Abstract
Objective
This study tests the hypothesis that certain MRI-based regional brain volumes will show reductions over time in a cohort exposed to repetitive head impacts (RHI).
Methods
Participants were drawn from the Professional Fighters Brain Health Study, a longitudinal observational study of professional fighters and controls. Participants underwent annual 3T brain MRI, computerized cognitive testing, and blood sampling for determination of neurofilament light (NfL) and tau levels. Yearly change in regional brain volume was calculated for several predetermined cortical and subcortical brain volumes and the relationship with NfL and tau levels determined.
Results
A total of 204 participants who had at least 2 assessments were included in the analyses. Compared to controls, the active boxers had an average yearly rate of decline in volumes of the left thalamus (102.3 mm3/y [p = 0.0004], mid anterior corpus callosum (10.2 mm3/y [p = 0.018]), and central corpus callosum (16.5 mm3/y [p = <0.0001]). Retired boxers showed the most significant volumetric declines compared to controls in left (32.1 mm3/y [p = 0.002]) and right (30.6 mm3/y [p = 0.008]) amygdala and right hippocampus (33.5 mm3/y [p = 0.01]). Higher baseline NfL levels were associated with greater volumetric decline in left hippocampus and mid anterior corpus callosum.
Conclusion
Volumetric loss in different brain regions may reflect different pathologic processes at different times among individuals exposed to RHI.

The relationship between high levels of exposure to repetitive head impacts (RHI) and long-term neurologic impairment has garnered much attention and debate in both the medical and lay literature. How often this exposure, or which level or severity of exposure, leads to a progressive neurodegenerative condition such as chronic traumatic encephalopathy (CTE) is unknown (figure 1).1,2 Hindering our ability to determine the natural history of exposure to RHI is the lack of biomarkers that can track change in brain structure and function over time.
Figure 1. Average yearly change in left thalamic and central corpus callosum (CC) volumes among retired fighters, active boxers, active mixed martial arts (MMA) fighters, and control group.
Average yearly change in (A) left thalamic and (B) central CC volumes (mL/y) among retired fighters, active boxers, active MMA fighters, and control group.
Previous work has suggested that monitoring regional brain volumes through MRI may serve as a potential tool to recognize and follow structural brain changes.3 Measurement of hippocampal volumes has been well-studied in Alzheimer disease (AD) and is commonly used as a secondary outcome measure in AD clinical trials.4 Similarly, we and others have reported associations between volumes of certain brain regions and exposure to RHI as well as clinical outcomes in cross-sectional studies.5,6 Less is known about using volumetric MRI techniques in a longitudinal manner in those exposed to RHI.
The Professional Fighters Brain Health Study (PFBHS) is a longitudinal study of professional fighters intended to better understand the long-term effects of exposure to RHI. Utilizing this cohort, where MRI is completed annually, we test the hypothesis that certain regional brain volumes will show reductions over time in those exposed to RHI and explore factors that are associated with or modify the effect of those reductions.
Methods
Participants in the PFBHS consist of active and retired professional fighters (boxers and mixed martial arts [MMA] fighters), along with age- and education-matched controls. Active fighters were required to have at least 1 professional fight within 2 years of enrollment and be training with the intent to compete; information about the study was disseminated by the Nevada Athletic Commission, fight promoters, and local training facilities. Retired fighters were included if they had been boxers, had a minimum of 10 professional fights, had no sanctioned fights for at least 2 years, and did not intend to return to competition (there were too few retired MMA fighters to include as a separate group). Control participants were recruited from outreach efforts in the community and could not have any history of neurologic disorders, head trauma, military service, or participation at a high school level or higher in a combat sport or a sport in which head trauma can be anticipated to occur, such as football, wrestling, hockey, rugby, soccer, or rodeo. Enrollment in the PFBHS began in 2011 and has been continuous since then. Each participant is seen on an annual basis, and for active fighters, not sooner than 45 days from a sanctioned fight. The PFBHS was approved by the Cleveland Clinic Institutional Review Board and written informed consent was obtained from all participants. More detailed methods of recruitment and study procedures have been described previously.7
At baseline and each annual visit, a battery of tests and information are acquired including MRI brain, computerized cognitive testing, and exposure history. Participants answer questionnaires with the assistance of the study coordinator that collect information on demographics; educational attainment; medical history including concurrent illnesses and prescribed medications; previous head trauma, both related and unrelated to athletic activities; and prior involvement in other contact sports. Number of professional fights was ascertained by review of commonly recognized databases (boxrec.com for boxers, sherdog.com for MMA fighters). Information on the amount of sparring the participant has engaged in, as well as whether there have been any concussions or head injuries within the 2 weeks prior to the study visit, is obtained through a structured questionnaire.
