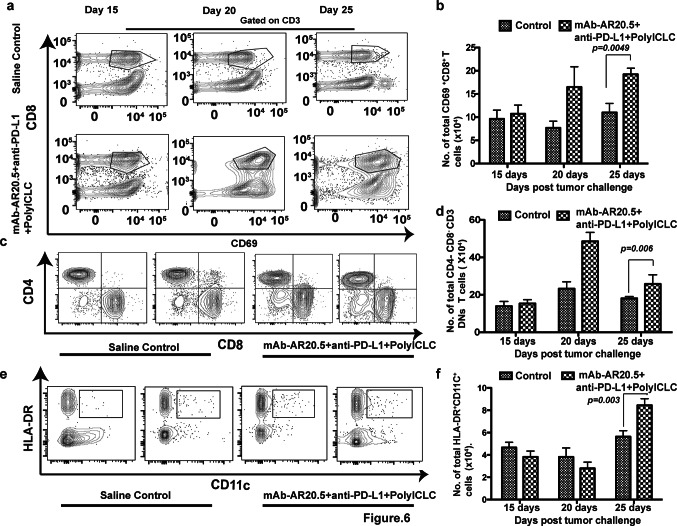
Increased levels of circulating activated CD8+, CD3+DN T cells and mature dendritic cells in mAb-AR20.5 + anti-PD-L1 + PolyICLC combination-treated, tumor-bearing MUC1.Tg mice
Results presented in the previous section encouraged us to investigate the phenotype of circulating lymphocytes and myeloid cells after implantation of Panc02.MUC1 tumors. Combination-treated mice displayed progressive and significant increases in activated CD8+ (CD69+CD8 T cells and KLRG1+CD8 T cells) (p = 0.0049), CD3+DN T cells (p = 0.006), and mature dendritic cells (p = 0.003), compared to saline control counterparts (Fig. 6a–f, Supplementary Fig. 3). Additionally, functional assessment of CD3+DN T cell revealed an effector phenotype (IFN-γ+ IL-2+ CD3+DN T cells) in treated mice as compared to control counterparts (IL-17+ IL-2+ CD3+DN T cells) (Supplementary Fig. 4). In contrast, other immune subtypes, including CD4 T cells and macrophages, were not significantly affected in combination-treated mice (Supplementary Fig. 5a, b). Interestingly, CD19+ cells (B cells) showed a progressive increase in saline-treated control mice compared to combination-treated mice (Supplementary Fig. 5c).
Fig. 6.
mAb-AR20.5 + anti-PD-L1 + PolyICLC-treated mice display high numbers of circulating activated CD8, CD3+DN T cell and mature dendritic cells (CD11c+ HLA-DR+). a Representative FACS plots for activated CD8 T cells at different days post-treatment with saline or mAb-AR20.5 + anti-PD-L1 + PolyICLC for Panc02.MUC1 tumor-bearing mice. b Histogram plot shows the frequency of activated CD8 T cells (p = 0.049) in circulation at indicated days post-treatment with saline or mAb-AR20.5 + anti-PD-L1 + PolyICLC. c, e Representative FACS plots for CD3+DN (gated on CD3, CD69) T cells (c) and mature dendritic cells (gated on CD11b− population) (e) for two mice at day 25 in the indicated treatment groups. d, f representative histogram plot show the frequency CD3+DN T cells (p = 0.006) (d), and mature dendritic cells (p = 0.003) (f), in the circulation at different days post-treatment with saline or mAb-AR20.5 + anti-PD-L1 + PolyICLC. (n = 5/gp), two-way ANOVA using the Bonferroni correction for multiple comparison
Human MUC1 expression in Panc02 tumors remains unchanged after rechallenge with Panc02.MUC1 tumor cells
To evaluate mechanisms of tumor escape from tumor immune responses in combination-treated mice post-rechallenge, we assessed MUC1 expression in the tumors that grew upon rechallenge. We observed no significant alterations in MUC1 expression in tumor post-first challenge (saline control mice) and second challenge (mAb-AR20.5 + anti-PD-L1 + PolyICLC) in MUC1.Tg mice (Supplementary Fig. 6a, b). These data suggest a possible suppressive role of other immune cells or pathways in mitigating CD8 T cell-mediated, protective immune responses in combination-treated mice. These results imply that further inhibition of immune suppression may be of benefit.
