Abstract
Objective
To estimate the risk of intracerebral hemorrhage (ICH) recurrence in a large, diverse, US-based population and to identify racial/ethnic and socioeconomic subgroups at higher risk.
Methods
We performed a longitudinal analysis of prospectively collected claims data from all hospitalizations in nonfederal California hospitals between 2005 and 2011. We used validated diagnosis codes to identify nontraumatic ICH and our primary outcome of recurrent ICH. California residents who survived to discharge were included. We used log-rank tests for unadjusted analyses of survival across racial/ethnic groups and multivariable Cox proportional hazards regression to determine factors associated with risk of recurrence after adjusting for potential confounders.
Results
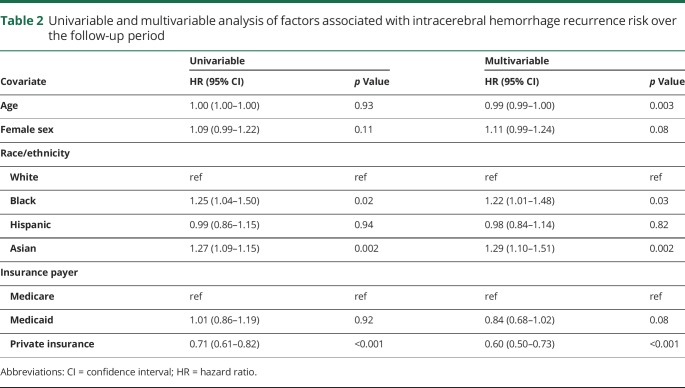
We identified 31,355 California residents with first-recorded ICH who survived to discharge, of whom 15,548 (50%) were white, 6,174 (20%) were Hispanic, 4,205 (14%) were Asian, and 2,772 (9%) were black. There were 1,330 recurrences (4.1%) over a median follow-up of 2.9 years (interquartile range 3.8). The 1-year recurrence rate was 3.0% (95% confidence interval [CI] 2.8%–3.2%). In multivariable analysis, black participants (hazard ratio [HR] 1.22; 95% CI 1.01–1.48; p = 0.04) and Asian participants (HR 1.29; 95% CI 1.10–1.50; p = 0.001) had a higher risk of recurrence than white participants. Private insurance was associated with a significant reduction in risk compared to patients with Medicare (HR 0.60; 95% CI 0.50–0.73; p < 0.001), with consistent estimates across racial/ethnic groups.
Conclusions
Black and Asian patients had a higher risk of ICH recurrence than white patients, whereas private insurance was associated with reduced risk compared to those with Medicare. Further research is needed to determine the drivers of these disparities.
Spontaneous, nontraumatic intracerebral hemorrhage (ICH) comprises 10%–15% of all strokes and constitutes the most devastating stroke subtype, with 40% 1-month case fatality and severe disability affecting the majority of survivors.1 Survivors are at risk of recurrent ICH, but estimates of this annual risk have varied widely from 1% to 24%.2–5 This variation may be explained by small sample sizes, referral bias of particularly high-risk patients with ICH into research registries, and short duration of follow-up to detect rare recurrence events with precision. Furthermore, the risk of ICH recurrence in a US population has not been well-described, as prior studies of ICH recurrence are older and were conducted in Asian and European populations.3,6–13 Therefore, nationally representative and diverse data are needed to study the risk of ICH recurrence in the US population.
Factors associated with the risk of incident ICH have been well-described, but few studies have examined factors associated with the risk of recurrent ICH.14 Race/ethnicity is known to influence the risk of first-ever ICH,15–17 but only one study has investigated racial and ethnic differences in the risk of recurrent ICH, and found that black and Hispanic patients were at higher risk than white patients.5 Blood pressure (BP) reduction is recommended for the secondary prevention of ICH,18 and higher achieved BPs are known to be associated with a higher risk of recurrent ICH,19 but this does not seem to completely explain racial/ethnic differences in ICH recurrence.5
We used US-based administrative claims data from the Healthcare Cost and Utilization Project (HCUP) California State Inpatient Database (SID), a large, racially and ethnically diverse population with administrative data that allows tracking of individuals' hospitalizations over time, to (1) estimate the long-term risk of ICH recurrence across different racial/ethnic groups in a multicenter hospital-based US sample and (2) identify racial/ethnic subgroups at an increased risk of ICH recurrence to inform targeted secondary prevention strategies.
