Outbreaks of Tembusu virus (TMUV) infection have caused huge economic losses in the production of domestic waterfowl since the virus was first recognized in China in 2010. To control TMUV infection, a live-attenuated vaccine candidate of TMUV was developed in our previous study, but the mechanisms of virulence attenuation are not fully understood. Here, we found that the Thr-to-Lys substitution at E367 is a crucial determinant of TMUV virulence attenuation in ducks. We demonstrated that the T367K mutation attenuates TMUV through reducing viral replication in the blood, brain, heart (ducklings), and ovaries. These data provide new insights into understanding the pathogenesis of TMUV and the rational development of novel TMUV vaccines.
KEYWORDS: Tembusu virus, flavivirus, amino acid substitution, envelope protein, virulence, duck
ABSTRACT
Tembusu virus (TMUV) is a flavivirus responsible for panzootic outbreaks of severe egg-drop and fatal encephalitis of domestic waterfowl in China. Although TMUV can be attenuated by in vitro passaging, experimental evidence supporting the role of specific genetic changes in virulence attenuation is currently lacking. Here, we performed site-directed mutagenesis on five envelope (E) protein amino acid residues in accordance with the attenuated TMUV generated in our recent study. Our results showed that the Thr-to-Lys mutation of residue 367 in E protein (E367) plays a predominant role in viral cell adaptation and virulence attenuation in ducks compared with mutations in other residues. We further demonstrated that the positively charged basic amino acid substitution at E367 enhanced the viral binding affinity for glycosaminoglycans (GAGs) and reduced viremia levels and the efficiency of replication in major target organs in subcutaneously inoculated ducks. Interestingly, the T367K mutation increased viral neutralization sensitivity to the early immune sera. Together, our findings provide the first evidence that a basic amino acid substitution at E367 strongly impacts the in vitro and in vivo infection of TMUV.
IMPORTANCE Outbreaks of Tembusu virus (TMUV) infection have caused huge economic losses in the production of domestic waterfowl since the virus was first recognized in China in 2010. To control TMUV infection, a live-attenuated vaccine candidate of TMUV was developed in our previous study, but the mechanisms of virulence attenuation are not fully understood. Here, we found that the Thr-to-Lys substitution at E367 is a crucial determinant of TMUV virulence attenuation in ducks. We demonstrated that the T367K mutation attenuates TMUV through reducing viral replication in the blood, brain, heart (ducklings), and ovaries. These data provide new insights into understanding the pathogenesis of TMUV and the rational development of novel TMUV vaccines.
INTRODUCTION
Tembusu virus (TMUV) is a member of the Ntaya virus group within the genus Flavivirus, family Flaviviridae (1), which was originally isolated from mosquitoes in Malaysia in 1955 (2). The first recognized disease caused by TMUV was reported in chicken flocks in Perak State, Malaysia, in 2000 and was characterized by encephalitis and retarded growth with hyperglycemia (3). In 2010, a panzootic outbreak of TMUV infection occurred in domestic duck and goose flocks throughout the waterfowl-producing regions of China, leading to huge economic losses for the poultry industry (4–6). Recently, this disease was also observed in duck flocks in Southeastern Asian countries (7, 8).
Currently, TMUV infection seems to be one of the most important flavivirus diseases of poultry, affecting almost all species of domestic ducks and geese (9–14), with sporadic cases in chickens (11, 15, 16). In laying flocks, TMUV has been shown to elicit greater pathogenic effects and long-term infection in the ovaries of laying ducks, as characterized by ovarian hemorrhage, ovaritis, and follicle atresia with severe egg drop (4–6, 17). While the clinical signs and mortality of young birds are age dependent, younger birds are more susceptible to fatal infection and severe lesions in the brain, heart, and spleen tissues and thus display a higher mortality rate (18, 19). Despite the distinct clinical syndromes observed in ducks infected with TMUV, the genetic basis for the pathogenesis of the virus in ducks is poorly understood. Furthermore, a remarkable characteristic of TMUV is that the virus can be efficiently transmitted via the aerosol route and direct contact, in addition to mosquito-borne transmission (20). Therefore, outbreaks of TMUV infection can occur throughout the year, and disease can spread rapidly among duck flocks (7). This is quite different from other vector-borne flavivirus infections.
TMUV contains a positive-sense single-stranded RNA genome of approximately 10.9 kb in length encoding a single large precursor polyprotein. The polyprotein is predicted to be proteolytically processed into three structural proteins (capsid [C], precursor membrane [prM], and envelope glycoprotein [E]), and seven nonstructural (NS) proteins, similar to those of other flaviviruses (1, 21). The E protein of flavivirus, the major surface protein of the viral particle, plays an important role in host-specific adaptation, cell tropism, virus attachment, and membrane fusion with target cells (22–25) and is the dominant target of neutralizing antibodies (26). It has been reported that a mutation(s) in E protein could modulate viral antigenicity, stability, and pathogenesis (27–31). For example, three residues in E protein domain III (EDIII) of yellow fever virus (YFV) 17D enhanced binding to glycosaminoglycans (GAGs), inhibited virus spread in extraneural tissues, and reduced virulence in mice (24). In addition, the Glu-to-Lys mutation at residue 138 of the E protein of Japanese encephalitis virus (JEV) enhanced susceptibility to heparin inhibition in vitro and strongly attenuated viral neurovirulence and neuroinvasiveness in IFNR–/– mice (28, 32). Studies of live-attenuated flavivirus vaccines, e.g., YFV 17D and JEV SA14-14-2, have also indicated that genetic changes in E protein are responsible for flavivirus cell adaptation as well as virulence attenuation in vivo (23, 24, 33–35).
We recently developed a highly attenuated TMUV vaccine candidate, P310, by sequential passaging in BHK-21 cells, resulting in a total of 23 amino acid substitutions during the adaptive process (36). The chimeric virus with the mutated E gene showed increased growth in BHK cells and significantly reduced pathogenicity in subcutaneously (s.c.) inoculated ducklings, highlighting the importance of these mutations to the phenotypic changes of attenuated TMUV. To further determine the specific role of mutations in the E protein in attenuating TMUV, the five in vitro passage-derived amino acid substitutions were individually engineered into the infectious cDNA of virulent TMUV using site-directed mutagenesis. The in vitro growth characteristics of these mutant viruses were determined, and the virulence of the mutant viruses was evaluated in ducks. We demonstrated that the T367K mutation of E protein confers TMUV cell-adaptation and attenuates virulence by affecting the levels of viremia, virus invasion, and/or replication in major target tissues.
RESULTS
Mutated E protein impairs the replication of TMUV in ducklings.
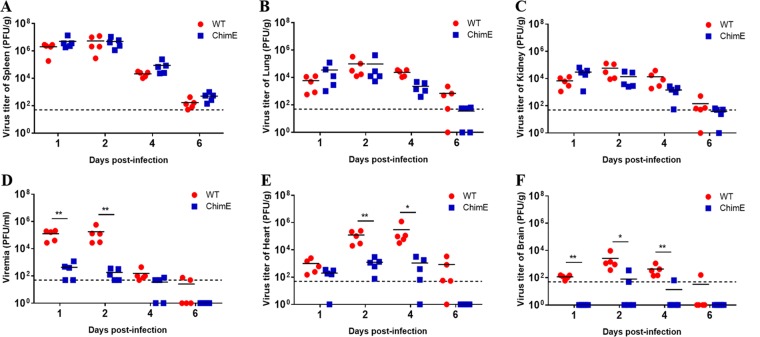
In our previous study, a chimeric virus (ChimE) was generated by introducing the mutated E genes of the attenuated P310-4C virus (a clone of the 310th passaged TMUV) into the backbone of a virulent TMUV strain (wild type [WT]). When subcutaneously (s.c.) injected into ducklings, this chimeric virus showed significant virulence attenuation compared with WT virus (36). However, it was unclear which stage(s) was affected by the mutated E gene during the TMUV infection process. We thus determined the in vivo replication kinetics of the ChimE virus by assessing the titers of infectious viruses in sera and tissues on days 1, 2, 4, and 6 postinoculation (dpi) of 4-day-old ducklings (Fig. 1). The virus titers in the spleen, lungs, and kidneys were found to be similar between the two groups (Fig. 1A to C). However, ChimE infection produced significantly lower levels of viremia (P < 0.01) (Fig. 1D) and >100-fold fewer infectious viruses in heart tissue (Fig. 1E) compared with the WT-infected ducklings. Furthermore, infectious viruses were only recovered from the brains of two (2/5) and one (1/5) of the ChimE-infected ducklings at 2 and 4 dpi, respectively (Fig. 1F). We also assessed the pathogenicity by infecting a group of 4-day-old ducklings in each group, as described above. Consistent with our previous study, ducklings infected with WT virus exhibited depression, loss of food uptake, and ataxia from 3 dpi, resulting in a mortality rate of 70% (36), whereas none of the ducklings infected with ChimE virus showed evident signs of illness throughout the test period. These results suggested that mutations in the E protein impaired virus replication in the blood, brain, and heart of ducklings, leading to virulence attenuation.
FIG 1.
Replication of ChimE virus in the different tissues of ducklings. Four-day-old ducklings were inoculated s.c. with WT and ChimE viruses (1 × 105 PFU/bird) and then euthanized at the indicated time points. The infectious virus titers in the spleen (A), lungs (B), kidney (C), blood (D), heart (E), and brain (F) were determined using a plaque assay on BHK-21 cells. Horizontal lines across data points indicate the mean virus titer. The data were tested for statistical significance by two-way analysis of variance (ANOVA) multiple comparisons. *, P < 0.05, **, P < 0.01. The dashed line represents the limit of detection of the assay.