Discussion
Due in part to its critical role in tumor progression, metastasis and chemo-resistance, MUC1 is regarded as an appropriate candidate for immunotherapeutic strategies for pancreatic cancer. There have been numerous attempts to target MUC1 for immunotherapy; however, these efforts met with only moderate success, in part because of the complexity and immunosuppressive nature of tumor microenvironment [20, 21]. Administration of mAb-AR20.5 produced MUC1-specific immune responses in a small study of advanced cancer patients. In the current study, we examined whether the addition of chemotherapy, checkpoint inhibitors, and adjuvants could escalate and sustain MUC1-specific immune responses against human MUC1-expressing tumors in MUC1.Tg mice.
MAb-AR20.5 produces a MUC1-specific immune response by forming immune complexes with circulating or cell-bound MUC1, in part by deriving strong dendritic induction of cell-mediated CD4 and CD8 T-cell responses [22–24]. ADCC activity for mAb-AR20.5 has been suggested in the past. Our in vitro results here showed a moderate, though not statistically significant, cytotoxic activity in CFSE-based ADCC assays (Supplementary Figure 7). These data suggest the possible induction of ADCC dependent and independent pathways against MUC1-expressing tumor cells by the mAb-AR20.5 in the in vivo models examined here. We investigated anti-tumor immune responses in a unique model system that includes accurate temporal and spatial expression of the target antigen in normal tissues and consequent immunological tolerance to human MUC1 [9]. We detected increased levels of circulating MUC1 15 days post-subcutaneous tumor challenge in MUC1.Tg mice. Therapeutic intervention with mAb-AR20.5 (in association with gemcitabine) as early as 7 days post-tumor challenge reduced tumor growth and prolonged overall survival of Panc02.MUC1 tumor-bearing mice. Our results were consistent with previous attempts to combine gemcitabine with anti-MUC1 immunotherapy, which showed moderate anti-tumor efficacy in mouse models of pancreatic cancer [25]. Data presented here support the concept that careful selection of dose and schedule can allow gemcitabine to enhance circulating levels of MUC1 through cytotoxic effects on tumor cells, which we posited would enhance formation of antigen–antibody complexes and thereby amplify the anti-tumor response produced by administering mAb-AR20.5.
Several lines of evidence suggest that passive immunization with anti-tumor antibodies can initiate antigen-specific T-cell response in tumor-bearing mice [26]. Efficient immune-complex uptake, processing and cross-presentation of tumor antigen is a prerequisite for productive anti-tumor immune response by T cells [27]. However, tumor-associated local and systemic immunosuppression is predicted to manipulate initial and subsequent aspects of antigen presentation and associated T-cell activation. MHC-TCR interactions and costimulatory signals promote T-cell activation; however, signaling through inhibitory receptors (PD-1/LAG-3/Tim3) subdues T-cell associated immune responses, which tumors use to evade anti-tumor immune control. Most therapies, including antibody-based approaches, falter in maintaining a persistent adaptive immune response against tumor antigens. PD-L1 status and density of tumor-infiltrating immune cell (TILs) directly correlates with therapeutic efficacy of checkpoint inhibitor therapy [28]. Interestingly, TILs are often not detected in pancreatic cancer, and clinical trials with checkpoint inhibitors have thus far failed when deployed in the settings of locally advanced and metastatic pancreatic cancer [2]. Partial responses for only 8% of patients were achieved with anti-PD-L1 (MEDI4736) in an ongoing trial for pancreatic cancer patients [4]. This has led some to suggest that addition of an active specific immune stimulant (vaccination) improves TIL activity in pancreatic cancer [29].
