Kaposi’s sarcoma-associated herpesvirus (KSHV) manipulates several cellular pathways for its survival advantage during its latency in the infected human host. Here, we demonstrate that KSHV infection upregulates the expression of genes related to neuronal and neuroendocrine (NE) functions that are characteristic of NE tumors, both in vitro and in KS patient tissues and the heterogeneity of neuroendocrine receptors having opposing roles in KSHV-infected cell proliferation. Induction of NE genes by KSHV could also provide a potential survival advantage, as the expression of proteins at immunologically privileged sites such as neurons on endothelial cells may be an avenue to escape host immune surveillance functions. The NE gene products identified here could serve as markers for KSHV-infected cells and could potentially serve as therapeutic targets to combat KSHV-associated KS.
KEYWORDS: endothelial cells, Kaposi’s sarcoma-associated herpesvirus, neuroendocrine genes, cell proliferation
ABSTRACT
Kaposi’s sarcoma-associated herpesvirus (KSHV) is etiologically associated with endothelial Kaposi’s sarcoma (KS) in immunocompromised individuals. KS lesion cells exhibit many similarities to neuroendocrine (NE) cancers, such as highly vascular and red/purple tumor lesions, spindle-shaped cells, an insignificant role for classic oncogenes in tumor development, the release of bioactive amines, and indolent growth of the tumors. However, the mechanistic basis for the similarity of KS lesion endothelial cells to neuroendocrine tumors remains unknown. Next-generation sequencing and bioinformatics analysis in the present study demonstrate that endothelial cells latently infected with KSHV express several neuronal and NE genes. De novo infection of primary dermal endothelial cells with live and UV-inactivated KSHV demonstrated that viral gene expression is responsible for the upregulation of five selected NE genes (adrenomedullin 2 [ADM2], histamine receptor H1 [HRH1], neuron-specific enolase [NSE] [ENO2], neuronal protein gene product 9.5 [PGP9.5], and somatostatin receptor 1 [SSTR1]). Immunofluorescence and immunohistochemistry examinations demonstrated the robust expression of the NE genes HRH1 and NSE/ENO2 in KSHV-infected KS tissue samples and KS visceral tissue microarrays. Further analysis demonstrated that KSHV latent open reading frame K12 (ORFK12) gene (kaposin A)-mediated decreased host REST/NRSF (RE1-silencing transcription factor/neuron-restrictive silencer factor) protein, a neuronal gene transcription repressor protein, is responsible for NE gene expression in infected endothelial cells. The NE gene expression observed in KSHV-infected cells was recapitulated in uninfected endothelial cells by the exogenous expression of ORFK12 and by the treatment of cells with the REST inhibitor X5050. When the neuroactive ligand-activating receptor HRH1 and inhibitory SSTR1 were knocked out by CRISPR, HRH1 knockout (KO) significantly inhibited cell proliferation, while SSTR1 KO induced cell proliferation, thus suggesting that HRH1 and SSTR1 probably counteract each other in regulating KSHV-infected endothelial cell proliferation. These results demonstrate that the similarity of KS lesion cells to neuroendocrine tumors is probably a result of KSHV infection-induced transformation of nonneuronal endothelial cells into cells with neuroendocrine features. These studies suggest a potential role of neuroendocrine pathway genes in the pathobiological characteristics of KSHV-infected endothelial cells, including a potential mechanism of escape from the host immune system by the expression of immunologically privileged neuronal-site NE genes, and NE genes could potentially serve as markers for KSHV-infected KS lesion endothelial cells as well as novel therapeutic targets to control KS lesions.
IMPORTANCE Kaposi’s sarcoma-associated herpesvirus (KSHV) manipulates several cellular pathways for its survival advantage during its latency in the infected human host. Here, we demonstrate that KSHV infection upregulates the expression of genes related to neuronal and neuroendocrine (NE) functions that are characteristic of NE tumors, both in vitro and in KS patient tissues and the heterogeneity of neuroendocrine receptors having opposing roles in KSHV-infected cell proliferation. Induction of NE genes by KSHV could also provide a potential survival advantage, as the expression of proteins at immunologically privileged sites such as neurons on endothelial cells may be an avenue to escape host immune surveillance functions. The NE gene products identified here could serve as markers for KSHV-infected cells and could potentially serve as therapeutic targets to combat KSHV-associated KS.
INTRODUCTION
Kaposi’s sarcoma-associated herpesvirus (KSHV), or human herpesvirus 8 (HHV-8), is etiologically associated with Kaposi’s sarcoma (KS), primary effusion B-cell lymphoma (PEL), and B-proliferative multicentric Castleman’s disease (MCD) (1–3). These cancers continue to be a major concern in immunocompromised individuals such as HIV-infected patients. The oncogenic processes underlying KSHV-associated cancers are governed by complex interactions between the expression of viral genes and host cellular genes (4, 5). KSHV can infect many different cell types, establishes a latent infection, and periodically enters a lytic infection phase (6, 7). Latent infection is a quiescent state characterized by the expression of a limited number of latent viral genes, which include latency-associated nuclear antigen 1 (LANA-1) (open reading frame 73 [ORF73]), viral cyclin (vCyclin) (ORF72), viral FLIP (vFLIP) (ORF71), kaposin A (ORFK12), and viral microRNAs. The expression of latent genes as well as KSHV infection-induced cytokines and growth factors released into tumor microenvironments play important roles in coordinating oncogenic signaling, antiapoptosis, cell proliferation, survival, and tumor progression (5, 8–10). Furthermore, a few KSHV lytic genes, such as ORF74 (vGPCR [viral G-protein-coupled receptor]), ORFK1, ORFK9 (vIRF1 [viral interferon regulatory factor 1]), and ORFK15, also contribute to oncogenic transformation by modulating the host cellular proteins involved in cell proliferation (5, 9, 11).
KS develops from endothelial cells latently infected with KSHV, and their proliferation leads to lesions in multiple sites of the body, including the skin, visceral organs, and lymph nodes. KS has been subcategorized into four epidemiological variants: classical, endemic, epidemic (AIDS-associated), and iatrogenic (transplantation-associated) KS (12–14). Our analysis of the literature indicate that the different forms of KS, especially classical KS, and neuroendocrine (NE) cancers share several distinct morphological and pathological features, such as (i) highly vascular and red/purple tumor lesions, (ii) a spindle-shaped cellular morphology, (iii) an insignificant role for classical oncogenes in tumor development, (iv) the release of bioactive amines, and, most importantly, (v) indolent growth of the tumors (15–20).
The amino acid glutamate is the most abundant excitatory neurotransmitter in the nervous system of vertebrates (21). Glutamate, via its interaction with specific cell surface receptors, also mediates key functions in several biological processes of a eukaryotic cell, which include cell proliferation, survival, and apoptosis (21–23). Glutamate released from the cell binds and activates ionotropic ligand-gated ion channels (iGluRs) as well as group I, II, and III G-protein-coupled metabotropic glutamate receptors (mGluRs). Group I mGluR1 and mGluR5 subtypes (mGluR1/5) are coupled to Gαq/11 proteins, and agonist stimulation of mGluRs induces phosphatidylinositol hydrolysis and increases diacylglycerol, cytoplasmic calcium, phospholipase C (PLC)- and protein kinase C (PKC)-dependent signaling pathways, and downstream extracellular signal-regulated kinase 1/2 (ERK1/2), resulting in cell proliferation (24, 25).
Interestingly, in addition to neuronal cells, mGluRs are also expressed in nonneuronal cells (26–28). Our previous studies showed that mGluRs are also expressed in human B cells and human microvascular dermal endothelial cells (HMVEC-d), which are biologically relevant for KSHV infection (29). We observed increased glutamate secretion and glutaminase expression during de novo KSHV infection of endothelial cells and in KSHV latently infected endothelial and B cells (29, 30). More importantly, we observed significantly increased mGluR1 expression in KSHV-infected KS and PEL tissue sections. KSHV latency-associated nuclear antigen 1 (LANA-1) mediated an increase in c-Myc expression, which in turn induced glutaminase expression in infected cells, and glutaminase mediated the conversion of glutamine to glutamate.
The expressions of mGluR1 and other neuroendocrine genes are regulated by host cell nuclear RE1-silencing transcription factor/neuron-restrictive silencer factor (REST/NRSF) (29). We observed that REST is localized in the nucleus of uninfected cells, but in contrast, it was localized in the cytoplasm of KSHV-infected endothelial and B cells (29). Western blot (WB) studies with cytoplasmic and nuclear fractions demonstrated that REST was undetectable in the cytoplasm of uninfected endothelial and B cells, while the REST level was significantly decreased in the nuclei of KSHV-infected endothelial and B cells, with a corresponding increase in the cytoplasm of infected cells. Our studies furthermore demonstrated that REST was retained in the cytoplasm of infected cells by the KSHV latent protein kaposin A (K12), which resulted in the phosphorylation of REST and interaction with the E3 ubiquitin ligase beta transducin repeats-containing protein (β-TRCP), leading to the ubiquitination of REST and degradation. Colocalization of kaposin A with REST was also observed in KSHV infection-positive KS and PEL patient tissue samples, which clearly indicated the potential importance of cytoplasmic REST retention in infected cells (29). We also reported that KSHV-infected endothelial and B-lymphoma cell proliferation was significantly inhibited by the glutamate release inhibitor riluzole and the mGluR1 antagonist A841720v (29). These results demonstrated that KSHV infection induces elevated glutamate secretion and mGluR1 expression to mediate key roles in KSHV biology, including infected cell proliferation.
Based on the similarities between KS and neuroendocrine tumors and the overexpression of mGluR1, here, we examined the expression of neuroendocrine genes in KSHV-infected endothelial cells to determine the functional link between their expression and the NE tumor characteristics of KS lesions. We carried out bioinformatics analysis of the next-generation sequencing (NGS) data derived from uninfected and KSHV-infected endothelial cells. Our data show that KSHV infection upregulates the expression of genes related to neuronal and neuroendocrine functions; highlight the existence of neuroendocrine receptor heterogeneity in KSHV-infected cells, with receptors having opposing roles in cell proliferation; and suggest that the similarity of KS lesion cells to neuroendocrine tumors is probably a result of KSHV infection-induced neuroendocrine features in endothelial cells.
RESULTS
Next-generation RNA sequencing analysis identifies the expression of neuroendocrine genes in endothelial cells latently infected with KSHV.
The telomerase-immortalized human umbilical vein endothelial (TIVE) cell line latently infected with KSHV (TIVE-LTC) has been widely used as an in vitro model for studying the biological characteristics of KS (30). To investigate whether KSHV-infected endothelial cells express genes related to NE cancers, we first performed NGS analysis for cellular genes in uninfected TIVE and KSHV-infected TIVE-LTC cells. In the NGS data analysis, 4,051 genes were upregulated, with a log fold change (FC) of 0.58 or higher (see Table S1 in the supplemental material), and 3,671 genes were downregulated (log FC of less than −0.58) in TIVE-LTC cells compared to uninfected TIVE cells (Table S2).