T367K mutation in E protein contributes to the cell-adaptation of TMUV.
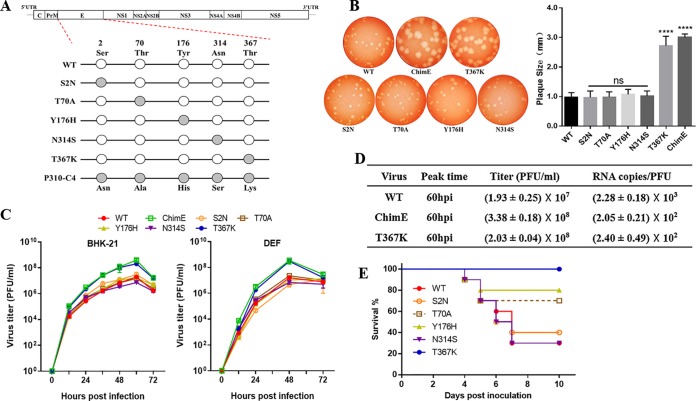
Five amino acid substitutions were detected in the mutated E protein, and ChimE virus was found to have increased growth adaptation on BHK-21 cells (36). To test the effect of specific amino acid substitutions on TMUV growth in vitro, five single-amino acid mutant viruses (designated S2N, T70A, Y176H, N314S, and T367K) were generated using site-directed mutagenesis based on the backbone of the WT virus (Fig. 2A). Morphological examination showed that the ChimE and T367K mutants produced larger plaques than the WT virus (P < 0.0001) in the BHK-21 cell monolayer (Fig. 2B), whereas the plaques produced by the mutant S2N, T70A, Y176H, and N314S viruses were of similar diameter to those of the WT. Growth kinetics measurements showed that the T367K mutant displayed enhanced growth adaptation in BHK-21 and primary duck fibroblast cells (DEF), resulting in an approximately 10-fold higher infectious virus titer than that of the WT virus at 60 hours postinoculation (hpi) (Fig. 2C and D). However, both the ChimE and T367K mutants showed an almost 10-fold-lower RNA copy number/PFU ratio than the WT (Fig. 2D), suggesting that the significantly enhanced in vitro growth adaptation of T367K should not be induced by increased viral RNA replication but should be attributed to the increased viral infectivity in vitro.
FIG 2.
Characteristics of viruses harboring E protein site-directed mutations in vitro and in vivo. (A) Schematics of the construction of five individual E protein site-directed mutant viruses based on the infectious WT clone. Site-directed mutations at E2, E70, E176, E314, and E367 were generated and constructed by fusion PCR. (B) Plaque phenotypes of the WT, ChimE, and E protein site-directed mutant viruses. The virus-infected BHK-21 monolayers were incubated under an overlay medium containing 1% agarose and then stained with neutral red at 3 dpi (left). Twenty plaques for each virus were chosen at random to calculate plaque sizes (right). The data are presented as means and standard deviation (SD) and were tested for statistical significance using the Student’s t test. ns, no significant difference; ****, P < 0.0001. (C) Virus growth kinetics. BHK-21 and DEF cells were infected with WT, ChimE, and E protein site-directed mutant viruses at an MOI of 0.01. At each indicated time point, the extracellular infectious viruses were quantified using a plaque assay. The data represent the means and SD of three independent cell cultures. The result is shown as a representative of three independent experiments. (D) Virus titer and RNA copy number/PFU ratios. From 12 to 96 hpi, supernatants were collected at an interval of 12 h. Infectious virions were determined using a plaque assay, and the copies of viral RNA at the peak titer time were quantified by qRT-PCR. (E) Survival curve for the groups of 4-day-old ducklings (n = 10 ducks per group) s.c. infected with the indicated viruses (1 × 105 PFU/bird).
T367K mutation plays a predominant role in TMUV attenuation.
To investigate whether individual amino acid substitutions in the E protein may result in TMUV attenuation in vivo, we performed a pathogenicity test by infecting 4-day-old ducklings with the amino acid mutant viruses individually, as described above. Compared with the mortality rate of 70% (7/10) in the WT-infected group, the mutant viruses T70A and Y176H showed decreased virulence with mortality rates of 3/10 and 2/10 ducklings, respectively. However, all ducklings inoculated with T367K survived until the end of the experiment (Fig. 2E). This result indicated that the amino acid mutation at E70 or E176 could partially attenuate TMUV virulence, whereas the E protein T367K mutation dramatically attenuated TMUV virulence in ducklings.
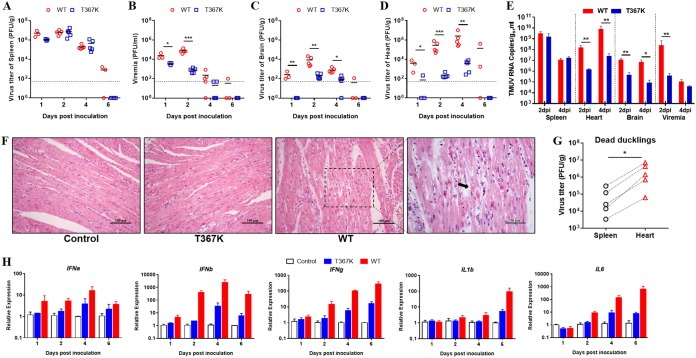
We further determined the virus growth kinetics of the T367K mutant virus in ducklings compared with the WT parental virus by s.c. inoculating them with 105 PFU of virus per bird. The plaque assay results showed that the infectious virus titers in the spleens of both groups were similar (Fig. 3A). However, the T367K mutant virus produced significantly lower levels of viremia and lower viral loads in the brain and heart compared with the WT virus (Fig. 3B to D). We further determined the viral RNA content in blood and indicated tissue of ducklings infected with WT and T367K by reverse transcription-quantitative PCR (qRT-PCR) (Fig. 3E), and the results were consistent with the plaque assays. Pathological histology analysis of heart tissue showed that the WT infection caused severe degeneration and necrosis of myocardial fibers at 4 dpi, whereas no significant lesions were observed in T367K virus-infected ducklings (Fig. 3F). Simultaneously, we compared the mRNA expression of immune-related genes in the heart tissue of ducklings infected with T367K and WT viruses by real-time quantification PCR (Fig. 3H). The results revealed that infection with the T367K mutant virus induced much lower-level expression of interferon alpha (IFN-α), IFN-β, IFN-γ, interleukin-1β (IL-1β), and IL-6 than the WT virus, especially at 2, 4, and 6 dpi, suggesting that the antiviral and proinflammatory responses might be related to the severity of the lesions found in the heart tissue of ducklings. We also noted that the infectious virus titers in the heart of ducklings that died following WT infection were significantly higher than those in the spleen (Fig. 3G). Overall, our data indicated that the heart may be one of the main target organs of TMUV infection and that the decreased replication in the heart of ducklings may partly explain the attenuation of the T367K mutant.
FIG 3.
T367K mutation reduces TMUV virulence in ducklings. Viral load in the spleen (A), blood (B), brain (C), and heart (D) in ducklings inoculated with WT and mutant virus T367K. Four-day-old ducklings were s.c. inoculated with WT or T367K viruses (1 × 105 PFU/bird) and then euthanized at the indicated time points. Infectious virus titers in different tissues were determined by a plaque assay on BHK-21 cells. Horizontal lines across data points indicate the mean of the virus titer. The data were tested for statistical significance by two-way ANOVA multiple comparisons. *, P < 0.05; **, P < 0.01; ***, P < 0.001. The dashed line represents the limit of detection of the assay. (E) Viral RNA levels in blood and tissue samples were determined by qRT-PCR. Statistical values were analyzed using two-way ANOVA multiple comparisons. *, P < 0.05; **, P < 0.01. (F) Histopathologic analysis of the hematoxylin and eosin (HE)-stained heart tissue of ducklings infected with WT or T367K viruses. T367K, showed no histopathological differences from the control; WT, degeneration and necrosis of myocardial fibers (black arrow). (G) Virus burden in the spleen and heart of dead ducklings infected with WT virus at 4 to 6 dpi. The dashed lines indicate the tissues from the same duckling. The data are presented as a single symbol and were tested for statistical significance using the Student’s t test. *, P < 0.05. (H) Expression of immune-related genes in the heart tissue of ducklings infected with WT and E367 viruses. The transcription of IFN-α, IFN-β, IFN-γ, IL-1β, and IL-6 was analyzed by qPCR and normalized to GADPH. The data are presented as the means and SEM (n = 3).