Methods
Study design
We performed longitudinal analyses using prospectively collected claims data from the HCUP California SID from January 2005 to December 2011. California was chosen because it is a large, racially and ethnically diverse population with administrative data that allow tracking of individuals' hospitalizations over time, providing a sufficient sample size to study rare events. Uniform data on all hospitalizations in nonfederal acute care California hospitals, regardless of payer, were collected using automated online reporting software.20
Standard protocol approvals, registrations, and patient consents
This study was approved under a Data Use Agreement with HCUP. Review by an institutional review board is not required for use of HCUP limited data sets.
Study population
We identified all patients with a first-recorded primary ICH using validated codes from the ICD-9-CM for ICH (431).21 Discharged survivors age 18 or older with available tracking information were included. Non-California residents were excluded to reduce bias introduced by lack of follow-up data. Patients presenting with a concurrent diagnosis of trauma were excluded (ICD-9-CM codes 800–804, 850–854).
Measurements
Outcomes
The primary outcome of this study was recurrent primary ICH. To ascertain the outcome in our study population, we identified all subsequent readmissions for a primary diagnosis of ICH using the same validated ICD-9-CM code (431) and excluded readmission for ICH with a concurrent diagnosis of trauma and those due to transfers between acute care centers using HCUP-defined criteria.22 We limited our analysis to first recurrence events because only a small number of patients had multiple recurrent hemorrhages. Death during the follow-up period was ascertained by identifying all recorded deaths in the inpatient, emergency department, and outpatient HCUP datasets.
Covariates
Race and ethnicity are coded in one data element in HCUP, with ethnicity taking precedence over race. Information on race and ethnicity is required at the time of discharge and could have been obtained from various sources, including patient self-designation, administrative personnel during the registration process, admitting providers, or nursing intake forms. For this study, we restricted the analysis to 4 patient groups: non-Hispanic white (referred to as white), non-Hispanic black (referred to as black), Hispanic regardless of race (referred to as Hispanic), and Asian. Patients who were classified as Pacific Islander, mixed race, or other were excluded. Insurance payer indicates the expected primary payer. We restricted our analysis to Medicare, Medicaid, and private insurance and excluded self-pay and other categories.
Statistical analysis
Categorical variables were compared using the Fisher exact test (2-tailed) and continuous variables using the Mann-Whitney rank sum or unpaired t test, as appropriate. Right-censored survival analysis (days-to-event) was used to determine the incidence of ICH recurrence. Patients were censored after their first ICH recurrence, at the time of in-hospital death during the follow-up period, or at the end of the follow-up period. We performed unadjusted analysis using the log-rank test and adjusted analysis using Cox proportional hazards regression modeling to identify factors associated with the risk of ICH recurrence. Race/ethnicity and insurance payer were analyzed as categorical variables using white and Medicaid, respectively, as the reference categories due to their numerical preponderance. Patients with missing data for race/ethnicity or insurance payer were excluded from analyses. Multivariable model building proceeded as follows: first, covariates with p < 0.1 in univariable analyses were included in the model; second, universal confounders (age and sex) were forced into the model; third, covariates with p > 0.1 were backward eliminated; and fourth, collinear covariates, as expressed by a variance inflation factor >5, were identified and removed from the model. The final multivariable model for risk of ICH recurrence included age, sex, race/ethnicity, and insurance payer. The proportional hazard assumption was tested via graphical inspection and calculation of Schoenfeld residuals. R (version 3.5.1) was used for all analyses.
Sensitivity analyses
To verify that our primary outcome reflects true ICH recurrence, we performed a series of sensitivity analyses. First, we excluded readmission events in the first 30 days after the incident ICH with a concurrent diagnosis of pneumonia, urinary tract infection, or bacteremia/sepsis, since it is possible patients could be readmitted for a complication of the incident event and receive a primary diagnosis of ICH.23 Second, we included only readmission events with concurrent procedure codes for external ventricular drain (EVD), craniotomy, or craniectomy (01.2, 02.2), as these interventions are likely to identify true ICH cases. Third, we performed 2 subgroup sensitivity analyses to identify populations likely to have higher recurrence rates: first, in patients over the age of 70 with likely cerebral amyloid angiopathy (CAA)–related ICH; second, in patients with existing diagnoses of atrial fibrillation or valvular heart disease likely to be on anticoagulation. In all analyses, we identified these diagnoses and procedures using validated ICD-9-CM codes.21,24–27
E-values were calculated for the observed hazard ratios (HRs) and confidence interval (CI) bound closest to the null from multivariable analysis for race/ethnicity and insurance payer to assess the potential contribution of unmeasured confounding.28
Data availability
The authors certify that the data, methods, and materials used to conduct the present research have been thoroughly documented. HCUP data are deidentified and publicly available through the Agency for Healthcare Research and Quality (hcup-us.ahrq.gov).
