Abstract
Meal ingestion increases body temperature in multiple species, an effect that is blunted by obesity. However, the mechanisms responsible for these phenomena remain incompletely understood. Here we show that refeeding increases plasma leptin concentrations approximately 8-fold in 48-hour-fasted lean rats, and this normalization of plasma leptin concentrations stimulates adrenomedullary catecholamine secretion. Increased adrenal medulla–derived plasma catecholamines were necessary and sufficient to increase body temperature postprandially, a process that required both fatty acids generated from adipose tissue lipolysis and β-adrenergic activation of brown adipose tissue (BAT). Diet-induced obese rats, which remained relatively hyperleptinemic while fasting, did not exhibit fasting-induced reductions in temperature. To examine the impact of feeding-induced increases in body temperature on energy balance, we compared rats fed chronically by either 2 carbohydrate-rich boluses daily or a continuous isocaloric intragastric infusion. Bolus feeding increased body temperature and reduced weight gain compared with continuous feeding, an effect abrogated by treatment with atenolol. In summary, these data demonstrate that leptin stimulates a hypothalamus–adrenal medulla–BAT axis, which is necessary and sufficient to induce lipolysis and, as a result, increase body temperature after refeeding.
Keywords: Endocrinology, Metabolism
Keywords: Leptin
Introduction
Within minutes of consuming a meal containing carbohydrate and/or protein, both humans and rodents exhibit an increase in energy expenditure and body temperature, typically described as diet-induced or meal thermogenesis. In lean individuals, postprandial energy expenditure typically correlates positively with meal size (1, 2), protein and carbohydrate content (3–8), and plasma insulin concentrations (9–13); however, most (12, 14–28) but not all (4, 9, 29–31) studies on this topic have indicated that meal thermogenesis — measured as energy expenditure — is diminished in obese individuals and have suggested that this reduction in meal thermogenesis may contribute to the pathogenesis of obesity by decreasing overall daily energy expenditure. Therefore, it is of great interest to understand the mechanism by which feeding increases body temperature and energy expenditure.
Recent studies have implicated brown adipose tissue (BAT) as a key driver of postprandial thermogenesis, although most studies have inferred this indirectly by measuring postprandial BAT blood flow (32), oxygen consumption (33, 34), glucose uptake (35), and/or ex vivo respiration (36), with implanted temperature sensors used only recently to demonstrate an increase in BAT temperature after feeding (37). In addition, the timing of BAT activation in the context of meal ingestion remains uncertain: several investigators have suggested that BAT thermogenesis precedes food intake, at least in rodents (38–41), calling into question whether BAT thermogenesis is a result of dietary intake or simply an associated phenomenon. In addition, the fractional contribution of BAT activation to postprandial increases in body temperature, as well as the mechanism by which meal ingestion may activate respiration in BAT or other tissues, have not been established.
β-Adrenergic activity has for decades been considered a candidate mediator of postprandial increases in body temperature, as serum catecholamine concentrations clearly increase both after meal ingestion (7, 26, 42–44) and under hyperinsulinemic-euglycemic conditions (45). This concept has been bolstered by studies demonstrating that β-adrenergic blockade limits increases in energy expenditure under postprandial (43, 46–49) and hyperinsulinemic-euglycemic conditions (50), although other studies have failed to discern an effect of beta blockers on meal thermogenesis (51, 52), leaving the potential impact of β-adrenergic activity on postprandial temperature regulation, as well as the physiologic mechanism by which feeding increases β-adrenergic activity, still uncertain.
Here we demonstrate that carbohydrate or protein ingestion, but not fat ingestion, increases plasma leptin concentrations in awake, unrestrained 48-hour-fasted rats, and that this raises body temperature by increasing the delivery of fatty acids from adipose tissue lipolysis and β3-adrenergic activation of BAT, in a manner dependent on secretion of catecholamines by the adrenal medulla. We further demonstrate that time-restricted feeding reduces weight gain by promoting leptin- and circulating catecholamine–mediated increases in postprandial temperature; in contrast, the time of day at which meals are administered had no effect on these parameters. Taken together, these data identify a leptin–adrenal medulla–adipose tissue axis as responsible for meal-induced increases in body temperature in rodents.
Results
Adrenalectomy abrogates feeding-induced increases in body temperature.