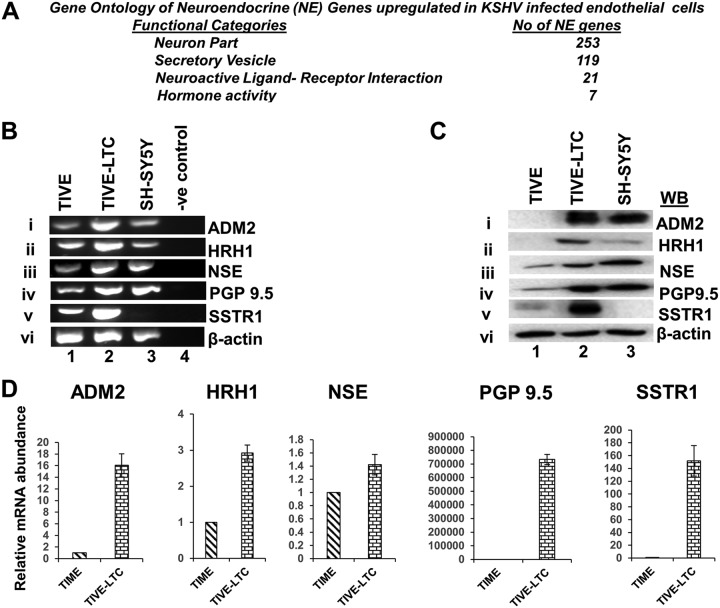
Although several markers are currently available as helpful indicators to screen for and diagnose NE cancers, NE-specific gene sets grouped together based on their involvement in NE functions are not available in the database. A neuroendocrine cell typically displays many distinct neuronal features and functions as an endocrine cell. As such, several characteristics that define neurons, endocrine cells, secretory cells, and neuroactive ligand-receptor binding are also considered hallmark features of a neuroendocrine cell (31, 32). To sort the NE-associated genes in KSHV-infected cells, the upregulated genes (Table S1) were subjected to gene set enrichment analysis (GSEA). Specifically, gene sets that control the NE phenotype and their functions were of interest (31, 32). This analysis resulted in the identification of 421 NE genes associated with KSHV-infected cells, and these genes were grouped into four categories, which included (i) 253 neuronal genes, (ii) 119 secretary vesicle genes, (iii) 21 neuroactive ligand-receptor genes, and (iv) 7 hormonal genes (Fig. 1A and Table S3).
FIG 1.
KSHV latently infected endothelial cells upregulate the expression of NE genes. (A) Gene ontology (GO) analysis showing the various functional categories and the number of NE genes upregulated in KSHV latently infected TIVE-LTC cells. (B and C) Expression of NE genes in KSHV-infected latent cells. (B) RNA isolated from TIVE, TIVE-LTC, and SH-SY5Y (positive-control neuroblastoma cell line) cells was used to determine the expression levels of the NE genes ADM2, HRH1, NSE, PGP9.5, and SSTR1 by RT-PCR. PCR without cDNA was used as a negative control. β-Actin was used as an endogenous control. (C) Lysates from TIVE, TIVE-LTC, and SH-SY5Y cell lines were analyzed by Western blotting for the expression of NE markers. β-Actin was used as a loading control. (D) Real-time qRT-PCR analysis of NE genes in KSHV latently infected TIVE-LTC cells.
Among the several NE genes identified (Table S3), for further investigation, we selected 5 genes that have been shown to be important for the growth and development of NE cancers and represent most of the well-known neuroendocrine markers (33). These genes are (i) adrenomedullin 2 (ADM2), (ii) histamine H1 receptor (HRH1), (iii) neuron-specific enolase (NSE) (ENO2), (iv) neuronal protein gene product 9.5 (PGP9.5) (UCHL1), and (v) somatostatin receptor type 1 (SSTR1).
Synaptophysin, chromogranin A, and neuron-specific enolase are well-documented markers of the NE phenotype. Synaptophysin is detected in all neurons, brain, and spinal cord; functions in synaptic transmission; and serves as a marker of neuroendocrine tumors. Chromogranin, synaptophysin, and NSE provide the necessary evidence for a definitive diagnosis of a tumor of neuroendocrine origin. However, we did not detect the expression of synaptophysin, secretogranin I, and secretogranin II in KSHV-infected TIVE-LTC cells (Tables S1 to S3). This is not surprising since it is well known that if a tumor develops from typical neuroendocrine cells, these typical markers are upregulated in the transformed cells. However, if a nonneuroendocrine cell transforms into a neuroendocrine cell, then synaptophysin, secretogranin I, and secretogranin II may or may not be upregulated (33). Therefore, we did not conduct further biochemical analyses of these genes in this study.
Confirmation of neuroendocrine gene expression in endothelial cells latently infected with KSHV.
The expression of the NE genes ADM2, HRH1, NSE (ENO2), PGP9.5, and SSTR1 was first confirmed by RT-PCR (reverse transcription-PCR) using RNA isolated from TIVE and TIVE-LTC cells (Fig. 1). As a positive control, we used the SH-SY5Y neuroblastoma cell line, an NE tumor of the neural type (34). RT-PCR analysis confirmed the upregulation of all 5 NE genes in KSHV-infected cells compared to uninfected TIVE cells, and the levels of most of them were comparable to the levels of gene expression in positive-control SH-SY5Y cells (Fig. 1Bi to v).
To verify the expression of NE genes at the protein level, WB analysis was performed with TIVE, TIVE-LTC, and SH-SY5Y cell lysates. Consistent with the RT-PCR data, compared to TIVE cells, high levels of NE proteins were detected in TIVE-LTC cells (Fig. 1Ci to v). The protein levels observed in KSHV-infected cells were either comparable to or higher than the levels detected in positive-control SH-SY5Y cells (Fig. 1Ci to v). These results were also verified by quantitative real-time RT-PCR, where we observed the upregulation of all 5 NE genes in cells latently infected with KSHV (TIVE-LTC) compared to uninfected TIME cells (Fig. 1D). Chromogranin gene expression was not detected (data not shown).
Neuroendocrine gene expression is induced by de novo infection of endothelial cells by live KSHV.
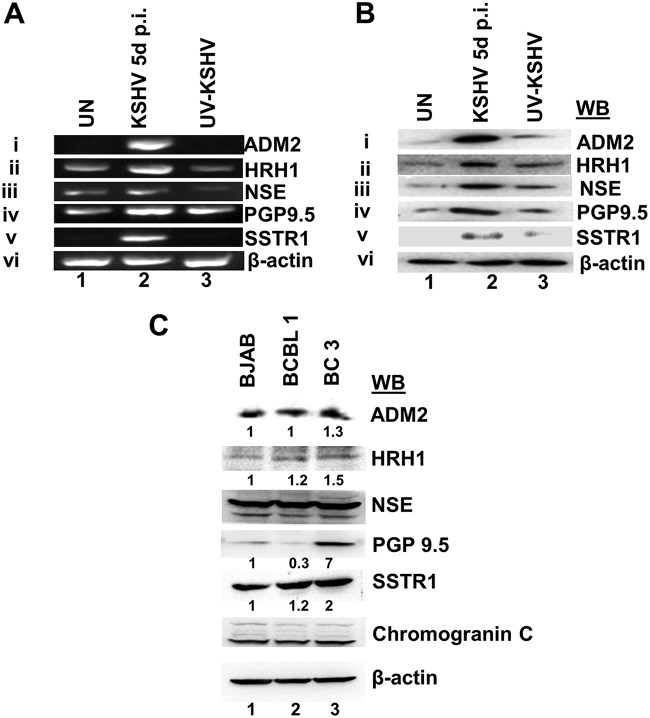
To ascertain whether the NE genes detected in latently infected endothelial cells are indeed the result of KSHV infection, we determined whether de novo KSHV-infected endothelial cells express these genes. Primary human microvascular dermal endothelial cells (HMVEC-d) were infected with purified KSHV or purified UV-inactivated KSHV (UV-KSHV) (50 DNA copies/cell) for 5 days, and the expression levels of NE genes were determined by RT-PCR. UV-KSHV was used as a replication negative control, as although UV-KSHV enters the target cells and viral DNA is delivered into the nucleus, it does not express any viral genes. As shown in Fig. 2A, all 5 NE genes were upregulated in KSHV-infected cells compared to uninfected cells. In contrast, we did not observe any significant induction of NE genes in UV-KSHV-infected cells (Fig. 1Ai to v), indicating that viral gene expression is required for the upregulation of NE genes. Consistent with the mRNA data, WB analysis confirmed higher expression levels of all 5 NE genes in KSHV-infected cells but not in UV-KSHV-infected cells (Fig. 2Bi to v). These results demonstrate that KSHV infection and viral gene expression are responsible for the upregulated NE gene expression in infected endothelial cells.
FIG 2.
Live KSHV de novo infection of primary endothelial cells induces the neuroendocrine gene expression. (A) Expression of NE genes following de novo KSHV infection. Primary HMVEC-d were left uninfected (UN) or infected with purified KSHV or UV-KSHV (50 DNA copies/cell) for 5 days. Isolated RNA was utilized to determine NE gene expression by RT-PCR. β-Actin was used as an endogenous control. p.i., postinfection. (B) Western blot determination of NE gene expression in HMVEC-d left uninfected or infected with KSHV or UV-treated KSHV (50 DNA copies/cell) for 5 days. β-Actin was used as a loading control. (C) Western blot determination of NE gene expression in KSHV latently infected primary effusion B-cell lymphoma BCBL-1 and BC-3 cells and the control KSHV-negative Burkitt’s lymphoma cell line BJAB. β-Actin was used as a loading control.
As we have shown previously that KSHV latently infected B-lymphoma BCBL-1 and BC3 cells express NE gene mGluRs (29), we next examined the levels of the NE genes ADM2, HRH1, NSE (ENO2), PGP9.5, and SSTR1. We observed various expression levels of these NE genes in B-lymphoma cells latently infected with KSHV (Fig. 2B). As the focus of the present study is to define the mechanistic basis for the similarity of KS lesion endothelial cells to neuroendocrine tumors, we did not conduct further studies with KSHV-infected B-lymphoma cells.
Neuroendocrine genes are expressed in KSHV-infected Kaposi’s sarcoma lesion endothelial cells.
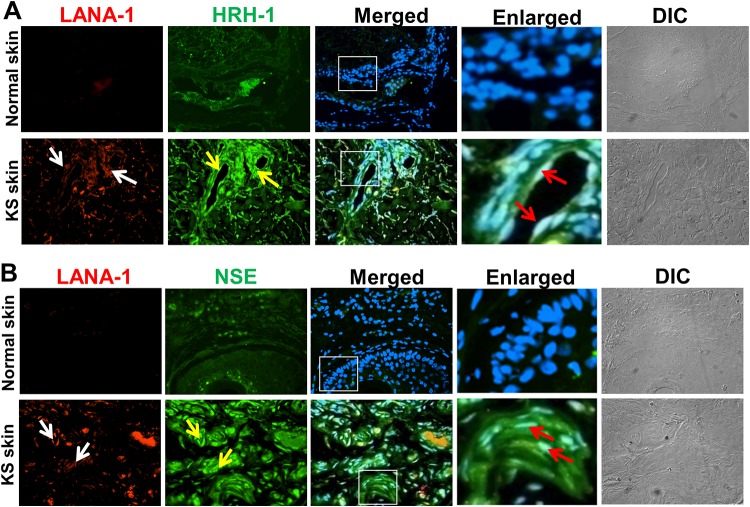
To analyze whether NE genes are expressed in patients with KS, we used an immunofluorescence assay to determine the expression levels of two NE proteins, HRH1 and NSE, in tissue sections from patients with skin KS (Fig. 3).
FIG 3.
Immunofluorescence costaining of the NE proteins HRH1 and NSE in KSHV-infected Kaposi’s sarcoma lesion tissue samples. (A) Normal and KS skin tissues were incubated with anti-HRH1 and anti-LANA-1 antibodies, followed by staining with either Alexa Fluor 488- or Alexa Fluor 594-conjugated secondary antibodies, respectively. (B) Normal and KS skin tissues were incubated with anti-NSE and anti-LANA-1 antibodies. NSE and LANA-1 were stained with Alexa Fluor 488- or Alexa Fluor 594-labeled secondary antibodies, respectively. In panels A and B, merged panels show NE markers and LANA-1 merged with DAPI. Enlarged boxed areas show magnified views of the merged panels. White arrows indicate LANA-1, yellow arrows indicate endothelial cells positive for the NE marker proteins HRH1 and NSE, and red arrows indicate spindle-shaped endothelial cells positive for both LANA-1 and NE markers. Differential interference contrast (DIC) images of the tissue sections are shown in the rightmost panels. Magnification, ×20.