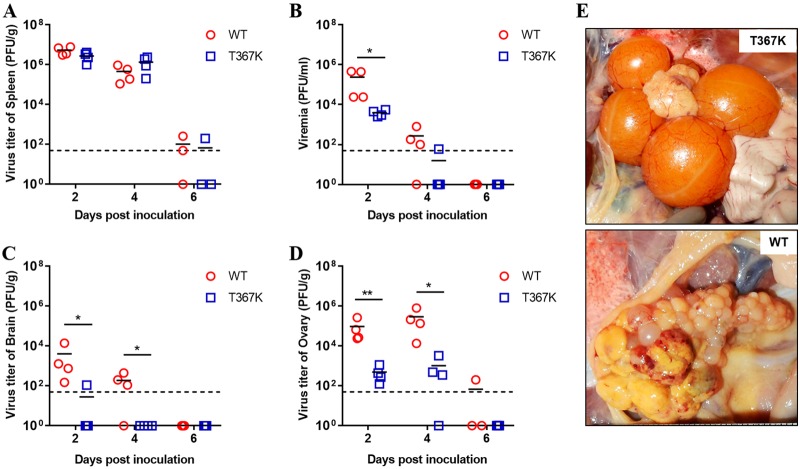
Because TMUV infection in adult ducks causes ovary damage and encephalitis, we further infected laying ducks with WT and T367K mutant viruses to verify the attenuation of TMUV mediated by the T367K mutation. As observed in ducklings, similar virus titers were observed in the spleen tissues of T367K- and WT-infected laying ducks (Fig. 4A), but the T367K mutant virus produced significantly lower viremia levels at 2 and 4 dpi (Fig. 4B), and infectious viruses were only recovered from the brain of one duck (1/4) infected with T367K mutant virus at 2 dpi (Fig. 4C). Remarkably, significantly lower virus titers were detected in the ovary tissues of ducks infected with T367K mutant virus than of those infected with WT virus (Fig. 4D). In agreement with the viral loads detected in the ovaries, ducks infected with WT virus exhibited hemorrhagic and ruptured ovarian follicles and vitelline peritonitis, whereas no evident gross lesions were observed in the ovaries of T367K-infected ducks (Fig. 4E). Taken together, these results indicated that a single amino acid substitution (Thr to Lys) at residue 367 of E protein significantly attenuates the virulence of TMUV in ducks by affecting viremia, neuroinvasion, and viral replication in the heart (ducklings) and ovaries.
FIG 4.
T367K mutation reduces TMUV virulence in laying ducks. Viral replication of WT and mutant virus T367K in the spleen (A), blood (B), brain (C), and ovaries (D) of laying ducks. Twenty-five-week-old laying ducks were s.c. inoculated with WT and T367K viruses (1 × 105 PFU/bird) and then euthanized at 2, 4, and 6 dpi. The infectious virus titer in different tissues was determined by a plaque assay on BHK-21 cells. Horizontal lines across data points indicate the mean of the virus titers. The data were tested for statistical significance by two-way ANOVA multiple comparisons. *, P < 0.05; **, P < 0.01. The dashed line represents the limit of detection of the assay. (E) Pathological changes in the ovaries of ducks infected with WT and T367K viruses. Upper image, the ducks infected with T367K had ovarian tissue of normal appearance. Lower image, the ducks infected with WT virus showed ruptured ovarian follicles and vitelline peritonitis.
Positively charged amino acid substitution at E367 is critical for TMUV attenuation.
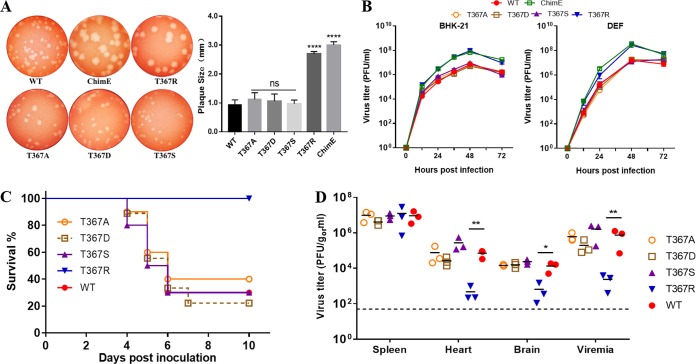
Adaptation of flaviviruses or alphaviruses to growth in cell culture has been shown to elicit amino acid substitutions that increase the net positive charge of surface envelope protein (34, 37–39). As shown in Fig. 2A, the residue at E367 of the WT virus is threonine (neutral/uncharged), whereas the residue at E367 in the attenuated virus P310-4C is lysine (basic/positively charged). To investigate whether the positively charged amino acid substitution at E367 is related to TMUV virulence attenuation, four individual mutant viruses (T367A, T367D, T367S, and T367R) were generated by replacing the Thr residue at E367 with Ala, Asp, Ser, or Arg in the WT background, respectively. The plaque morphology and growth kinetics of these mutant viruses were first measured. Mutant viruses T367A, T367D, and T367S produced plaques of similar size to those of the WT virus. However, mutant virus T367R formed larger plaques than the WT virus, but the plaques were similar to those of the T367K and ChimE viruses (Fig. 5A). In vitro growth kinetic assays showed that the T367R mutant virus grew faster than the WT virus on both BHK-21 cells and DEF cells, whereas the growth curves for T367A, T367D, and T367S were similar to that of the WT virus (Fig. 5B), suggesting that the positively charged basic amino acid substitution at E367 enhances the cell culture growth adaptation of TMUV.
FIG 5.
Characteristics of the E367 site-directed mutant virus in vitro and in vivo. (A) Plaque phenotypes of the WT, ChimE, and the four E367 site-directed mutant viruses. The virus-infected BHK-21 monolayers were incubated under an overlay medium containing 1% agarose and then stained with neutral red at 3 dpi (left). Twenty plaques for each virus were chosen at random to calculate plaque sizes (right). The data are presented as the means and SD and were tested for statistical significance using the Student’s t test. ns, no significant difference; ****, P < 0.0001. (B) Virus growth kinetics. BHK-21 and DEF cells were infected with WT, ChimE, and mutant viruses T367A, T367D, T367S, and T367R at an MOI of 0.01. Extracellular infectious virions were quantified using a plaque assay. The value represents the means and SD of three independent cultures. The data shown are representative of three independent experiments. (C) Survival curves of groups of 4-day-old ducklings (n = 10 ducks per group) s.c. infected with the indicated viruses (1 × 105 PFU/bird). (D) Virus burden in the spleen, heart, brain, and blood of ducklings infected with the indicated mutant and WT viruses at 2 dpi. Horizontal lines across data points indicate the mean virus titer. The data were tested for statistical significance by one-way ANOVA multiple comparisons. *, P < 0.05; **, P < 0.01.
We further compared the pathogenicity of these E367 site-mutated viruses with the parental WT virus by s.c. inoculating 4-day-old ducklings, as above. As shown in Fig. 5C, all ducklings inoculated with the T367R mutant virus survived throughout the experiment, whereas ducklings inoculated with the T367A, T367D, and T367S mutant viruses had mortality rates comparable to those inoculated with the WT virus. Furthermore, infectious virus quantification in different tissues revealed that mutant virus T367R exhibited replicative deficiency in vivo, with significantly lower titers of infectious viruses in the heart, brain, and blood of ducklings (Fig. 5D). Our results revealed that mutant viruses T367K and T367R, both with positively charged basic amino acid substitutions at E367, showed significantly attenuated virulence in ducklings. Together, these findings suggest that the positively charged amino acid substitution at residue 367 of E protein is the crucial molecular determinant of TMUV attenuation in ducks.
T367K mutation accelerates virus clearance from the bloodstream.
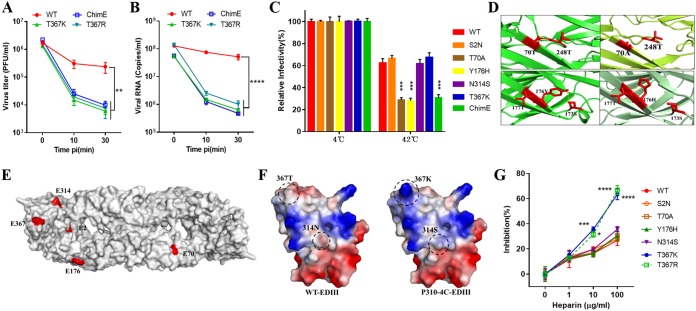
Next, we examined whether virulence attenuation of the T367K mutant involves the common mechanism of rapid virus clearance from the blood as described for other flaviviruses, such as Murray Valley encephalitis (MVE), West Nile virus (WNV), and Japanese encephalitis virus (JEV) (23, 24, 34, 40). The in vivo kinetics of virus clearance from the duck bloodstream was determined by comparing clearance of the WT virus with clearance of the ChimE, T367K, and T367R mutant viruses. Groups of 1-week-old ducklings were intravenously (i.v.) injected with 107 PFU of virus, blood samples were collected 10 and 30 min after inoculation, and infectious virus titers were measured by plaque assay on BHK-21 cells (Fig. 6A). The parental WT virus showed a <15-fold reduction in the blood over a 30-min interval. In contrast, infectious virus titers in ducklings inoculated with ChimE, T367K, or T367R dropped >100-fold in the bloodstream over the same period. Similar to the plaque assay, the level of RNA copies of the mutant virus in the blood samples decreased faster than that of the parental WT virus after ducklings were inoculated with 109 copies of virus particles (Fig. 6B), indicating that virions were removed from the bloodstream, not just inactivated. These data suggest that a basic amino acid (Thr or Arg) mutation at E367 results in rapid virus clearance from the duck bloodstream, which may account to some extent for the low level of viremia.
FIG 6.