Cognitive function was assessed by 2 computerized cognitive test batteries. One consists of 4 subtests of the CNS Vital Signs including verbal memory, symbol digit coding, Stroop, and a finger-tapping test. CNS Vital Signs offers robust and reliable measurements of cognition, which are computerized, but are supervised by a technician.8 Results from these tests are used to make up scores in various clinical domains: verbal memory, processing speed, psychomotor speed, and reaction time. The other cognitive assessment is the C3, an iPad-based test that includes symbol digit, Trails A and B, simple and choice reaction time, and a balance measure.9
A high-resolution T1-weighted anatomical MRI was obtained on all fighters at each visit. A 3T MRI scanner (Siemens [Munich, Germany] Verio from April 2011 through October 2015 and Siemens Skyra from December 2016 to the present) with a 32-channel head coil was used to acquire structural 3D T1-weighted magnetization-prepared rapid acquisition gradient echo images (repetition time ms/echo time ms, 2,300/2.98; resolution, 1 × 1 × 1.2 mm3). Only data with high-quality cortical reconstruction from FreeSurfer were utilized. This was ensured through a quality control procedure outlined by FreeSurfer's quality analysis tools (surfer.nmr.mgh.harvard.edu/fswiki/QATools), and only data with signal-to-noise ratio (SNR) of at least 16 were included in this study (surfer.nmr.mgh.harvard.edu/pub/dist/freesurfer/tutorial_packages/OSX/freesurfer/bi n/wm-anat-snr). This SNR number was decided upon based on our observation from randomly selected participants that the segmented images with an SNR of 16 or above yielded maps that require no operator-controlled edits. Therefore, no manual edits were performed.
Volumes of the hippocampus and amygdala and subcortical gray matter including thalamus, caudate, and putamen, along with corpus callosum, were calculated using the automated full brain segmentation process in Freesurfer v.6 software. These regions have been shown in pathologic series and our prior work to be affected in those with extensive RHI.5,10 The volumes of each structure were measured in both hemispheres separately and adjusted for total intracranial volume.
Blood samples were collected annually in EDTA tubes and centrifuged at 3,200 rpm for 10 minutes to separate plasma from blood cells. The supernatant was aliquoted in 2 mL portions that were immediately frozen and stored at −80° pending analysis. Plasma tau and neurofilament light (NfL) concentrations were measured using ultrasensitive single molecule array (Simoa) assays as previously described in detail.11,12 The lower limits of quantification for tau and NfL were 1.22 and 6.9 pg/mL, respectively, and intra-assay coefficients of variation were just above or below 10%. All analyses were performed by board-certified laboratory technicians who were blinded to clinical data.
