Cdk2-dependent TopBP1-treslin interaction is critical for DNA replication initiation. However, it remains unclear how this association is terminated after replication initiation is finished. Here, we demonstrate that phosphorylation of TopBP1 by Akt coincides with cyclin A activation during S and G2 phases and switches the TopBP1-interacting partner from treslin to E2F1, which results in the termination of replication initiation. Premature activation of Akt in G1 phase causes an early switch and inhibits DNA replication.
KEYWORDS: TopBP1, treslin, Akt, cyclin A, Cdk2
ABSTRACT
Cdk2-dependent TopBP1-treslin interaction is critical for DNA replication initiation. However, it remains unclear how this association is terminated after replication initiation is finished. Here, we demonstrate that phosphorylation of TopBP1 by Akt coincides with cyclin A activation during S and G2 phases and switches the TopBP1-interacting partner from treslin to E2F1, which results in the termination of replication initiation. Premature activation of Akt in G1 phase causes an early switch and inhibits DNA replication. TopBP1 is often overexpressed in cancer and can bypass control by Cdk2 to interact with treslin, leading to enhanced DNA replication. Consistent with this notion, reducing the levels of TopBP1 in cancer cells restores sensitivity to a Cdk2 inhibitor. Together, our study links Cdk2 and Akt pathways to the control of DNA replication through the regulation of TopBP1-treslin interaction. These data also suggest an important role for TopBP1 in driving abnormal DNA replication in cancer.
INTRODUCTION
During G1-to-S phase transition, there is a cascade of cyclin activation: first Cdk4/Cdk6/cyclin D in G1 phase, followed by Cdk2/cyclin E in the G1/S transition, and Cdk2/cyclin A activation in S phase and the S/G2 transition. Orderly activation of cyclins is widely believed to be important for proper cell cycle control (1). In particular, the timing of DNA replication initiation is tightly controlled, with a cascade of events termed the restriction point (2). The key kinase of the restriction point is Cdk2/cyclin E. Cdk2/cyclin E can phosphorylate treslin and induce its binding to TopBP1 (topoisomerase IIβ-binding protein 1) (3). This event plays an important role in DNA replication initiation (4).
TopBP1 contains multiple BRCT (BRCA1 C terminus) domains that mediate protein-protein interactions. TopBP1 is involved in DNA replication, ATR checkpoint activation, DNA repair, mitosis, and transcriptional regulation (5). In normal cells, the expression and functions of TopBP1 are tightly regulated. TopBP1 is an E2F target, and its expression is induced during G1/S transition (6). Different functions of TopBP1 can be regulated by posttranslational modifications. Akt phosphorylates TopBP1 at Ser1159 and induces its oligomerization (7). Oligomerization of TopBP1 inhibits its binding to chromatin and ATR and therefore inhibits its ATR-activating function (8). However, at the same time, oligomerization of TopBP1 induces its binding to E2F1 and inhibits E2F1-dependent apoptosis (7). Thus, phosphatidylinositol 3-kinase (PI3K)/Akt survival signaling can inhibit the proapoptotic activity of E2F1 through phosphorylation of TopBP1. Whether oligomerization of TopBP1 also affects other functions of TopBP1, such as interaction with treslin and subsequent initiation of DNA replication, is not known yet. Recently, it has been shown that Akt is activated by Cdk2/cyclin A in S phase (9). It is unclear whether S1159 phosphorylation of TopBP1 is regulated during cell cycle progression.
TopBP1 is overexpressed in many types of cancer, such as breast cancer (10, 11), ovarian cancer, and lung cancer (The Cancer Genome Atlas [TCGA] database). This is in part due to the fact that TopBP1 is regulated by retinoblastoma (Rb)/E2F (6, 10, 12). Many E2F target genes are overexpressed in cancers due to deregulation of the Rb pathway. TopBP1 overexpression can contribute to tumor progression in multiple ways. In cancer cells harboring mutant p53, overexpressed TopBP1 can bind several hot spot mutant p53 proteins and mediate the gain of function of mutant p53 through NF-Y and/or p63/p73 (11). On the other hand, mutant p53 proteins are stable in cancer cells, and the accumulated mutant p53 proteins may bind TopBP1 and perturb its ATR-activating function (13). Some forms of mutant p53 (e.g., contact mutants) can bind both TopBP1 and treslin and promote DNA replication, even in the presence of a Cdk2 inhibitor (13). Given the role of TopBP1 in replication, whether abnormally high levels of TopBP1 directly promote DNA replication in cancer cells deserves to be investigated.
In this report, we show that phosphorylation of TopBP1 at Ser1159 is induced when cells enter S phase, corresponding to the time when Akt and Cdk2/cyclin A are activated. Importantly, we show that this phosphorylation switches the TopBP1 binding partner from treslin to E2F1 in S phase. Premature activation of Akt by a specific Akt activator in G1 phase can trigger the switch and result in inhibition of DNA replication, demonstrating a role for Akt in the control of DNA replication. We also show that, in some cancer cells, TopBP1 is expressed at such high levels that it can force its interaction with treslin even under serum starvation conditions or in the presence of Cdk2 inhibitors and thus bypass Cdk2-mediated normal regulation and promote DNA replication initiation. These data unveil the sequential regulation of the treslin/TopBP1 complex in G1/S transition by Cdk2/cyclin E, which induces treslin/TopBP1 interaction and subsequent initiation of DNA replication; followed by Cdk2/cyclin A, which activates Akt and inhibits treslin/TopBP1 interaction, thereby turning off replication initiation. Our data also demonstrate the direct impact of TopBP1 on DNA replication in cancer cells overexpressing TopBP1.
RESULTS
Akt regulates the switch of TopBP1 binding partners during cell cycle progression.
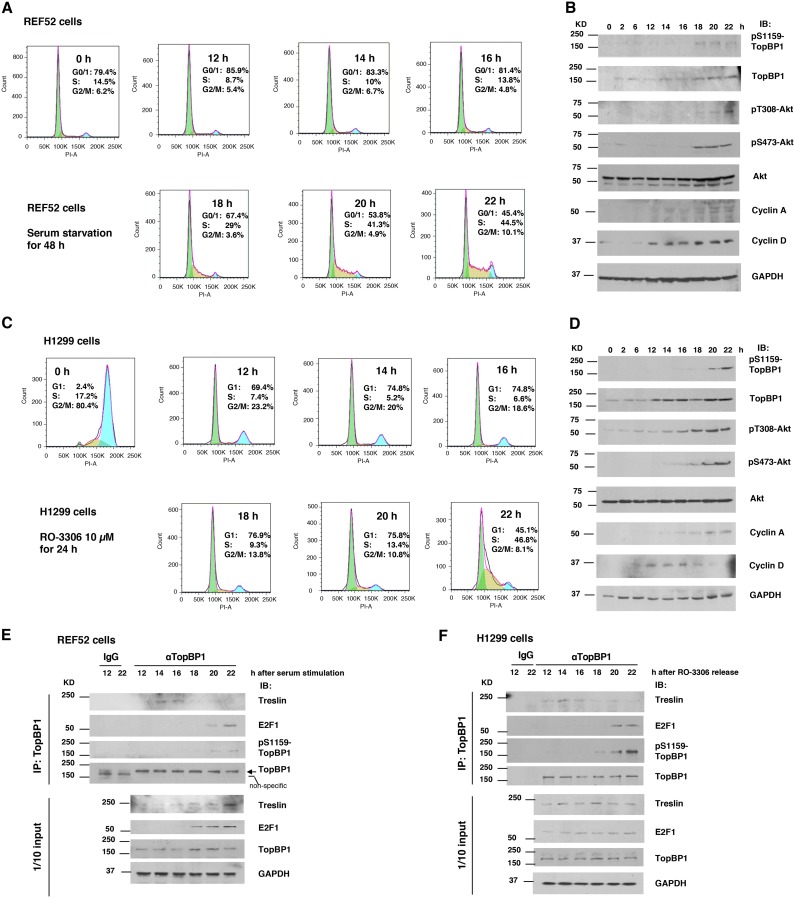
To examine whether phosphorylation of TopBP1 at S1159 by Akt is regulated during the cell cycle, we synchronized REF52 rat embryonic fibroblasts by serum starvation, followed by serum stimulation (6, 14). After serum stimulation for 18 h, the REF52 cells started to enter S phase (Fig. 1A). Consistent with a prior report (9), phosphorylation of Akt coincided with S phase entry and cyclin A induction (Fig. 1A and B). While there was a gradual increase of the total TopBP1 level when REF52 cells progressed from G1 to S phase, pS1159-TopBP1 could be detected only in S phase (18, 20 and 22 h), coinciding with the timing of activated Akt (Fig. 1B), suggesting that Akt phosphorylates TopBP1 in S phase. A similar result was also observed in the H1299 human lung cancer cell line (Fig. 1C and D), which was synchronized by a Cdk1 inhibitor, RO-3306, for 24 h, followed by release for 12 to 22 h to progress from G1 to S phase (13).
FIG 1.
Akt regulates interaction between TopBP1 and treslin during cell cycle progression. (A to D) Cell cycle regulation of TopBP1 phosphorylation. (A and B) REF52 cells were starved in DMEM containing 0.25% FBS for 48 h and then stimulated with 15% FBS. Cells were collected at the indicated time points. (A) Portions of the cells were fixed and stained with propidium iodide for flow cytometry. Green shading, G0/G1; yellow shading, S; blue shading, G2/M. (B) The rest of the cells were harvested for Western blot analysis (IB). (C and D) H1299 cells were synchronized at G2 phase by treatment with a Cdk1 inhibitor (10 μM RO-3306; Tocris) for 24 h and then released into fresh serum-containing medium without RO-3306. Cells were collected at the indicated time points and subjected to flow cytometry and Western blot analyses as described above. (E) REF52 cells were synchronized at G0 phase by serum starvation, followed by serum stimulation as for panels A and B, and then harvested in TNN buffer at the indicated time points. Coimmunoprecipitation (IP) was performed using an anti-TopBP1 mouse monoclonal antibody or control mouse IgG, followed by immunoblotting as indicated. One-tenth of the cell lysates were subjected to Western blot analysis. (F) H1299 cells were synchronized at G2 phase by RO-3306 as for panels C and D. After release from RO-3306 block, cells were harvested at the indicated time points, and procedures similar to those described for panel E were performed to determine the interaction of TopBP1 with either treslin or E2F1.