T367K mutation enhances virus clearance from the duck bloodstream as well as virus susceptibility to heparin inhibition. (A) Virus clearance from the blood was determined for the WT, ChimE, T367K, and T367R viruses. Seven-day-old ducklings were i.v. inoculated with WT, ChimE, T367K, and T367R (107 PFU/bird). At 10 and 30 min postinoculation, blood was collected and infectious virus titer was determined using a plaque assay. Data were tested for statistical significance by one-way ANOVA multiple comparisons. **, P < 0.01. (B) Virus clearance from the blood measured by qRT-PCR. Seven-day-old ducklings were inoculated as above, at a dose of 109 copies of virus per bird. Levels of viral RNA in the blood were quantified by qRT-PCR. Data were tested for statistical significance by one-way ANOVA multiple comparisons. ****, P < 0.0001. (C) Thermostability analysis of the WT, ChimE, and E protein site-directed mutant viruses at 42°C. The y axis shows the percentage of PFU at 42°C divided by that of the same virus at 4°C. The values represent the means and SD of triplicates and were tested for statistical significance by one-way ANOVA multiple comparisons. ****, P < 0.0001. The data shown are representative of three independent experiments. (D) Upper panels: the predicted hydrogen bonds formed between 70T and 248T were broken by the T70A mutation; lower panels: the hydrogen bonds between 176Y and 173S were changed to 176H and 177T. Hydrogen bonds are shown as yellow dotted lines. (E) Location of amino acid mutations on the external surface of the TMUV E protein antiparallel dimer. The protein-dimer structure of TMUV E protein was created using Swiss-Model online using the crystal structure of the JEV E protein (PDB identifier [ID] 3P54). The locations of E2, E70, E176, E314, and E367 are highlighted in red. (F) Molecular modeling and the electrostatic surface of TMUV EDIII in the indicated viruses. Blue and red denote positive and negative charges, respectively. Thr-to-Lys mutation at E367 enhances the net positive charge of E protein. (G) Inhibition by heparin of virus infectivity in BHK-21 cells. WT and E protein site-directed mutant viruses were incubated with heparin prior to the addition to cells. Agar overlay was added to allow for plaque formation after 1 h of adsorption at 37°C. The values represent the means and SD of triplicates and are representative of three independent experiments. Statistically significant differences were determined using two-way ANOVA multiple comparisons. **, P < 0.01; ****, P < 0.0001.
T367K mutation does not affect the thermal stability of TMUV.
As reported in our previous study (36), the mutations in attenuated TMUV, including Thr to Lys at E367, occurred during viral passage in BHK-21 cells under a conventional temperature of 37°C; however, the normal body temperature of ducks is about 42°C (41). Hence, we hypothesized that the rapid clearance of the T367K mutation from the bloodstream was related to the thermal stability of the virus at high physiological temperatures in ducks. To test this, we compared the stability of the WT virus and its derivative mutants at 42°C. After treatment for 60 min, the infectivity of the virus samples was measured by a plaque assay on BHK-21 cells. As shown in Fig. 6C, the ChimE and mutant viruses T70A and Y176H lost significantly more infectivity than the WT virus at 42°C, whereas the mutant S2N, N314S, and T367K viruses retained infectivity to levels similar to that of the WT virus. Therefore, it is apparent that the rapid clearance of T367K from the blood of ducks is not caused by alterations in viral thermal stability.
We next performed homology modeling analysis of TMUV E protein based on the crystal structure of JEV envelope protein. The predicted structure showed that the T70A mutation resulted in the breakage of a hydrogen bond between 70T and 248T, whereas the Y176H mutation transferred a hydrogen bond from 176Y-173S to 176H-177T (Fig. 6D). This suggested that the changes in hydrogen bonds in E protein are responsible for the altered thermal stability of TMUV, which may account for the partial attenuation of mutant viruses T70A and Y176H in ducklings (Fig. 2E).
T367K mutation enhances TMUV susceptibility to heparin inhibition.
It has been reported that the enhanced affinity for glycosaminoglycans (GAGs), which are ubiquitously present on the cell surface and extracellular matrix, is associated with the rapid clearance of viruses from the blood (23, 24, 34, 40). Our homology modeling analysis suggested that residue E367 was exposed on the outer surface of EDIII (Fig. 6E), and a Thr-to-Lys substitution at E367 could increase the net positive charge on the surface of E protein (Fig. 6F). To assess the effect of the T367K and T367R mutation on virus GAG-binding affinity, a heparin (a highly sulfated GAG) inhibition assay was performed for the WT and E protein mutant viruses on BHK-21 cells (Fig. 6G). Both T367K and T367R were found to be more sensitive to inhibition by heparin at 10 and 100 μg/ml during the infection of BHK-21 cells, but no significant difference was observed for other mutant viruses compared with the WT virus. Our data suggested that the enhanced susceptibility of the T367K/R mutant to inhibition by heparin may be due to a common mechanism, namely, that the positively charged amino acid substitution in the E protein enhances viral binding affinity for GAG and simultaneously results in rapid virus clearance from circulation, attenuating virulence in vivo (23, 24, 33–35).
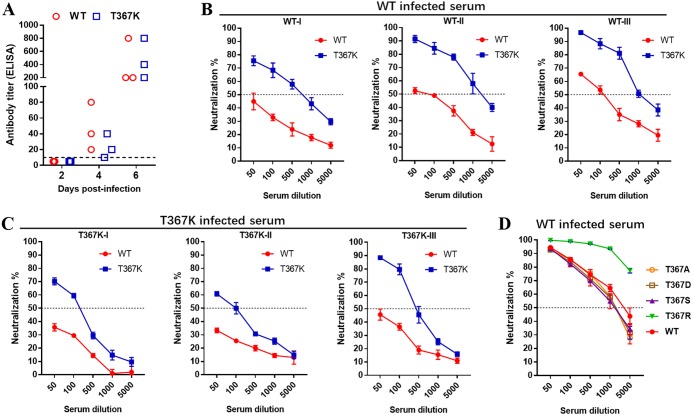
T367K mutation increases TMUV sensitivity to neutralizing antibodies.
EDIII of flavivirus is a major target of neutralizing antibodies, which play an important role in controlling virus infection (42). As the E367 residue is predicted to be exposed on the surface of E protein (Fig. 6E), we hypothesized that the T367K mutation may affect the interaction between viral EDIII and neutralizing antibodies. To verify this, we compared the susceptibility of the WT and T367K mutant viruses to duck sera collected at days 2 and 4 after infection with WT or T367K virus by a plaque reduction neutralization test (PRNT). TMUV-specific antibody titer was detected by blocking enzyme-linked immunosorbent assay (ELISA), which was developed in our previous study (43). The result indicated that TMUV-specific antibody was not detected in serum of ducklings at 2 dpi but was detected starting from 4 days after infection with WT or T367K, and antibody titers significantly increased at 6 dpi (Fig. 7A). Consistent with the ELISA antibody titer result, the in vitro infectivity of the WT or T367K mutant virus was not affected by treatment with the sera collected at 2 dpi (data not shown). However, sera collected at 4 dpi from either WT- or T367K-infected ducks displayed potent neutralizing activity against the WT and T367K viruses (Fig. 7B and C). It was notable that the same neutralizing serum displayed greater potency against the T367K mutant virus than the WT virus, with around 10- to 20-fold differences in the PRNT50. We further tested the sensitivity of the mutant viruses T367A, T367D, T367S, and T367R to the neutralizing sera collected from WT-infected duck at 6 dpi, as described above (Fig. 7D). Minimal PRNT50 differences (<2-fold) were observed between the T367A, T367D, and T367S mutants and the WT virus. Interestingly, the T367R mutant virus also displayed increased sensitivity to these neutralizing sera in a similar manner to T367K, suggesting that a positively charged amino acid substitution at E367 can affect the antibody accessibility of epitopes on the E protein of TMUV. Taken together, these results indicate that the Thr-to-Lys change at residue 367 of E protein increases neutralizing antibody accessibility to TMUV without substantially reducing viral immunogenicity.
FIG 7.
The T367K mutation increases TMUV sensitivity to neutralization serum. (A) TMUV-specific antibody titers in the sera of infected ducklings. Ducks were infected individually with WT or T367K (105 PFU) by s.c. inoculation. Sera were collected at indicated time points, and TMUV-specific antibody titers were determined by a blocking ELISA. Dotted lines indicated the limits of detection. (B and C) Neutralization activity of duck sera. Duck sera collected at day 4 after infecting with WT virus and T367K mutant. The neutralization activity of the sera to WT and T367K was tested individually by PRNT. (D) Neutralization sensitivity of WT and four E367 site-directed mutant viruses to duck serum. The test serum was collected 6 days after WT infection. Data are presented as the means and SD (n = 3).
DISCUSSION
With the emergence of TMUV causing severe disease in domestic ducks and geese (4–8, 12, 13), attention has focused not only on developing a vaccine to prevent infection (44–47), but also on understanding the genetic determinants responsible for virus transmission and enhanced pathogenicity (20, 48, 49). In our previous study, a highly attenuated and immunogenic TMUV was developed after sequential passaging on BHK-21 cells, and 23 amino acid substitutions were identified in the viral genome compared with the parental TMUV (36). Animal infection studies with chimeric viruses provided preliminary evidence that multiple gene mutations could potentially lead to decreased pathogenicity in ducklings. Here, the ChimE virus, harboring the mutated E gene from an attenuated virus, displayed increased adaptation to cell culture with larger plaque sizes and higher virus titers but lower viremia and less efficient invasion of brain and heart tissue in s.c. infected ducklings. For the first time, our data provide evidence that the mutations in E protein, which accumulated during in vitro passage, not only conferred TMUV adaptations in cell culture but also reduced viral virulence in ducks.