In this study we posited that opsonization of the host’s own tumor antigen through binding of mAb-AR20.5 in combination with PolyICLC would result in activation of large pool of T cells, whose anti-tumor activity could be further enhanced by removal of immune suppression through treatment with anti-PD-L1. The anti-PD-L1 relieves tumor-mediated immunosuppression on cytotoxic T cells and augments tumor-specific immune response in tumor-bearing hosts [30, 31]. PolyICLC assists in dendritic cell maturation and tumor antigen presentation in murine models of cancer [32]. Tumor protective effects have been described for anti-PD-L1 and PolyICLC combinations in pre-clinical models of melanoma, lung, and colon cancer [33]. We hypothesized that mAb-AR20.5 + PolyICLC + anti-PD-L1 combination would generate robust MUC1-specific immune responses and restrain pancreatic tumor growth in MUC1.Tg mice. In support of this hypothesis, mAb-AR20.5 + PolyICLC + anti-PD-L1 combination induced immune-mediated rejection of Panc02.MUC1 tumors in a significant fraction of mice, as compared to controls and other groups. Importantly, several mAb-AR20.5 + PolyICLC + anti-PD-L1 treated mice completely rejected primary challenge (Panc02.MUC1) and then exhibited antigen-specific rejection or delayed progression of a secondary challenge with Panc02.MUC1 cells, but did not reject antigen-negative control tumor cells (Panc02.Neo). These data strongly support the hypothesis that this combination therapy activated antigen specific tumor immune recognition in tumor-bearing MUC1.Tg mice that could be recalled upon a second round of tumor insult. Furthermore, we noted that MUC1-specific immune responses generated in the mAb-AR20.5 + PolyICLC + anti-PD-L1 treated mice could be adoptively transferred to confer anti-tumor immunity on tumor-naïve syngeneic mice. A similar robust anti-tumor immune response was produced in mice bearing a more aggressive tumor cell line (KPC.MUC1) by immunization with mAb-AR20.5 + PolyICLC + anti-PD-L1, where treated mice displayed a delay in tumor progression and tumor growth as compared to saline-treated mice. Failure of the mAb-AR20.5 combination to induce complete rejection of KPC tumor cells could be due to the highly aggressive growth properties of this line, which may have allowed fast growing KPC.MUC1 tumors to “outrun” the developing immune response. It is also possible that there are differences in the immunosuppressive milieu associated with these two tumor cell lines. Nevertheless, we conclude that the mAb-AR20.5 + PolyICLC + anti-PD-L1 combination produced immune responses capable of restricting tumor growth against different pancreatic tumor cell lines.