Normal skin and KS skin tissues were immunostained by dual fluorescence for the KSHV latent protein LANA-1 as a marker for infection and the individual NE markers HRH1 (Fig. 3A) and NSE (Fig. 3B). In normal skin tissue, the expression levels of HRH1 and NSE were very low or completely absent (Fig. 3A and B, top), whereas the KS skin sections showed high levels of HRH1 and NSE protein expression (Fig. 3A and B, bottom), indicative of NE characteristics. The immunoreactivities of the markers were present in both LANA-1-positive spindle-shaped endothelial cells throughout the cross sections as well as spindle cells surrounding vascular slits and microvessels (Fig. 3A and B, bottom, white and yellow arrows), representing the characteristic histological features of KS lesions (14).
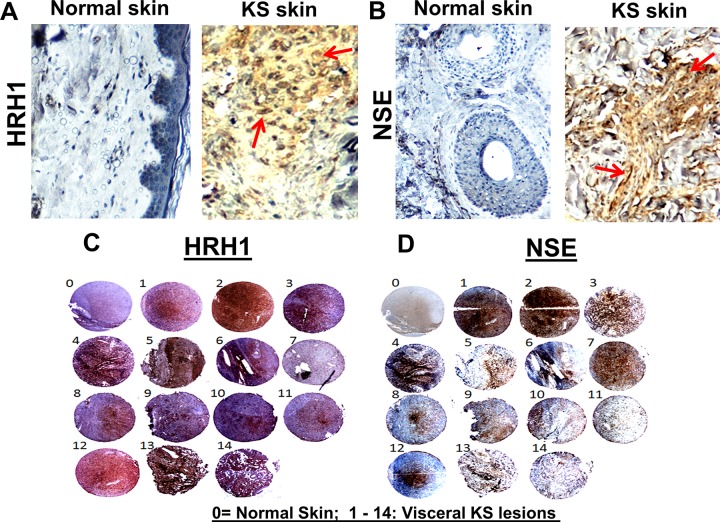
To support the immunofluorescence data for KS tissues, HRH1 and NSE protein expressions were also assessed in normal skin and KS skin tissue samples by immunohistochemistry. Compared to normal skin tissue, we observed strong positive staining for HRH1 (Fig. 4A) and NSE (Fig. 4B) in KS skin tissue, which correlated with the immunofluorescence results. The immunohistochemistry expression results were further extended to a larger set of KS tissue samples as a means to detect and confirm the significance of NE genes in KS tumors. We performed immunohistochemical staining for the HRH1 (Fig. 4C) and NSE (Fig. 4D) proteins in the KS tissue microarray (TMA) (from the ASCR [AIDS and Cancer Specimen Resource]) with control tissue (sample 0) and 14 visceral KS tissues (samples 1 to 14), and positive and negative staining were assessed. Visceral KS tissues demonstrated positive staining for both the HRH1 and NSE proteins, and the control tissues were negative for both proteins (Fig. 4C and D). These results confirmed that the NE genes upregulated by KSHV in vitro infection identified by NGS analysis are also expressed in in vivo patient KS lesion endothelial cells.
FIG 4.
Immunohistochemical detection of NE proteins in Kaposi’s sarcoma lesion tissue samples. (A and B) Immunohistochemical analyses were performed on normal skin and KS skin tissue samples using HRH1 (A) and NSE (B) antibodies, and slides were developed with DAB as the substrate. The tissues were counterstained for nuclei with hematoxylin (blue). Red arrows indicate the locations of positive staining appearing as brown for HRH1 and NSE in KS tissues. Magnifications, ×20. (C and D) Immunohistochemical analysis for HRH1 and NSE proteins in visceral KS tissue microarrays. Two tissue microarrays comprising normal skin tissue (sample 0) and 14 cores of visceral KS lesions (samples 1 to 14) were assessed for HRH1 (C) and NSE (D) staining using immunohistochemistry. Positive staining appears as shades of brown for HRH1 and NSE proteins.
The KSHV latent gene K12 induces the expression of neuroendocrine genes in endothelial cells.
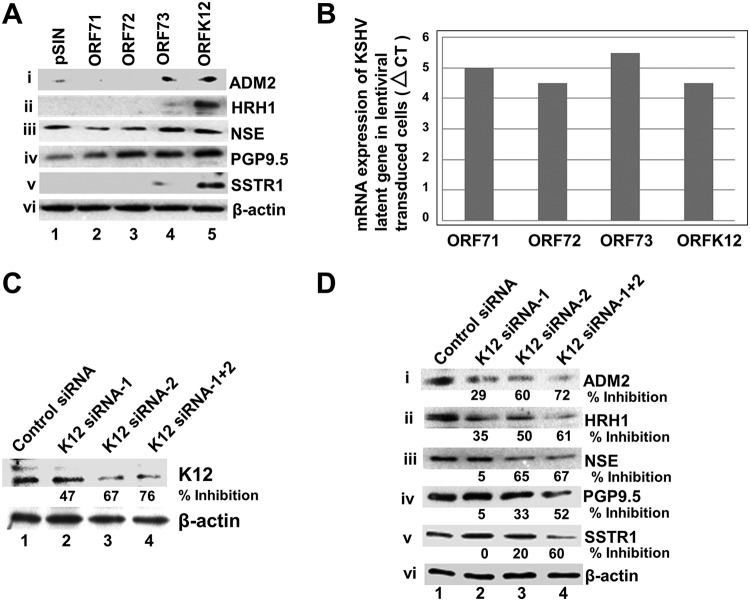
We next sought to identify the KSHV gene responsible for the expression of the NE genes. The expression of the KSHV latency genes ORF71, -72, -73, and -K12 play a central role in the development of KSHV-related cancers (5, 9). To identify the latent gene(s) responsible for the expression of NE genes, HMVEC-d were transduced with lentiviral constructs for the KSHV latency genes ORF71, -72, -73, and -K12; the expression of NE genes was evaluated by Western blotting (Fig. 5A); and the expression of viral genes was measured by real-time RT-PCR (Fig. 5B). We observed that all 5 NE genes, ADM2, HRH1, NSE, PGP9.5, and SSTR1, were highly expressed in ORFK12-transduced cells (Fig. 5A, lane 5). In addition, low levels of these 5 genes were also expressed in ORF73-transduced cells (Fig. 5Ai to v, lane 4) (see Discussion). These results suggested that the NE genes are predominantly upregulated by the KSHV latent gene K12.
FIG 5.
The KSHV latent gene K12 regulates the expression of NE genes in endothelial cells. (A) HMVEC-d were transduced with the control lentivirus vector pSIN or lentiviral vectors expressing ORFs 71, 72, 73, and K12. After 72 h posttransduction, cell lysates were Western blotted for ADM2, HRH1, NSE, PGP9.5, and SSTR1. β-Actin was used as a loading control. (B) qRT-PCR 72 h after transduction of HMVEC-d with lentiviral vectors showing the mRNA expression levels of latent ORFs 71, 72, 73, and K12. The comparative CT method was used to determine the expression levels of latent genes. (C) Knockdown efficiencies of K12 siRNAs. KSHV latently infected endothelial L1T2 cells were transfected with the siRNA negative control, K12 siRNA1, K12 siRNA2, and a mix of K12 siRNA1 and -2 for 72 h, and the cell lysates were Western blotted with anti-K12 antibodies. Percent inhibition indicates the inhibition of K12 expression in K12 siRNA-treated cells compared to control siRNA-treated cells. (D) K12 siRNA inhibits the expression of NE genes in KSHV latently infected L1T2 endothelial cells. NE protein levels in cells transfected with control siRNA, K12 siRNA1, K12 siRNA2, and a mix of siRNA1 and -2 for 72 h were measured by Western blotting. β-Actin was used as a loading control. Percent inhibition indicates the inhibition of NE gene expression in K12 siRNA-treated cells over that of control siRNA-treated cells.
As most of the NE genes were induced by the expression of K12, we next determined whether depleting K12 by small interfering RNA (siRNA)-mediated knockdown (KD) can downregulate the NE genes in KSHV-infected cells. To perform K12 KD, KSHV latently infected endothelial L1T2 cells (30) were transiently transfected with two independent siRNAs targeting K12 and a mix of the two siRNAs, and the efficiency of KD was evaluated by WB. K12 siRNA1, K12 siRNA2, and a mix of K12 siRNA1 and -2 reduced K12 expression levels by 47%, 67%, and 76%, respectively, compared to the control siRNA (Fig. 5C). NE gene expression analysis in siRNA KD L1T2 cells by WB showed that K12 siRNA2 downregulated the expression of NE genes more efficiently than siRNA1, with a reduction in expression of ∼20% to 60% compared to the control siRNA (Fig. 5Di to v, lanes 1 to 3). Combinations of K12 siRNA1 and -2 resulted in robust synergistic decreases (∼52% to 72%) in the levels of the respective proteins compared to individual siRNAs (Fig. 5Di to v, lane 4). These results suggested that the decreased expression of NE genes coincided with the decreased expression of K12, which further confirmed that the expression of NE genes is either directly or indirectly regulated by the KSHV latent gene K12.
The KSHV latent gene K12 regulates the expression of neuroendocrine genes by decreasing the level of the transcription factor REST and the binding of REST to neuroendocrine genes in endothelial cells.
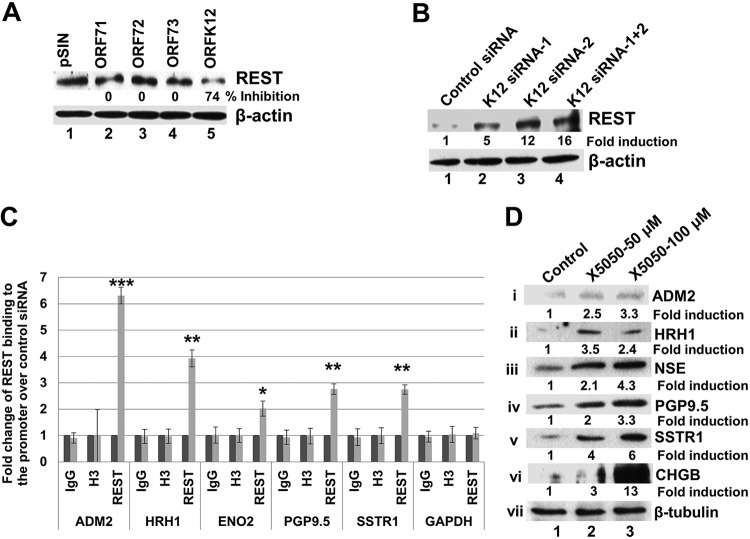
We next determined the potential molecular mechanism by which these NE genes are induced by K12 in KSHV-infected cells. RE1-silencing transcription factor (REST), or neuron-restrictive silencer factor (NRSF), is a transcriptional repressor. It is known that in nonneuronal cells, several neuron-specific genes are silenced by the transcription factor REST protein. This gene-silencing effect is achieved by the binding of REST to neuron-restrictive silencer element (NRSE), also known as RE1, sequence motifs present in the promoter region of its target genes (35). To determine whether the expression of NE genes occurs through a REST-mediated mechanism, we analyzed the levels of REST protein in both K12-overexpressing and KD cells by WB.
When HMVEC-d were transduced with lentiviral constructs for ORF71, -72, -73, and -K12, the level of the REST protein was decreased only in cells transduced with the K12 construct (Fig. 6A). This suggested that REST protein level reduction may play a role in the expression of NE genes in K12-overexpressing cells. Conversely, when we determined the REST protein level in K12 siRNA-transfected KSHV latently infected endothelial L1T2 cells, we observed an increase in the level of REST protein (Fig. 6B), indicating that K12 siRNA prevented the reduction in REST protein levels, which may lead to the decreased NE gene expression observed in K12 siRNA-transfected cells (Fig. 5D).