Results
Study population
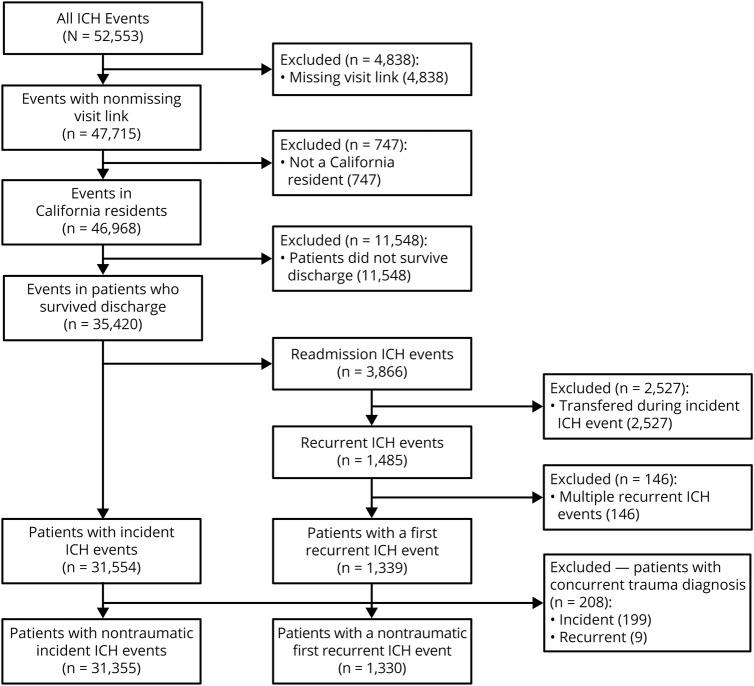
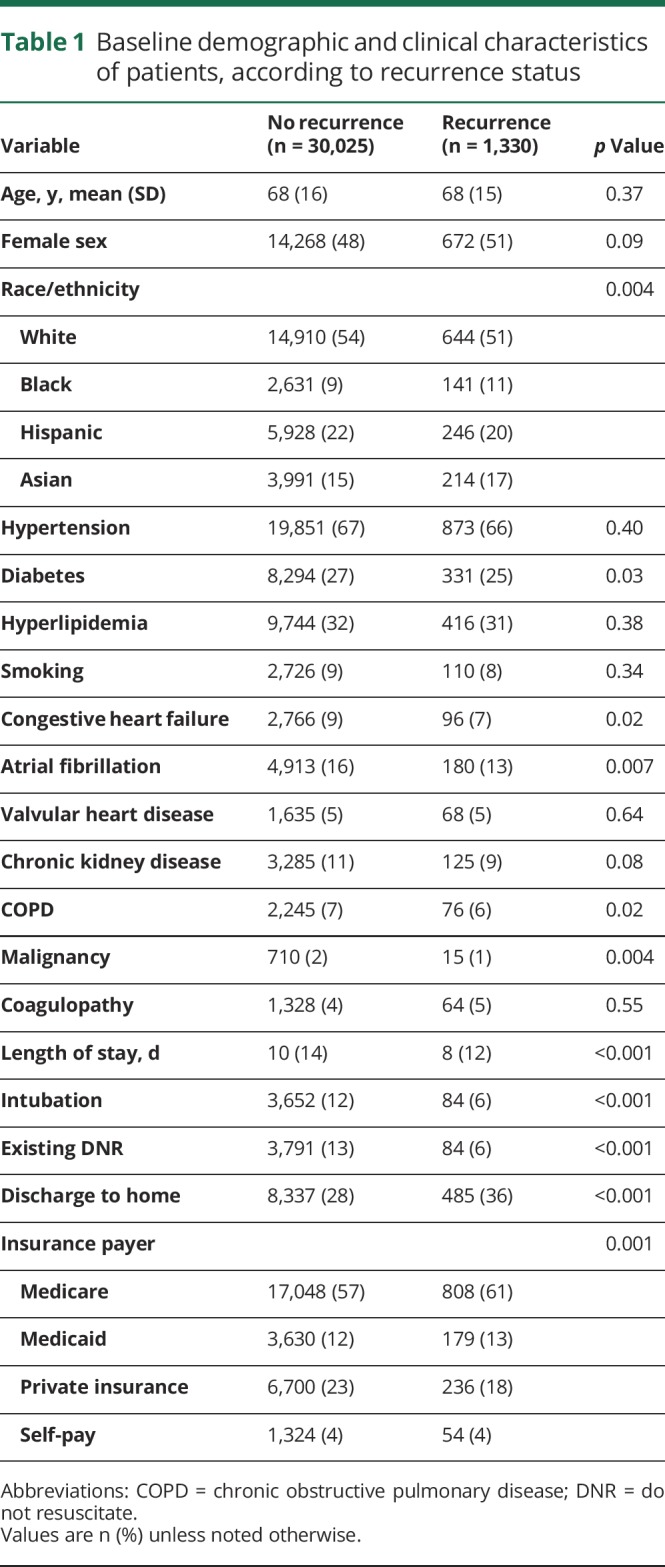
We identified 43,088 California residents with a first-recorded admission for ICH between January 1, 2005, and December 31, 2011. Of these, 11,733 (27%) did not survive to discharge. Of the 31,355 patients who survived to discharge, the mean age was 68 years (SD 16) and 15,175 (48%) were female (figure 1). A total of 1,330 (4.1%) patients experienced a recurrent ICH over a median follow-up of 2.9 years (interquartile range 3.8). Compared to those who did not experience recurrence, those with recurrent ICH were more likely to be black (11% vs 9%) or Asian (17% vs 14%, p = 0.004), were less likely to have private insurance (18% vs 23%, p = 0.001), and had fewer medical comorbidities. Hypertension prevalence at diagnosis was similar between those who did and not experience recurrence (66% vs 67%, p = 0.40) (table 1).
Figure 1. Study selection criteria.
Flowchart summarizing sequential application of selection criteria for this study. ICH = intracerebral hemorrhage.
Table 1.
Baseline demographic and clinical characteristics of patients, according to recurrence status
Incidence of ICH recurrence
The 1-year estimated risk of recurrence was 3.0% (95% CI 2.8%–3.2%). At 5 years, the cumulative risk was 5.5% (95% CI 5.2%–5.8%). These estimates were slightly lower in sensitivity analyses excluding readmissions with concurrent diagnosis of infection within the first 30 days (1 year: 2.7% [95% CI 2.5%–2.9%]; 5 years: 5.2% [95% CI 4.9%–5.5%]). In age-stratified sensitivity analyses comparing ICH recurrence in patients over the age of 70 vs those age 70 or younger, we found an increased 1-year risk of recurrence in the over 70 group (3.3% [95% CI 3.0%–3.6%] vs 2.7% [95% CI 2.5%–3.0%], p = 0.04) in univariable analysis. The recurrence rate was not significantly different in a sensitivity analysis comparing ICH recurrence in patients with and without a history of atrial fibrillation or valvular heart disease (2.7% [2.3%–3.1%] vs 3.1% [95% CI 2.9%–3.4%]; p = 0.50).
Variations in ICH recurrence risk by race/ethnicity and insurance payer
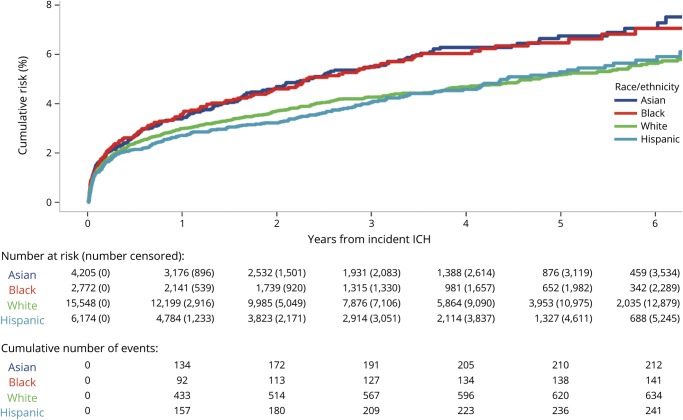
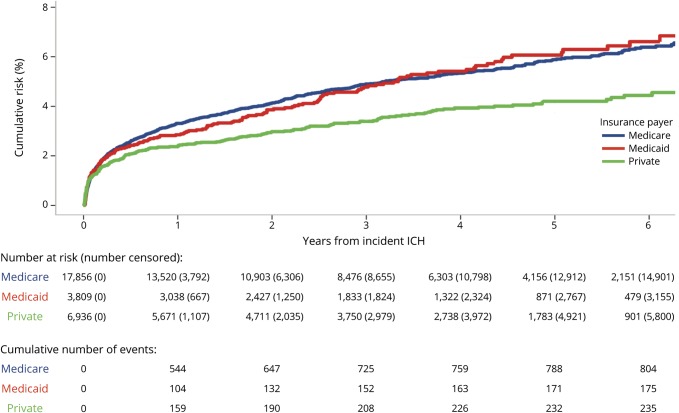