The E protein of flavivirus plays an important role in cell culture adaptation and virus virulence (23–25). A single amino acid mutation in the E protein of other flaviviruses has been reported to affect viral pathogenesis in animal models (27–30). Recently, a study revealed that an S156P mutation in the E protein of TMUV resulted in the loss of N-linked glycosylation and abrogated vector-free transmission in ducks (49). There are five amino acid changes in the E protein of attenuated TMUV compared with the WT parental virus (36). With the availability of TMUV infectious clones, it is possible to examine accurately the specific mutations in the viral genome that are responsible for viral virulence. In fact, the generation and characterization of E protein site-directed mutant viruses demonstrated that the T367K mutation significantly reduced TMUV virulence in ducks, exhibiting low-level viremia and impaired virus replication in the brain, heart (ducklings), and ovaries, similar to the ChimE virus. Furthermore, the positively charged basic amino acid substitution at E367 is sufficient to cause these phenotype changes. This was confirmed by replacing the E367-Thr residue with different amino acids. The Arg substitution, but not the Ala, Asp, or Ser residues, resulted in phenotype changes comparable to those of the T367K mutant virus. We noted that mutant viruses T70A and Y176H exhibited partial attenuation with decreased mortality rates in s.c. infected ducklings (Fig. 2E), but no significant difference in viral load was detected between these two mutants and the parental virus in the tested tissues (data not shown), suggesting that the T367K mutation should be the predominant determinant in E protein for TMUV attenuation.
GAGs, negatively charged polysaccharides expressed ubiquitously on the surfaces and extracellular matrices of multicellular organisms, are attachment factors for flaviviruses, alphaviruses, and picornaviruses that enhance virion attachment at the target cell surface before their interaction with primary receptors (37, 50–52). The binding of viruses to GAG involves primarily the electrostatic interaction of clusters of basic amino acids on the virus with negatively charged sulfate groups on the polysaccharide (24). The acquisition of a net positive charge in E protein could enhance viral binding affinity for GAGs, which is frequently accompanied by reduced efficiency of virus spread through circulation, as well as advantage virus growth in cell culture. It has been reported that the rapid clearance of virus from the bloodstream most likely is the result of nonproductive binding of virus by extracellular matrices, which are rich in GAG, or readsorption of progeny virus by infected cells (24). The lateral edge of E protein domain III of flaviviruses was identified as a GAG-binding region (34, 37–39). Consistent with other cell-adapted flaviviruses, the predicted crystal structure of TMUV E protein showed that the E367 residue exposed on the outer surface of EDIII (Fig. 6E) and the T367K substitution increased the net positive charge of E protein (Fig. 6F). Actually, the positively charged amino acid substitution (Lys or Arg) at E367 increased viral binding affinity for heparin (a highly sulfated GAG) and enhanced TMUV growth in cell culture, resulting in rapid viral clearance from the bloodstream and impaired viscerotropism, which is consistent with the common mechanism of virulence attenuation for cell-adapted flaviviruses (23, 24, 33–35).
The severe damage to ovaries and ovarian follicles induced by the high titer of viral replication results in a considerable drop in egg production, the most significant clinical manifestation observed in TMUV-infected laying ducks (4–6, 17). The T367K mutation significantly reduced the viral load in the ovaries, avoiding evident damage to the ovaries or ovarian follicles, and thereby indicated that the E367 residue could be an important determinant for TMUV ovary tropism. It has been reported that in WNV-infected birds, the most severely affected tissues were the brain and heart (53–55). High virus titers have been detected in the spleen, brain, and heart of ducklings infected with virulent TMUV, with evident microscopic lesions (18, 19, 56, 57). However, the relationship between heart damage and the pathogenesis of TMUV infection remained unclear. In this study, virus titers in the heart tissue were significantly higher than in the tissue of the spleen of ducklings that succumbed to TMUV infection, supporting the proposal that the heart is one of the main target organs in TMUV-infected ducklings. Interestingly, the replication of the T367K mutant or the ChimE virus in the heart of s.c. inoculated ducklings was remarkably reduced compared with the WT virus, with fewer lesions evident and reduced expression of proinflammatory genes in the heart. These data suggest that a decreased ability to replicate in the duckling heart could contribute to the virulence attenuation of TMUV.
Antibodies are critical for the control of flavivirus infection, and this protection has been correlated with neutralizing activity in vitro (26, 58, 59). The flavivirus E protein is the dominant antigen inducing immunologic responses in infected hosts and eliciting virus-neutralizing antibodies (42, 60, 61). Recent studies reported that the recombinant E protein of TMUV could induce high-level neutralizing antibodies and provide protection against TMUV infection (62, 63). In this study, the T367K mutant was neutralized more potently than its parental virus by infected duck serum collected at an earlier time point (4 dpi), suggesting that the T367K mutation may affect the exposure of epitopes, thus modulating antibody recognition and neutralization of the virus. However, viremia was reduced as early as 1 to 2 days after T367K infection, before TMUV-specific antibodies became detectable, indicating that early viral suppression is not likely due to the humoral immune responses. The increased susceptibility of the T367K mutant virus to neutralizing serum is not directly related to virulence attenuation, but this amino acid substitution may redirect antibody responses toward the more protective neutralizing epitopes in EDIII of TMUV.
In conclusion, we demonstrated that a single amino acid substitution (Thr to Lys) at position E367 is the predominant determinant of TMUV cell adaptation as well as virulence attenuation in ducks. Our data highlight that a gain in net positive charge at E367 enhances viral binding affinity for GAG, concomitantly resulting in rapid viral clearance from the bloodstream and impaired viscerotropism in ducks. Additionally, the T367K mutation increased viral sensitivity to neutralizing antibodies, which may redirect antibody responses toward the more protective neutralizing epitopes.
MATERIALS AND METHODS
Ethics statement.
The animal experiments were carried out according to the Chinese Regulations of Laboratory Animals, the Guidelines for the Care of Laboratory Animals (Ministry of Science and Technology of the People’s Republic of China), and Laboratory Animal Requirements of Environment and Housing Facilities (GB 14925-2010, National Laboratory Animal Standardization Technical Committee). The animal trials in this study were approved by the Laboratory Animal Ethical Committee of China Agricultural University.
Cells and viruses.
BHK-21 (baby hamster kidney) cells were obtained from the American Type Culture Collection (ATCC), and primary DEF cells were prepared from 9-day-old duck embryos. Both BHK-21 and DEF cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin and were incubated at 37°C with 5% CO2. Duck TMUV strain JXSP (GenBank accession no. JQ920423.1) was isolated from an infected duck flock as described previously (1). The attenuated virus P310 was obtained by serial passage of JXSP strain in BHK-21 cells (36).
Construction of the mutant TMUV cDNA clone.
The general procedure for the construction of TMUV infectious cDNA clones has been described elsewhere (36). Briefly, the first-strand cDNA of JXSP virus was used to amplify five overlapping fragments covering the whole genome. Each fragment was cloned into respective plasmid vectors using pEASY-Blunt simple cloning vectors (TransGen, Beijing, China). To introduce mutations into the E protein gene, PCR-based site-directed mutagenesis was used along with plasmid p-1 (carrying T7 sequences followed by 1 to 2,479 nucleotides [nt] of the JXSP genome) and the indicated primers (Table 1). Each fragment containing a single-site mutation was cloned into a plasmid vector. All plasmids were sequenced to confirm the correct sequence. Five overlapping fragments were amplified from the respective plasmids (p-1, p-2, p-3, p-4, and p-5). The full-length cDNA was produced by fusion PCR using PrimeSTAR Max DNA polymerase (TaKaRa, Dalian, China).
TABLE 1.
Primers used for construction of TMUV cDNA clones
| Primer name | Sequence (5′-3′)a | Purpose |
|---|---|---|
| T7 + 1F | CCCGGGTAATACGACTCACTATAGGGAGAAGTTCATCTGTGTGAACTTATTCC | T7 promoter introduction |
| 2459R | GTCGATTGAGCACCCCGTGTC | PCR amplification fragment-1 |
| 2459F | GACACGGGGTGCTCAATCGAC | PCR amplification fragment-2 |
| 3514R | TTCCATGCCACCCCCTTGAAA | |
| 3514F | TTTCAAGGGGGTGGCATGGAA | PCR amplification fragment-3 |
| 5851R | CGACTATCTATGACCCGTTGC | |
| 5285F | ATAGCGGAAGCACTGAAAGGA | PCR amplification fragment-4 |
| 8164R | CAACGCCCCTAGCTAACCATT | |
| 8164F | AATGGTTAGCTAGGGGCGTTG | PCR amplification fragment-5 |
| 10990R | AGACTCTGTGTTCTACCACCACCAGCCACACTTTCGGCGATCTGTGCCAA | |
| 955F | TGTTAATTGCCCCAGCGTACAGC | E protein gene substitution |
| 955R | GCTGTACGCTGGGGCAATTAACA | |
| E2-F | GTACAGCTTCAaCTGTCTGG | Mutation PCR of E2(S→N) |
| E2-R | CCAGACAGtTGAAGCTGTAC | |
| E70-F | TCGGACGTGACGgCAGAATCC | Mutation PCR of E70(T→A) |
| E70-R | GGATTCTGCcGTCACGTCCGA | |
| E176-F | AGTCCCGTCcACACCGCTGAG | Mutation PCR of E176(Y→H) |
| E176-R | CTCAGCGGTGTgGACGGGACT | |
| E314-F | AGTGAAGAgTCCTACCGACAC | Mutation PCR of E314(N→S) |
| E314-R | GTGTCGGTAGGAcTCTTCACT | |
| E376-F | ACCTCCTCCAaGGGTGCCAAG | Mutation PCR of E367(T→K) |
| E376-R | CTTGGCACCCtTGGAGGAGGT | |
| 367A-F | ACCTCCTCCgCGGGTGCCAAG | Mutation PCR of E367(T→A) |
| 367A-R | CTTGGCACCCGcGGAGGAGGT | |
| 367D-F | ACCTCCTCCgacGGTGCCAAG | Mutation PCR of E367(T→D) |
| 367D-R | CTTGGCACCgtcGGAGGAGGT | |
| 367S-F | ACCTCCTCCtCGGGTGCCAAG | Mutation PCR of E367(T→S) |
| 367S-R | CTTGGCACCCGaGGAGGAGGT | |
| 367R-F | ACCTCCTCCAgGGGTGCCAAG | Mutation PCR of E367(T→R) |
| 367R-R | CTTGGCACCCcTGGAGGAGGT |
Lowercase letters indicate mutated nucleotides.