FIG 6.
K12 overexpression decreases REST levels and REST binding to NE gene promoters in endothelial cells. (A) HMVEC-d were transduced with a control lentivirus vector or lentiviral vectors expressing ORFs 71, 72, 73, and K12. At 72 h posttransduction, cell lysates were harvested to determine the protein levels of REST by Western blotting. β-Actin was used as a loading control. Percent inhibition shows the inhibition of REST protein levels compared to pSIN-transduced cells. (B) K12 knockdown increases REST protein levels and REST binding to NE genes. KSHV latently infected L1T2 cells were transfected with control siRNA, K12 siRNA1, K12 siRNA2, and a mix of K12 siRNA1 and -2, and the cell lysates were harvested after 48 h. The expression levels of REST in the cell lysates were analyzed by Western blotting. (C) ChIP-qPCR analysis of REST binding to NE gene promoters in K12 knockdown cells. L1T2 cells were transduced with control siRNA or K12 siRNA. Cell lysates were subjected to chromatin shearing and immunoprecipitated with ChIP-specific REST antibody or control H3 or IgG antibody. After immunoprecipitation, DNA was isolated from the immunoprecipitates and subjected to qPCR using primers specific for the NE genes. Error bars represent the means ± standard deviations (SD) of data from three experiments. *, P < 0.01; **, P < 0.001; ***, P < 0.0001 (compared to control siRNA-treated cells). (D) Treatment of uninfected HMVEC-d with the REST inhibitor X5050 induces NE gene expression. HMVEC-d were left untreated or treated with 50 and 100 μM X5050 for 24 h, and the cell lysates were Western blotted for the expression of ADM2, HRH1, NSE, PGP9.5, and SSTR1. The NE-specific protein CHGB was used as a positive control. β-Actin was used as a loading control. Fold induction of expression was calculated compared to the control.
Since the upregulation of NE genes is associated with decreased REST activity, we hypothesized that it may also be associated with decreased binding of REST to the RE1 elements present in the promoter regions of the above-mentioned NE genes in infected cells. It is known that REST can bind to the canonical 21-bp RE1 consensus sequence or the noncanonical bipartite RE1 consensus sequence separated by 10 to 16 bp, or half of the RE1 sequence (35–37). Therefore, we first searched for whether the promoter regions of the upregulated NE genes contain any of these RE1 sequences. The promoter sequences were retrieved from the EPD (Eukaryotic Promoter Database), and the potential RE1-like sequences for each of the five NE genes were deduced by comparing the gene sequences with the canonical 21-bp RE1 consensus sequence (TTCAGCACCACGGACAGCGCC/NNCAGCACCNNGGACAGNNNC). This sequence comparison revealed the identification of RE1-like sequences in the NE genes.
To assess whether REST can bind to RE1-like sequences in NE genes and whether K12 can regulate this binding, we designed primers flanking the RE1-like sequences in the NE genes, and chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR) was subsequently performed using these primers (see Materials and Methods). We first transduced KSHV latently infected L1T2 cells with control siRNA and K12 siRNA, and cell lysates were prepared. The cell lysates were subjected to chromatin shearing and immunoprecipitated using a ChIP-specific REST antibody or control immunoglobulin G (IgG) or histone H3 antibody. DNAs isolated from the immunoprecipitates were subjected to qPCR. The PCR data analysis revealed that binding of REST to the NE genes ADM2, HRH1, NSE, PGP9.5, and SSTR1 was significantly increased by ∼2- to 6-fold in K12 siRNA-transduced cells compared to control siRNA-transduced cells (Fig. 6C). These results suggested that decreased binding of REST to the RE1 domains is associated with higher expression levels of the NE genes in control cells and that increased binding is associated with lower expression levels in K12 siRNA-transfected cells. In addition, these results confirmed that all 5 NE genes tested have functional RE1 binding sites with various affinities.
Based on the above-described results, which linked the reduction in the REST level to the upregulation of NE gene expression in KSHV-infected cells, we next sought to determine whether the loss of REST would recapitulate NE gene expression in uninfected endothelial cells. To determine this, we treated uninfected HMVEC-d with a REST inhibitor, X5050, which specifically targets REST protein degradation without affecting its gene expression (38). Western blot analysis of untreated and treated cells for the NE genes showed that REST inhibitor-treated cells effectively upregulated the transcription of NE genes compared to untreated cells (Fig. 6Di to v, lanes 1 to 3). We used the NE-specific REST target gene chromogranin B (CHGB) (39) as a positive control, which was highly upregulated in X5050-treated cells (Fig. 6Dvi, lanes 1 to 3). These results suggested that REST degradation is adequate to drive the expression of NE genes in uninfected endothelial cells.
We previously demonstrated that REST was retained in the cytoplasm of KSHV-infected endothelial and B cells by the kaposin A (K12) protein, which resulted in the phosphorylation of REST and interaction with the E3 ubiquitin ligase β-TRCP leading to the ubiquitination of REST and degradation. We have also shown that kaposin A colocalizes with REST in KSHV-infected patient samples of KS lesion endothelial cells (29). Together with the results presented here, those studies demonstrated that the upregulation of the NE genes ADM2, HRH1, NSE, PGP9.5, and SSTR1 in KSHV latently infected cells is mediated by the K12 gene product, which decreases the protein levels of the NE gene transcription repressor REST.
Neuroendocrine HRH1 and SSTR1 genes exhibit opposing roles in the regulation of endothelial cell proliferation.
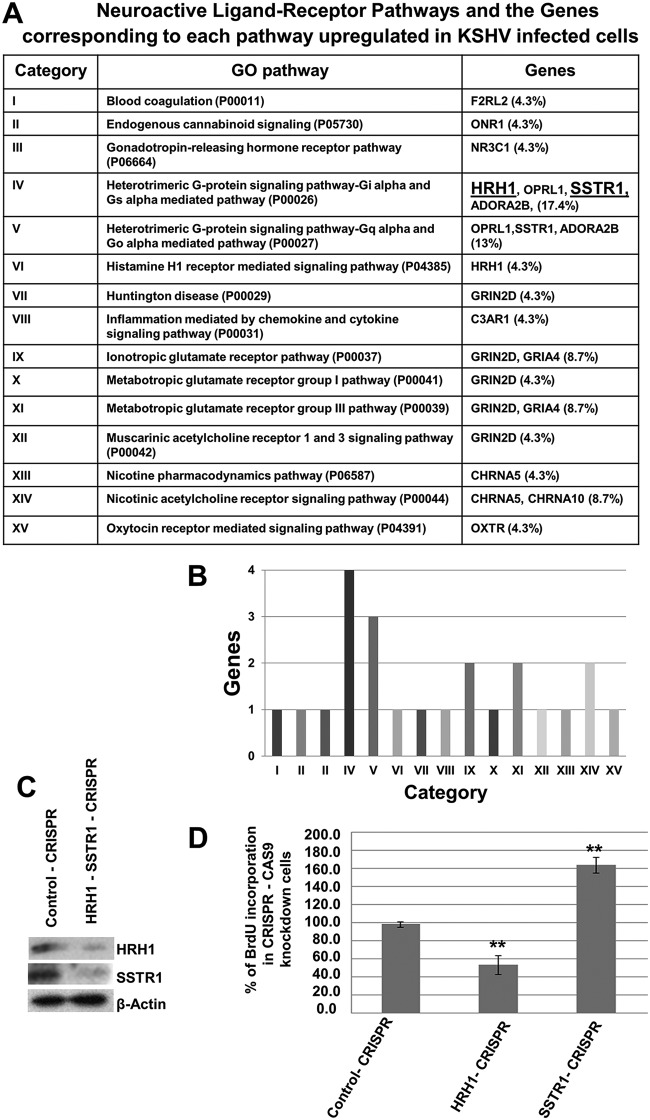
Among the four categories of NE genes that were identified by our bioinformatics analysis (Table S3), the neuroactive ligand-receptor interaction pathways are known to be significant in cancer development, as they transduce signals to the intracellular environment and regulate biological processes. We chose the neuroactive ligand-receptor interaction to examine the significance of NE gene expression in KSHV-infected cells. To identify key candidate genes in the neuroactive-receptor pathway, the list of neuroactive ligand-receptor interactions upregulated in KSHV-infected cells was subjected to gene ontology (GO) pathway analysis (http://www.geneontology.org/). The total number of pathways and the number and percentage of genes that participate in each pathway were tabulated (Fig. 7A).
FIG 7.
Neuroactive ligand-binding receptors counteract KSHV-infected endothelial cell proliferation. (A) GO analysis of neuroactive ligand-receptor interaction pathways in KSHV-infected cells. (B) Bar graph showing the neuroactive ligand-receptor pathways and the number of genes corresponding to each receptor pathway. (C) L1T2 cells were transduced with CRISPR-Cas9 HRH1 and CRISPR-Cas9 SSTR1 plasmids. Knockout (KO) cells were selected using puromycin hydrochloride, and the efficiencies of HRH1 and SSTR1 knockout were measured by Western blotting. β-Actin was used as a loading control. (D) To determine the effect of HRH1 and SSTR1 KO on cell proliferation, KO L1T2 cells were cultured for 48 h and pulsed with BrdU for 2 h, and BrdU incorporation was measured by using a BrdU cell proliferation ELISA kit. Error bars indicate the means ± SD of data from three experiments. *, P < 0.01; **, P < 0.001 (compared to the control).
Pathway analysis predicted the existence of 15 neuroactive pathways in the infected cells, with a high percentage of genes in the heterodimeric G-protein-coupled Gi/o, Gs, and Gq pathways (Fig. 7A and B, categories IV and V). The genes filtered out from these pathways included SSTR1, HRH1, ADORA2B, and OPRL1, and among these, the SSTR1 and HRH1 genes were the highly upregulated ones (log FCs of 4.8- and 2.3-fold increases, respectively). SSTR1 receptors are linked to Gi/o (inhibitory), while HRH1 receptors are Gq (stimulatory)-linked receptors. As we identified two receptors with Gi and Gq receptor activity, we hypothesized that the intrinsic signaling pathways associated with these receptors and their altered functions may be important regulators of the proliferation of KSHV-infected cells. To determine their effect on signaling pathways and cellular fates, we knocked out the two receptors HRH1 and SSTR1, individually or in combination, in L1T2 cells using the CRISPR-Cas9 system (Fig. 7C), and their role in cell proliferation was determined.
For the cell proliferation assay, HRH1 and SSTR1 knockout (KO) KSHV latently infected L1T2 cells and their control cells were cultured for 2 days and then pulsed with bromodeoxyuridine (BrdU) for 2 h, and BrdU incorporation was measured using a BrdU enzyme-linked immunosorbent assay (ELISA) kit. As shown in Fig. 7D, knockout of HRH1 significantly inhibited cell proliferation by >50%, whereas knockout of SSTR1 accelerated cell proliferation by >60% (Fig. 7D), compared to control CRISPR cells. These results suggest that in KSHV-infected cells, HRH1 may stimulate cellular signaling and cell proliferation, while the SSTR1-mediated negative signaling pathway may inhibit cell proliferation and consequently may counteract the proliferation of KSHV-infected cells.