Statistical analysis
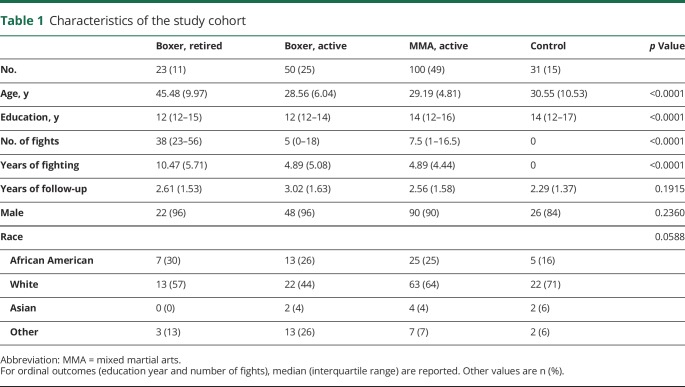
In the table for the characteristics of the study cohort (table 1), mean and SD were computed for continuous variables (e.g., age, number of fights) and the number of events and proportion were calculated for dichotomous variables. Analysis of variance was used to compare the group difference for each continuous variable, nonparametric Kruskal-Wallis test was used for comparing groups when the outcome is ordinal, and the χ2 test was used when the outcome is dichotomized.
Table 1.
Characteristics of the study cohort
Linear mixed models were used for the repeated measurements. When the 4 groups (retired boxer, active boxer, active MMA, and control) were compared with regards to each brain volume, the main effects were visit, group, and their interaction, after adjustment for age, education years, ethnicity, scanner type, and total intracranial volume. The variable scanner type was added in the model because the scanner was upgraded during the study. When only the fighters were included in the model, the number of fights was added as another covariate.
Yearly percentage change of volume for each brain region was determined by calculating the slope of all MRI time points; this value was utilized for all the analyses. However, to categorize the active fighters by rate of volume decline of the caudate, only the volume at the first visit and that at the last visit from the same scanner was used. A 1.5% yearly percentage decrease was considered as the cutoff to separate the participants into 2 subgroups: a stable group with the decreasing rate being less than the cutoff and a declining group with the decreasing rate being more than 1.5% yearly. The repeated cognitive measurements were assessed in linear mixed models with the main effects of group (decliner or not), visit, and their interaction, adjusted by age, education years, ethnicity, scanner type, and number of fights.
All these tests were 2-sided at α level of 0.05. All statistical analyses were performed using SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC).
Data availability
Anonymized data will be shared by request from any qualified investigator.
Results
In total, 204 participants who had at least 2 assessments and complete data for those visits were included in the analyses. Characteristics of the study group are shown in table 1. As expected, the retired boxers were older and had more professional fights and years of fighting. Demographics for active boxers and MMA fighters were similar. The controls were matched for age but tended to have a higher level of education and were more likely to be Caucasian.
Compared to controls, the active boxers had a notable average yearly rate of decline in volumes of the left thalamus (102.3 mm3/y [p = 0.0004]), mid anterior corpus callosum (10.2 mm3/y [p = 0.018]), and central corpus callosum (16.5 mm3/y [p < 0.0001]) (figure 1). For the MMA fighters, the pattern was similar but to a slightly lesser extent in left thalamic decline (57.5 mm3/y [p = 0.036]) and central corpus callosum (9.7 mm3/y [p = 0.007]). Retired boxers, on the other hand, showed the most significant volumetric declines compared to controls in left (32.1 mm3/y [p = 0.002]) and right (30.6 mm3/y [p = 0.008]) amygdala and right hippocampus (33.5 mm3/y [p = 0.01]). Several other structures showed a trend toward decreasing volumes compared to controls (table 2).
Table 2.
Average regional yearly rate of volume change in mm3
To assess whether degree and timing of exposure to RHI was influencing the pattern of regional volumetric decline seen between active and retired fighters, we compared the different fighter groups, controlling for number of professional fights. Among types of fighters, with active MMA fighters as the reference, active boxers showed greater yearly volumetric decline in left thalamus (44.7 mm3/y [p = 0.0211]), right hippocampus (19.1 mm3/y [p = 0.0067]), and central (7.17 mm3/y [p = 0.0053]), mid anterior (7.36 mm/y [p = 0.0114]), and mid posterior corpus callosum (6.30 mm3/y [p = 0.0007]); retired fighters showed greater decline in left amygdala (27.4 mm3/y [p = 0.0011]), left hippocampus (32.7 mm3/y [p = 0.0055]), and right hippocampus (36.9 mm3/y [p = 0.0002]).