Next we investigated the cellular nature of immune response against Panc02.MUC1 tumor cells and noted that depletion of CD8 (but not CD4 or NK) cells abrogated the anti-tumor potential of the mAb-AR20.5 + PolyICLC + anti-PD-L1 therapy. There was a commensurate increase in peripheral activated CD8 T cells in mAb-AR20.5 + PolyICLC + anti-PD-L1 treated mice, suggesting a critical role for CD8 T cells as effectors of the observed MUC1-specific immune responses. The development of CD8 T cell-dependent tumor immunity against Panc02.MUC1 tumor cells in C57BL/6 mice has already been reported [34]. Our study also demonstrated a progressive increase of activated CD3+DN T cells in mAb-AR20.5 + PolyICLC + anti-PD-L1 treated mice, which peaked at day 25. γδ T cells, and iNKT cells are major constituents of the CD3+DN T-cell fraction and have been recognized as important immunotherapeutic candidates for treating cancer [35–37]. These cells are not MHC restricted and are known to mediate ADCC with monoclonal Abs in different tumor models [38]. Cytokine analysis of circulating CD3+DN T cells further supports the hypothesis that these have an effector phenotype in mAb-AR20.5 + PolyICLC + anti-PD-L1 treated mice [39, 40]. Perhaps these effector cells in association with CD8 T cells, may contribute to tumor rejection in the tumor cells. This supposition is supported by the fact that the CD3+DN T cell subsets are early players of tumor immune responses and regulates CD8 T-cell function in virus models of immunity [41, 42]. Additionally, of potential importance to findings in this model system, is the possibility that some CD3+DN T cells (i.e., γδ T cells) can be co-stimulated directly by PolyICLC, which culminates in IFN-γ production and CD69 expression [43]. This lends support to our findings of increased rejection of Panc02.MUC1 tumor cells in the mice treated with mAb-AR20.5 + anti-PD-L1 + PolyICLC and mAb-AR20.5 + PolyICLC as compared to other groups. The observation of reduced tumor growth rates in CD8-depleted and mAb-AR20.5 + anti-PD-L1 + PolyICLC treated mice suggests a possible role of these subsets in restraining initial MUC1.Panc02 tumor growth. Though the current study was focused principally on CD3+DN T cells, future studies characterizing this immune subtype should evaluate the potential of cross talk between the CD3+DN T cells and classical CD8 T cells during the induction of MUC1-specific immune responses. Supplemental to the observed alterations of frequency of CD8 and CD3+DN T cells, it was notable that mature dendritic cells (CD11c+ HLA-DR+) cells peaked at similar time points in the peripheral circulation of mAb-AR20.5 + PolyICLC + anti-PD-L1 treated mice compared to control mice. Given the posited role of PolyICLC in boosting dendritic cells maturation, this was not surprising, and further corroborates our hypothesis that mAb-AR20.5 + PolyICLC + anti-PD-L1 triggers both innate and adaptive immune responses against MUC1-expressing tumors in mice. Interestingly, there were no significant changes in the percentages of macrophages and CD4 T cells among different treatment groups. B cells, however, showed an inverse pattern in mAb-AR20.5 combination-treated mice: their numbers peaked on day 25 in control saline-treated, tumor-bearing MUC1.Tg mice. This is not surprising, considering the pro-tumorigenic and possible immunosuppressive role of B cells in pancreatic cancer, where it regulates CD8 T cell migration and function [44]. Our observation of decreased levels of B cells in mAb-AR20.5 combination-treated mice (as compared to controls) suggests that these cells diminish CD8-mediated tumor rejection and promote tumor growth and progression in saline-treated mice compared to mAb-AR20.5 combination-treated counterparts. Future studies are warranted to illuminate the exact contribution of B cells or their subtypes in mitigating anti-tumor response in Panc02.MUC1 tumor-bearing mice.
In the present study, a fraction of mAb-AR20.5 + PolyICLC + anti-PD-L1 treated, tumor-free mice failed to completely reject a second round of Panc02.MUC1 tumor challenge (though delays in tumor onset and reductions in tumor growth rates were observed). We investigated the possibility that tumor cells escaped CD8 T cell-mediated immunosurveillance by reducing MUC1 expression during rechallenge studies (or that antigen-negative variants were selected). However, we observed moderate but not significant reduction in MUC1 expression in tumors after both the first (saline control group) and second challenges. Interestingly, regulatory T cells (Tregs; Foxp3+ CD4 T cells), important immunosuppressive players, also remained indistinguishable in both saline and mAb-AR20.5 combination-treated mice (Supplementary Figure 8). These data support the hypothesis that immunosuppression (other than Tregs), immune exhaustion, or immune anergy compromised tumor rejection in tumor rechallenge studies. Though CD3+DN T cells are early responders in tumor-associated insults, they are rapidly turned over in circulation. Perhaps the loss of PolyICLC activated CD3+DN T cells in rechallenged (70 days after first challenge) mice compromised CD8 T cell-mediated tumor killing. Nevertheless, these mice exhibited reduced tumor growth rates and delayed onset, which implies the persistence of MUC1-specific immune response after rechallenge with Panc02.MUC1 tumor cells mAb-AR20.5 + PolyICLC + anti-PD-L1 treated mice.