DISCUSSION
Our comprehensive studies here demonstrate that in vitro KSHV-infected endothelial cells and in vivo KSHV-infected KS tissue cells express several neuronal and neuroendocrine genes, which are mediated mainly by the KSHV latent ORFK12 gene and by decreased host neuron-restrictive silencer factor (REST/NRSF) levels (Fig. 8). Our previous studies demonstrating the retention of the REST/NRSF protein in the cytoplasm of KSHV-infected endothelial and B cells by kaposin A (K12) protein resulting in the phosphorylation of REST, interaction with the E3 ubiquitin ligase β-TRCP, ubiquitination of REST, degradation, and colocalization of kaposin A with REST in KSHV-infected KS lesion endothelial cells (29) together with the results here demonstrating the upregulation of the NE genes ADM2, HRH1, NSE, PGP9.5, and SSTR1 in KSHV latently infected cells suggest that KSHV has evolved to induce neuroendocrine features in nonneuronal endothelial cells. KSHV-induced neuroendocrine pathways could be playing critical roles in the pathobiological characteristics of KSHV-infected endothelial cells, such as the slow proliferation of infected KS lesion endothelial cells, and this is supported by our observations that KSHV infection induces two neuroactive ligand receptors, HRH1 and SSTR1, probably to counteract each other in regulating infected cell proliferation (Fig. 8). NE gene induction by KSHV may also provide a potential survival advantage, as the induction of proteins of immunologically privileged sites such as neurons on endothelial cells may provide an avenue for KSHV to escape the host immune surveillance functions and, thus, the survival of infected cells.
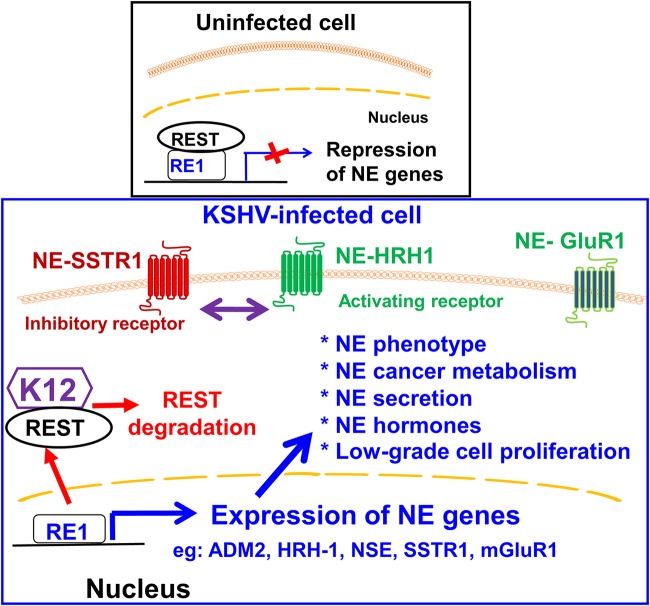
FIG 8.
Model figure showing the expression of neuroendocrine genes and their effect on KSHV-infected cells. In uninfected cells, REST binds to the RE1 motif in NE genes and silences their expression (29). In infected cells, K12 (KSHV latent protein) binds and retains REST in the cytoplasm and targets REST for proteasomal degradation (29). This results in the abolition of the REST-mediated silencing effect on NE genes and the upregulation of >400 NE genes, including mGluR1 (glutamine receptor), ADM2, HRH1, NSE, PGP9.5, and SSTR1, etc., which are known to mediate neuronal and neuroendocrine functions characteristic of neuroendocrine tumors. The expression of these genes in KS patient tissues suggests that they probably provide an advantage for KSHV such as the low-grade proliferation of infected cells by the functions of NE inhibitory and activating receptors and potential escape from host immune surveillance by the expression of proteins of immunologically privileged sites such as neurons on endothelial cells. The NE gene products identified here could serve as markers for KSHV-infected endothelial cells and could potentially serve as therapeutic targets to combat KSHV-associated KS.
Viruses need to tackle several molecular and biological challenges in the infected host, and they might have evolved a way to protect infected host cells by activating certain signaling pathways that could slow down cellular activities while maintaining successful survival inside host cells. Such cellular and metabolic adaptations may help prolong the survival of the virus in the host organism. Unlike many other cancers, KS lesions from all four epidemiologically distinct KS types contain spindle-shaped endothelial cells with low-grade proliferative potential, and patients with classical KS can survive for a long period without any treatment (40–42), which may be due to the expression of NE genes, especially neuroendocrine receptors having opposing roles in cell proliferation. However, depending on the host and the nature of the complications, such as immunosuppression and HIV infection, KS can spread to other organs and can be fatal, as AIDS patients are more prone to secondary infections and other risk factors (43, 44). Our studies suggest that the similarity of KS lesion cells to neuroendocrine tumors is probably a result of KSHV infection-induced neuroendocrine features in endothelial cells.
The NE genes identified by NGS in KSHV-infected endothelial cells were sorted into 4 functional categories, neuronal, hormonal, secretory, and neuroactive ligand-receptor interaction, based on their association with the functional aspects of NE cells. Interestingly, we observed that most of the well-known NE proteins that are used as practical markers for the detection of NE cancers, including NSE, PGP9.5, SSTR, neuropeptides (e.g., VGF), ADM2, human chorionic gonadotropin gene (CGB7), synaptic vesicle protein 2A (SV2A), and secretogranin 5 (SCG5), are highly expressed in KSHV-infected TIVE-LTC cells (33). Among the 4 categories, the first category consists of neuronal part-related genes (GO neural part), which are critical constituents of neurons. The identification of clusters of 253 genes for the neuronal part indicates that infected cells differentiate toward a neuronal and NE phenotype. These genes may be interconnected with KSHV infection and may influence essential cellular or metabolic functions associated with the various aspects of virus-induced cancer development. For example, the expression of genes involved in energy metabolism and growth in neurons and NE cells, NSE and PGP9.5 (45–48), and additional molecules such as VGF, a nerve growth factor which regulates metabolism and homeostasis in NE cancers (49), indicates that the metabolic activities of infected cells shift toward NE differentiation. The expression of these molecules is one of the characteristic phenotypic symptoms of NE cancers (46, 48, 49).
The second category of genes is the secretory vesicle genes, which include all genes that encode proteins involved in neuronal and endocrine secretory pathways. As KSHV-associated cancers depend on the cytokines and proteins secreted by the infected cells (10, 50), the expression of the secretory vesicle genes indicates the existence of secretory granule-mediated peptides and cytokine release in KSHV-infected cells. The upregulations of secretory vesicle-associated genes such as SCG5 and SV2A in KSHV-infected cells are important constituents of endocrine secretory granules and secretory functions (51). The third category includes hormonal genes, which are normally found to be expressed in NE cancers. Although being a small group of hormone-coding genes, this group imparts distinct features to NE tumors. For example, adrenomedullin is an endogenous peptide hormone that is known to function as a vasodilator (52). Its increased expression in infected cells may increase the blood flow to the site of tumor formation and may contribute to the reddish color of KS tumors. Adrenomedullin may be a new addition to the factors that have already been reported to be responsible for the formation of red KS lesions (53).
The fourth category of genes includes genes involved in neuroactive ligand-receptor interactions. This category includes 21 neuroactive ligand-receptor interaction genes, and from this list, we identified two genes, HRH1 and SSTR1, that contribute significantly to the biological activity of infected endothelial cells. Generally, SSTR1 is a Gi-coupled receptor, and its activation leads to the inhibition of adenylyl cyclase as well as intracellular Ca2+ levels that eventually inhibit ERK1/2, the cell cycle, and the proliferation of cells (54, 55). Therefore, SSTRs may be crucial molecules involved in the inhibition of KSHV-infected endothelial cell proliferation. On the other hand, HRH1 expression largely indicates that it functions as a growth factor receptor, which triggers cancer cell proliferation, tumor development, and progression (55). HRH1 is a Gq/11-linked receptor that stimulates the phospholipase C-inositol trisphosphate (IP3)-protein kinase C (PKC) signaling pathway to mobilize calcium release from the endoplasmic reticulum, which may be coupled with proproliferative activities. The HRH1 receptor and its ligand histamine are involved in tumor growth and metastasis of many cancers, such as gastric cancer, breast cancer, and melanoma (55). As reported by many previous studies, KSHV-infected cells receive stimulatory signals from several cell surface receptors in an overlapping manner, and multiple signaling pathways contribute to the proliferation of infected cells (56). It is likely that these receptors that have previously been shown to stimulate proliferation may cooperate with SSTRs in maintaining a slow proliferative status of infected cells and tumor development.
Mechanistically, we demonstrate that K12 induced the expression of NE genes by decreasing the level of host REST, which negatively regulates NE gene expression in nonneuronal cells by binding to an RE1 element present in the NE genes. Since REST is a negative transcription factor, a decreased level of REST in infected cells abrogates binding to RE1 elements and the upregulation of NE genes. These findings are consistent with several previous studies that have shown that the loss of REST expression and function via proteasomal degradation, mutations, and alternative splicing leads to the derepression of REST target genes (57, 58). Additionally, host molecules such as Aurora kinase A (AURKA), which has been reported to be upregulated in KSHV-infected cells (59), are also known to play an important role in the regulation of NE pathways by various studies (60). The effect of these genes on regulating the expression of NE genes in KSHV-infected cells needs to be investigated further.
In addition to the KSHV K12-induced upregulation of NE genes via its degradation of the NE gene transcription repressor protein REST, the KSHV latent ORF73 gene also weakly stimulated most of the NE genes induced by K12 (Fig. 5A). Since ORF73 expression did not affect the REST protein level (Fig. 6A), this suggested that ORF73 may be operating via a different mechanism. The ORF73 gene product, LANA-1, has been known to interact with a number of molecules to suppress gene expression, including the mSin3 corepressor molecules associated with the negative regulatory effect of REST (61). We speculate that the ability of LANA-1 to bind the mSin3 corepressor complex molecules may partially relieve the suppressive effect of REST on their target genes, leading to the weak expression of NE genes. However, further investigation is required to understand the mechanism involved in the ORF73-mediated activation of NE genes, which is beyond the focus of the present study. Nevertheless, our results emphasize that K12 is crucial for the expression of NE genes in infected cells.
Overall, our studies demonstrate that KSHV-infected endothelial cells exhibit the molecular features of NE cancers, such as the upregulation and expression of neuronal and NE molecules. This is the first experimental evidence to explain the concept that virus-infected cells and virus-induced cancers have the ability to transform nonneuronal endothelial cells into cells with neuroendocrine features. The translocation of the REST protein between the cytoplasm and nucleus of infected cells is probably mediated by an as-yet-unknown molecular candidate. Further studies are required in order to understand the molecular mechanisms involved in the translocation of REST to the cytoplasm, which is beyond the scope of this study.
The differential functioning of NE receptor genes with opposite effects on cell proliferation suggests that a high relative abundance of stimulatory receptors might reverse the effect of inhibitory receptors. Although the stimulatory receptors can compensate for the effect of inhibitory receptors, their overall effect on cell proliferation might lead to slow growth of the cells. However, further extensive studies are essential to understand how the expressions of the NE molecules and their interconnected pathways participate in KSHV-KS pathogenesis, which is beyond the scope of the present study. The expression of NE genes and proteins that are normally expressed in immunologically privileged sites such as the central nervous system may also provide a survival advantage for endothelial (KS) cells latently infected with KSHV. Further studies are essential to determine whether NE gene expression provides an avenue for KSHV-infected endothelial cells to escape host immune responses.