In vitro transcription and RNA transfection.
The assay protocols used were the same as those described previously (36). Briefly, in vitro transcription was performed using the T7 RiboMAX Express large-scale RNA production system (Promega, WI, USA) according to the manufacturer’s instructions. RNA was transfected into BHK-21 cells with Lipofectamine 3000 transfection reagent (Invitrogen, CA, USA) according to the manufacturer’s instructions. The cell culture medium was changed to fresh medium (DMEM with 2% FBS) at 4 h posttransfection. The rescued viruses were harvested 3 to 4 days posttransfection when typical cytopathic effects were observed. All rescued viruses were confirmed by full-genome sequencing. Working virus stocks were amplified from the harvested transfection of one passage in BHK-21 cells. Virus titers were determined by standard plaque assays on BHK-21 cells. The amplified virus stocks were stored in aliquots at −80°C until further use.
Virus growth kinetics and plaque assays.
Virus replication kinetics were measured by infecting confluent BHK-21 and DEF cells in six-well plates at a multiplicity of infection (MOI) of 0.01. After infection at 37°C for 1 h, the virus inoculum was removed and the cells were washed with phosphate-buffered saline (PBS) to eliminate unbound virus. Then, 3 ml of fresh medium (DMEM plus 2% FBS) was added to each well. Cell culture supernatants were collected at the indicated times postinfection. The yields of progeny virus were quantitated by a plaque assay on BHK-21 cells.
Serial 10-fold dilutions of virus samples were prepared in DMEM (plus 2% FBS) and inoculated onto BHK-21 monolayers in 12-well plates. The plates were incubated at 37°C for 1 h with gentle rocking every 15 min. After removal of the inoculum, the cells were washed twice with PBS and overlaid with DMEM containing 1% (wt/vol) low-melting-point agarose (Amresco, WA, USA) and 2% FBS. After further incubation at 37°C for 3 days, the cells were stained with 0.02% neutral red to visualize the plaques.
Duck experiments.
Pekin ducklings and laying ducks, purchased from Beijing Golden Star Duck Co., Ltd., were used to examine the virulence of TMUV and mutant viruses. Groups of 4-day-old healthy Pekin ducklings were s.c. inoculated with 105 PFU of WT or mutant viruses. Duck survival was monitored for 10 days. Three or five ducks from each group were euthanized at the indicated time points, and blood and tissue samples from the heart, spleen, lungs, kidney, and brain were collected for viral titer determination. Tissue samples were homogenized and diluted in DMEM to a final concentration of 20% (wt/vol). After three cycles of freeze-thawing, the tissue suspensions were centrifuged at 5,000 × g at 4°C for 15 min. The virus titers in the supernatant were determined using a plaque assay on BHK-21 cells.
Groups of 25-week-old laying ducks were s.c. inoculated with 105 PFU of TMUV. At 2, 4, and 6 dpi, 3 or 4 ducks from each group were euthanized, and blood and tissue samples were collected. The virus titers in different tissues were determined as described above.
Viral RNA quantification by qRT-PCR.
For viral RNA quantification, viral RNAs in culture fluids, serum, and tissue samples were extracted using a viral RNA kit (Omega, GA, USA) according to the manufacturer’s instructions. The cDNA was synthesized using a reverse transcription system (Promega, WI, USA) with random primers following the user guide. Real-time PCR was carried out in a Bio-Rad CFX Connect real-time system using SYBR green SuperReal PreMix Plus (Tiangen, Beijing, China) with primers (forward, 5′-TTGGGACACCTTGCAAAACG-3′; reverse, 5′-TTGCCACGATGTTCATGACC-3′) targeting for the TMUV NS5 region. The 20-μl reaction mixtures were set up with 10 μl SuperReal PreMix Plus, 0.3 μM specific primer, and 2 μl cDNA template. Cycling conditions were as follows: 95°C for 15 min; 40 cycles of 95°C for 10 s and 60°C for 30 s. A plasmid fragment containing genome sequence of the NS5 region was used to establish a standard curve for quantification of viral genome copy numbers.
Virus clearance assays.
With reference to a previously described method (23), virus clearance from the duck bloodstream was assayed by monitoring the intravenous (i.v.) inoculation of 107 PFU of virus into 7-day-old ducklings. Blood was taken from the jugular vein at 10 and 30 min postinoculation, and the infectious virus content in blood samples was determined using a plaque assay. To verify that the virus particles were actually removed from the blood, not only inactivated or neutralized, we performed an additional experiment by infecting ducklings with 109 RNA copies of the virus as above. The blood samples were collected at 10 and 30 min postinoculation, and viral genome copy numbers were determined using qRT-PCR. The virus content at 0 min was calculated as the input PFU or genome copies of virus/blood volume and was assumed to be 10% of the body weight (64).
Virus thermal stability assays.
Equal amounts (about 500 PFU/ml) of WT virus, chimeric virus, and mutant viruses were preincubated for 60 min at 4°C and 42°C in DMEM containing 2% FBS. Subsequently, each virus at different temperatures was used to infect BHK-21 cells seeded in 12-well plates for 1 h at 37°C before being washed with PBS. A plaque assay was then performed as described above. PFU were then tabulated as percentages of the control (4°C). Experiments were performed three times in triplicate.
Heparin inhibition assays.
The inhibitory effect of heparin on TMUV binding in BHK-21 cells was examined using a previously described method (31). Ten-fold serial dilutions of heparin (MCE, USA) plus 100 PFU of TMUV mutant virus were preincubated at 37°C for 1 h. The virus-heparin mixture was then incubated on confluent BHK-21 cells in 12-well plates at 4°C for 1 h. The inoculums were removed and the cells were washed three times with precooled PBS to remove any unbound virus. Cells were overlaid with 1% agarose-containing overlay medium, and the plates were incubated in a 5% CO2 incubator at 37°C for 3 days to allow for plaque formation. Cells inoculated without heparin were used as controls. The inhibition rate was calculated as (no. of plaques [controls] – no. of plaques [heparin treatment]/no. of plaques [controls]). Experiments were performed three times in triplicate.
Serum neutralization tests.
Virus sensitivity to neutralizing antibody was determined using a plaque reduction neutralization test (PRNT). Briefly, duck TMUV antisera were heat-inactivated for 30 min at 56°C and then serially diluted in DMEM supplemented with 2% FBS. The serum dilution was mixed with an equal volume of virus in DMEM with 2% FBS to yield a mixture containing approximately 500 PFU of virus/ml. After incubation at 37°C for 1 h, 200 μl of the mixtures were added to 12-well plates containing confluent monolayers of BHK-21 cells. Then a plaque assay was performed as described above. The 50% endpoint neutralization titer was calculated with the method of Reed and Muench.
Statistical analysis.
All statistical analyses were performed with GraphPad Prism version 7.0 (GraphPad Software, Inc., CA, USA).
ACKNOWLEDGMENTS
This study was supported by the National Key Research and Development Program of China (2016YFD0500106) and the National Natural Science Foundation of China (NSFC; grants 31672567 and 31802200). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
J.S. designed the study. M.S., L.Z., Y.C., J.W., Z.Y., X.S., F.L., and Z.L. performed the experiments. M.S., P.L., and J.S. analyzed the data and wrote the paper.
We thank Kate Fox from Liwen Bianji, Edanz Group, China, for editing the English text of a draft of the manuscript.