Next, we performed a coimmunoprecipitation assay to determine the interaction of TopBP1 with its binding partners during cell cycle progression in the REF52 and H1299 cell lines, following the synchronization procedures shown in Fig. 1A and C. Interestingly, TopBP1 interacted with treslin during late G1 phase. However, when cells entered S phase, its interacting partner was switched to E2F1 in both cell lines, albeit in the presence of treslin (Fig. 1E and F). The interaction of TopBP1 with E2F1 in S phase also coincided with the appearance of pS1159-TopBP1, which is consistent with our previous report that phosphorylation of TopBP1 at S1159 is required for its interaction with E2F1 (7).
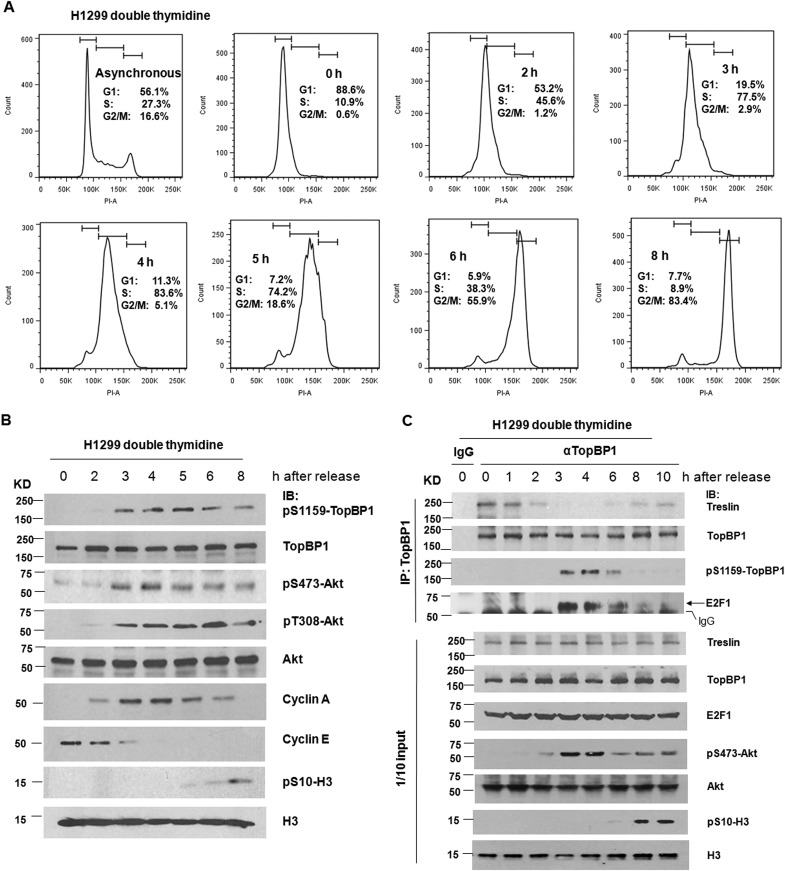
To further examine regulation during S phase progression, we performed a double thymidine block procedure to synchronize H1299 cells at the G1/S border, followed by release of the cells into S phase. The signals of pS1159-TopBP1 were not detectable until 3 h after release, coinciding with the appearance of phospho-Akt and cyclin A, as well as the enrichment of S phase (Fig. 2). In the first 2 h after release, TopBP1 bound exclusively to treslin, but the binding partner was switched to E2F1 between hours 3 and 6 when TopBP1 was phosphorylated at S1159. At 8 h, when the cells entered mitosis, as indicated by a mitosis marker, phospho-Ser10 histone H3, and cell cycle analysis with propidium iodide (PI) flow cytometry, pS1159-TopBP1 disappeared, and so did its binding to E2F1 (Fig. 2C). This result indicates that Akt phosphorylates TopBP1 in S phase and switches the interacting partner of TopBP1 from treslin to E2F1. Our previous data showed that phosphorylation of TopBP1 by Akt induces its oligomerization and subsequent interaction with E2F1. These new data suggest that monomeric TopBP1 interacts with treslin in late G1 phase, whereas oligomeric TopBP1 interacts with E2F1 in S phase.
FIG 2.
Phosphorylation of TopBP1 by Akt coincides with cyclin A activation during S and G2 phases and switches the TopBP1-interacting partner from treslin to E2F1. (A and B) H1299 cells were synchronized at the G1/S border by treatment with thymidine (2 mM). After double thymidine block, the cells were released into fresh serum-containing medium and then collected at the indicated time points for flow cytometry analysis and Western blot analysis. The bars at the tops of the graphs in panel A represent gates defining, from left to right, the G1, S, and G2/M subpopulations. Histone H3 phosphorylation at Ser10 served as a marker for mitosis. (C) H1299 cells were synchronized by double thymidine block/release as for panel A. The cells were then harvested in TNN buffer at the indicated time points. Coimmunoprecipitation was performed as described for Fig. 1E.
Premature activation of Akt in G1 phase inhibits TopBP1-treslin interaction and DNA replication.
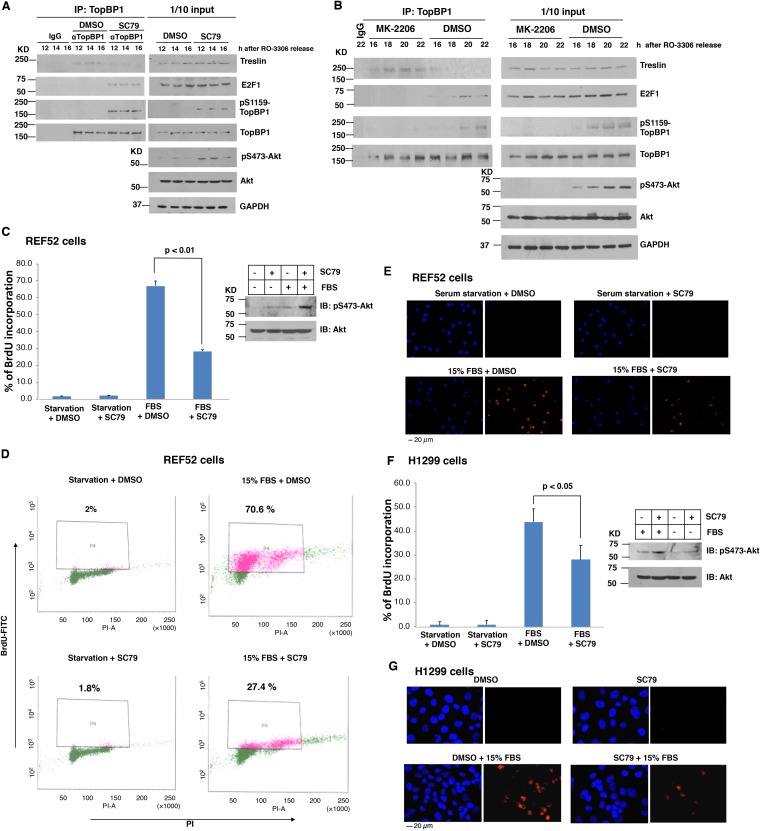
The biological significance of cell cycle regulation by Akt has not been fully explored. While full Akt activation is governed by Cdk2/cyclin A in S phase (9), it is unclear whether Akt activity needs to be kept low in G phase for proper cell cycle progression. Based on the results shown in Fig. 1 and 2, we postulated that Akt must be kept in an inactive state during late G1 phase to allow interaction between TopBP1 and treslin. To investigate this possibility, we used an Akt activator, SC79 (15), to activate Akt in G1 phase in the synchronized H1299 cells and tested whether premature activation of Akt would affect TopBP1-treslin interaction and therefore inhibit S phase entry. We synchronized H1299 cells at the G2/M phase border with a Cdk1 inhibitor, RO-3306, and then released the cells to enter G1 phase. When the cells were in mid-G1 phase (11.5 h after release), they were treated with SC79 and then harvested for coimmunoprecipitation of TopBP1 and treslin at 12 h, 14 h, and 16 h after release. As shown in Fig. 3A, interaction between TopBP1 and treslin in mid- to late G1 phase was indeed inhibited by SC79. Using a pS1159-TopBP1-specific antibody, we confirmed that, in addition to activation of Akt, SC79 also induced TopBP1 phosphorylation at S1159, as anticipated (Fig. 3A, right), and induced interaction between TopBP1 and E2F1. Thus, by activating Akt in mid- to late G1 phase, SC79 could switch the TopBP1-interacting partner from treslin to E2F1. In contrast, when synchronized H1299 cells started to enter S phase, treatment with an allosteric Akt inhibitor, MK-2206, inhibited TopBP1 phosphorylation at S1159 and blocked the switch of TopBP1 binding from treslin to E2F1 during G1/S phase transition (Fig. 3B).
FIG 3.