The detection of NE genes in KSHV-infected endothelial cells suggests that they could potentially serve as markers for KSHV-infected KS lesion endothelial cells as well as novel therapeutic targets to control KS lesions. For example, glutamate and glutamate receptors are associated with the pathogenesis of several human disorders, including cancers of brain, skin, lung, colon, and breast (62–65). The release and accumulation of glutamate in the extracellular space lead to aberrant glutamate receptor expression and receptor signaling in melanoma and glioma. The mGluR1/5-activating intracellular signaling pathway has been shown to be involved in the development of melanoma and glioma. A number of glutamate receptor antagonists and glutamate release inhibitors were shown to be effective in suppressing the proliferation of cancer cells (66). The glutamate release inhibitor riluzole has shown high effectiveness in controlling melanoma tumor growth by inhibiting mGluR1/5 activity and signaling (66). The glutamate release inhibitors talampanel and riluzole are in clinical trials for the treatment of glioma and melanoma, respectively (66–68). Riluzole is also an FDA-approved drug for the treatment of amyotrophic lateral sclerosis (ALS), which involves the overactivation of glutamate receptors (69). In this context, it is relevant to note that our studies show that KSHV-infected endothelial and B-lymphoma cell proliferation could be significantly inhibited by the glutamate release inhibitor riluzole and the mGluR1 antagonist A841720 (29).
Histamine acts by binding to the G-protein-coupled histamine receptors HRH1, HRH2, HRH3, and HRH4. Altered regulation of histamine receptors and an increase or decrease in proliferation have been reported for different types of cells. In many cancer cells, the activation of HRH1 leads to increased proliferation through the activation of Gαq protein and the IP3/Ca2+ cascade mechanism (70–72). However, in CHO cells stably expressing the HRH1 receptor, the H1 receptor inhibits proliferation by activating a pathway that involves PLC, RhoA, and Jun N-terminal protein kinase (JNK), indicating that the bimodal activity of HRH1 receptors may depend on the characteristic properties of the cell type (73). It is interesting to note that a recent study reported that treatment of a KS patient with the HRH1 receptor antagonist cetirizine and the HRH2 receptor antagonist ranitidine along with the leukotriene receptor antagonist montelukast resulted in the resolution of KS lesions (74). Thus, in addition to our present study, the above-mentioned studies demonstrated that histamine serves as a stimulator of cell proliferation in KSHV-associated KS by activating the receptors HRH1 and HRH2, which are coupled to the Gαq protein; however, the mechanism of action remains unclear and needs further studies. Those studies, together with our studies demonstrating a high level of HRH1 receptor expression in KS lesion cells, suggest that the induction of NE genes by KSHV has broad implications for the design of novel therapeutic drugs against KSHV-associated KS as well as advance our knowledge of the pathobiological characteristics of KSHV-associated KS lesions.
MATERIALS AND METHODS
Cells.
Primary human dermal microvascular endothelial cells (HMVEC-d) (CC-2543; Clonetics, Walkersville, MD), telomerase-immortalized vein endothelial (TIVE) cells and TIVE cells carrying KSHV in a latent state (TIVE-LTC) (gifts from Rolf Renne, University of Florida), and KSHV latently infected L1T2 cells (ATCC, Manassas, VA, USA) were cultured in EBM2 (endothelial cell growth basal medium 2) supplemented with growth factors. The human neuroblastoma SH-SY5Y (ATCC CRL-1821; ATCC, Manassas, VA, USA) cell line was maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 20% FBS (fetal bovine serum; Atlanta Biologicals). KSHV-positive PEL BCBL-1 cells were cultured in RPMI 1640 GlutaMAX (Gibco Life Technologies, Grand Island, NY) supplemented with 10% (vol/vol) FBS and penicillin-streptomycin (Gibco Life Technologies).
Virus.
For viral stock preparation, the KSHV lytic cycle was activated by treating BCBL-1 cells with 20 ng/ml TPA (12-O-tetradecanoylphorbol-13-acetate). Supernatants from TPA-treated cells were collected, and virus purification was done as described previously (75). UV-inactivated KSHV was prepared by exposing the purified virus stock to UV light (365 nm) for 20 min at a 10-cm distance. DNA extracted from KSHV and UV-inactivated KSHV was used to quantify viral copy numbers by real-time DNA PCR as described previously (7).
Antibodies and reagents.
Rabbit anti-ADM2 and SSTR1 antibodies were obtained from MyBioSource, San Diego, CA (Table 1). Rabbit anti-PGP9.5, -HRH1, and -CHGB antibodies were obtained from the Proteintech Group, Chicago, IL. Rabbit anti-NSE and -β-actin antibodies were obtained from Sigma, St. Louis, MO. Rabbit anti-REST antibodies were obtained from Santa Cruz Biotechnology, Santa Cruz, CA. Rat anti-kaposin A/C antibody was obtained from Millipore, Temecula, CA. Mouse anti-ORF73 monoclonal antibody 1D10C3 (immunoglobulin G1 [IgG1]) against the ORF73 amino acid sequence CEPQQREPQQREPQQ conjugated with keyhole limpet hemocyanin (KLH) was generated by the Chandran laboratory. Anti-mouse and anti-rabbit antibodies conjugated to horseradish peroxidase (HRP) were obtained from KPL Inc., Gaithersburg, MD. Alexa Fluor 594- and 488-conjugated secondary antibodies were obtained from Invitrogen. The REST inhibitor X5050 was obtained from Millipore, Temecula, CA. Protein A- and G-Sepharose CL-4B beads were obtained from Amersham Pharmacia Biotech, Piscataway, NJ.
TABLE 1.
Antibodies used
| Antibody | Type | Company (catalogue no.) | Dilution used |
|---|---|---|---|
| SSTR1 | Rabbit polyclonal | MyBioSource (MBS127455) | 1:500 |
| NSE | Rabbit polyclonal | Sigma (SAB4500768-100UG) | 1:500 |
| PGP9.5 | Rabbit polyclonal | EMD Millipore Sigma (AB1761-1) | 1:500 |
| HRH1 | Rabbit polyclonal | Proteintech (13413-1-AP) | 1:500 |
| ADM2 | Rabbit polyclonal | Invitrogen (PA5-72030) | 1:500 |
| Chromogranin C | Rabbit polyclonal | Gene Tex (GTX116446) | 1:500 |
| β-Actin | Mouse monoclonal | Sigma-Aldrich (A5441) | 1:5,000 |
Immunohistochemical staining and scoring of biomarkers.
Tissue microarrays (TMAs) from patients with skin and visceral KS and healthy individuals were obtained from the ACSR (AIDS and Cancer Specimen Resource). The TMA slides were subjected to deparaffinization with xylene, rehydration with alcohol, and antigen retrieval. The slides were then incubated with primary antibodies, followed by rabbit/mouse polymer-HRP, and developed using a DAB (3,3′-diaminobenzidine) kit according to the manufacturer’s instructions (Invitrogen). The tissue microarrays were examined after staining with hematoxylin. Images were acquired using a confocal microscope (Nikon), and the staining intensity was quantified with ImageJ analysis software (NIH). The percentage of the area stained was analyzed and scored as 0 (no staining), 1 (<10%, weak staining), 2 (11 to 50%, moderate staining), or 3 (51 to 100%, strong staining). Values of 0 to 1 were considered negative, and values of 2 to 4 were considered positive staining.
Immunofluorescence staining of tissue sections.
Formalin-fixed, paraffin-embedded tissue samples from patients with skin KS and healthy individuals were obtained from the ACSR. Following deparaffinization with xylene, the sections were rehydrated through decreasing grades of alcohol. The sections were then subjected to antigen retrieval by heating in citric acid buffer for 10 min in a microwave. After permeabilization with 0.5% Triton X-100 for 5 min, the samples were blocked with Image-iT FX signal enhancer blocking solution (Invitrogen) for 30 min at room temperature (RT). Immunofluorescence staining of biomarkers in KS tissues and normal tissue samples was performed using anti-rabbit HRH1 and NSE and anti-mouse LANA-1 antibodies, followed by Alexa Fluor 488- and Alexa Fluor 594-conjugated secondary antibodies. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probes, Invitrogen), and stained samples were viewed under a fluorescence microscope with the Nikon MetaMorph digital imaging system.
Western blotting.
Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer, with 15 mM NaCl, 1 mM MnCl2, 1 mM MgCl2, 2 mM phenylmethylsulfonyl fluoride (PMSF), 2 mM CaCl2, and a protease inhibitor cocktail (Sigma). Equal protein amounts were boiled at 95°C for 5 min in sample buffer containing β-mercaptoethanol, and the samples were separated by SDS-PAGE, followed by transfer to a nitrocellulose membrane. The membranes were then incubated with primary antibodies, followed by incubation with HRP-tagged secondary antibodies. An enhanced chemiluminescence (ECL) system (Pierce, Rockford, IL) was used for detection according to the manufacturer’s instructions. The bands were quantified by using NIH ImageJ analysis software. Percent inhibition or the fold change was calculated after normalization of samples to β-actin levels.
Lentivirus-mediated KSHV latent gene overexpression.
Lentiviruses expressing KSHV latent genes were produced by transfecting HEK293T cells with lentiviral constructs and the plasmid packaging vectors Gag-Pol, Rev, and vesicular stomatitis virus G (VSV-G) as described previously (76). Infections were carried out by incubating the virus preparation with cells in the presence of Polybrene. Green fluorescent protein (GFP)-expressing lentiviral vectors were used as positive controls to analyze and estimate the infection efficiency. The expression levels of transduced viral genes were evaluated by real-time PCR, and the expression levels of host genes were assessed by Western blotting.
K12 siRNA knockdown.
K12 knockdown in L1T2 cells was achieved using two individual siRNAs and a pool of two siRNAs synthesized by Integrated DNA Technologies (IDT). The sequences used for siRNA knockdown were 5′-r-UUGCAACUCGUGUCCUGAAUGCUACGG-3′ for siRNA1 and 5′-r-CCACAAACACCGUUAAGCCUCUAUCCA-3′ for siRNA2.
Cells were transfected for 48 h with the two individual siRNAs and a mix of the two siRNAs (final concentration, 100 pmol) using siLentFect transfection reagent (Bio-Rad) according to the manufacturer’s instructions. After 48 h, cell lysates were collected and used for Western blot analysis.
Reverse transcription-PCR.
The NE gene expression profiles were confirmed using conventional RT-PCR as well as real-time RT-PCR. Total RNA was isolated from uninfected and infected cells using the RNeasy minikit (Qiagen) and treated with DNase I at 37°C for 30 min. cDNA was synthesized from 2 μg RNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems) and random hexamers. PCR for the NE genes was performed using 2 μl of the cDNA and appropriate forward and reverse primers. Amplified products were resolved by agarose gel electrophoresis, and the bands were visualized and the image was captured using a gel documentation system (Alpha Innotech Corporation, San Leonardo, CA). For real-time reverse transcription-quantitative PCR (qRT-PCR), the synthesized cDNA was used as a template with Power SYBR green PCR master mix (Applied Biosystems) on an ABI StepOnePlus detection system (Applied Biosystems). All mRNA levels were normalized to cellular RNase P mRNA levels and calculated by the delta-delta threshold cycle (ΔΔCT) method. Primers used are listed in Table 2. All real-time experiments were performed in triplicate.
TABLE 2.
Primers used for conventional RT-PCR and real-time qRT-PCR
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| Conventional RT-PCR | ||
| ADM2 | GGAGCCTAAACACCCTGAAA-HairpinBlastHairpin | TGCATGAGAGCAGGAACAG |
| HRH1 | CCTCTGCTGGATCCCTTATTTC-HairpinBlast | CCAGGTCTGATTCTCTCTCTCT |
| NSE | CAGGACTTTGTCAGGGACTATC | GTAATCCCAGTGTGCTGTGA |
| PGP9.5 | CTGGGATTTGAGGATGGATCAG | TAATGCTCTGTGGGAGGGA |
| SSTR1 | GGAGGAGCCGGTTGACTATT | AAGGTAGCCTGAAAGCCTTCC |
| β-Actin | GCTCACCATGGATGATGATATCGCC | GGATGCCTCTCTTGCTCTGGGCCTC |
| Real-time qRT-PCR | ||
| ADM2 | CTGAGCCCCATCTGAAGCC | CAGCACTGCGTGTAGACCAG |
| HRH1 | GCACAGCGTCCATTTTCAGTG | TCGGGTCTTGGTACGATACTT |
| NSE/ENO2 | AGGTGCAGAGGTCTACCATAC | AGCTCCAAGGCTTCACTGTTC |
| UCHL1 | AATGTCGGGTAGATGACAAGGT | GGCATTCGTCCATCAAGTTCATA |
| SSTR1 | CCAGCATCTACTGTCTGACTGT | ATGACGAGCAGCGATAGCAC |
| CHGA | TAAAGGGGATACCGAGGTGATG | TCGGAGTGTCTCAAAACATTCC |
| SYP | CTCAGCATCGAGGTCGAGTTC | GAGGAGTAGTCCCCAACTAAGAA |
CRISPR-Cas9 knockout of HRH1 and SSTR1.