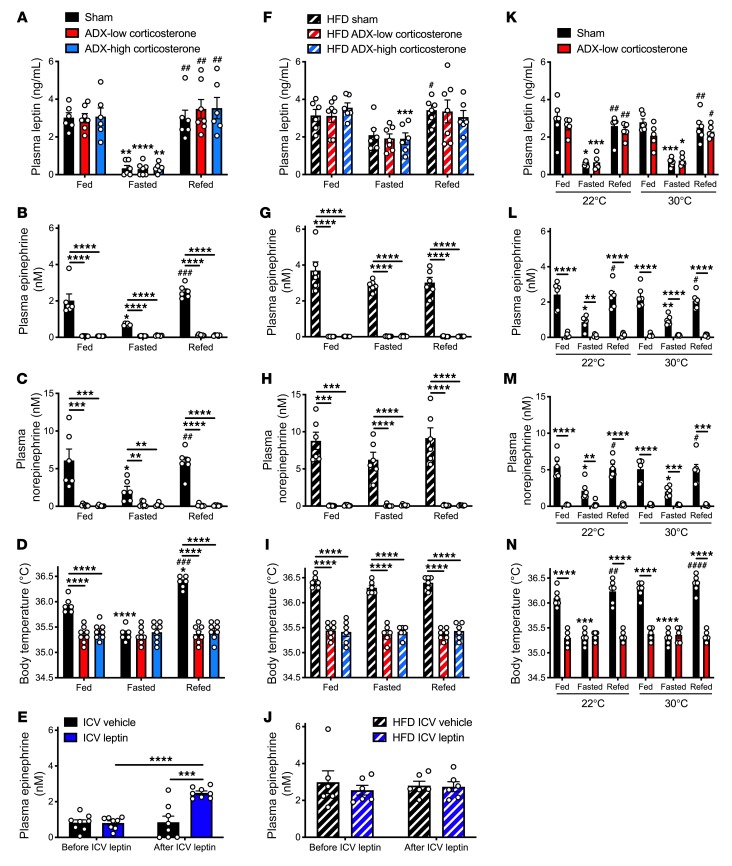
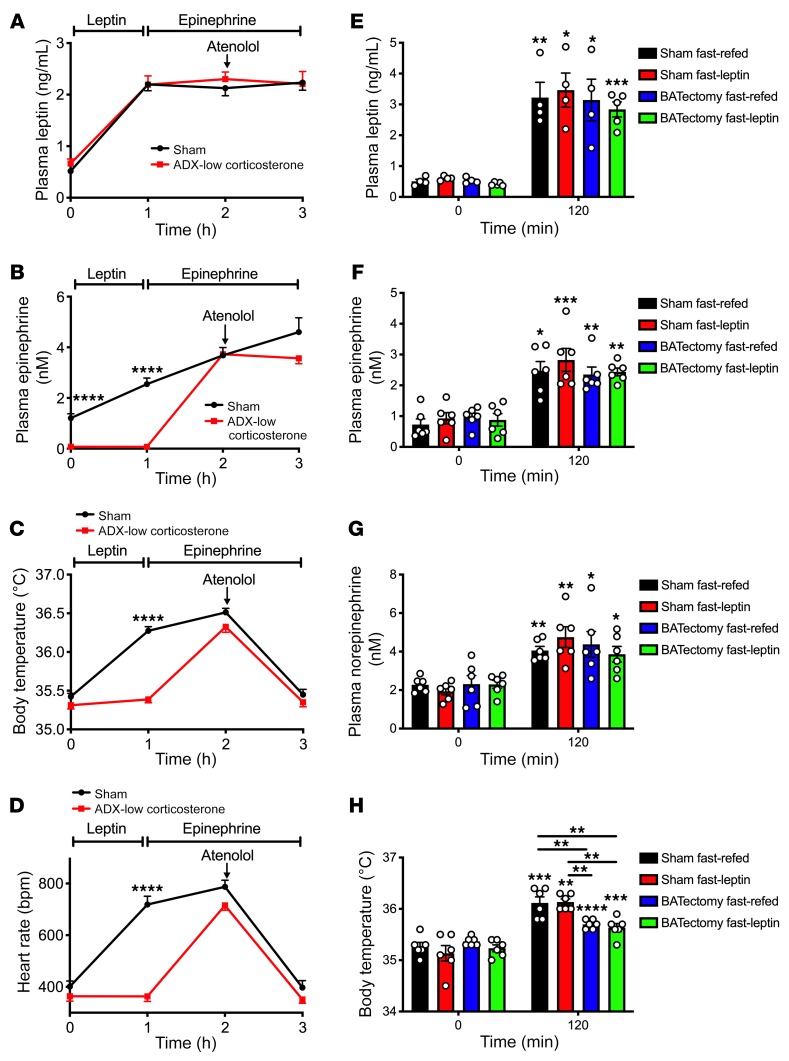
First, we verified that body temperatures measured in the colon reflected core body temperatures: in 48-hour fasted rats treated with or without epinephrine, measured temperatures did not change across measurements taken with the probe inserted at 2 to 6 cm from the anus (Supplemental Figure 1A; supplemental material available online with this article; https://doi.org/10.1172/JCI134699DS1). In order to examine the potential role of the adrenal glands in meal-induced increases in body temperature, we studied weight-matched sham-operated and adrenalectomized (ADX) rats infused with low and high levels of corticosterone to mimic the fed and fasted conditions (53, 54) (Supplemental Figure 1, B and C). Following a 48-hour fast, all groups manifested reductions in plasma glucose and insulin concentrations and increases in nonesterified fatty acid (NEFA) concentrations, each of which rebounded following refeeding; however, under fed conditions values for each parameter were lower in ADX low-corticosterone rats than sham-operated rats, demonstrating a significant role for adrenocortical secretion of glucocorticoids in regulating postprandial glycemia (Supplemental Figure 1, D–F). In contrast, plasma triiodothyronine (T3) and thyroxine (T4) concentrations were reduced in fasted rats and restored by refeeding in a manner independent of adrenocortical glucocorticoid secretion (Supplemental Figure 1G). Consistent with previous studies (53, 55–60), plasma leptin concentrations were reduced by approximately 90% in 48-hour-fasted lean rats, regardless of adrenalectomy, an effect that was reversed within 2 hours of refeeding (Figure 1A). Plasma catecholamine concentrations correlated with plasma leptin in sham-operated rats: both epinephrine and norepinephrine levels were reduced by approximately 70% with fasting in sham-operated rats and restored upon refeeding; however, both epinephrine and norepinephrine were undetectable in ADX animals, demonstrating that the adrenal glands are the major source of both circulating epinephrine and norepinephrine in healthy