Premature activation of Akt in G1 phase inhibits DNA replication. (A) H1299 cells were synchronized at G2 phase by treatment with RO-3306 for 24 h and then released into fresh serum-containing medium without RO-3306. After 11.5 h, an Akt activator, SC79 (4 μg/ml; Calbiochem) or control DMSO was added to the medium, and cells were harvested in TNN buffer at 12 h, 14 h, and 16 h. Coimmunoprecipitation of TopBP1 with treslin or E2F1 and Western blot analysis were performed as described for Fig. 1E. (B) H1299 cells were synchronized by treatment with RO-3306 for 24 h and then released into fresh serum-containing medium. After RO-3306 release, an Akt inhibitor (2 μM MK-2206 [MK]; Chemietek) or control DMSO was added to the medium, and cells were harvested in TNN buffer at 16, 18, 20, and 22 h. Coimmunoprecipitation was performed using an anti-TopBP1 mouse monoclonal antibody or control mouse IgG, followed by immunoblotting, as indicated. One-tenth of the cell lysates were subjected to Western blot analysis. (C and D) REF52 cells were starved in DMEM containing 0.25% FBS for 48 h and then stimulated or not stimulated with 15% FBS. Fourteen hours later, the cells were treated with an Akt activator, SC79 (4 μg/ml), or DMSO vehicle for 2 h and then labeled with BrdU (10 μM) for another 2 h. Cells were collected and fixed for anti-BrdU antibody staining. Flow cytometric analysis was performed to quantify BrdU incorporation from at least 10,000 cells per sample. (C) The data shown represent means and standard deviations from three biological replicates. The P values are based on a two-tailed t test. The effect of SC79 on Akt activity was verified by immunoblotting. (D) Representative profiles of BrdU incorporation. (E) REF52 cells were serum starved and then stimulated with 15% FBS. While the cells progressed to mid-G1 phase, they were treated with vehicle or SC79 as for panel C for 2 h and then labeled with BrdU (10 μM) for another 6 h. Incorporated BrdU was detected with anti-BrdU mouse antibody and Texas Red X-conjugated anti-mouse secondary antibody. Nuclei were stained with Hoechst 33258. The images were taken with a fixed exposure time by fluorescence microscopy. Shown are representative images at ×20 magnification from each indicated group. (F and G) H1299 cells were starved in medium containing 0.25% FBS for 48 h or synchronized by RO-3306 followed by release into fresh medium for 11.5 h as described for panel A. After treatment with SC79 or DMSO vehicle for 2 h, the cells were labeled with BrdU for 6 h and then fixed. Incorporated BrdU was detected with anti-BrdU mouse monoclonal antibody and Texas Red X-conjugated anti-mouse secondary antibody. Nuclei were stained with Hoechst 33258. At least 300 nuclei were scored on each sample to determine BrdU incorporation by fluorescence microscopy. (G) Representative images at ×20 magnification from each indicated group.
Given the pivotal role of TopBP1-treslin interaction in DNA replication initiation (3, 4), the inhibition of TopBP1-treslin interaction by SC79 was expected to perturb S phase entry. This prediction was indeed verified in two different cell lines, REF52 and H1299. We synchronized REF52 cells in G0 phase by serum starvation and then stimulated the cells with 15% fetal bovine serum (FBS), as shown in Fig. 1A. Fourteen hours later, when the cells were in mid- to late G1 phase (Fig. 1A), SC79 was added for 2 h, followed by 5-bromo-2-deoxyuridine (BrdU) incorporation. The incorporated BrdU was quantified by either flow cytometry (Fig. 3C and D) or fluorescence microscopy (Fig. 3E). The data showed that activation of Akt by SC79 significantly inhibited serum-induced DNA replication. The effect of SC79 on DNA replication was also observed in H1299 cells (Fig. 3F and G). Thus, premature activation of Akt in mid- to late G1 phase leads to inhibition of S phase entry.
Phosphorylation of TopBP1 by Akt inhibits interaction between TopBP1 and treslin.
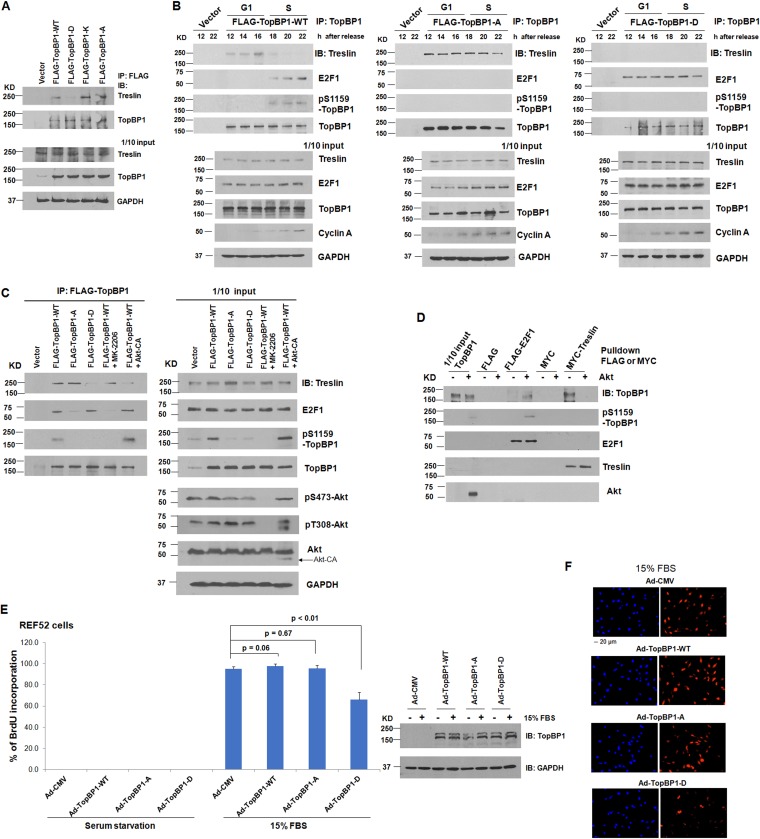
To investigate whether phosphorylation of TopBP1 by Akt plays a direct role in inhibiting its binding to treslin, we next examined the interaction of treslin with either TopBP1 S1159 mutants or a TopBP1 mutant defective in oligomerization (7). Indeed, a coimmunoprecipitation assay showed that, unlike wild-type (WT) TopBP1, the phosphomimetic TopBP1 S1159D mutant failed to interact with treslin in H1299 cells (Fig. 4A). In contrast, both K1317M and S1159A mutants that are defective in oligomerization (7, 8) were able to interact with treslin (Fig. 4A). We also examined the interaction between treslin and these TopBP1 mutants during cell cycle progression. We transfected WT or mutant TopBP1 in H1299 cells, synchronized the cells with RO-3306, and then released the cells to enter G1 and S phases, as shown in Fig. 1C. Indeed, contrary to WT TopBP1, the S1159A mutant bound treslin constitutively without switching its partner to E2F1 in S phase, whereas the S1159D mutant constitutively bound E2F1 but not treslin (Fig. 4B).
FIG 4.
Akt phosphorylation switches TopBP1 binding partners from treslin to E2F1. (A) H1299 cells were transfected with a control vector, FLAG-TopBP1-WT, or one of the FLAG-TopBP1 mutants (S1159D [D], K1317M [K], or S1159A [A]). Coimmunoprecipitation was performed using anti-FLAG M2 monoclonal antibody-conjugated agarose beads, followed by immunoblotting as indicated. One-tenth of the cell lysates were subjected to Western blot analysis. (B) H1299 cells were transfected with a control vector, FLAG-TopBP1-WT, or one of the FLAG-TopBP1 mutants (S1159A [A] or S1159D [D]). After 24 h, the cells were synchronized with RO-3306 as described for Fig. 1C. The cells were then harvested in TNN buffer at the indicated time points after RO-3306 release. Coimmunoprecipitation was performed using anti-FLAG M2 monoclonal antibody-conjugated agarose beads, followed by immunoblotting as indicated. One-tenth of the cell lysates were subjected to Western blot analysis. (C) Myc-treslin was cotransfected or not cotransfected with one of the FLAG-TopBP1 constructs (WT or the D or A mutant) and a constitutively active Myr-Akt1-ΔPH mutant (CA) in H1299 cells as indicated. After 24 h, one of the transfectants that expressed FLAG-TopBP1-WT was treated with 1 μM Akt inhibitor MK-2206 for 24 h. Coimmunoprecipitation was performed as described for panel A. (D) Purified recombinant TopBP1 protein was phosphorylated with recombinant Akt kinase as described previously (7) and then incubated with FLAG-E2F1 or Myc-treslin. To prepare E2F1 and treslin, H1299 cells were transfected with a FLAG control vector, FLAG-E2F1; a Myc control vector; or Myc-TopBP1. After 48 h, cells were harvested in radioimmunoprecipitation assay (RIPA) buffer. FLAG-E2F1 and Myc-treslin were immunoprecipitated using anti-FLAG M2 or anti-Myc monoclonal antibody-conjugated agarose beads. The beads were washed with TNN buffer and then incubated with Akt-phosphorylated purified TopBP1. The beads were washed again and subjected to SDS-PAGE, followed by immunoblotting with the indicated antibodies. (E and F) REF52 cells were starved for 24 h and then infected with Ad-CMV, Ad-TopBP1-WT, Ad-TopBP1-S1159A (A), or Ad-TopBP1-S1159D (D) at a multiplicity of infection of 400 for another 24 h. The cells were stimulated or not stimulated with 15% FBS for 14 h and then subjected to BrdU labeling for 6 h. After fixation, immunostaining of incorporated BrdU was performed as described for Fig. 3F. Expression of Ad-TopBP1-WT, -A, or -D was verified by immunoblotting. (F) Representative images at ×20 magnification of the serum-stimulated group. The data are presented as means and SD.
To further investigate the Akt-dependent switch, we treated H1299 cells with an Akt inhibitor, MK-2206, or transfected the cells with a constitutively active Myr-Akt1-ΔPH construct (designated Akt-CA). The interaction between TopBP1 and E2F1 was inhibited by MK-2206, whereas the interaction between TopBP1 and treslin was inhibited by coexpression of Akt-CA (Fig. 4C). The Akt-dependent switch of TopBP1 binding partners between treslin and E2F1 was further confirmed by an in vitro binding assay (Fig. 4D).
We then investigated whether S1159D-TopBP1 could serve as a dominant-negative mutant to interfere with S phase entry. We synchronized REF52 cells at G0 phase by serum starvation and then infected the cells with recombinant adenovirus (Ad) harboring either an empty vector (Ad-CMV [cytomegalovirus]) or a TopBP1 construct (Ad-TopBP1-WT, -S1159A, or -S1159D). The cells were then stimulated with serum to enter G1 and S phases, and a BrdU incorporation assay was performed to quantify the cell population with S phase entry. The result showed that expression of wild-type TopBP1 or S1159A-TopBP1 did not affect S phase entry compared with the Ad-CMV control, whereas expression of S1159D-TopBP1 decreased the fraction of BrdU-positive (BrdU+) cells (Fig. 4E and F).