There were no significant differences in trajectory of cognitive test scores between controls and active or retired fighters at a group level. However, after separating the active fighters into those with and without yearly caudate volumetric decline as described above, one additional variable (dichotomized variable: decliner or nondecliner) was created and included in the repeated measures statistical model with all visits included. We found that decliners had significant yearly worsening in both Trails A (2.67 s/y [p = 0.004]) and Trails B (4.72 s/y [p = 0.015]) tests (figure 2). No differences in symbol digit or simple or choice reaction time tests were seen between decliners and nondecliners.
Figure 2. Average yearly change in performance on Trail-Making Test (TMT) parts A and B between active fighters who showed >1.5% average yearly decline in caudate volumes (decliners) and those who did not exceed this rate of decline.
Average yearly change in performance on TMT parts A (A) and B (B) in seconds between active fighters who showed >1.5% average yearly decline in caudate volumes (decliners) compared to those who did not exceed this rate of decline.
Higher baseline NfL levels were associated with greater volumetric decline in left hippocampus (33.2 mm3/y, p < 0.001) and mid anterior corpus callosum (7.68 mm3, p = 0.015), with a trend seen in the left amygdala, right thalamus, and mid corpus callosum. Higher baseline tau levels were not correlated with regional volumetric changes. Moreover, there was no difference in change over time in plasma tau or NfL levels between the active fighter decliners and nondecliners.
Discussion
Identifying biomarkers that can track change in brain structure or function over time in those exposed to RHI may have important implications prognostically and in clinical trials as a means to select cohorts or as a possible outcome measure. One potential modality that is already commercially available is MRI-based regional volumetric measures.13 This study examined longitudinal change in MRI volumes in a cohort of active and retired professional fighters. Among the active fighters, thalamic and corpus callosum volumes significantly declined over time compared to controls, whereas in the retired fighters, the amygdala and hippocampus were the structures that showed the most decline, controlling for age. Moreover, the group of fighters that had significant decline in caudate volume also demonstrated reduction in processing speed on a cognitive measure. Higher baseline NfL levels were associated with declining volumes in certain cortical and subcortical volumes.
Previous studies, including our own work, have reported differences compared to controls in thalamic and corpus callosum volumes in disorders that affect the white matter, including traumatic brain injury and multiple sclerosis.5,14–16 Animal studies of head trauma indicate that blows to the head can result in physical injury to axons.17 Because the thalamus has abundant afferent and efferent fibers, the progressive loss of volume seen may reflect Wallerian degeneration of the axons with subsequent neuronal dropout, though other mechanisms that may reduce thalamic volume are also possible.18
In the retired fighters, the structures that showed the greatest decline in volumes over time differed from those in the younger, active fighters. In this retired group, who were generally older, the most significant changes were in the amygdala and hippocampus. Postmortem studies of CTE suggest that the characteristic tau pathology is more scattered in frontal and temporal regions in lower stages and with more advanced disease, and tends to accumulate in the temporal lobe structures with accompanying gross atrophy.19 One interpretation of our findings is that MRI volumetric changes in thalamus and corpus callosum in younger individuals, particularly those actively exposed to RHI, is primarily the result of accumulating axonal injury, whereas in the older, retired cohort, change in hippocampal and amygdala volumes may be reflective of a different process such as CTE or other types of neurodegeneration, such as Alzheimer-type pathology.
The relationship between decline in regional volumes and longitudinal performance on cognitive measures requires scrutiny. We did not find any differences at a group level between active and retired fighters and controls after adjusting for age and education. This may be due to the limited scope of our cognitive test battery, lack of sensitivity to the type of cognitive changes one might encounter in those exposed to head trauma, or variability of the tests themselves.
