Abstract
Chronic graft-versus-host disease (GVHD) is a major complication of allogeneic hematopoietic cell transplantation that resembles autoimmunity, with unclear pathogenesis and few effective therapeutic options. In this issue of the JCI, Dertschnig et al. used mouse models to investigate the basis of T cell autoreactivity following GVHD. Notably, GVHD caused irreversible damage to a population of tolerogenic stromal cells that display peripheral tissue–restricted antigens in lymph nodes, which impaired their capacity to purge and suppress autoreactive T cells. Together with damage to central tolerance mechanisms in the thymus, these findings outline a critical one-two punch injury that profoundly disrupts immune tolerance in this devastating disease.
Burden and pathogenesis of GVHD
Chronic graft-versus-host disease (GVHD) complicates allogeneic hematopoietic cell transplantation (allo-HCT) in a large fraction of transplant recipients (1). Extensive, chronic GVHD can induce life-long morbidity and markedly impair the quality of life of patients, even if they have been cured of their underlying malignancy. Chronic GVHD is typically poorly responsive to treatment, and efforts to identify new therapeutic strategies have been frustratingly unsuccessful so far. Thus, chronic GVHD remains an unmet medical need, and fresh insights into its pathogenesis are critically needed to rationally design effective interventions.
Acute GVHD, which typically occurs during the first weeks and months after transplantation, is characterized by acute inflammation and infiltration of alloreactive donor T cells into host tissues. In contrast, chronic GVHD presents either de novo or following acute GVHD with clinical features reminiscent of autoimmune disorders such as scleroderma and Sjögren’s syndrome. Chronic GVHD often affects the skin (rashes, fibrosis) and the oral cavity (ulcers, lichenoid hyperkeratosis), but can involve multiple other organs such as the eyes, gastrointestinal tract, lungs, joints, and liver. More than 50% of patients with chronic GVHD still require immunosuppression five years after their initial diagnosis, often with inadequate control of disease manifestations in addition to immunodeficiency and an increased risk of opportunistic infections (2).
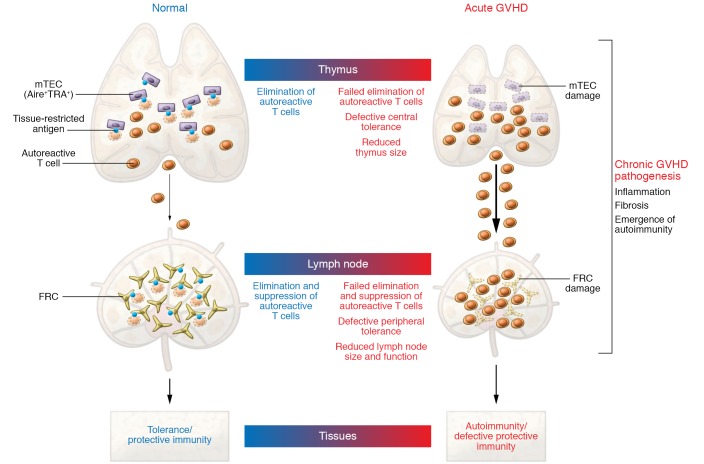
To date, the mechanisms underlying the transition from acute to chronic GVHD remain poorly understood. In mice, multiple research groups reported that acute GVHD damages thymic epithelial cells (TECs), which disrupts central tolerance induced by medullary TECs (mTECs) (3–8). In steady-state conditions, mTECs play a critical role in the negative selection of autoreactive T cells and in the development of thymus-derived regulatory T cells. Under the control of the autoimmune regulator (AIRE), mTECs promiscuously express a diverse set of peripheral tissue–restricted antigens usually confined to other organs, thereby eliminating or tolerizing developing T cells that recognize these antigens (9). In acute GVHD, mTEC damage leads to the emergence of donor-derived autoreactive T cells that fail negative selection in the thymus (3–8). This situation continuously generates a new population of pathogenic T cells in addition to the bolus of alloreactive T cells present in the initial hematopoietic graft, thus propagating immune injury. However, despite experimental data in mice, to what extent mTEC damage might impact chronic GVHD pathogenesis in human patients remains unknown, especially in the large group of older transplant recipients who have age-related thymic involution and yet remain most susceptible to GVHD. In addition, immune tolerance is maintained through multiple mechanisms both centrally (in the thymus) and peripherally (in secondary lymphoid organs), and it has remained unclear why a defect in central tolerance during GVHD could not be compensated by fail-safe mechanisms of peripheral tolerance.