Taken together, these data demonstrate that Akt negatively regulates the interaction of TopBP1 and treslin, and therefore premature activation of Akt at G1 phase leads to inhibition of their interaction, as well as S phase entry.
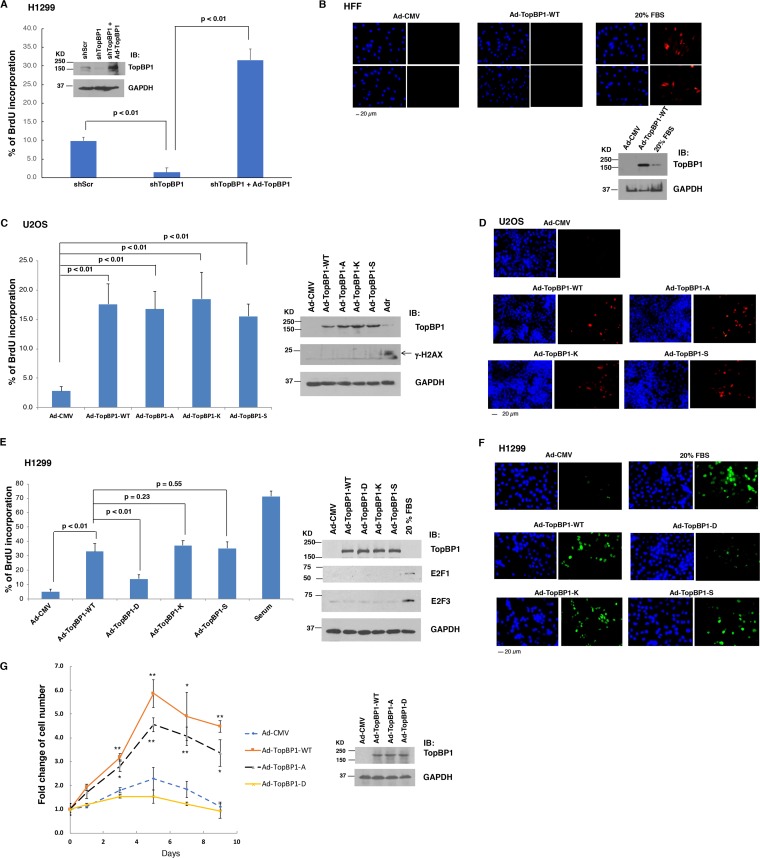
TopBP1 promotes DNA replication in cancer cells expressing high levels of TopBP1.
Many cancer cells express high levels of TopBP1, in part due to deregulation of the Rb pathway and the fact that TopBP1 is an E2F target (12, 14). Cdk2 phosphorylates treslin and induces its interaction with TopBP1. The interaction of TopBP1 with treslin plays a critical role in initiating DNA replication (3, 4). Therefore, we next investigated whether TopBP1 expressed at high levels might drive DNA replication in cancer cells even when Cdk2 activity is low, such as under low-serum conditions. We reconstituted TopBP1 with Ad-TopBP1 infection in TopBP1-depleted H1299 cells (13) and then performed a BrdU incorporation assay under serum starvation conditions. As shown in Fig. 5A, there was a small but significant fraction (around 10%) of a BrdU-positive population in control H1299 cells even after serum starvation for 48 h. However, it was barely detectable when TopBP1 was depleted. Nonetheless, adding TopBP1 back to the TopBP1-depleted H1299 cells at a high level dramatically elevated the BrdU-positive cell population under serum starvation conditions (Fig. 5A), suggesting that TopBP1 can drive DNA synthesis in the absence of serum in H1299 cells. It should be noted that Ad-TopBP1 infection did not induce BrdU incorporation under serum starvation conditions in nontransformed REF52 cells (Fig. 4E) (14) or in primary human foreskin fibroblasts (HFFs) even when TopBP1 was overexpressed at much higher levels than that stimulated with 20% FBS (Fig. 5B). Thus, to confirm the specific effect of TopBP1 on promoting DNA replication in serum-starved cancer cells, we next performed similar experiments in another cancer cell line, U2OS osteosarcoma cells. Indeed, overexpression of wild-type TopBP1 or an oligomerization-defective mutant TopBP1, i.e., S1159A (7), K1317M, or S1273A (8), could induce DNA synthesis comparably in serum-starved U2OS cells (Fig. 5C and D). Likewise, both K1317M and S1273A TopBP1 mutants also showed activity comparable to that of wild-type TopBP1 in inducing DNA synthesis in serum-starved H1299 cells; however, this activity was significantly attenuated by the phosphomimetic S1159D mutation (Fig. 5E and F). The effect of TopBP1 on BrdU incorporation in these starved cancer cells was not due to DNA damage/repair in the processes, since we did not detect any γ-H2AX signal by Western blotting analysis (Fig. 5C, right). Ad-TopBP1 infection also did not induce the expression of E2F1 and E2F3 (Fig. 5E, right), which are known to be able to induce S phase entry in serum-starved cells (16). Thus, the induction of BrdU incorporation by TopBP1 in serum-starved cancer cells reflects the replication-promoting activity of TopBP1. As a consequence, U2OS cells overexpressing WT or S1159A TopBP1 showed an increase of total cell numbers after 1 week of incubation in the absence of serum compared to control cells or cells expressing S1159D TopBP1 (Fig. 5G). The fact that expression of TopBP1 alone in these serum-starved cancer cells was able to enhance replication reveals a pivotal role for TopBP1 in the regulation of DNA replication in cancer cells.
FIG 5.
TopBP1 overexpression promotes DNA replication and cell proliferation in cancer cells. (A) H1299 cells stably expressing either a shScr or a TopBP1 shRNA were starved for 48 h, followed or not followed by infection with Ad-TopBP1 at a multiplicity of infection of 400 for another 18 h. The cells were labeled with BrdU for 17 h and then fixed for BrdU incorporation assay. The data shown represent means and standard deviations from three biological replicates. The P values are based on a two-tailed t test. Knockdown and overexpression of TopBP1 were verified by immunoblotting. (B) HFFs were starved in DMEM containing 0.25% FBS for 48 h and then infected with Ad-CMV or Ad-TopBP1-WT at a multiplicity of infection of 400. (Top) After 18 h, the cells were labeled with BrdU (10 μM) for 17 h and then fixed for BrdU incorporation assay. As a positive control, a group of cells were incubated with 20% FBS when BrdU was added. (Bottom) Overexpression of TopBP1 was verified by immunoblotting. (C and D) U2OS cells were starved for 48 h and then infected with Ad-CMV, Ad-TopBP1-WT, Ad-TopBP1-A, Ad-TopBP1-K, or Ad-TopBP1-S (S1273A [S]) at a multiplicity of infection of 400. After 18 h, a BrdU incorporation assay was performed as described for panel A. Representative images are presented in panel D. As a positive control for γ-H2AX immunoblotting, some cells were treated with 1 μM adriamycin (Adr) for 5 h before harvesting. (E and F) Serum-starved H1299 cells were infected with adenovirus as indicated, followed by a BrdU incorporation assay. Additionally, parental H1299 cells were serum starved for 48 h, followed by stimulation with 20% FBS to serve as a positive control. Representative images are presented in panel F. Equal expression of TopBP1-WT and various mutants was verified by immunoblotting. (G) U2OS cells were serum starved for 48 h, infected with Ad-CMV or Ad-TopBP1-WT or one of the Ad-TopBP1 mutants (S1159A or S1159D) at a multiplicity of infection of 200, and then cultured in medium containing 0.25% FBS. The cells were counted using a Countess II automatic cell counter on the indicated days. The data shown represent means and standard deviations from three biological replicates. The P values are based on a two-tailed t test. *, P < 0.05; **, P < 0.005 compared with the corresponding Ad-CMV controls. Overexpression of TopBP1 was verified by immunoblotting.
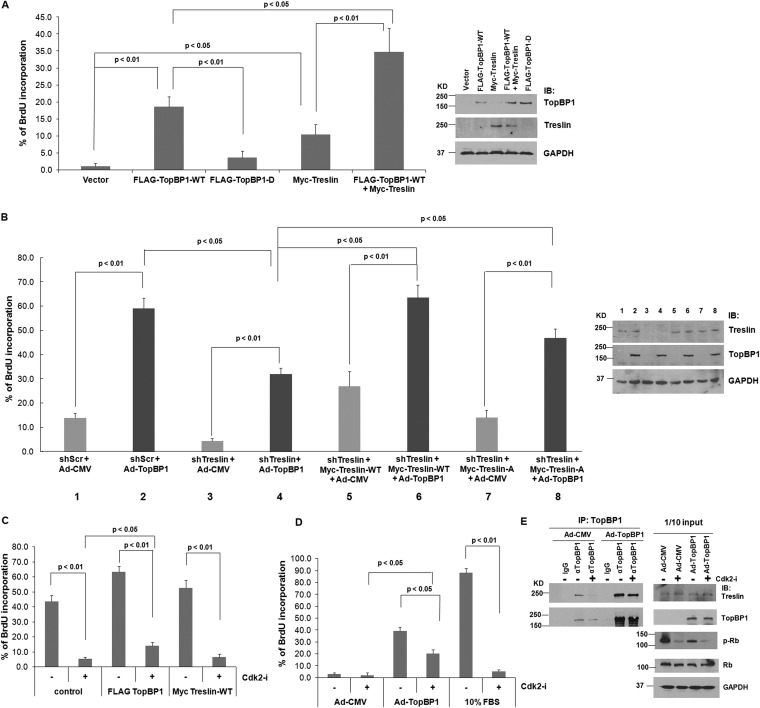
TopBP1 and treslin cooperate to induce DNA replication under serum starvation condition in cancer cells.