Experience with other neurodegenerative diseases has shown that more obvious clinically apparent cognitive decline may lag behind structural changes.20,21 To investigate this possibility in our cohort, we dichotomized the study participants into decliners and nondecliners using a greater than 1.5 SD per year drop in caudate volume compared to controls. The caudate was chosen because damage to this structure can result in cognitive and behavioral impairment; we have previously reported a relationship between caudate volumes and cognitive performance and it provided a reasonable number of participants in each group (decliners and nondecliners).5,22,23 Those who had decline in caudate volumes showed a greater yearly lowering in scores on the Trails A and B tests. Trail-making tests are thought to reflect a wide range of cognitive functions including attention, sequencing and shifting, cognitive flexibility, abstraction, psychomotor speed, and ability to simultaneously maintain 2 trains of thought.24
NfL is highly expressed in large-caliber myelinated axons of the white matter and is an established biochemical marker of axonal injury.25,26 Elevations in NfL have been reported not only after concussion, but also after cardiac arrest, as well as in a number of chronic neurologic disorders.27–30 We have previously reported that serum NfL levels are higher in active fighters compared to retired fighters and controls, with very little overlap between NfL levels in active fighters and controls.31 Thus, the current finding that, within the active fighter group, those with higher baseline NfL levels were more likely to show regional volumetric decline suggests that this measure may have predictive properties in identifying individuals who may be at risk of ongoing structural brain injury. Whether cessation of exposure to RHI in those who have elevated NfL levels will halt this volume loss over time needs further investigation.
While this study benefits from having well-characterized participants who either are, or have been, exposed to repetitive head impacts, along with a control group free from reported head trauma, there are certain limitations to be acknowledged. For one, the cohort examined was not a random sample of active and retired fighters, introducing potential bias in the results; that is, it may be that participants who are the most symptomatic and have more concern about their health might be more likely to continue in the study. Arguing against this is the finding that only a small proportion showed decline in cognitive function. Though our study group included both men and women, the number of women was small, making it difficult to determine if sex influences volumetric change. However, repeat analyses that were restricted to men did not significantly change any results. The timeframe for follow-up was brief; although some participants had up to 6 years between baseline and last visit, the average follow-up time was 2.6 years. In addition, we used a brief computerized cognitive battery and may have found more correlations between volumetric decline and cognitive change if we employed more exhaustive testing. In a clinical setting, though, it may not be realistic to obtain full neuropsychological testing on a frequent basis, so that identifying brief, readily available tests that correlate with structural change may be more practical.
One major concern with studies using MRI volumetrics is the variability or noise that may occur with repeated measures. Studies of test and retest stability of volumes consistently report a certain amount of variability including gain or loss of volume.32,33 Our sample is no exception, though we benefit from including multiple imaging data points to determine yearly rate of change. We employed an automated quality control procedure for the MRI volumetrics in this study and there may be some imperfections in exact reconstruction of the brain regions. However, we speculate that the statistical effects due to lack of manually editing those minor imperfections on brain regions utilized in this study should be minimal.34 In addition, we have processed the longitudinal data with cross-sectional analysis in FreeSurfer. To minimize the effect of brain volume at different time points, this study utilized intracranial volume as a nuisance regressor.
Inclusion of this nuisance variable does not eliminate the bias inherent due to lack of longitudinal reconstruction and future studies will evaluate the effects of cross-sectional and longitudinal reconstruction on the results we report. Finally, the average yearly percentage change in regional brain volumes is relatively small. Yet the magnitude of volume change in the brain regions we found to be significant exceed the within-scanner test–retest variability reported in the literature. Still, it is not known if this reliability would apply in general clinical practice, and further work is needed to determine how useful this could be on an individual basis, or if these small changes are prognostic.
Despite these limitations, this study is one of the largest to longitudinally follow MRI brain volumetric changes among active and retired professional athletes exposed to RHI. The findings reported shed some light on how longitudinal regional volumetric MRI may be employed in tracking the long-term neurologic effects of repetitive head trauma. Volumetric loss in different brain regions may reflect different pathologic processes at different times among individuals exposed to RHI. Further research is needed to determine if this pattern of volumetric change continues over a longer follow-up period and can be replicated in other cohorts exposed to RHI.