In summary, our data support the hypothesis that multi-tier targeting of immune responses by administration of appropriate adjuvants and blockade of checkpoint-based immunosuppression together with administration of an antigen specific stimulus, in this case mAb-AR20.5, produces efficient MUC1-specific immune responses that reject pancreatic tumors. Furthermore our data serve as a proof of principle for future interventions with different tumor antigens, and for future studies into the effects of dose and schedule of chemotherapy in combination with this method of immunization. The overall strategy investigated here (antibody-based opsonization of patients tumor antigen to prime immune responses) has the potential to circumvent the problem of heterogeneity in tumor antigens [45] that exist within individual patients and among different patients, because it seeks to immunize patients with their own tumor antigens. These results support further investigation of the mechanisms that underpin this method to produce immune responses and the translation of this strategy into clinical trials for pancreatic cancer patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- ANOVA
Analysis of variance
- BSA
Bovine serum albumin
- CFSE
Carboxyfluorescein succinimidyl ester
- DN T cells
Double negative T cells
- ELISA
Enzyme-linked immunosorbent assay
- FACS
Fluorescence-activated cell sorting
- gp
Group
- γδT cells
Gamma delta T cells
- GVAX
Granulocyte-macrophage colony-stimulating factor (GM-CSF) gene-transfected tumor cell vaccine
- HLA-DR
Human leukocyte antigen–antigen D related
- IACUC
Institutional Animal Care and Use Committee
- IFN-γ
Interferon gamma
- i.p
Intraperitoneal injection
- iNKT cells
Invariant natural killer T cells
- KPC
LSL-KrasG12D/+; LSL-Trp53R172H/+; Pdx-1-Cre
- KPC.MUC1
KPC tumors expressing human MUC1
- LAG-3
Lymphocyte activation gene 3
- MFI
Mean fluorescence intensity
- MHC
Major histocompatibility complex
- MsIgG1
Mouse IgG1
- MUC1
Mucin 1
- MUC1.Tg
Human MUC1 transgenic
- NK cells
Natural killer cells
- PDAC
Pancreatic ductal adenocarcinoma
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed death-Ligand 1
- PMA
Phorbol 12-myristate 13-acetate
- PolyICLC
Polyinosinic-polycytidylic acid
- TCR
T-cell receptor
- TILs
Tumor-infiltrating lymphocytes
- TLR3
Toll-like receptor 3
- TTP
Time-to-tumor progression
- Type 1 IFN
Type 1 interferon
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest.
Funding
This study was supported by the grants from the National Cancer Institute (CA127297, CA163120, CA036727, and CA163649). Kamiya Mehla is supported by Project Purple Jayne Snyder Pancreatic Cancer Research Fellowship Grant.