In normal cells, the function of TopBP1 in replication is mediated by Cdk2-induced interaction with treslin (4). Therefore, we next investigated whether treslin also plays a role in DNA replication in serum-starved cancer cells. Indeed, overexpression of treslin in H1299 cells induced BrdU incorporation under serum starvation conditions, although to a lesser degree than with TopBP1 (Fig. 6A). Importantly, this effect could be further enhanced by cooverexpression of TopBP1 and treslin. Again, overexpression of S1159D mutant TopBP1 barely induced BrdU incorporation in serum-starved H1299 cells. On the other hand, depletion of treslin reduced BrdU incorporation in H1299 cells and further attenuated the effect of TopBP1 on promoting DNA replication (Fig. 6B). Nonetheless, this effect could be rescued by reconstitution of treslin with a treslin short hairpin RNA (shRNA) (shTreslin)-resistant treslin expression construct (Fig. 6B). To further evaluate the role of treslin phosphorylation in the observed replication-promoting activity of TopBP1 in cancer cells under low-serum conditions, we next transfected the treslin-depleted H1299 cells with an S1000A-treslin mutant defective in phosphorylation by Cdk (Fig. 6B), which has been shown to interact poorly with TopBP1 under normal conditions (3, 13). While S1000A-treslin was less active than wild-type treslin, it could still cooperate with overexpressed TopBP1 to promote DNA replication (Fig. 6B). These data suggest that TopBP1 and treslin can cooperate and bypass the requirement for Cdk2 to promote DNA replication in cancer cells expressing both proteins at high levels.
FIG 6.
TopBP1 and treslin cooperate and bypass the regulation of Cdk to promote DNA replication in cancer. (A) H1299 cells were transfected with an empty vector, FLAG-TopBP1-WT, FLAG-TopBP1-D, and/or Myc-treslin, as indicated. Thirty hours later, the cells were serum starved for 48 h and then labeled with BrdU for 17 h. The cells were fixed, and a BrdU incorporation assay was performed. The data shown represent means and standard deviations from three biological replicates. The P values are based on a two-tailed t test. (Right) Expression of the indicated constructs was verified by immunoblotting. (B) H1299 cells stably expressing either shScr or shTreslin were established. Treslin-depleted cells were then transfected with a control vector or an shRNA-resistant Myc-treslin-WT or Myc-treslin-A mutant (bar 3). Twenty-four hours later, the cells were serum starved for 48 h and then infected with either Ad-CMV or Ad-TopBP1 at a multiplicity of infection of 400. After 18 h, the cells were labeled with BrdU for 17 h and then fixed for BrdU incorporation assay. The data shown represent means and standard deviations from three biological replicates. The P values are based on a two-tailed t test. (Right) Knockdown of treslin and overexpression of TopBP1 were verified by immunoblotting. (C) H1299 cells were transfected with an empty vector, FLAG-TopBP1-WT, or Myc-treslin. After 48 h, the cells were treated with vehicle (DMSO) or 2 μM Cdk2 inhibitor II (Cdk2-i) for 24 h. After BrdU labeling for 17 h, the cells were fixed, followed by a BrdU incorporation assay. The data shown represent means and standard deviations from three biological replicates. The P values are based on a two-tailed t test. (D) H1299 cells were serum starved for 48 h and then left alone or infected with Ad-CMV or Ad-TopBP1-WT at a multiplicity of infection of 400. After 18 h, the cells were treated or not treated with Cdk2 inhibitor II, followed by a BrdU incorporation assay. A group of parental cells were treated with 10% FBS with or without Cdk2 inhibitor II at the time when BrdU was added to serve as a positive control. (E) H1299 cells were serum starved for 48 h and then infected with either Ad-CMV or Ad-TopBP1-WT at a multiplicity of infection of 400. After 18 h, the cells were treated with Cdk2 inhibitor II or not treated for 4 h and then harvested in TNN buffer. Coimmunoprecipitation of TopBP1 with treslin and Western blot analysis were performed as described for Fig. 1E.
Overexpression of TopBP1 promotes its interaction with treslin even in the presence of a Cdk2 inhibitor and renders cancer cells partially resistant to a Cdk2 inhibitor.
To further investigate the role of Cdk2 in TopBP1-induced DNA replication, we treated TopBP1- or treslin-transfected H1299 cells with a specific Cdk2 inhibitor. In the presence of serum, treatment with Cdk2 inhibitor II (designated Cdk2-i) greatly reduced BrdU incorporation under all conditions (Fig. 6C). However, we could still find a significantly larger fraction of BrdU+ cells by overexpression of TopBP1. The effect of TopBP1 was more striking when H1299 cells were infected with Ad-TopBP1 under serum starvation conditions (Fig. 6D). We found that Cdk2 inhibitor treatment almost completely blocked serum-induced BrdU incorporation but only partially decreased TopBP1-promoted BrdU incorporation under serum starvation conditions. Thus, by expressing TopBP1 at high levels, cancer cells can partially bypass regulation by Cdk2 and become less susceptible to Cdk2 inhibition. This notion is further supported on a molecular basis by coimmunoprecipitation analysis. While Cdk2 inhibitor II blocked the interaction between endogenous TopBP1 and treslin, as shown in Fig. 6E and prior studies (3, 4), it failed to do so when TopBP1 was overexpressed (Fig. 6E). The effect of Cdk2 inhibitor II on blocking Cdk2 activity was confirmed by the inhibition of Rb phosphorylation. We conclude that overexpression of TopBP1 can override the requirement for Cdk2 phosphorylation and interact with treslin to promote DNA replication.
Expression levels of TopBP1 correlate with the proliferation index, particularly in tumors expressing high levels of TopBP1, such as lymphoma and triple-negative breast cancer (TNBC).
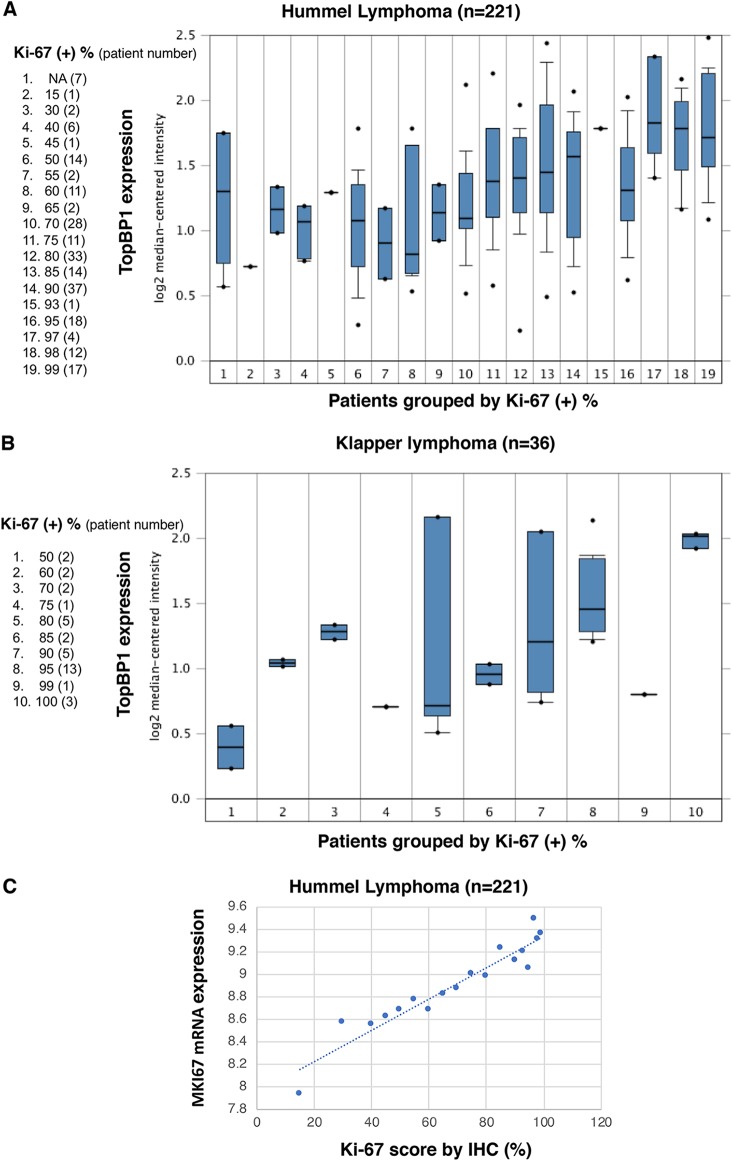
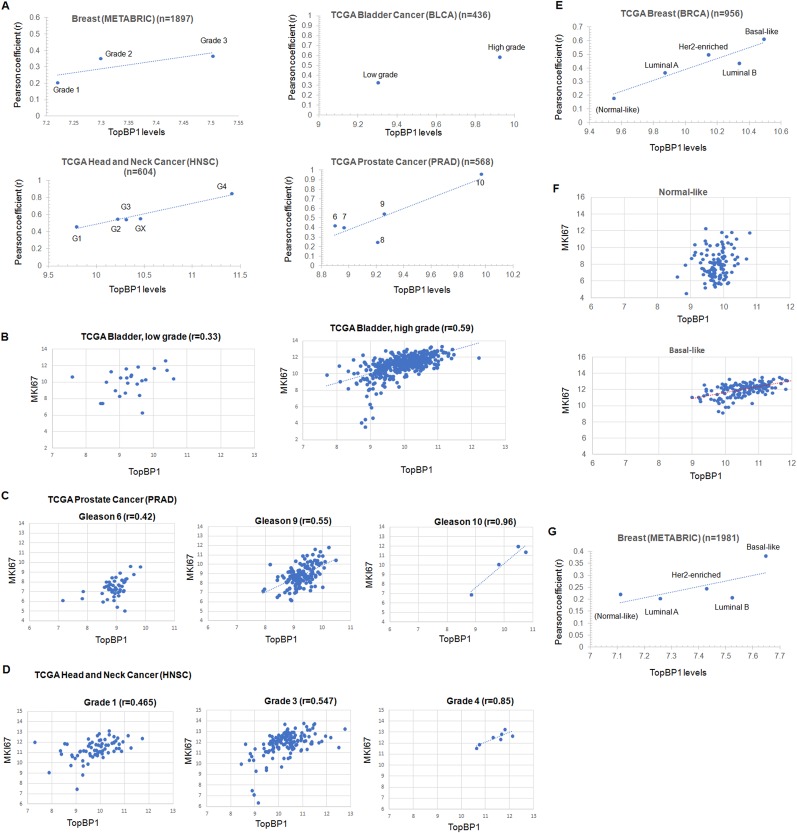
Based on our study showing that TopBP1 overexpression can drive DNA replication, we expected that high levels of TopBP1 would be associated with a high Ki-67 (encoded by the MKI67 gene) proliferation index in tumors. Indeed, The Ki-67 scores correlated very well with TopBP1 mRNA levels in two lymphoma data sets (Fig. 7A and B). Except for lymphoma, Ki-67 immunohistochemistry (IHC) is not routinely performed in clinics. However, MKI67 mRNA levels correlated very well with Ki-67 protein-staining levels, as determined by IHC (17) (Fig. 7C). As such, we used MKI67 mRNA expression as a surrogate marker for Ki-67 positivity and analyzed its correlation with TopBP1 in other data sets. We examined several clinical cancer data sets with annotation of the tumor grade, an indicator for how quickly a tumor grows or spreads. Indeed, TopBP1 mRNA levels correlated with the tumor grade in breast, bladder, prostate, and head and neck cancers, and the correlation between TopBP1 expression and MKI67 increased in higher-grade tumors (Fig. 8A to D).