The risk of ICH recurrence varied by race/ethnicity and insurance payer. At 5 years, the cumulative risk was 5.2% (95% CI 4.7%–5.6%) in white patients, 5.3% (95% CI 4.6%–6.0%) in Hispanic patients, 6.5% (95% CI 5.4%–7.6%) in black patients, and 6.7% (95% CI 5.7%–7.6%) in Asian patients (p = 0.007) (figure 2). The 5-year recurrence risk also varied by insurance payer: 4.2% (95% CI 3.6%–4.7%) in those with private insurance, 5.9% (95% CI 5.5%–6.3%) in those with Medicare, and 6.1% (95% CI 5.1%–7.0%) in those with Medicaid (p = 0.001) (figure 3). In multivariable analysis, black (HR 1.22; 95% CI 1.01–1.48; p = 0.03) and Asian (HR 1.29; 95% CI 1.10–1.51; p = 0.002) patients were at an increased risk compared to white patients, and patients with private insurance had a decreased risk compared to those with Medicare (HR 0.60; 95% CI 0.50–0.73; p < 0.001) (table 2). These associations remained significant in a sensitivity analysis excluding recurrence events with a concurrent diagnosis of infection within the first 30 days; black (HR 1.26; 95% CI 1.04–1.53; p = 0.017) and Asian (HR 1.28; 95% CI 1.09–1.51; p = 0.003) patients remained at an increased risk compared to white patients, and those with private insurance remained at a reduced risk compared to patients with Medicare (HR 0.74; 95% CI 0.63–0.86; p < 0.001). In a sensitivity analysis including only recurrence events with procedure codes for EVD, craniotomy, or craniectomy (n = 69 [5.2%]), black (HR 2.56; 95% CI 1.30–5.03; p = 0.007) and Asian (HR 2.14; 1.12–4.08; p = 0.02) patients remained at an increased risk of recurrence compared to white patients, but private insurance was not significantly associated with a reduced risk of recurrence (HR 0.47; 95% CI 0.21–1.03; p = 0.06). The E-values for the HRs from the multivariable model for ICH recurrence risk were 1.73 (lower 95% CI 1.11) for black race, 1.90 (lower 95% CI 1.43) for Asian race, and 2.72 (upper 95% CI 2.08) for private insurance.
Figure 2. Risk of intracerebral hemorrhage (ICH) recurrence by race/ethnicity.
Survival curve summarizing the risk of ICH recurrence over time by race/ethnicity.
Figure 3. Risk of intracerebral hemorrhage (ICH) recurrence by insurance payer.
Survival curve summarizing the risk of ICH recurrence over time by insurance payer.
Table 2.
Univariable and multivariable analysis of factors associated with intracerebral hemorrhage recurrence risk over the follow-up period