Glossary
- AD
Alzheimer disease
- CTE
chronic traumatic encephalopathy
- MMA
mixed martial arts
- NfL
neurofilament light
- PFBHS
Professional Fighters Brain Health Study
- RHI
repetitive head impacts
- SNR
signal-to-noise ratio
Appendix. Authors

Footnotes
Editorial, page 101
Podcast: NPub.org/0jqjfy
Study funding
K.B. holds the Torsten Söderberg Professorship in Medicine at the Royal Swedish Academy of Sciences and is supported by the Swedish Research Council (2017–00915), the Swedish Alzheimer Foundation (AF-742881), Hjärnfonden, Sweden (FO2017-0243), and a grant (ALFGBG-715986) from the Swedish state under the agreement between the Swedish government and the County Councils, the ALF agreement. H.Z. is a Wallenberg Academy Fellow supported by grants from the Swedish Research Council (2018-02532), the European Research Council (681712), the UK Dementia Research Institute at UCL, and a grant (ALFGBG-720931) from the Swedish state under the agreement between the Swedish government and the County Councils, the ALF agreement. The Article Processing Charge was funded by the August Rapone Family Fund.
Disclosure
C. Bernick received research funding from UFC, Top Rank Promotions, Haymon Boxing, Bellator/Spike TV, and UCLA Dream Fund. G. Shan reports no disclosures relevant to the manuscript. H. Zetterberg has served on scientific advisory boards for Roche Diagnostics, Samumed, CogRx, and Wave, and is a cofounder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures–based platform company at the University of Gothenburg, all unrelated to the work presented. S. Banks and V.R. Mishra report no disclosures relevant to the manuscript. L. Bekris is supported by a grant from the Department of Defense Peer Reviewed Alzheimer's Research Program (PRARP) Convergence Science Research (AZ160058). J.B. Leverenz has consulted for Acadia, Aptinyx, Eisai, Sanofi, and Takeda. He has research support from GE Healthcare and Sanofi unrelated to work presented. K. Blennow has served as a consultant or on advisory boards for Alector, Alzheon, CogRx, Biogen, Lilly, Novartis, and Roche Diagnostics, and is a cofounder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures–based platform company at the University of Gothenburg, all unrelated to the work presented. Go to Neurology.org/N for full disclosures.
References
- 1.Asken BM, Sullan MJ, DeKosky ST, Jaffe MS, Bauer RM. Research gaps and controversies in chronic traumatic encephalopathy: a review. JAMA Neurol 2017;74:11256–11262. [DOI] [PubMed] [Google Scholar]
- 2.Gardner BE, Iverson GL, McCrory P. Chronic traumatic encephalopathy in sport: a systematic review. Br J Sports Med 2014;48:84–90. [DOI] [PubMed] [Google Scholar]
- 3.Reiter K, Nielson KA, Durgerian S, et al. Five-year longitudinal brain volume change in healthy elders at genetic risk for Alzheimer's disease. J Alzheimers Dis 2017;55:1363–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Platero C, Lin L, Tobar MC. Longitudinal neuroimaging hippocampal markers for diagnosing Alzheimer's disease. Neuroinformatics 2019;17:43–61. [DOI] [PubMed] [Google Scholar]
- 5.Bernick C, Banks SJ, Shin W, et al. Repeated head trauma is associated with smaller thalamic volumes and slower processing speed: the Professional Fighters Brain Health Study. Br J Sports Med 2015;9:1007–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Misquitta K, Dadar M, Tarazi A, et al. The relationship between brain atrophy and cognitive-behavioural symptoms in retired Canadian football players with multiple concussions. Neuroimage Clin 2018;19:551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernick C, Banks S, Phillips M, et al. Professional Fighters Brain Health Study: rationale and methods. Am J Epidemiol 2013;15:280–286. [DOI] [PubMed] [Google Scholar]
- 8.Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS vital signs. Arch Clin Neuropsychol 2006;21:623–643. [DOI] [PubMed] [Google Scholar]
- 9.Alberts JL, Hirsch JR, Koop MM, et al. Using accelerometer and gyroscopic measures to quantify postural stability. J Athl Train 2015;50:578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKee AC, Stein TD, Kiernan PT, Alvarez VE. The neuropathology of chronic traumatic encephalopathy. Brain Pathol 2015;25:350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rohrer JD, Woollacott IO, Dick KM, et al. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology 2016;87:1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattsson N, Zetterberg H, Janelidze S, et al. Plasma tau in Alzheimer disease. Neurology 2016;87:1827–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross DE, Ochs AL, Tate DF, et al. High correlations between MRI brain volume measurements based on NeuroQuant® and FreeSurfer. Psychiatry Res Neuroimaging 2018;278:69–76. [DOI] [PubMed] [Google Scholar]