FIG 7.
TopBP1 expression is associated with Ki-67 positivity in lymphoma. (A and B) Expression of TopBP1 mRNA is correlated with Ki-67 positivity in the Hummel lymphoma cohort (25) and Klapper lymphoma cohort (26). The data were extracted from and analyzed with Oncomine (www.oncomine.org). NA, not analyzed. (A) The mean values of MKI67 mRNA expression levels within each group (except the NA group) were plotted against the percentage of Ki-67 positivity by IHC. The bars represent values between the 25th and 75th percentiles. The horizontal lines in the bars represent medians. The whiskers indicate the 10th and 90th percentiles. The dots represent the minimum and maximum values. (C) Ki-67 positivity scored by IHC correlated very well with the MKI67 mRNA level in each sample. The data were extracted from and analyzed with the Hummel lymphoma data set (25).
FIG 8.
TopBP1 overexpression is associated with a high proliferation index in high-grade human cancers and basal-like subtype breast cancer. (A) TopBP1 mRNA levels were correlated with tumor grades or Gleason scores (for prostate cancer) of the tumors in several cancer data sets, including the METABRIC breast cancer (n = 1897), TCGA bladder cancer (n = 436), TCGA head and neck cancer (n = 604), and TCGA prostate cancer (n = 568) data sets. The Pearson coefficient (r) between TopBP1 mRNA and MKI67 mRNA levels within each tumor grade group was also calculated. High-grade tumors have higher TopBP1 mRNA levels and better correlation between TopBP1 and MKI67. (B to D) Representative scatterplots. TopBP1 expression and its correlation with MKI67 mRNA levels in different grades of tumors from TCGA data sets are shown. The scales of the graphs within the same type of cancer are set the same. The expression of TopBP1 is higher in higher-grade tumors and becomes more associated with MKI67. (E) Analysis similar to that in panel A was performed in TCGA breast cancer data set according to the molecular subtypes of breast cancer. Basal-like breast cancers express higher TopBP1 levels and have a better correlation between TopBP1 and MKI67. (F) Correlation between TopBP1 and MKI67 mRNA levels in normal-like and basal-like subtypes of breast cancer in TCGA data set. (G) Analysis similar to that in panel E was performed in the METABRIC breast cancer data set according to the molecular subtypes of breast cancer.
By analyzing TCGA breast cancer data set, we also found that TopBP1 expression was lowest in normal-like subtype and highest in basal-like subtype (triple-negative) breast cancer (Fig. 8E). Consistent with the tumor grade, the basal-like subtype showed the best correlation between TopBP1 expression and MKI67. There was no significant correlation in normal-like subtype breast cancer (Fig. 8F). Similar results were observed by analyzing another breast cancer data set, METABRIC (Molecular Taxonomy of Breast Cancer International Consortium) (Fig. 8G).
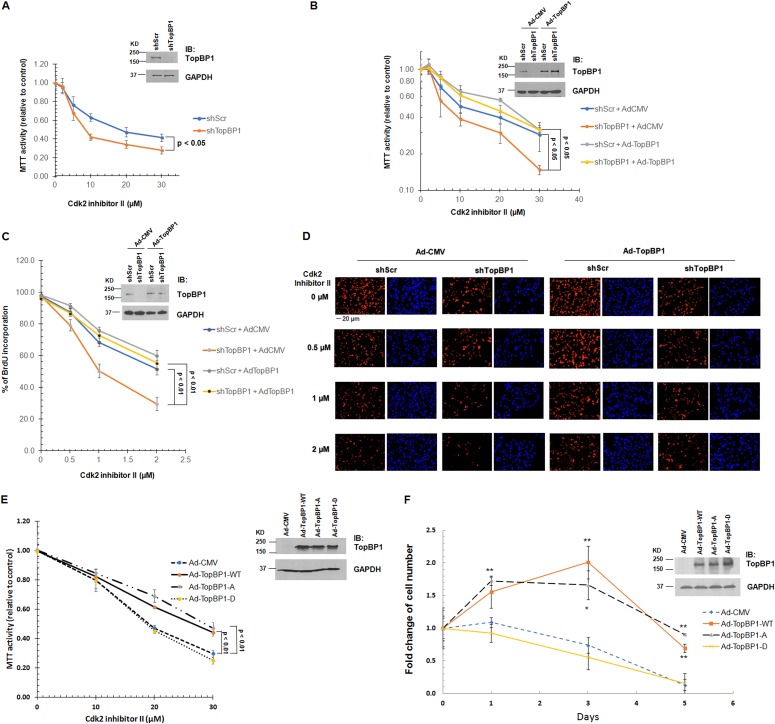
Knockdown of TopBP1 increases sensitivity to Cdk2 inhibitor treatment in cancer cells expressing TopBP1 at high levels.
If high TopBP1 expression in cancer cells contributes to the overriding of Cdk control, depletion of TopBP1 in these cancer cells would resensitize the cancer cells to Cdk inhibition. To investigate this possibility, we depleted TopBP1 in MDA-MB468 triple-negative breast cancer cells, which express TopBP1 at high levels (18), and measured the effect of Cdk2 inhibitor II on cell viability and DNA replication by MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assay and BrdU incorporation assay, respectively. Indeed, TopBP1 knockdown sensitized MDA-MB468 cells to Cdk2 inhibitor II treatment (Fig. 9A, C, and D). This effect was reversed by reconstitution of TopBP1 in the cells (Fig. 9B, C, and D), indicating that the effect is specific to TopBP1. Moreover, only WT and S1159A, but not S1159D, TopBP1 reconstitution in these TopBP1-depleted MDA-MB468 cells could increase resistance to Cdk2 inhibition (Fig. 9E) or serum starvation (Fig. 9F).
FIG 9.
Knockdown of TopBP1 increases sensitivity to a Cdk2 inhibitor. (A) MDA-MB468 cells stably expressing either shScr or shTopBP1 were treated with Cdk2 inhibitor II at the indicated concentrations for 48 h, followed by MTT assay. The data shown represent means and standard deviations from three biological replicates. The P values are based on a two-tailed t test. Knockdown of TopBP1 was verified by immunoblotting. (B) MDA-MB468 cells stably expressing either shScr or shTopBP1 were infected with either Ad-CMV or Ad-TopBP1-WT at a multiplicity of infection of 400. After 18 h, the cells were treated with Cdk2 inhibitor II at the indicated concentrations for 48 h, followed by an MTT assay as described for panel A. (C) MDA-MB468 cells stably expressing either shScr or shTopBP1 were infected with either Ad-CMV or Ad-TopBP1-WT at a multiplicity of infection of 100. After 18 h, the cells were treated with Cdk2 inhibitor II at the indicated concentrations for 36 h, following by BrdU labeling for 12 h, and then fixed for BrdU incorporation assay. The data shown represent means and standard deviations from three biological replicates. The P values are based on a two-tailed t test. Knockdown of treslin and overexpression of TopBP1 were verified by immunoblotting. (D) Representative immunostaining profiles of BrdU incorporation. Shown are representative images at ×20 magnification from each indicated group. (E) TopBP1-depleted MDA-MB468 cells were infected with either Ad-CMV, Ad-TopBP1-WT, or one of the Ad-TopBP1 mutants (S1159A or S1159D) at a multiplicity of infection of 400. After 18 h, the cells were treated with Cdk2 inhibitor II at the indicated concentrations for 48 h, followed by MTT assay. (F) TopBP1-depleted MDA-MB468 cells were serum starved for 48 h, infected with Ad-CMV, Ad-TopBP1-WT, or one of the Ad-TopBP1 mutants (S1159A or S1159D) at a multiplicity of infection of 200 and then cultured in medium containing 0.25% FBS. The cells were counted using a Countess II automatic cell counter on the indicated days. The data shown represent means and standard deviations from three biological replicates. The P values are based on a two-tailed t test. *, P < 0.01; **, P < 0.005 compared with the corresponding Ad-CMV controls. Overexpression of TopBP1 was verified by immunoblotting.
Collectively, our data indicate that when levels of TopBP1 are high enough in cancer cells, such as MDA-MB468 cells, it can promote DNA replication even in the absence of activated Cdk2.