Discussion
In this large US-based, racially and ethnically diverse study, we found the estimated 1-year risk of ICH recurrence was 3.0% (95% CI 2.8%–3.2%) among first-ever ICH survivors. We found that patients who did not experience recurrence had a higher burden of medical comorbidities and indicators of greater severity, likely reflecting an increased risk of death before recurrence. Furthermore, we highlight important disparities: we found that black and Asian patients have an increased risk of ICH recurrence compared to white patients and that patients with private insurance have a significant reduction in risk compared to those with Medicare.
Our findings extend the reports of prior small studies. Only one study has evaluated differences in recurrence risk by race and ethnicity, reporting higher recurrence rates in black and Hispanic patients.5,19 While we confirm the finding that black patients are at an increased risk of recurrence compared to white patients, we did not find an increased risk of recurrence in Hispanic patients. These differences may reflect the small number of racial/ethnic minorities and recruitment bias in prior studies and may represent differences in underlying pathophysiology among racial/ethnic minorities. A recent study has shown an association between APOE ε4 and risk of ICH in Hispanic patients but not black patients after controlling for the burden of hypertension in this population.29 We also add that Asian patients, a group not well-studied in the United States, are at an increased risk for recurrence. While prior studies have shown a 2-fold increase in the risk of first-ever ICH in Asian populations compared to other ethnic groups,1 no studies have evaluated ICH recurrence in this population. The mechanisms underlying these racial/ethnic differences remain unclear but are likely mediated by socioeconomic factors that disproportionately affect minorities. Socioeconomic status may influence access to health care, early detection of hypertension, and compliance with antihypertensive medications, which in turn affects the risk of recurrent ICH. Prior studies have shown that racial and ethnic minorities are less likely to achieve recommended BP goals.5 Further study of interventions to control BP in ICH survivors at both the individual and population level is warranted.