The role of lymph node fibroblastic reticular cells
Fibroblastic reticular cells (FRCs) are nonendothelial stromal cells whose precursors play a critical role during embryogenesis in assembling functional secondary lymphoid organs. In adult tissues, FRCs are strategically dispatched throughout lymphoid tissues to control their architecture, compartmentalization, and function. FRCs create a supportive network regulating immune cell recruitment, trafficking, and activation in lymphoid organs, with specialized regional immunological functions (10–12). For example, T zone FRCs produce the cytokine IL-7 and the chemokine CCL19, which is essential to attract CCR7+ dendritic cells and naive T cells. B zone FRCs and follicular dendritic cells express CXCL13 to attract both CXCR5+ B cells and T follicular helper cells, facilitating T cell–B cell interactions. Additional FRC subsets currently being discovered through heterogeneous expression of surface markers and single-cell transcriptomics will undoubtedly emerge, refining our understanding of their specialized molecular functions (12). Importantly, functions of FRC subsets in enhancing immune responses coexist with their capacity to enforce peripheral tolerance through multiple mechanisms that remain under investigation, but at least in part through a tolerogenic display of selected tissue-restricted antigens (refs. 13–18 and Figure 1).
Figure 1. Model of impaired tolerance in GVHD pathogenesis.
Acute GVHD simultaneously damages nonhematopoietic cells enforcing central and peripheral tolerance in the thymus and lymph nodes. In the thymus, mTECs ectopically express a broad range of tissue-restricted antigens under the control of the autoimmune regulator AIRE. mTECs promote the negative selection of autoreactive T cells and development of central tolerance. In the periphery, lymph node FRCs also show promiscuous expression of tissue-restricted antigens and facilitate the suppression of mature autoreactive T cells, thereby maintaining peripheral tolerance. Acute GVHD targets both mTECs in the thymus and FRCs in lymph nodes, thereby creating a dangerous situation in which large numbers of autoreactive T cells are left to sustain tissue damage during chronic GVHD. TRA, tissue-restricted antigen; GVHD, graft-versus-host disease; mTECs, medullary thymic epithelial cells; AIRE, autoimmune regulator; FRCs, fibroblastic reticular cells.
In this issue of the JCI, Dertschnig et al. provide a critical perspective into the pathogenesis of chronic GVHD by focusing on the role of lymph node FRCs in the maintenance of peripheral immune tolerance after allo-HCT (19). Within weeks after performing allo-HCT in several mouse models, and starting during the acute phase of GVHD, the authors noted selective, profound, and durable loss of FRCs in the recipient lymph nodes. FRC loss resulted from CD4+ T cell–mediated or CD8+ T cell–mediated alloreactivity in polyclonal GVHD models, as well as in models driven by T cell receptor–transgenic T cells specific for a prototypical minor histocompatibility antigen relevant to human transplantation. Although others had previously described T cell–mediated FRC damage in GVHD (20), Dertschnig et al. explored in detail the impact of this injury on the maintenance of peripheral tolerance (19). Even before profound physical loss of FRCs was apparent, they observed decreased abundance of multiple transcripts encoding peripheral tissue–restricted antigens normally displayed by FRCs, as well as decreased expression of Deaf1, encoding a transcription factor previously shown to regulate expression of tissue-restricted antigens in lymph node stromal cells (by analogy with the effects of Aire in the thymus) (15, 21). Moreover, the authors elegantly investigated the functional impact of FRC damage. They studied T cell immune responses using a previously developed model in which a tissue antigen expressed in gut epithelial cells was found to be displayed in lymph node FRCs to maintain peripheral tolerance (13, 14). Indeed, acute GVHD and FRC loss were associated with impaired purging and increased effector functions of T cells autoreactive to this model antigen, leading to gut injury (19). In additional support for this concept, FRCs were characterized by gene expression signatures enriched for transcripts also present in key target organs of chronic GVHD. Thus, donor-derived alloreactive T cells not only damage TECs during acute GVHD, but also lymph node FRCs — a one-two punch to populations of nonhematopoietic cells in central and secondary lymphoid organs that cooperate to maintain immune tolerance (Figure 1).