DISCUSSION
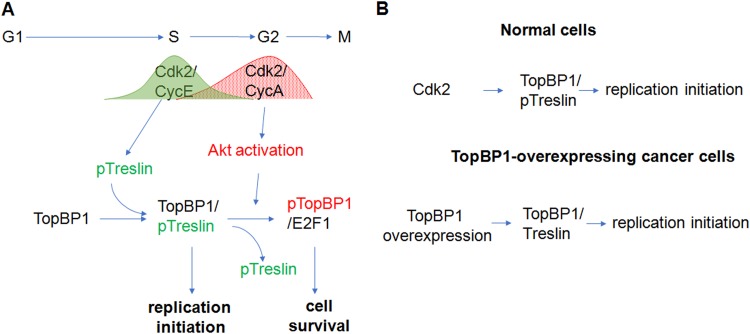
Our prior study revealed a role for Akt in the regulation of TopBP1 oligomerization, which induces its binding to E2F1 and also inhibits its ATR-activating function during replicative stress (7, 8). A recent study demonstrated that Akt is regulated by Cdk2/cyclin A during S phase of the cell cycle (9), raising the question whether Akt may regulate cell cycle progression through the phosphorylation of TopBP1. It has been shown that phosphorylation of treslin by Cdk2/cyclin E induces its interaction with TopBP1 and subsequent initiation of DNA replication (3, 4). Once the replication is complete, the association of TopBP1 with treslin needs to be terminated. However, it is completely unknown whether Akt-dependent phosphorylation of TopBP1 regulates its dissociation from treslin. The current study provides an integrated understanding of how Cdk2 and Akt sequentially regulate the multiple functions of TopBP1 during G1/S transition and S phase and places Akt/TopBP1 regulation in the context of cell cycle control. To sum up, the data support a model in which sequential activations of Cdk2/cyclin E and Akt play opposing roles in the control of complex formation between TopBP1 and either treslin or E2F1 during cell cycle progression, so that TopBP1 forms a complex with treslin only in mid- to late G1 and the G1/S junction for replication initiation but switches to a complex with E2F1 in S phase for cell survival (6, 7) (Fig. 10A).
FIG 10.
Models for the switch of TopBP1 functions in the cell cycle and its deregulation in cancer. (A) During late G1 phase to S phase entry, Cdk2/cyclin E phosphorylates treslin and induces its binding to TopBP1. Their binding promotes initiation of DNA replication. Once cells enter S phase, Cdk2/cyclin A activates Akt, which in turn phosphorylates TopBP1. Phosphorylation of TopBP1 by Akt changes its binding partner from treslin to E2F1 and switches its function from replication initiation to cell survival in S phase. (B) The initiation of DNA replication in normal cells is tightly controlled by Cdk2 activity through phosphorylation of treslin, which induces its binding to TopBP1. However, overexpressed TopBP1 in some cancer cells decreases the dependency of Cdk2, probably by mass action with a high concentration of TopBP1 that drives its interaction with unphosphorylated treslin and leads to uncontrolled DNA replication.
In support of this notion, our data show that premature activation of Akt in mid- to late G1 phase disrupts the TopBP1-treslin interaction and instead induces TopBP1/E2F1 complex formation and therefore inhibits initiation of DNA replication. On the other hand, when TopBP1 is overexpressed, it can bind treslin even in the absence of activated Cdk2 and therefore bypasses control by Cdk2 (Fig. 10B). As a consequence, cancer cells expressing high levels of TopBP1 are more resistant to Cdk2 inhibition (Fig. 9).
Low CDK activity is required for assembly of the prereplication complex (19). Corresponding to the low CDK activity in G1, Akt is not activated until S phase (Fig. 1) (9). Our study demonstrates the importance of keeping Akt activity low in G1 phase, since premature Akt activation can lead to TopBP1 oligomerization, thereby inhibiting its function in replication initiation (Fig. 3). Our data show that S1159 phosphorylation of TopBP1 and the switch of binding from treslin to E2F1 occur 3 to 6 h after release from double thymidine block (Fig. 2), suggesting that TopBP1 still binds to treslin in early S phase, when the initiation of DNA replication occurs, but the binding is switched to E2F1 in mid-S phase to prevent reinitiation of DNA replication. Interestingly, inhibition of CDK activity in G2 can cause rereplication, suggesting that maintenance of a moderate level of CDK activity after DNA replication can prevent reinitiation of S phase (20). Our results raise the possibility that the activation of Akt in S and G2 phases through phosphorylation by Cdk2/cyclin A induces TopBP1 oligomerization, which may contribute to the prevention of DNA rereplication. While downregulation of Cdt1 activity and displacement of MCM2-MCM7 complexes from replicated DNA are known to prevent rereplication (21), our data identify Akt-dependent TopBP1 oligomerization as another mechanism to prevent DNA rereplication by blocking TopBP1-treslin interaction, which is necessary for Cdc45 loading (4). It is worth noting that degradation of Cdt1 is initiated by Cdk2/cyclin E at the onset of S phase (22, 23), but Akt-dependent TopBP1 oligomerization is initiated by Cdk2/cyclin A in mid-S and G2 phases (Fig. 2). Thus, cells employ multiple mechanisms to ensure the prevention of DNA rereplication before the onset of mitosis.
One prediction from our study is that cancers expressing high levels of TopBP1 might be more resistant to Cdk inhibitors. Indeed, unlike estrogen receptor (ER)-positive breast cancer, TNBCs, which typically overexpress TopBP1, do not respond to Cdk4/Cdk6 inhibitors (24). This is thought to be due to the loss of Rb function in many TNBCs. Loss of Rb function almost invariably leads to high levels of TopBP1, since TopBP1 is an E2F target (12, 14). By reducing TopBP1 expression in MDA-MB468 TNBC cells, we can resensitize the cells to Cdk inhibitors (Fig. 9). Therefore, it would be interesting to investigate in future clinical studies whether resistance to Cdk4/Cdk6 inhibitors in some TNBCs can be attributed to high TopBP1 expression.
MATERIALS AND METHODS
Cell culture and transfection.
REF52, MDA-MB468, U2OS, and H1299 cells and HFFs were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, penicillin (50 IU/ml), and streptomycin (50 μg/ml). All the cells were grown in a humidified incubator at 37°C with 5% CO2 and 95% air. The cells were transfected with polyethylenimine (PEI) or Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. After transfection, the cells were incubated for 48 h before analysis.
Double thymidine block.
Subconfluent H1299 cells were treated with 2 mM thymidine for 18 h. After washing twice in phosphate-buffered saline (PBS), the cells were cultured in fresh DMEM with 10% FBS for 9 h and then treated with 2 mM thymidine for another 18 h. After being washed twice in PBS, the cells were incubated in DMEM containing 10% FBS and then collected at the designated time points for assays.
Establishment of stable cell lines.
H1299 and MDA-MB468 cell lines were infected with lentivirus harboring a scrambled shRNA (shScr) or TopBP1 shRNA (shTopBP1) (11), followed by selection with puromycin (2 μg/ml) to establish stable cell lines expressing shScr or shTopBP1. Treslin cDNA constructs with silent mutations conferring resistance to treslin small interfering RNA (siRNA) no. 1 (5′-GACCUGAGAGAAGAUUCAGAAGUUA-3′) were kindly provided by William Dunphy (3). A pair of DNA oligonucleotides were designed and cloned into the pLKO.1 vector according to the standard protocol (https://www.addgene.org/tools/protocols/plko/) to target the same sequence as treslin siRNA no. 1 (5′-GACCTGAGAGAAGATTCAGAAG-3′). The effect of knockdown was confirmed by Western blotting using antibodies specific for TopBP1 and treslin, respectively.
Immunoprecipitation and Western blot analysis.
After cell cycle synchronization, cells were harvested in TNN buffer at different time points as described previously (18). Immunoprecipitation was performed by incubating cell lysates with appropriate antibodies or anti-FLAG M2 monoclonal antibody-conjugated agarose beads for 3 to 16 h at 4°C. After three washes, the immunoprecipitates were fractionated by SDS-PAGE and electrotransferred to an Imobilon-P membrane (Millipore). Equal protein loading was verified with Ponceau S staining. Immunoblotting was performed with appropriate antibodies. Antibodies specific for E2F1 (C-20 or KH-95), c-Myc (A-14), or GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (6C5) were from Santa Cruz Biotechnology. Anti-TopBP1 monoclonal antibody was from BD Transduction Laboratories. Anti-TopBP1 (BL893) rabbit polyclonal antibody and antitreslin antibody were from Bethyl Laboratories. Anti-phospho-TopBP1 (S1159) antibody was from Abgent. Antibodies specific for phosphorylated Akt (p-Akt) (S473), p-Akt (T308), Akt (pan), cyclin D1 (9262), cyclin A2 (BF683), Rb (4H1), p-Rb (S807/811), histone γ-H2AX (20E3), histone H3 and phospho-histone H3 (Ser10) (6G3) were from Cell Signaling. Anti-FLAG (F7425) rabbit antibody was from Sigma.
Bromodeoxyuridine incorporation assay and flow cytometry.
For flow cytometric analysis, cells were labeled with BrdU (10 μM) for 2 h and then fixed with 70% ethanol. The cells were treated with 2 N HCl–Triton X-100 for 30 min and then neutralized with 0.1 M sodium tetraborate, pH 8.5. The cells were then stained with anti-BrdU–fluorescein isothiocyanate (FITC) (BD Biosciences) and propidium iodide, followed by flow cytometry. At least 10,000 cells were analyzed for each sample. All experiments were performed at least in triplicate. For microscopic analysis, cells were labeled with BrdU for 6 or 17 h. After fixation with 4% formaldehyde, the incorporated BrdU was detected with anti-BrdU antibody (Ab-3; Calbiochem) followed by Texas Red X-conjugated secondary antibody (Invitrogen). Nuclei were stained with Hoechst 33258 dye. Images were captured with a Zeiss fluorescence microscope (Axio Observer inverted microscope). The DNA content profiles of REF52 and H1299 cells were determined by propidium iodide staining followed by flow cytometry and analyzed using FlowJo software (Tree Star).
MTT assay.
MDA-MB468 cells or H1299 cells were seeded on 96-well plates at 3,000 cells per well and treated with various concentrations of a specific Cdk inhibitor, Cdk2 inhibitor II (Cayman), for 48 h. The MTT assay was performed by adding 20 μl of MTT reagent (thiazolyl blue tetrazolium bromide; 5 mg/ml; Sigma) to each well, followed by incubation at 37°C for 2 to 4 h. The medium and reagent were removed, and 100 μl of dimethyl sulfoxide (DMSO) was added to each well. After incubation at 37°C for 0.5 to 1 h, absorbance was read at 490 nm on a plate reader (BioTek Synergy HT). Each experiment was performed at least in triplicate.