In addition to describing racial/ethnic differences in ICH recurrence risk, our study highlights the important finding that private insurance is associated with a significant reduction in the risk of ICH recurrence compared to Medicare across age and racial/ethnic groups. These results are consistent with several studies that have reported worse health outcomes in stroke patients with Medicaid and Medicare compared to those with private insurance.30–32 However, it remains unknown whether these disparities represent true differences in the quality of care between insurance providers or if they represent confounding by baseline socioeconomic and health differences between groups. One possible mediator of quality differences among insurance payers is BP control, as previous studies have shown an association between inadequate BP control and risk of ICH recurrence.5,15,19 While we do not have data on hypertension control or medications to test this hypothesis, our results suggest that patients with and without ICH recurrence are similarly affected by hypertension. Moreover, our calculated E-values indicate that an unmeasured confounder, such as BP, would have to have a strength of association with both the exposure and the outcome of at least 1.73, 1.90, or 2.72 to fully explain away the association of black race, Asian race, or insurance payer, respectively, with risk of recurrence. It is possible that BP is a mediator of the association between race/ethnicity and recurrence risk, but it is unlikely that unmeasured confounding could have completely accounted for these findings. Further research is needed to determine drivers of these disparities and to reduce secondary stroke burden due to hypertension.
While this is the largest study of ICH recurrence in a United States–based, racially and ethnically diverse population, our study has several limitations related to the use of administrative data that require consideration. First, there is a possibility of misclassification of the exposures and outcomes. The attribution of race/ethnicity that is not based on direct self-report may not be accurate; for example, patients who belong to 2 or more racial/ethnic categories may be classified based on phenotypic descriptions and may not reflect true ancestry.33 In terms of outcome classification, we relied on ICD-9-CM codes to identify our outcome of recurrent ICH. However, we used previously validated diagnosis codes that have high positive predictive values for identifying primary ICH. Second, in the absence of prospective case ascertainment, some outcome events may represent sequelae of the incident ICH event. For example, readmission related to a complication, such as infection, may be coded with a diagnosis of ICH, which may bias our results toward higher recurrence rates. However, we took several steps to mitigate the effect of this limitation. First, we identified our outcome using only ICH diagnosis codes in the primary diagnosis position and excluded transfers during the incident hospitalization that may appear as readmissions for recurrent ICH. Second, we performed sensitivity analyses excluding readmissions in the first 30 days with concurrent diagnoses of infectious complications (pneumonia, urinary tract infection, and bacteremia/sepsis) that support our findings. Third, our estimated recurrence rates are consistent with previously published rates. A recent review provided estimates of annual recurrence rates from 1.3% to 7.4% per year, and our 1-year recurrence rate of 3% falls within this range.4 A further limitation of this study is the lack of data on out-of-hospital deaths and emigration from California, which may cause us to overestimate the population at risk and underestimate the risk of recurrence. While the above limitations may decrease our confidence in the absolute rates, they do not affect the relative differences in recurrence risk among racial and ethnic groups. The results of our 2 sensitivity analyses, first, excluding readmissions with concurrent infection in the first 30 days, and, second, in only recurrence events with procedure codes for EVD, craniotomy, or craniectomy, demonstrated the persistence of these racial/ethnic disparities. A final limitation is the lack of data on clinical and radiographic phenotypes of ICH to further study the association of clinical factors such as BP control, anticoagulation, and hemorrhage location (deep vs lobar) on recurrence. Several studies have suggested an increased risk of recurrence in lobar ICH, with estimates from 5% to 24% per year,4,34 thought to be mediated by APOE genotype and underlying CAA.35–38 Furthermore, CAA-related ICH has been shown to have a higher recurrence rate than non-CAA-related ICH.39 Our sensitivity analyses support these observations, demonstrating an increased risk of recurrence in patients over the age of 70. However, additional studies of ICH recurrence in large, well-phenotyped clinical datasets are needed to determine differences in recurrence based on underlying biological mechanisms.
We present the largest, United States–based study of ICH recurrence in a racially and ethnically diverse population. We provide evidence that black and Asian patients are at a higher risk of recurrence than white patients, and that private insurance is associated with a significant reduction in risk. Further research is needed to determine drivers of these significant disparities and to identify targeted secondary ICH prevention strategies.