REFERENCES
- 1.Liu P, Lu H, Li S, Moureau G, Deng YQ, Wang Y, Zhang L, Jiang T, de Lamballerie X, Qin CF, Gould EA, Su J, Gao GF. 2012. Genomic and antigenic characterization of the newly emerging Chinese duck egg-drop syndrome flavivirus: genomic comparison with Tembusu and Sitiawan viruses. J Gen Virol 93:2158–2170. doi: 10.1099/vir.0.043554-0. [DOI] [PubMed] [Google Scholar]
- 2.Mackenzie JS, Williams DT. 2009. The zoonotic flaviviruses of southern, south-eastern and eastern Asia, and Australasia: the potential for emergent viruses. Zoonoses Public Health 56:338–356. doi: 10.1111/j.1863-2378.2008.01208.x. [DOI] [PubMed] [Google Scholar]
- 3.Kono Y, Tsukamoto K, Abd HM, Darus A, Lian TC, Sam LS, Yok CN, Di KB, Lim KT, Yamaguchi S. 2000. Encephalitis and retarded growth of chicks caused by Sitiawan virus, a new isolate belonging to the genus Flavivirus. Am J Trop Med Hyg 63:94. doi: 10.4269/ajtmh.2000.63.94. [DOI] [PubMed] [Google Scholar]
- 4.Yan P, Zhao Y, Zhang X, Xu D, Dai X, Teng Q, Yan L, Zhou J, Ji X, Zhang S, Liu G, Zhou Y, Kawaoka Y, Tong G, Li Z. 2011. An infectious disease of ducks caused by a newly emerged Tembusu virus strain in mainland China. Virology 417:1–8. doi: 10.1016/j.virol.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Su J, Li S, Hu X, Yu X, Wang Y, Liu P, Lu X, Zhang G, Hu X, Liu D, Li X, Su W, Lu H, Mok NS, Wang P, Wang M, Tian K, Gao GF. 2011. Duck egg-drop syndrome caused by BYD virus, a new Tembusu-related flavivirus. PLoS One 6:e18106. doi: 10.1371/journal.pone.0018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Z, Zhang C, Liu Y, Liu Y, Ye W, Han J, Ma G, Zhang D, Xu F, Gao X, Tang Y, Shi S, Wan C, Zhang C, He B, Yang M, Lu X, Huang Y, Diao Y, Ma X, Zhang D. 2011. Tembusu virus in ducks, china. Emerg Infect Dis 17:1873–1875. doi: 10.3201/eid1710.101890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Homonnay ZG, Kovacs EW, Banyai K, Albert M, Feher E, Mato T, Tatar-Kis T, Palya V. 2014. Tembusu-like flavivirus (Perak virus) as the cause of neurological disease outbreaks in young Pekin ducks. Avian Pathol 43:552–560. doi: 10.1080/03079457.2014.973832. [DOI] [PubMed] [Google Scholar]
- 8.Thontiravong A, Ninvilai P, Tunterak W, Nonthabenjawan N, Chaiyavong S, Angkabkingkaew K, Mungkundar C, Phuengpho W, Oraveerakul K, Amonsin A. 2015. Tembusu-related flavivirus in ducks, Thailand. Emerg Infect Dis 21:2164–2167. doi: 10.3201/eid2112.150600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang Y, Diao Y, Chen H, Ou Q, Liu X, Gao X, Yu C, Wang L. 2015. Isolation and genetic characterization of a Tembusu virus strain isolated from mosquitoes in Shandong, China. Transbound Emerg Dis 62:209–216. doi: 10.1111/tbed.12111. [DOI] [PubMed] [Google Scholar]
- 10.Tang Y, Diao Y, Yu C, Gao X, Ju X, Xue C, Liu X, Ge P, Qu J, Zhang D. 2013. Characterization of a Tembusu virus isolated from naturally infected house sparrows (Passer domesticus) in Northern China. Transbound Emerg Dis 60:152–158. doi: 10.1111/j.1865-1682.2012.01328.x. [DOI] [PubMed] [Google Scholar]
- 11.Liu M, Chen S, Chen Y, Liu C, Chen S, Yin X, Li G, Zhang Y. 2012. Adapted Tembusu-like virus in chickens and geese in China. J Clin Microbiol 50:2807–2809. doi: 10.1128/JCM.00655-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yun T, Zhang D, Ma X, Cao Z, Chen L, Ni Z, Ye W, Yu B, Hua J, Zhang Y, Zhang C. 2012. Complete genome sequence of a novel flavivirus, duck Tembusu virus, isolated from ducks and geese in china. J Virol 86:3406–3407. doi: 10.1128/JVI.07132-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han K, Huang X, Li Y, Zhao D, Liu Y, Zhou X, You Y, Xie X. 2013. Complete genome sequence of goose Tembusu virus, isolated from Jiangnan white geese in Jiangsu, China. Genome Announc 1:e0023612. doi: 10.1128/genomeA.00236-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang X, Han K, Zhao D, Liu Y, Zhang J, Niu H, Zhang K, Zhu J, Wu D, Gao L, Li Y. 2013. Identification and molecular characterization of a novel flavivirus isolated from geese in China. Res Vet Sci 94:774–780. doi: 10.1016/j.rvsc.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Wang S, Li Z, Lin F, Cheng X, Zhu X, Wang J, Chen S, Huang M, Zheng M. 2014. Isolation and characterization of a Chinese strain of Tembusu virus from Hy-Line Brown layers with acute egg-drop syndrome in Fujian, China. Arch Virol 159:1099–1107. doi: 10.1007/s00705-013-1931-0. [DOI] [PubMed] [Google Scholar]
- 16.Yu G, Lin Y, Tang Y, Diao Y. 2018. Evolution of Tembusu virus in ducks, chickens, geese, sparrows, and mosquitoes in northern China. Viruses 10:485. doi: 10.3390/v10090485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L, Liu J, Chen P, Jiang Y, Ding L, Lin Y, Li Q, He X, Chen Q, Chen H. 2014. The sequential tissue distribution of duck Tembusu virus in adult ducks. Biomed Res Int 2014:703930. doi: 10.1155/2014/703930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun XY, Diao YX, Wang J, Liu X, Lu AL, Zhang L, Ge PP, Hao DM. 2014. Tembusu virus infection in Cherry Valley ducks: the effect of age at infection. Vet Microbiol 168:16–24. doi: 10.1016/j.vetmic.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Li N, Lv C, Yue R, Shi Y, Wei L, Chai T, Liu S. 2015. Effect of age on the pathogenesis of duck Tembusu virus in Cherry Valley ducks. Front Microbiol 6:581. doi: 10.3389/fmicb.2015.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Shi Y, Liu Q, Wang Y, Li G, Teng Q, Zhang Y, Liu S, Li Z. 2015. Airborne transmission of a novel Tembusu virus in ducks. J Clin Microbiol 53:2734–2736. doi: 10.1128/JCM.00770-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slon Campos JL, Mongkolsapaya J, Screaton GR. 2018. The immune response against flaviviruses. Nat Immunol 19:1189–1198. doi: 10.1038/s41590-018-0210-3. [DOI] [PubMed] [Google Scholar]
- 22.Mukhopadhyay S, Kuhn RJ, Rossmann MG. 2005. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol 3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 23.Lee E, Hall RA, Lobigs M. 2004. Common E protein determinants for attenuation of glycosaminoglycan-binding variants of Japanese encephalitis and West Nile viruses. J Virol 78:8271–8280. doi: 10.1128/JVI.78.15.8271-8280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee E, Lobigs M. 2008. E protein domain III determinants of yellow fever virus 17D vaccine strain enhance binding to glycosaminoglycans, impede virus spread, and attenuate virulence. J Virol 82:6024–6033. doi: 10.1128/JVI.02509-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Li L, Woodson SE, Huang YH, Kinney RM, Barrett ADT, Beasley D. 2006. A mutation in the envelope protein fusion loop attenuates mouse neuroinvasiveness of the NY99 strain of West Nile virus. Virology 353:35–40. doi: 10.1016/j.virol.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 26.Dai L, Wang Q, Qi J, Shi Y, Yan J, Gao GF. 2016. Molecular basis of antibody-mediated neutralization and protection against flavivirus. IUBMB Life 68:783–791. doi: 10.1002/iub.1556. [DOI] [PubMed] [Google Scholar]
- 27.Duggal NK, McDonald EM, Weger-Lucarelli J, Hawks SA, Ritter JM, Romo H, Ebel GD, Brault AC. 2019. Mutations present in a low-passage Zika virus isolate result in attenuated pathogenesis in mice. Virology 530:19–26. doi: 10.1016/j.virol.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng X, Zheng H, Tong W, Li G, Wang T, Li L, Gao F, Shan T, Yu H, Zhou Y, Qiu Y, Ma Z, Tong G. 2018. Acidity/alkalinity of Japanese encephalitis virus E protein residue 138 alters neurovirulence in mice. J Virol 92. doi: 10.1128/JVI.00108-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goo L, VanBlargan LA, Dowd KA, Diamond MS, Pierson TC. 2017. A single mutation in the envelope protein modulates flavivirus antigenicity, stability, and pathogenesis. PLoS Pathog 13:e1006178. doi: 10.1371/journal.ppat.1006178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guirakhoo F, Zhang Z, Myers G, Johnson BW, Pugachev K, Nichols R, Brown N, Levenbook I, Draper K, Cyrek S, Lang J, Fournier C, Barrere B, Delagrave S, Monath TP. 2004. A single amino acid substitution in the envelope protein of chimeric yellow fever-dengue 1 vaccine virus reduces neurovirulence for suckling mice and viremia/viscerotropism for monkeys. J Virol 78:9998–10008. doi: 10.1128/JVI.78.18.9998-10008.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Li SH, Zhu L, Nian QG, Yuan S, Gao Q, Hu Z, Ye Q, Li XF, Xie DY, Shaw N, Wang J, Walter TS, Huiskonen JT, Fry EE, Qin CF, Stuart DI, Rao Z. 2017. Near-atomic structure of Japanese encephalitis virus reveals critical determinants of virulence and stability. Nat Commun 8:14. doi: 10.1038/s41467-017-00024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Z, Date T, Li Y, Kato T, Miyamoto M, Yasui K, Wakita T. 2005. Characterization of the E-138 (Glu/Lys) mutation in Japanese encephalitis virus by using a stable, full-length, infectious cDNA clone. J Gen Virol 86:2209–2220. doi: 10.1099/vir.0.80638-0. [DOI] [PubMed] [Google Scholar]