The important role of lymph node FRCs in immune homeostasis has been an emerging and rapidly evolving field of study. FRC damage as observed in GVHD was previously associated with humoral immunodeficiency and defective responses to T cell–dependent antigens (20), while Dertschnig et al. focused mostly on the loss of peripheral tolerance in similar circumstances (19). These observations are by no means a contradiction, as FRCs exert versatile functions to support and to limit immune responses, and GVHD patients experience both increased immune reactivity with features of autoimmunity, as well as functional immunodeficiency out of proportion to their degree of lymphopenia. In acute GVHD, we previously reported that FRCs expressing a Ccl19-Cre transgene (22) function as a critical cellular source of Delta-like Notch ligands to prime the pathogenic reactivity of alloreactive T cells within days after allo-HCT, with loss of Notch ligands specifically in FRCs leading to profound protection from GVHD (23). It is interesting to speculate whether this initial proinflammatory function of FRCs and their intimate interaction with T cells may also tag them as prime cellular targets of alloreactivity. It also remains to be established precisely which FRC subsets are most susceptible to immune-mediated damage and if these overlap with stromal cells expressing Notch ligands.
The FRC network shows little evidence of repair in GVHD
Strikingly, Dertschnig et al. reported little evidence of repair in the FRC network after initial damage during acute GVHD, even when applying potent immunosuppressive regimens to deplete or inactivate offending T cells (19). Defective repair contrasts with previous observations made in models of viral infection. Initial virus-mediated FRC damage is followed by the engagement of repair mechanisms that borrow from the playbook of lymph node ontogeny, with innate lymphoid cells stimulating FRCs and their precursors through lymphotoxin-β (LT-β) (22, 24). Several mechanisms could account for insufficient FRC regeneration during chronic GVHD. First, low levels of persistent T cell alloreactivity could be sufficient to inhibit repair or to induce ongoing damage to the FRC network. Second, innate lymphoid cells or other cellular sources of LT-β could be inadequate after allo-HCT, either in numbers, function, or microanatomical location. Third, damaged FRCs may acquire hyporesponsiveness to LT-β and other critical pathways needed for their recovery. Finally, it is possible that acute GVHD damages the very stromal cell subsets with progenitor potential that are needed to rebuild a functional FRC network, although the precise identity of these progenitors has not been established in adult lymphoid organs. In any case, long-lasting FRC damage could be a cardinal feature of the dysregulated immune reactivity characteristic of chronic GVHD. New insights into FRC biology and regulation are critically needed to understand their functions during acute GVHD, their demise during chronic GVHD, and the regeneration mechanisms that might be tapped into to promote healing of the stromal network in secondary lymphoid organs.
Twin cellular mechanisms of tolerance are under attack
Taken together, these exciting results provide some answers as to why the immune dysfunction and autoimmune manifestations of chronic GVHD may be so extensive and unresponsive to treatment. Twin cellular mechanisms that maintain central and peripheral tolerance are under attack during GVHD. In lymph nodes, damaged FRCs may lead to a profound disorganization of the immune cell choreography that sustains effective immune responses. This results in immune deficiency, as well as loss of the mechanisms that purge and restrain T cells with autoreactive potential, or that support regulatory T cells. Of note, B cells have also been reported to display a combination of defective function and increased autoreactivity during chronic GVHD (25). Thus, persistent damage to the FRC network could underlie multiple critical aspects of the complex immune dysregulation that our patients struggle with during chronic GVHD.
Acknowledgments
Research on graft-versus-host disease in the Maillard laboratory is supported by funding from National Institute of Allergy and Infectious Diseases (R01-AI091627) and the Leukemia and Lymphoma Society (TRP 6583-20).
Version 1. 03/09/2020
Electronic publication
Version 2. 04/01/2020
Print issue publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2020, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2020;130(4):1625–1628. https://doi.org/10.1172/JCI136139.
Contributor Information
Léolène J. Carrington, Email: Leolene.Carrington@pennmedicine.upenn.edu.
Ivan Maillard, Email: imaillar@pennmedicine.upenn.edu.