Glossary
- CAA
cerebral amyloid angiopathy
- CI
confidence interval
- BP
blood pressure
- EVD
external ventricular drain
- HCUP
Healthcare Cost and Utilization Project
- HR
hazard ratio
- ICD-9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- ICH
intracerebral hemorrhage
- SID
State Inpatient Database
Appendix. Authors
Study funding
No targeted funding reported.
Disclosure
A. Leasure is supported by the NIH (T35HL007649) and the American Heart Association Student Scholarship in Cerebrovascular Diseases and Stroke. Z. King and V. Torres-Lopez report no disclosures relevant to the manuscript. S. Murthy is supported by the NIH (K23NS105948) and the Leon Levy Fellowship. H. Kamel is supported by the NIH (R01NS097443, K23NS082367, U01NS095869). A. Shoamanesh and R. Al-Shahi Salman report no disclosures relevant to the manuscript. J. Rosand is supported by the NIH (R01NS036695, UM1HG008895, R01NS093870, R24NS092983). W. Ziai is supported by the NIH (U01NS080824). D. Hanley is supported by the NIH (U01NS080824, U24TR001609). D. Woo and C. Matouk report no disclosures relevant to the manuscript. L. Sansing is supported by the NIH (R01NS095993, R01NS097728). G. Falcone is supported by the NIH (K76AG059992), the American Heart Association (18IDDG34280056), the Yale Pepper Scholar Award (P30AG021342), and the Neurocritical Care Society Research Fellowship. K. Sheth is supported by the NIH (U24NS107136, U24NS107215, R01NR018335, U01NS106513) and the American Heart Association (18TPA34170180, 17CSA33550004). Go to Neurology.org/N for full disclosures.
References
- 1.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010;9:167–176. [DOI] [PubMed] [Google Scholar]
- 2.Bailey RD, Hart RG, Benavente O, Pearce LA. Recurrent brain hemorrhage is more frequent than ischemic stroke after intracranial hemorrhage. Neurology 2001;56:773–777. [DOI] [PubMed] [Google Scholar]
- 3.Hanger HC, Wilkinson TJ, Fayez‐Iskander N, Sainsbury R. The risk of recurrent stroke after intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 2007;78:836–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poon MTC, Fonville AF, Salman RAS. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2014;85:660–667. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Torres A, Murphy M, Kourkoulis C, et al. Hypertension and intracerebral hemorrhage recurrence among white, black, and Hispanic individuals. Neurology 2018;91:e37–e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae HG, Jeong DS, Doh JW, Lee KS, Yun IG, Byun BJ. Recurrence of bleeding in patients with hypertensive intracerebral hemorrhage. Cerebrovasc Dis 1999;9:102–108. [DOI] [PubMed] [Google Scholar]
- 7.Vermeer SE, Algra A, Franke CL, Koudstaal PJ, Rinkel GJE. Long-term prognosis after recovery from primary intracerebral hemorrhage. Neurology 2002;59:205–209. [DOI] [PubMed] [Google Scholar]
- 8.Arakawa S, Saku Y, Ibayashi S, Nagao T, Fujishima M. Blood pressure control and recurrence of hypertensive brain hemorrhage. Stroke 1998;29:1806–1809. [DOI] [PubMed] [Google Scholar]
- 9.Passero S, Burgalassi L, D'Andrea P, Battistini N. Recurrence of bleeding in patients with primary intracerebral hemorrhage. Stroke 1995;26:1189–1192. [DOI] [PubMed] [Google Scholar]
- 10.Hill MD, Silver FL, Austin PC, Tu JV. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke 2000;31:123–127. [DOI] [PubMed] [Google Scholar]
- 11.Huhtakangas J, Löppönen P, Tetri S, et al. Predictors for recurrent primary intracerebral hemorrhage: a retrospective population-based study. Stroke 2013;44:585–590. [DOI] [PubMed] [Google Scholar]
- 12.Weimar C, Benemann J, Terborg C, et al. Recurrent stroke after lobar and deep intracerebral hemorrhage: a hospital-based cohort study. Cerebrovasc Dis Basel Switz 2011;32:283–288. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt LB, Goertz S, Wohlfahrt J, Melbye M, Munch TN. Recurrent intracerebral hemorrhage: associations with comorbidities and medicine with antithrombotic effects. PLoS One 2016;11:e0166223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke 2017;19:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh KB, Woo D, Sekar P, et al. Untreated hypertension: a powerful risk factor for lobar and nonlobar intracerebral hemorrhage in whites, blacks, and Hispanics. Circulation 2016;134:1444–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labovitz DL, Halim A, Boden-Albala B, Hauser WA, Sacco RL. The incidence of deep and lobar intracerebral hemorrhage in whites, blacks, and Hispanics. Neurology 2005;65:518–522. [DOI] [PubMed] [Google Scholar]