Statistical analysis.
Two-tailed t tests were performed to compare experimental groups. Data are presented as means and standard deviations (SD) from at least three biological replicates. P values of less than 0.05 were considered statistically significant. Gene expression data in TCGA RNA sequencing (RNA-Seq) database were extracted from the UCSC Xena server (https://xena.ucsc.edu/). Gene expression data in the METABRIC breast cancer data set were extracted from the Synapse server (https://www.synapse.org/). Ki-67 scores and TopBP1 mRNA and MKI67 mRNA data in the Hummel lymphoma data set (GSE4475) were extracted from the NIH GEO2R server. Pearson correlation coefficients were calculated to evaluate correlations. This study used public gene expression in the METABRIC breast cancer data set and TCGA data sets, including bladder cancer (BLCA), head and neck cancer (HNSC), prostate carcinoma (PRAD), and TCGA breast cancer (BRCA). The information on breast cancer subtypes classified according to PAM50 RNA-Seq in TCGA breast cancer cohort was also obtained from the Xena server.
ACKNOWLEDGMENTS
We thank William Dunphy for providing treslin constructs.
This work was supported by NIH grants R01CA100857 (to W.-C.L.) and R01CA203824 and Department of Defense grants W81XWH-14-1-0306, W81XWH-14-1-0339, W81XWH-18-1-0329, and W81XWH-19-1-0369 (to W.-C.L. and F.-T.L.). J.D.G. was supported by T32 Fellowship T32DK060445. We also acknowledge support from the Cytometry and Cell Sorting Core (NIAID P30AI036211, NCI P30CA125123, and NCRR S10RR024574) at Baylor College of Medicine.
W.-C.L. conceived the project; K.L. and W.-C.L. designed research; K.L. performed most experiments; J.D.G., and Y.-J.L. performed BrdU/PI flow cytometry analysis; F.-T.L. and W.-C.L. contributed new reagents/analytic tools; W.-C.L. performed data mining; K.L., J.D.G., Y.-J.L., F.-T.L. and W.-C.L. analyzed data; and K.L., F.-T.L., and W.-C.L. wrote the paper. W.-C.L. and F.-T.L. acquired funding.
We all declare that we have no financial interests that pose a conflict of interest regarding the article.
REFERENCES
- 1.Gerard C, Goldbeter A. 2009. Temporal self-organization of the cyclin/Cdk network driving the mammalian cell cycle. Proc Natl Acad Sci U S A 106:21643–21648. doi: 10.1073/pnas.0903827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blagosklonny MV, Pardee AB. 2002. The restriction point of the cell cycle. Cell Cycle 1:103–110. [PubMed] [Google Scholar]
- 3.Kumagai A, Shevchenko A, Shevchenko A, Dunphy WG. 2011. Direct regulation of Treslin by cyclin-dependent kinase is essential for the onset of DNA replication. J Cell Biol 193:995–1007. doi: 10.1083/jcb.201102003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumagai A, Shevchenko A, Shevchenko A, Dunphy WG. 2010. Treslin collaborates with TopBP1 in triggering the initiation of DNA replication. Cell 140:349–359. doi: 10.1016/j.cell.2009.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wardlaw CP, Carr AM, Oliver AW. 2014. TopBP1: a BRCT-scaffold protein functioning in multiple cellular pathways. DNA Repair 22:165–174. doi: 10.1016/j.dnarep.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Liu K, Luo Y, Lin FT, Lin WC. 2004. TopBP1 recruits Brg1/Brm to repress E2F1-induced apoptosis, a novel pRb-independent and E2F1-specific control for cell survival. Genes Dev 18:673–686. doi: 10.1101/gad.1180204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu K, Paik JC, Wang B, Lin FT, Lin WC. 2006. Regulation of TopBP1 oligomerization by Akt/PKB for cell survival. EMBO J 25:4795–4807. doi: 10.1038/sj.emboj.7601355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu K, Graves JD, Scott JD, Li R, Lin WC. 2013. Akt switches TopBP1 function from checkpoint activation to transcriptional regulation through phosphoserine binding-mediated oligomerization. Mol Cell Biol 33:4685–4700. doi: 10.1128/MCB.00373-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu P, Begley M, Michowski W, Inuzuka H, Ginzberg M, Gao D, Tsou P, Gan W, Papa A, Kim BM, Wan L, Singh A, Zhai B, Yuan M, Wang Z, Gygi SP, Lee TH, Lu KP, Toker A, Pandolfi PP, Asara JM, Kirschner MW, Sicinski P, Cantley L, Wei W. 2014. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature 508:541–545. doi: 10.1038/nature13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu K, Bellam N, Lin HY, Wang B, Stockard CR, Grizzle WE, Lin WC. 2009. Regulation of p53 by TopBP1: a potential mechanism for p53 inactivation in cancer. Mol Cell Biol 29:2673–2693. doi: 10.1128/MCB.01140-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu K, Ling S, Lin WC. 2011. TopBP1 mediates mutant p53 gain of function through NF-Y and p63/p73. Mol Cell Biol 31:4464–4481. doi: 10.1128/MCB.05574-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida K, Inoue I. 2004. Expression of MCM10 and TopBP1 is regulated by cell proliferation and UV irradiation via the E2F transcription factor. Oncogene 23:6250–6260. doi: 10.1038/sj.onc.1207829. [DOI] [PubMed] [Google Scholar]
- 13.Liu K, Lin FT, Graves JD, Lee YJ, Lin WC. 2017. Mutant p53 perturbs DNA replication checkpoint control through TopBP1 and Treslin. Proc Natl Acad Sci U S A 114:E3766–E3775. doi: 10.1073/pnas.1619832114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu K, Lin FT, Ruppert JM, Lin WC. 2003. Regulation of E2F1 by BRCT-domain containing protein TopBP1. Mol Cell Biol 23:3287–3304. doi: 10.1128/mcb.23.9.3287-3304.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jo H, Mondal S, Tan D, Nagata E, Takizawa S, Sharma AK, Hou Q, Shanmugasundaram K, Prasad A, Tung JK, Tejeda AO, Man H, Rigby AC, Luo HR. 2012. Small molecule-induced cytosolic activation of protein kinase Akt rescues ischemia-elicited neuronal death. Proc Natl Acad Sci U S A 109:10581–10586. doi: 10.1073/pnas.1202810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeGregori J, Leone G, Miron A, Jakoi L, Nevins JR. 1997. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc Natl Acad Sci U S A 94:7245–7250. doi: 10.1073/pnas.94.14.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schleifman EB, Desai R, Spoerke JM, Xiao Y, Wong C, Abbas I, O'Brien C, Patel R, Sumiyoshi T, Fu L, Tam RN, Koeppen H, Wilson TR, Raja R, Hampton GM, Lackner MR. 2014. Targeted biomarker profiling of matched primary and metastatic estrogen receptor positive breast cancers. PLoS One 9:e88401. doi: 10.1371/journal.pone.0088401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowdhury P, Lin GE, Liu K, Song Y, Lin FT, Lin WC. 2014. Targeting TopBP1 at a convergent point of multiple oncogenic pathways for cancer therapy. Nat Commun 5:5476. doi: 10.1038/ncomms6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diffley JF. 2001. DNA replication: building the perfect switch. Curr Biol 11:R367–R370. doi: 10.1016/s0960-9822(01)00196-8. [DOI] [PubMed] [Google Scholar]
- 20.Stern B, Nurse P. 1996. A quantitative model for the cdc2 control of S phase and mitosis in fission yeast. Trends Genet 12:345–350. doi: 10.1016/S0168-9525(96)80016-3. [DOI] [PubMed] [Google Scholar]
- 21.Blow JJ, Dutta A. 2005. Preventing re-replication of chromosomal DNA. Nat Rev Mol Cell Biol 6:476–486. doi: 10.1038/nrm1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arias EE, Walter JC. 2005. Replication-dependent destruction of Cdt1 limits DNA replication to a single round per cell cycle in Xenopus egg extracts. Genes Dev 19:114–126. doi: 10.1101/gad.1255805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishitani H, Taraviras S, Lygerou Z, Nishimoto T. 2001. The human licensing factor for DNA replication Cdt1 accumulates in G1 and is destabilized after initiation of S-phase. J Biol Chem 276:44905–44911. doi: 10.1074/jbc.M105406200. [DOI] [PubMed] [Google Scholar]
- 24.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, Los G, Slamon DJ. 2009. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hummel M, Bentink S, Berger H, Klapper W, Wessendorf S, Barth TFE, Bernd H-W, Cogliatti SB, Dierlamm J, Feller AC, Hansmann M-L, Haralambieva E, Harder L, Hasenclever D, Kühn M, Lenze D, Lichter P, Martin-Subero JI, Möller P, Müller-Hermelink H-K, Ott G, Parwaresch RM, Pott C, Rosenwald A, Rosolowski M, Schwaenen C, Stürzenhofecker B, Szczepanowski M, Trautmann H, Wacker H-H, Spang R, Loeffler M, Trümper L, Stein H, Siebert R, Molecular Mechanisms in Malignant Lymphomas Network Project of the Deutsche Krebshilfe. 2006. A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med 354:2419–2430. doi: 10.1056/NEJMoa055351. [DOI] [PubMed] [Google Scholar]
- 26.Klapper W, Szczepanowski M, Burkhardt B, Berger H, Rosolowski M, Bentink S, Schwaenen C, Wessendorf S, Spang R, Moller P, Hansmann ML, Bernd HW, Ott G, Hummel M, Stein H, Loeffler M, Trumper L, Zimmermann M, Reiter A, Siebert R, Molecular Mechanisms in Malignant Lymphomas Network Project of the Deutsche Krebshilfe. 2008. Molecular profiling of pediatric mature B-cell lymphoma treated in population-based prospective clinical trials. Blood 112:1374–1381. doi: 10.1182/blood-2008-01-136465. [DOI] [PubMed] [Google Scholar]