- 33.Janice N, Maria C, Droll DA, Yan L, Wold WSM, Chambers TJ. 2008. Neuroadapted yellow fever virus strain 17D: a charged locus in domain III of the E protein governs heparin binding activity and neuroinvasiveness in the SCID mouse model. J Virol 82:12510–12519. doi: 10.1128/JVI.00458-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee E, Lobigs M. 2002. Mechanism of virulence attenuation of glycosaminoglycan-binding variants of Japanese encephalitis virus and Murray Valley encephalitis virus. J Virol 76:4901–4911. doi: 10.1128/jvi.76.10.4901-4911.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozlovskaya LI, Osolodkin DI, Shevtsova AS, Romanova L, Rogova YV, Dzhivanian TI, Lyapustin VN, Pivanova GP, Gmyl AP, Palyulin VA, Karganova GG. 2010. GAG-binding variants of tick-borne encephalitis virus. Virology 398:262–272. doi: 10.1016/j.virol.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Sun M, Zhang Q, Wang J, Cao Y, Cui S, Su J. 2019. Long-term passage of duck Tembusu virus in BHK-21 cells generates a completely attenuated and immunogenic population with increased genetic diversity. Vaccine 38:933–941. doi: 10.1016/j.vaccine.2019.10.080. [DOI] [PubMed] [Google Scholar]
- 37.Goto A, Hayasaka D, Yoshii K, Mizutani T, Kariwa H, Takashima I. 2003. A BHK-21 cell culture-adapted tick-borne encephalitis virus mutant is attenuated for neuroinvasiveness. Vaccine 21:4043–4051. doi: 10.1016/s0264-410x(03)00269-x. [DOI] [PubMed] [Google Scholar]
- 38.Mandl CW, Kroschewski H, Allison SL, Kofler R, Holzmann H, Meixner T, Heinz FX. 2001. Adaptation of tick-borne encephalitis virus to BHK-21 cells results in the formation of multiple heparan sulfate binding sites in the envelope protein and attenuation in vivo. J Virol 75:5627–5637. doi: 10.1128/JVI.75.12.5627-5637.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klimstra WB, Ryman KD, Johnston RE. 1998. Adaptation of Sindbis Virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J Virol 72:7357–7366. doi: 10.1128/JVI.72.9.7357-7366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee E, Wright PJ, Davidson A, Lobigs M. 2006. Virulence attenuation of Dengue virus due to augmented glycosaminoglycan-binding affinity and restriction in extraneural dissemination. J Gen Virol 87:2791–2801. doi: 10.1099/vir.0.82164-0. [DOI] [PubMed] [Google Scholar]
- 41.Smith EN, Peterson C, Thigpen K. 1976. Body temperature, heart rate and respiration rate of an unrestrained domestic mallard duck, Anas platyrhynchos domesticus. Comp Biochem Physiol A Comp Physiol 54:19–20. doi: 10.1016/S0300-9629(76)80064-3. [DOI] [PubMed] [Google Scholar]
- 42.Chávez JH, Silva JR, Amarilla AA, Figueiredo L. 2010. Domain III peptides from flavivirus envelope protein are useful antigens for serologic diagnosis and targets for immunization. Biologicals 38:613–618. doi: 10.1016/j.biologicals.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Zhanhong L, Huan J, Xueying H, Jingliang S. 2018. Development and application of a monoclonal antibody-based blocking ELISA for detection of antibodies to Tembusu virus in multiple poultry species. BMC Vet Res 14:201. doi: 10.1186/s12917-018-1537-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, Su J. 2017. Duck Tembusu virus infection, p 237–249. In Bayry J. (ed), Emerging and re-emerging infectious diseases of livestock. Springer International Publishing, Cham, Switzerland. [Google Scholar]
- 45.Zhang L, Li Z, Zhang Q, Sun M, Li S, Su W, Hu X, He W, Su J. 2017. Efficacy assessment of an inactivated Tembusu virus vaccine candidate in ducks. Res Vet Sci 110:72–78. doi: 10.1016/j.rvsc.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 46.He D, Zhang X, Chen L, Tang Y, Diao Y. 2019. Development of an attenuated live vaccine candidate of duck Tembusu virus strain. Vet Microbiol 231:218–225. doi: 10.1016/j.vetmic.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 47.Li G, Gao X, Xiao Y, Liu S, Peng S, Li X, Shi Y, Zhang Y, Yu L, Wu X, Yan P, Yan L, Teng Q, Tong G, Li Z. 2014. Development of a live attenuated vaccine candidate against duck Tembusu viral disease. Virology 450–451:233–242. doi: 10.1016/j.virol.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 48.Lei W, Guo X, Fu S, Feng Y, Tao X, Gao X, Song J, Yang Z, Zhou H, Liang G. 2017. The genetic characteristics and evolution of Tembusu virus. Vet Microbiol 201:32–41. doi: 10.1016/j.vetmic.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Yan D, Shi Y, Wang H, Li G, Li X, Wang B, Su X, Wang J, Teng Q, Yang J, Chen H, Liu Q, Ma W, Li Z. 2018. A single mutation at position 156 in the envelope protein of Tembusu virus is responsible for virus tissue tropism and transmissibility in ducks. J Virol 92. doi: 10.1128/JVI.00427-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kroschewski H, Allison SL, Heinz FX, Mandl CW. 2003. Role of heparan sulfate for attachment and entry of tick-borne encephalitis virus. Virology 308:92–100. doi: 10.1016/s0042-6822(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 51.Lin YL, Lei HY, Lin YS, Yeh TM, Chen SH, Liu HS. 2002. Heparin inhibits Dengue-2 virus infection of five human liver cell lines. Antiviral Res 56:93–96. doi: 10.1016/S0166-3542(02)00095-5. [DOI] [PubMed] [Google Scholar]
- 52.Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem 68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 53.Gamino V, Höfle U. 2013. Pathology and tissue tropism of natural West Nile virus infection in birds: a review. Veterinary Res 44:39–15. doi: 10.1186/1297-9716-44-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Senne DA, Pedersen JC, Hutto DL, Taylor WD, Schmitt BJ, Panigrahy B. 2000. Pathogenicity of West Nile virus in chickens. Avian Dis 44:642–649. doi: 10.2307/1593105. [DOI] [PubMed] [Google Scholar]
- 55.Himsworth CG, Gurney KEB, Neimanis AS, Wobeser GA, Leighton FA. 2009. An outbreak of West Nile virus infection in captive lesser scaup (Aythya affinis) ducklings. Avian Dis 53:129–134. doi: 10.1637/8387-063008-Case.1. [DOI] [PubMed] [Google Scholar]
- 56.Lu Y, Dou Y, Ti J, Wang A, Cheng B, Zhang X, Diao Y. 2016. The effect of Tembusu virus infection in different week-old Cherry Valley breeding ducks. Vet Microbiol 192:167–174. doi: 10.1016/j.vetmic.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 57.Li N, Wang Y, Li R, Liu J, Zhang J, Cai Y, Liu S, Chai T, Wei L. 2015. Immune responses of ducks infected with duck Tembusu virus. Front Microbiol 6:425–425. doi: 10.3389/fmicb.2015.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goo L, Debbink K, Kose N, Sapparapu G, Doyle MP, Wessel AW, Richner JM, Burgomaster KE, Larman BC, Dowd KA, Diamond MS, Crowe JE, Pierson TC. 2019. A protective human monoclonal antibody targeting the West Nile virus E protein preferentially recognizes mature virions. Nat Microbiol 4:71–77. doi: 10.1038/s41564-018-0283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.VanBlargan LA, Goo L, Pierson TC. 2016. Deconstructing the antiviral neutralizing-antibody response: implications for vaccine development and immunity. Microbiol Mol Biol Rev 80:989–1010. doi: 10.1128/MMBR.00024-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang S, Kostyuchenko VA, Ng TS, Lim XN, Ooi JSG, Lambert S, Tan TY, Widman DG, Shi J, Baric RS, Lok SM. 2016. Neutralization mechanism of a highly potent antibody against Zika virus. Nat Commun 7:13679. doi: 10.1038/ncomms13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gallichotte EN, Young EF, Baric TJ, Yount BL, Metz SW, Begley MC, de Silva AM, Baric RS. 2019. Role of Zika virus envelope protein domain III as a target of human neutralizing antibodies. mBio 10:e01485-19. doi: 10.1128/mBio.01485-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li L, Zhang Y, Dong J, Zhang J, Zhang C, Sun M, Cao Y. 2019. The truncated E protein of DTMUV provide protection in young ducks. Vet Microbiol 240:108508. doi: 10.1016/j.vetmic.2019.108508. [DOI] [PubMed] [Google Scholar]
- 63.Ma T, Liu Y, Cheng J, Liu Y, Fan W, Cheng Z, Niu X, Liu J. 2016. Liposomes containing recombinant E protein vaccine against duck Tembusu virus in ducks. Vaccine 34:2157–2163. doi: 10.1016/j.vaccine.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 64.Olayemi D, Olayemi F, Oyewale J, Rahman S, Omolewa O. 2003. Comparative assessment of the white blood cell values, plasma volume and blood volume in the young and adult Nigerian duck (Anas platyrhynchos). Veterinarski Arhiv 73:271–276. [Google Scholar]