CRISPR-Cas9-directed knockouts of HRH1 and SSTR1 in L1T2 cells were performed using an HRH1 CRISPR-Cas9 plasmid (catalogue no. sc-403410-NIC-2; Santa Cruz) and an SSTR1 CRISPR-Cas9 plasmid (catalogue no. sc-403410-NIC-2; Santa Cruz). The knockout cells were selected using puromycin hydrochloride, and the efficiencies of HRH1 and SSTR1 knockdown in double-knockout cells were measured by Western blot analyses.
Chromatin immunoprecipitation assay.
Chromatin immunoprecipitation (ChIP) assays were performed on cell lysates from K12 siRNA- and control siRNA-transfected cells using a ChIP assay kit (Upstate Biotechnology, Lake Placid, NY) according to the manufacturer’s protocol. Briefly, the cell lysates were immunoprecipitated using an antibody against REST or nonspecific mouse IgG or histone H3 antibody overnight. DNA was purified from immunoprecipitated complexes and analyzed by qPCR with primers designed to amplify the RE1 element-containing regions of the NE genes. PCR was performed with SYBR green on an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA). REST binding to RE1 sequences at NE genes was normalized to IgG binding to the DNA and calculated as a percentage of the total input. The fold increase in REST binding to NE genes in K12 siRNA was calculated compared to control siRNA. The primer sequences used for ChIP-qPCR are given in Table 3.
TABLE 3.
Primer sequences used for ChIP-qPCR
| Genea | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| ADM2 | AGCCGTGGGATCAGCTC | CGCCAGCCTCACTCCAA |
| HRH1 | GCGAGGCTCTTTCCCATAAC | CGTCAGGGATTCCCGAAATAAG |
| NSE | GTGAGACATTGAGGCTGTCTTG | CCGACCCACTCGACAGTAA |
| PGP9.5 | TCCAGAAACTTCGCCCAAA | ATAATCTGGTGGTTGTGGAGAC |
| SSTR1 | CTGGTTGAGGAGAAGGAGAAAC | AGATCCCTGCAAGCCTTTATT |
| GAPDH | TCTTTCCTTTCGCGCTCTG | CTGCGCACTAGCATCCC |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistical analyses.
Statistical significance was calculated using two-tailed Student’s t test. A P value of <0.01 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank Chris Boschoff (UCL Cancer Institute) for providing the lentiviral constructs of the KSHV latent genes and Rolf Renne (University of Florida) for TIVE and TIVE-LTC cells.
This study was supported in part by Department of Biotechnology (India)-Ramalingaswamy Re-entry grant BT/RLF/Re-entry/60/2013 to M.V.V. and by Public Health Service grant R01 CA180758 to B.C.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Chang Y, Cesarman E, Pessin M, Lee F, Culpepper J, Knowles D, Moore P. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 2.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. 1995. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 3.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d’Agay MF, Clauvel JP, Raphael M, Degos L, Sigaux F. 1995. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 86:1276–1280. doi: 10.1182/blood.V86.4.1276.bloodjournal8641276. [DOI] [PubMed] [Google Scholar]
- 4.Ganem D. 2010. KSHV and the pathogenesis of Kaposi sarcoma: listening to human biology and medicine. J Clin Invest 120:939–949. doi: 10.1172/JCI40567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mesri EA, Cesarman E, Boshoff C. 2010. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer 10:707–719. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandriani S, Ganem D. 2010. Array-based transcript profiling and limiting-dilution reverse transcription-PCR analysis identify additional latent genes in Kaposi’s sarcoma-associated herpesvirus. J Virol 84:5565–5573. doi: 10.1128/JVI.02723-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnan HH, Naranatt PP, Smith MS, Zeng L, Bloomer C, Chandran B. 2004. Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi’s sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J Virol 78:3601–3620. doi: 10.1128/jvi.78.7.3601-3620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai Q, Verma SC, Lu J, Robertson ES. 2010. Molecular biology of Kaposi’s sarcoma-associated herpesvirus and related oncogenesis, p 87–142. In Maramorosch K, Shatkin AJ, Murphy FA (ed), Advances in virus research, vol 78 Academic Press, San Diego, CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen KW, Damania B. 2010. Kaposi sarcoma-associated herpesvirus (KSHV): molecular biology and oncogenesis. Cancer Lett 289:140–150. doi: 10.1016/j.canlet.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ensoli B, Sturzl M. 1998. Kaposi’s sarcoma: a result of the interplay among inflammatory cytokines, angiogenic factors and viral agents. Cytokine Growth Factor Rev 9:63–83. doi: 10.1016/s1359-6101(97)00037-3. [DOI] [PubMed] [Google Scholar]
- 11.Sun Q, Zachariah S, Chaudhary PM. 2003. The human herpes virus 8-encoded viral FLICE-inhibitory protein induces cellular transformation via NF-κB activation. J Biol Chem 278:52437–52445. doi: 10.1074/jbc.M304199200. [DOI] [PubMed] [Google Scholar]
- 12.Roth WK, Brandstetter H, Sturzl M. 1992. Cellular and molecular features of HIV-associated Kaposi’s sarcoma. AIDS 6:895–913. doi: 10.1097/00002030-199209000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Dourmishev LA, Dourmishev AL, Palmeri D, Schwartz RA, Lukac DM. 2003. Molecular genetics of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) epidemiology and pathogenesis. Microbiol Mol Biol Rev 67:175–212. doi: 10.1128/mmbr.67.2.175-212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antman K, Chang Y. 2000. Kaposi’s sarcoma. N Engl J Med 342:1027–1038. doi: 10.1056/NEJM200004063421407. [DOI] [PubMed] [Google Scholar]
- 15.Fenoglio-Preiser CM. 2001. Gastrointestinal neuroendocrine/neuroectodermal tumors. Am J Clin Pathol 115(Suppl):S79–S93. [DOI] [PubMed] [Google Scholar]
- 16.Rindi G, Klöppel G. 2004. Endocrine tumors of the gut and pancreas tumor biology and classification. Neuroendocrinology 80(Suppl 1):12–15. doi: 10.1159/000080733. [DOI] [PubMed] [Google Scholar]
- 17.Oberg K. 2005. Neuroendocrine tumors of the gastrointestinal tract: recent advances in molecular genetics, diagnosis, and treatment. Curr Opin Oncol 17:386–391. doi: 10.1097/01.cco.0000167739.56948.a9. [DOI] [PubMed] [Google Scholar]
- 18.Rindi G, Villanacci V, Ubiali A. 2000. Biological and molecular aspects of gastroenteropancreatic neuroendocrine tumors. Digestion 62(Suppl 1):19–26. doi: 10.1159/000051851. [DOI] [PubMed] [Google Scholar]
- 19.Kulke MH. 2008. Neuroendocrine tumors: is there a standard treatment? Gastrointest Cancer Res 2:152–153. [PMC free article] [PubMed] [Google Scholar]
- 20.Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. 2010. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 39:707–712. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 21.Castiglione M, Calafiore M, Costa L, Sortino MA, Nicoletti F, Copani A. 2008. Group I metabotropic glutamate receptors control proliferation, survival and differentiation of cultured neural progenitor cells isolated from the subventricular zone of adult mice. Neuropharmacology 55:560–567. doi: 10.1016/j.neuropharm.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Genever PG, Skerry TM. 2001. Regulation of spontaneous glutamate release activity in osteoblastic cells and its role in differentiation and survival: evidence for intrinsic glutamatergic signaling in bone. FASEB J 15:1586–1588. doi: 10.1096/fj.00-0594fje. [DOI] [PubMed] [Google Scholar]
- 23.Conn PJ, Pin JP. 1997. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 24.Dingledine R, Borges K, Bowie D, Traynelis SF. 1999. The glutamate receptor ion channels. Pharmacol Rev 51:7–61. [PubMed] [Google Scholar]
- 25.Marin YE, Namkoong J, Cohen-Solal K, Shin SS, Martino JJ, Oka M, Chen S. 2006. Stimulation of oncogenic metabotropic glutamate receptor 1 in melanoma cells activates ERK1/2 via PKCepsilon. Cell Signal 18:1279–1286. doi: 10.1016/j.cellsig.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Kalariti N, Pissimissis N, Koutsilieris M. 2005. The glutamatergic system outside the CNS and in cancer biology. Expert Opin Investig Drugs 14:1487–1496. doi: 10.1517/13543784.14.12.1487. [DOI] [PubMed] [Google Scholar]
- 27.Skerry TM, Genever PG. 2001. Glutamate signalling in non-neuronal tissues. Trends Pharmacol Sci 22:174–181. doi: 10.1016/s0165-6147(00)01642-4. [DOI] [PubMed] [Google Scholar]
- 28.Pacheco R, Ciruela F, Casado V, Mallol J, Gallart T, Lluis C, Franco R. 2004. Group I metabotropic glutamate receptors mediate a dual role of glutamate in T cell activation. J Biol Chem 279:33352–33358. doi: 10.1074/jbc.M401761200. [DOI] [PubMed] [Google Scholar]
- 29.Valiya Veettil M, Dutta D, Bottero V, Bandyopadhyay C, Gjyshi O, Sharma-Walia N, Dutta S, Chandran B. 2014. Glutamate secretion and metabotropic glutamate receptor 1 expression during Kaposi’s sarcoma-associated herpesvirus infection promotes cell proliferation. PLoS Pathog 10:e1004389. doi: 10.1371/journal.ppat.1004389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.An F-Q, Folarin HM, Compitello N, Roth J, Gerson SL, McCrae KR, Fakhari FD, Dittmer DP, Renne R. 2006. Long-term-infected telomerase-immortalized endothelial cells: a model for Kaposi’s sarcoma-associated herpesvirus latency in vitro and in vivo. J Virol 80:4833–4846. doi: 10.1128/JVI.80.10.4833-4846.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tischler AS. 1989. The dispersed neuroendocrine cells: the structure, function, regulation and effects of xenobiotics on this system. Toxicol Pathol 17:307–316. doi: 10.1177/019262338901700207. [DOI] [PubMed] [Google Scholar]
- 32.Oronsky B, Ma PC, Morgensztern D, Carter CA. 2017. Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia 19:991–1002. doi: 10.1016/j.neo.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lloyd RV. 2003. Practical markers used in the diagnosis of neuroendocrine tumors. Endocr Pathol 14:293–301. doi: 10.1385/EP:14:4:293. [DOI] [PubMed] [Google Scholar]
- 34.Biedler JL, Helson L, Spengler BA. 1973. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res 33:2643–2652. [PubMed] [Google Scholar]
- 35.Schoenherr CJ, Anderson DJ. 1995. Silencing is golden: negative regulation in the control of neuronal gene transcription. Curr Opin Neurobiol 5:566–571. doi: 10.1016/0959-4388(95)80060-3. [DOI] [PubMed] [Google Scholar]