References
- 1.Kotera Y, Fontenot JD, Pecher G, Metzgar RS, Finn OJ. Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients. Cancer Res. 1994;54:2856–2860. [PubMed] [Google Scholar]
- 2.Barnd DL, Lan MS, Metzgar RS, Finn OJ. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. PNAS. 1989;86:7159–7163. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heller A, Zornig I, Muller T, et al. Immunogenicity of SEREX-identified antigens and disease outcome in pancreatic cancer. Cancer Immunol Immunother. 2010;59:1389–1400. doi: 10.1007/s00262-010-0870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le DT, Wang-Gillman A, Picozzi V, Greten TF, et al. Saftey and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33:1325–1333. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bono JS, Rha SY, Stephenson J, et al. Phase I trial of a murine antibody to MUC1 in patients with metastatic cancer: evidence for the activation of humoral and cellular antitumor immunity. Ann Oncol. 2004;15:1825–1833. doi: 10.1093/annonc/mdh472. [DOI] [PubMed] [Google Scholar]
- 9.Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ. Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res. 1998;58:315–321. [PubMed] [Google Scholar]
- 10.Morikane K, Tempero R, Sivinski CL, Kitajima S, Gendler SJ, Hollingsworth MA. Influence of organ site and tumor cell type on MUC1-specific tumor immunity. Int Immunol. 2001;13:233–40. doi: 10.1093/intimm/13.2.233. [DOI] [PubMed] [Google Scholar]
- 11.Tempero RM, VanLith ML, Morikane K, Rowse GJ, Gendler SJ, Hollingsworth MA. CD4+ lymphocytes provide MUC1-specific tumor immunity in vivo that is undetectable in vitro and is absent in MUC1 transgenic mice. J Immunol. 1998;161:5500–5506. [PubMed] [Google Scholar]
- 12.Qi W, Schultes BC, Liu D, Kuzma M, Decker W, Madiyalakan R. Characterization of an anti-MUC1 monoclonal antibody with potential as a cancer vaccine. Hybrid Hybridomics. 2001;20:313–324. doi: 10.1089/15368590152740716. [DOI] [PubMed] [Google Scholar]
- 13.Bunt SK, Mohr AM, Bailey JM, Grandgenett PM, Hollingsworth MA. Rosiglitazone and gemcitabine in combination reduces immune suppression and modulates T cell populations in pancreatic cancer. Cancer Immunol Immunother. 2013;62:225–236. doi: 10.1007/s00262-012-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riches JC, Davies JK, McClanahan F, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121:1612–1621. doi: 10.1182/blood-2012-09-457531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karagiannis SN, Wang Q, East N, et al. Activity of human monocytes in IgE antibody-dependent surveillance and killing of ovarian tumor cells. Eur J Immunol. 2003;33:1030–1040. doi: 10.1002/eji.200323185. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125:3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73:6900–6912. doi: 10.1158/0008-5472.CAN-13-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. 2015;15:405–414. doi: 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim NK, Yariz KO, Bondarenko I, et al. Randomized phase II trial of letrozole plus anti-MUC1 antibody AS1402 in hormone receptor-positive locally advanced or metastatic breast cancer. Clin Cancer Res. 2011;17:6822–6830. doi: 10.1158/1078-0432.CCR-11-1151. [DOI] [PubMed] [Google Scholar]
- 21.Roulois D, Gregoire M, Fonteneau JF. MUC1-specific cytotoxic T lymphocytes in cancer therapy: induction and challenge. Biomed Res Int. 2013 doi: 10.1155/2013/871936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultes B, Hou F, Smith L, Nicodemus C (2007) Immunization with MUC1-anti-MUC1 immune complexes induces CD4 and CD8 T cell responses and provides tumor control in MUC1-tg mice. In: AACR annual meeting proceedings. Cancer Res 67 (Abstr 5097)
- 23.Schultes BC, Eng H, Agopsowicz K, Nicodemus CF (2004) Potent helper and cytolytic T cell response by dendritic cells armed with MUC1-anti-MUC1 immune complexes. In: 12th International congress of immunology and 4th annual conference of FOCIS, CIM, 27(4) (Abstr 53.102)
- 24.Schultes BC, Kuzma ML, Agopsowicz K, et al. Antibodies as vaccines immune complexes allow for efficient uptake and processing of antigens on MHC class I and II and induce maturation of dendritic cells. Experimental biology (AAI meeting) FASEB J. 2002;16:A334. [Google Scholar]