- 17.Shen AYJ, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol 2007;50:309–315. [DOI] [PubMed] [Google Scholar]
- 18.Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:2032–2060. [DOI] [PubMed] [Google Scholar]
- 19.Biffi A, Anderson CD, Battey TWK, et al. Association between blood pressure control and risk of recurrent intracerebral hemorrhage. JAMA 2015;314:904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.HCUP-US SID Overview [online]. Available at: hcup-us.ahrq.gov/sidoverview.jsp#about. Accessed September 5, 2018. [Google Scholar]
- 21.McCormick N, Bhole V, Lacaille D, Avina-Zubieta JA. Validity of diagnostic codes for acute stroke in administrative databases: a systematic review. PLoS One 2015;10:e0135834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.User Guide: HCUP Supplemental Variables for Revisit Analyses [online]. Healthcare Cost and Utilization Project; Available at: hcup-us.ahrq.gov/toolssoftware/revisit/UserGuide_SuppRevisitFilesCD.pdf. [Google Scholar]
- 23.Lord AS, Lewis A, Czeisler B, et al. The majority of 30-day readmissions after intracerebral hemorrhage are related to infections. Stroke J Cereb Circ 2016;47:1768–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouza C, Lopez-Cuadrado T, Amate-Blanco JM. Use of explicit ICD9-CM codes to identify adult severe sepsis: impacts on epidemiological estimates. Crit Care 2016;20:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drahos J, Vanwormer JJ, Greenlee RT, Landgren O, Koshiol J. Accuracy of ICD-9CM codes in identifying infections of pneumonia and herpes simplex virus in administrative data. Ann Epidemiol 2013;23:291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tieder JS, Hall M, Auger KA, et al. Accuracy of administrative billing codes to detect urinary tract infection hospitalizations. Pediatrics 2011;128:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care 2005;43:480–485. [DOI] [PubMed] [Google Scholar]
- 28.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017;167:268. [DOI] [PubMed] [Google Scholar]
- 29.Marini S, Crawford K, Morotti A, et al. Association of apolipoprotein E with intracerebral hemorrhage risk by race/ethnicity: a meta-analysis. JAMA Neurol 2019;76:480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fargen KM, Neal D, Blackburn SL, Hoh BL, Rahman M. Health disparities and stroke: the influence of insurance status on the prevalence of patient safety indicators and hospital-acquired conditions. J Neurosurg 2015;122:870–875. [DOI] [PubMed] [Google Scholar]
- 31.Medford-Davis LN, Fonarow GC, Bhatt DL, et al. Impact of insurance status on outcomes and use of rehabilitation services in acute ischemic stroke: findings from get with the guidelines-stroke. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James ML, Grau-Sepulveda MV, Olson DM, et al. Insurance status and outcome after intracerebral hemorrhage: findings from Get With The Guidelines–Stroke. J Stroke Cerebrovasc Dis 2014;23:283–292. [DOI] [PubMed] [Google Scholar]
- 33.Marini S, Lena UK, Crawford KM, et al. Comparison of genetic and self-identified ancestry in modeling intracerebral hemorrhage risk. Front Neurol 2018;9:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Donnell HC, Rosand J, Knudsen KA, et al. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N Engl J Med 2000;342:240–245. [DOI] [PubMed] [Google Scholar]
- 35.Biffi A, Sonni A, Anderson CD, et al. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol 2010;68:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Misra UK, Kalita J, Somarajan BI. Recurrent intracerebral hemorrhage in patients with hypertension is associated with APOE gene polymorphism: a preliminary study. J Stroke Cerebrovasc Dis 2013;22:758–763. [DOI] [PubMed] [Google Scholar]
- 37.Sawyer RP, Sekar P, Osborne J, et al. Racial/ethnic variation of APOE alleles for lobar intracerebral hemorrhage. Neurology 2018;91:e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rannikmäe K, Kalaria RN, Greenberg SM, et al. APOE associations with severe CAA-associated vasculopathic changes: collaborative meta-analysis. J Neurol Neurosurg Psychiatry 2014;85:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Charidimou A, Imaizumi T, Moulin S, et al. Brain hemorrhage recurrence, small vessel disease type, and cerebral microbleeds: a meta-analysis. Neurology 2017;89:820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors certify that the data, methods, and materials used to conduct the present research have been thoroughly documented. HCUP data are deidentified and publicly available through the Agency for Healthcare Research and Quality (hcup-us.ahrq.gov).