- 36.Johnson DS, Mortazavi A, Myers RM, Wold B. 2007. Genome-wide mapping of in vivo protein-DNA interactions. Science 316:1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- 37.Otto SJ, McCorkle SR, Hover J, Conaco C, Han J-J, Impey S, Yochum GS, Dunn JJ, Goodman RH, Mandel G. 2007. A new binding motif for the transcriptional repressor REST uncovers large gene networks devoted to neuronal functions. J Neurosci 27:6729–6739. doi: 10.1523/JNEUROSCI.0091-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charbord J, Poydenot P, Bonnefond C, Feyeux M, Casagrande F, Brinon B, Francelle L, Aurégan G, Guillermier M, Cailleret M, Viegas P, Nicoleau C, Martinat C, Brouillet E, Cattaneo E, Peschanski M, Lechuga M, Perrier AL. 2013. High throughput screening for inhibitors of REST in neural derivatives of human embryonic stem cells reveals a chemical compound that promotes expression of neuronal genes. Stem Cells 31:1816–1828. doi: 10.1002/stem.1430. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z, Liu M, Niu G, Cheng Y, Fei J. 2009. Genome-wide identification of target genes repressed by the zinc finger transcription factor REST/NRSF in the HEK 293 cell line. Acta Biochim Biophys Sin (Shanghai) 41:1008–1017. doi: 10.1093/abbs/gmp095. [DOI] [PubMed] [Google Scholar]
- 40.Krithivas A, Young DB, Liao G, Greene D, Hayward SD. 2000. Human herpesvirus 8 LANA interacts with proteins of the mSin3 corepressor complex and negatively regulates Epstein-Barr virus gene expression in dually infected PEL cells. J Virol 74:9637–9645. doi: 10.1128/jvi.74.20.9637-9645.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dittmer DP, Richards KL, Damania B. 2012. Treatment of Kaposi sarcoma-associated herpesvirus-associated cancers. Front Microbiol 3:141. doi: 10.3389/fmicb.2012.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fatahzadeh M. 2012. Kaposi sarcoma: review and medical management update. Oral Surg Oral Med Oral Pathol Oral Radiol 113:2–16. doi: 10.1016/j.tripleo.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 43.Andreoni M, Goletti D, Pezzotti P, Pozzetto A, Monini P, Sarmati L, Farchi F, Tisone G, Piazza A, Pisani F, Angelico M, Leone P, Citterio F, Ensoli B, Rezza G. 2001. Prevalence, incidence and correlates of HHV-8/KSHV infection and Kaposi’s sarcoma in renal and liver transplant recipients. J Infect 43:195–199. doi: 10.1053/jinf.2001.0899. [DOI] [PubMed] [Google Scholar]
- 44.Martellotta F, Berretta M, Vaccher E, Schioppa O, Zanet E, Tirelli U. 2009. AIDS-related Kaposi’s sarcoma: state of the art and therapeutic strategies. Curr HIV Res 7:634–638. doi: 10.2174/157016209789973619. [DOI] [PubMed] [Google Scholar]
- 45.Isgrò MA, Bottoni P, Scatena R. 2015. Neuron-specific enolase as a biomarker: biochemical and clinical aspects, p 125–143. In Scatena R. (ed), Advances in cancer biomarkers: from biochemistry to clinic for a critical revision. Springer, Dordrecht, Netherlands. doi: 10.1007/978-94-017-7215-0_9. [DOI] [PubMed] [Google Scholar]
- 46.Vizin T, Kos J. 2015. Gamma-enolase: a well-known tumour marker, with a less-known role in cancer. Radiol Oncol 49:217–226. doi: 10.1515/raon-2015-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spataro V, Norbury C, Harris AL. 1998. The ubiquitin-proteasome pathway in cancer. Br J Cancer 77:448–455. doi: 10.1038/bjc.1998.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Day INM, Thompson RJ. 1987. Molecular cloning of cDNA coding for human PGP 9.5 protein. FEBS Lett 210:157–160. doi: 10.1016/0014-5793(87)81327-3. [DOI] [PubMed] [Google Scholar]
- 49.Lewis JE, Brameld JM, Jethwa PH. 2015. Neuroendocrine role for VGF. Front Endocrinol 6:3. doi: 10.3389/fendo.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boshoff C, Schulz TF, Kennedy MM, Graham AK, Fisher C, Thomas A, McGee JO, Weiss RA, O’Leary JJ. 1995. Kaposi’s sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat Med 1:1274–1278. doi: 10.1038/nm1295-1274. [DOI] [PubMed] [Google Scholar]
- 51.Mendoza-Torreblanca JG, Vanoye-Carlo A, Phillips-Farfán BV, Carmona-Aparicio L, Gómez-Lira G. 2013. Synaptic vesicle protein 2A: basic facts and role in synaptic function. Eur J Neurosci 38:3529–3539. doi: 10.1111/ejn.12360. [DOI] [PubMed] [Google Scholar]
- 52.Patel P, Mishra A, Sheikh AA, Kumar K, Bhagat R, Maibam U. 2017. Adrenomedullin: a novel peptide hormone. A review. J Pharmacogn Phytochem 6:2068–2073. [Google Scholar]
- 53.Stebbing J, Wildfire A, Portsmouth S, Powles T, Thirlwell C, Hewitt P, Nelson M, Patterson S, Mandalia S, Gotch F, Gazzard BG, Bower M. 2003. Paclitaxel for anthracycline-resistant AIDS-related Kaposi’s sarcoma: clinical and angiogenic correlations. Ann Oncol 14:1660–1666. doi: 10.1093/annonc/mdg461. [DOI] [PubMed] [Google Scholar]
- 54.Cakir M, Dworakowska D, Grossman A. 2010. Somatostatin receptor biology in neuroendocrine and pituitary tumours: part 1—molecular pathways. J Cell Mol Med 14:2570–2584. doi: 10.1111/j.1582-4934.2010.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grozinsky-Glasberg S, Shimon I, Korbonits M, Grossman AB. 2008. Somatostatin analogues in the control of neuroendocrine tumours: efficacy and mechanisms. Endocr Relat Cancer 15:701–720. doi: 10.1677/ERC-07-0288. [DOI] [PubMed] [Google Scholar]
- 56.Medina VA, Rivera ES. 2010. Histamine receptors and cancer pharmacology. Br J Pharmacol 161:755–767. doi: 10.1111/j.1476-5381.2010.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giffin L, Damania B. 2014. KSHV: pathways to tumorigenesis and persistent infection, p 111–159. In Maramorosch K, Murphy FA (ed), Advances in virus research, vol 88 Academic Press, San Diego, CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bruce AW, Donaldson IJ, Wood IC, Yerbury SA, Sadowski MI, Chapman M, Göttgens B, Buckley NJ. 2004. Genome-wide analysis of repressor element 1 silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) target genes. Proc Natl Acad Sci U S A 101:10458–10463. doi: 10.1073/pnas.0401827101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conaco C, Otto S, Han J-J, Mandel G. 2006. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc Natl Acad Sci U S A 103:2422–2427. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cai Q, Xiao B, Si H, Cervini A, Gao J, Lu J, Upadhyay SK, Verma SC, Robertson ES. 2012. Kaposi’s sarcoma herpesvirus upregulates Aurora A expression to promote p53 phosphorylation and ubiquitylation. PLoS Pathog 8:e1002566. doi: 10.1371/journal.ppat.1002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, Wang Y, Sheikh KL, Terry S, Tagawa ST, Dhir R, Nelson JB, de la Taille A, Allory Y, Gerstein MB, Perner S, Pienta KJ, Chinnaiyan AM, Wang Y, Collins CC, Gleave ME, Demichelis F, Nanus DM, Rubin MA. 2011. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov 1:487–495. doi: 10.1158/2159-8290.CD-11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pollock PM, Cohen-Solal K, Sood R, Namkoong J, Martino JJ, Koganti A, Zhu H, Robbins C, Makalowska I, Shin SS, Marin Y, Roberts KG, Yudt LM, Chen A, Cheng J, Incao A, Pinkett HW, Graham CL, Dunn K, Crespo-Carbone SM, Mackason KR, Ryan KB, Sinsimer D, Goydos J, Reuhl KR, Eckhaus M, Meltzer PS, Pavan WJ, Trent JM, Chen S. 2003. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet 34:108–112. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- 63.Stepulak A, Luksch H, Gebhardt C, Uckermann O, Marzahn J, Sifringer M, Rzeski W, Staufner C, Brocke KS, Turski L, Ikonomidou C. 2009. Expression of glutamate receptor subunits in human cancers. Histochem Cell Biol 132:435–445. doi: 10.1007/s00418-009-0613-1. [DOI] [PubMed] [Google Scholar]
- 64.Choi KY, Chang K, Pickel JM, Badger JD II, Roche KW. 2011. Expression of the metabotropic glutamate receptor 5 (mGluR5) induces melanoma in transgenic mice. Proc Natl Acad Sci U S A 108:15219–15224. doi: 10.1073/pnas.1107304108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lyons SA, Chung WJ, Weaver AK, Ogunrinu T, Sontheimer H. 2007. Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res 67:9463–9471. doi: 10.1158/0008-5472.CAN-07-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doble A. 1996. The pharmacology and mechanism of action of riluzole. Neurology 47:S233–S241. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- 67.Iwamoto FM, Kreisl TN, Kim L, Duic JP, Butman JA, Albert PS, Fine HA. 2010. Phase 2 trial of talampanel, a glutamate receptor inhibitor, for adults with recurrent malignant gliomas. Cancer 116:1776–1782. doi: 10.1002/cncr.24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yip D, Le MN, Chan JL, Lee JH, Mehnert JA, Yudd A, Kempf J, Shih WJ, Chen S, Goydos JS. 2009. A phase 0 trial of riluzole in patients with resectable stage III and IV melanoma. Clin Cancer Res 15:3896–3902. doi: 10.1158/1078-0432.CCR-08-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bensimon G, Lacomblez L, Meininger V. 1994. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med 330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 70.Iwata K, Luo J, Penn RB, Benovic JL. 2005. Bimodal regulation of the human H1 histamine receptor by G protein-coupled receptor kinase 2. J Biol Chem 280:2197–2204. doi: 10.1074/jbc.M408834200. [DOI] [PubMed] [Google Scholar]
- 71.Francis HL, Demorrow S, Franchitto A, Venter JK, Mancinelli RA, White MA, Meng F, Ueno Y, Carpino G, Renzi A, Baker KK, Shine HE, Francis TC, Gaudio E, Alpini GD, Onori P. 2012. Histamine stimulates the proliferation of small and large cholangiocytes by activation of both IP3/Ca2+ and cAMP-dependent signaling mechanisms. Lab Invest 92:282–294. doi: 10.1038/labinvest.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Massari NA, Nicoud MB, Medina VA. 10 November 2018. Histamine receptors and cancer pharmacology: an update. Br J Pharmacol doi: 10.1111/bph.14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Notcovich C, Diez F, Tubio MR, Baldi A, Kazanietz MG, Davio C, Shayo C. 2010. Histamine acting on H1 receptor promotes inhibition of proliferation via PLC, RAC, and JNK-dependent pathways. Exp Cell Res 316:401–411. doi: 10.1016/j.yexcr.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 74.Ayers LW, Barbachano-Guerrero A, McAllister SC, Ritchie JA, Asiago-Reddy E, Bartlett LC, Cesarman E, Wang D, Rochford R, Martin JN, King CA. 2018. Mast cell activation and KSHV infection in Kaposi sarcoma. Clin Cancer Res 24:5085–5097. doi: 10.1158/1078-0432.CCR-18-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naranatt PP, Akula SM, Zien CA, Krishnan HH, Chandran B. 2003. Kaposi’s sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-zeta-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J Virol 77:1524–1539. doi: 10.1128/jvi.77.2.1524-1539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tiscornia G, Singer O, Verma IM. 2006. Production and purification of lentiviral vectors. Nat Protoc 1:241–245. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.