- 25.Mukherjee P, Basu GD, Tinder TL, et al. Progression of pancreatic adenocarcinoma is significantly impeded with a combination of vaccine and COX-2 inhibition. J Immunol. 2009;182:216–224. doi: 10.4049/jimmunol.182.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mortenson ED, Park S, Jiang Z, Wang S, Fu YX. Effective anti-neu-initiated antitumor responses require the complex role of CD4+ T cells. Clin Cancer Res. 2013;19:1476–1486. doi: 10.1158/1078-0432.CCR-12-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rafiq K, Bergtold A, Clynes R. Immune complex-mediated antigen presentation induces tumor immunity. J Clin Invest. 2002;110:71–79. doi: 10.1172/JCI0215640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soares KC, Rucki AA, Wu AA, et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother. 2015;38:1–11. doi: 10.1097/CJI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gajewski TF, Corrales L. New perspectives on type I IFNs in cancer. Cytokine Growth Factor Rev. 2015;26:175–178. doi: 10.1016/j.cytogfr.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagato T, Lee YR, Harabuchi Y, Celis E. Combinatorial immunotherapy of polyinosinic-polycytidylic acid and blockade of programmed death-ligand 1 induce effective CD8 T-cell responses against established tumors. Clin Cancer Res. 2014;20:1223–1234. doi: 10.1158/1078-0432.CCR-13-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohlgraf KG, Gawron AJ, Higashi M, et al. Tumor-specific immunity in MUC1.Tg mice induced by immunization with peptide vaccines from the cytoplasmic tail of CD227 (MUC1) Cancer Immunol Immunother. 2004;53:1068–1084. doi: 10.1007/s00262-004-0557-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Acquisto F, Crompton T. CD3+ CD4CD8(double negative) T cells: saviours or villains of the immune response? Biochem Pharmacol. 2011;82:333–340. doi: 10.1016/j.bcp.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 36.Young KJ, Kay LS, Phillips MJ, Zhang L. Antitumor activity mediated by double-negative T cells. Cancer Res. 2003;63:8014–8021. [PubMed] [Google Scholar]
- 37.Gomes AQ, Martins DS, Silva-Santos B. Targeting gamma delta T lymphocytes for cancer immunotherapy: from novel mechanistic insight to clinical application. Cancer Res. 2010;70:10024–10027. doi: 10.1158/0008-5472.CAN-10-3236. [DOI] [PubMed] [Google Scholar]
- 38.Seidel UJ, Vogt F, Grosse-Hovest L, Jung G, Handgretinger R, Lang P. gamma delta T cell-mediated antibody-dependent cellular cytotoxicity with CD19 antibodies assessed by an impedance-based label-free real-time cytotoxicity assay. Front Immunol. 2014;5:618. doi: 10.3389/fimmu.2014.00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hossain MS, Takimoto H, Ninomiya T, et al. Characterization of CD4– CD8– CD3+ T-cell receptor-alpha beta + T cells in murine cytomegalovirus infection. Immunology. 2000;101:19–29. doi: 10.1046/j.1365-2567.2000.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Vliet HJ, Molling JW, Nishi N, et al. Polarization of Valpha24+ Vbeta11+ natural killer T cells of healthy volunteers and cancer patients using alpha-galactosylceramide-loaded and environmentally instructed dendritic cells. Cancer Res. 2003;63:4101–4106. [PubMed] [Google Scholar]
- 41.Deniger DC, Moyes JS, Cooper LJ. Clinical applications of gamma delta T cells with multivalent immunity. Front Immunol. 2014;5:636. doi: 10.3389/fimmu.2014.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao Y, Yang W, Pan M, et al. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198:433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wesch D, Beetz S, Oberg HH, Marget M, Krengel K, Kabelitz D. Direct costimulatory effect of TLR3 ligand poly(I:C) on human gamma delta T lymphocytes. J Immunol. 2006;176:1348–1354. doi: 10.4049/jimmunol.176.3.1348. [DOI] [PubMed] [Google Scholar]
- 44.Pylayeva-Gupta Y, Das S, Handler JS, et al. IL35-producing b cells promote the development of pancreatic neoplasia. Cancer Discov. 2016;6:247–255. doi: 10.1158/2159-8290.CD-15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.