References
- 1.Lee SJ. Classification systems for chronic graft-versus-host disease. Blood. 2017;129(1):30–37. doi: 10.1182/blood-2016-07-686642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarantopoulos S, Cardones AR, Sullivan KM. How I treat refractory chronic graft-versus-host disease. Blood. 2019;133(11):1191–1200. doi: 10.1182/blood-2018-04-785899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holländer GA, Widmer B, Burakoff SJ. Loss of normal thymic repertoire selection and persistence of autoreactive T cells in graft vs host disease. J Immunol. 1994;152(4):1609–1617. [PubMed] [Google Scholar]
- 4.Tivol E, Komorowski R, Drobyski WR. Emergent autoimmunity in graft-versus-host disease. Blood. 2005;105(12):4885–4891. doi: 10.1182/blood-2004-12-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Hexner E, Frank D, Emerson SG. CD4+ T cells generated de novo from donor hemopoietic stem cells mediate the evolution from acute to chronic graft-versus-host disease. J Immunol. 2007;179(5):3305–3314. doi: 10.4049/jimmunol.179.5.3305. [DOI] [PubMed] [Google Scholar]
- 6.Na IK, et al. The cytolytic molecules Fas ligand and TRAIL are required for murine thymic graft-versus-host disease. J Clin Invest. 2010;120(1):343–356. doi: 10.1172/JCI39395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu T, et al. Thymic damage, impaired negative selection, and development of chronic graft-versus-host disease caused by donor CD4+ and CD8+ T cells. J Immunol. 2013;191(1):488–499. doi: 10.4049/jimmunol.1300657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dertschnig S, Hauri-Hohl MM, Vollmer M, Holländer GA, Krenger W. Impaired thymic expression of tissue-restricted antigens licenses the de novo generation of autoreactive CD4+ T cells in acute GVHD. Blood. 2015;125(17):2720–2723. doi: 10.1182/blood-2014-08-597245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson MS, et al. Projection of an immunological self shadow within the thymus by the Aire protein. Science. 2002;298(5597):1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 10.Link A, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8(11):1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 11.Cremasco V, et al. B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat Immunol. 2014;15(10):973–981. doi: 10.1038/ni.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodda LB, et al. Single-cell RNA sequencing of lymph node stromal cells reveals niche-associated heterogeneity. Immunity. 2018;48(5):1014–1028.e6. doi: 10.1016/j.immuni.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JW, et al. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8(2):181–190. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher AL, et al. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 2010;207(4):689–697. doi: 10.1084/jem.20092642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen JN, et al. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med. 2010;207(4):681–688. doi: 10.1084/jem.20092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegert S, et al. Fibroblastic reticular cells from lymph nodes attenuate T cell expansion by producing nitric oxide. PLoS One. 2011;6(11):e27618. doi: 10.1371/journal.pone.0027618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan O, Headley M, Gerard A, Wei W, Liu L, Krummel MF. Regulation of T cell priming by lymphoid stroma. PLoS One. 2011;6(11):e26138. doi: 10.1371/journal.pone.0026138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukacs-Kornek V, et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol. 2011;12(11):1096–1104. doi: 10.1038/ni.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dertschnig S, et al. Graft-versus-host disease reduces lymph node display of tissue-restricted self-antigens and promotes autoimmunity. J Clin Invest. 2020;130(4):1896–1911. doi: 10.1172/JCI133102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suenaga F, et al. Loss of lymph node fibroblastic reticular cells and high endothelial cells is associated with humoral immunodeficiency in mouse graft-versus-host disease. J Immunol. 2015;194(1):398–406. doi: 10.4049/jimmunol.1401022. [DOI] [PubMed] [Google Scholar]
- 21.Yip L, et al. Deaf1 isoforms control the expression of genes encoding peripheral tissue antigens in the pancreatic lymph nodes during type 1 diabetes. Nat Immunol. 2009;10(9):1026–1033. doi: 10.1038/ni.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chai Q, et al. Maturation of lymph node fibroblastic reticular cells from myofibroblastic precursors is critical for antiviral immunity. Immunity. 2013;38(5):1013–1024. doi: 10.1016/j.immuni.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung J, et al. Fibroblastic niches prime T cell alloimmunity through Delta-like Notch ligands. J Clin Invest. 2017;127(4):1574–1588. doi: 10.1172/JCI89535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scandella E, et al. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol. 2008;9(6):667–675. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- 25.McManigle W, Youssef A, Sarantopoulos S. B cells in chronic graft-versus-host disease. Hum Immunol. 2019;80(6):393–399. doi: 10.1016/j.humimm.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]