- 14.Minagar A, Barnett MH, Benedict RHB. The thalamus and multiple sclerosis: modern views on pathologic, imaging and clinical aspects. Neurology 2013;80:210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu L, Zhou F, Zhang Y, et al. Thalamic atrophy and dysfunction in patients with mild-to-moderate traumatic diffuse axonal injury: a short-term and mid-term MRI study. Neuroreport 2018;29:1282–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pischiutta F, Micotti E, Hay JR, et al. Single severe traumatic brain injury produces progressive pathology with ongoing contralateral white matter damage one year after injury. Exp Neurol 2018;300:167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fehily B, Fitzgerald M. Repeated mild traumatic brain injury: potential mechanisms of damage. Cel Transpl 2017;26:1131–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behrens TE, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 2003;6:750–757. [DOI] [PubMed] [Google Scholar]
- 19.Stein TD, Alvarez VE, McKee AC. Chronic traumatic encephalopathy: a spectrum of neuropathological changes following repetitive brain trauma in athletes and military personnel. Alzheimers Res Ther 2014;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010;9:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fotenos AF, Mintun MA, Snyder AZ, Morris JC, Buckner RL. Brain volume decline in aging: evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve. Arch Neurol 2008;65:113–120. [DOI] [PubMed] [Google Scholar]
- 22.Mori E. Impact of subcortical ischemic lesions on behavior and cognition. Ann NY Acad Sci 2002;977:141–148. [DOI] [PubMed] [Google Scholar]
- 23.De Simoni S, Jenkins PO, Bourke NJ, et al. Altered caudate connectivity is associated with executive dysfunction after traumatic brain injury. Brain 2018;141:148–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salthouse TA. Cognitive correlates of cross-sectional differences and longitudinal changes in trail making performance. J Clin Exp Neuropsychol. 2011;33:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zetterberg H, Blennow K. Fluid biomarkers for mild traumatic brain injury and related disorders. Nat Rev 2016;12:563–574. [DOI] [PubMed] [Google Scholar]
- 26.Shahim P, Zetterberg H, Tegner Y, Blennow K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 2017;88:1788–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rojas JC, Karydas A, Bang J, et al. Plasma neurofilament light chain predicts progression in progressive supranuclear palsy. Ann Clin Transl Neurol 2016;3:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu CH, Macdonald-Wallis C, Gray E, et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology 2015;84:2247–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattsson N, Andreasson U, Zetterberg H, Blennow K. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer's disease. JAMA Neurol 2017;74:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moseby-Knappe M, Mattsson N, Nielsen N, et al. Serum neurofilament light chain for prognosis of outcome after cardiac arrest. JAMA Neurol 2019;76:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernick C, Zetterberg H, Shan G, Banks S, Blennow K. Longitudinal performance of plasma neurofilament light and tau in professional fighters: the Professional Fighters Brain Health Study. J Neurotrauma 2018;35:2351–2356. [DOI] [PubMed] [Google Scholar]
- 32.Jovicich J, Czanner S, Han X, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage 2009;46:177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maclaren J, Han Z, Vos S, Fischbein N, Bammer R. Reliability of brain volume measurements: a test-retest dataset. Sci Data 2014;1:140037. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guenette JP, Stern RA, Tripodis Y, et al. Automated versus manual segmentation of brain region volumes in former football players. Neuroimage Clin 2018;18:888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.