rats (Figure 1, B and C). Plasma catecholamine concentrations predicted the thermal response to fasting and to refeeding: fed body temperatures were lower in ADX rats, regardless of corticosterone replacement, whereas fasting reduced body temperature in sham-operated rats to levels measured in both ADX groups (Figure 1D). Based on the observed ability of refeeding to increase plasma leptin, catecholamines, and body temperature, we hypothesized that leptin exerts its effect on these parameters centrally. To test this hypothesis, we performed an intracerebroventricular (ICV) injection of leptin (10 μg) in 48-hour-fasted lean rats during an infusion of somatostatin to avoid any confounding effect of insulin on the parameters analyzed. ICV leptin administration increased plasma epinephrine and norepinephrine, glucose, and NEFA concentrations and modestly increased T3 and T4 without altering plasma leptin or insulin concentrations (Figure 1E and Supplemental Figure 2, A–F). These increases in plasma catecholamine concentrations were associated with a 1°C increase in body temperature (Supplemental Figure 2G), mimicking postprandial conditions.
Figure 1. Lack of postprandial increases in body temperature in ADX rats.
(A–C) Plasma leptin, epinephrine, and norepinephrine in ad libitum fed, 24-hour-fasted (with access to 2% sucrose drinking water), and 24-hour fasted/2-hour refed rats. (D) Body temperature. (E) Plasma epinephrine concentrations before and 30 minutes after an ICV injection of leptin (10 μg). (F–H) Plasma leptin, epinephrine, and corticosterone concentrations in the same rats shown in A–D after 10 days on a high-fat diet. (I) Body temperature. (J) Plasma epinephrine concentrations before and 30 minutes after an ICV injection of leptin (10 μg) in the same rats shown in E after 10 days on a high-fat diet. (K–M) Plasma leptin, epinephrine, and norepinephrine concentrations in rats housed for one week at 22 or 30°C. (N) Body temperature. In all panels, if no symbol appears, groups and time points are not statistically different. Data are presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001. In A–D, F–I, and K–N, asterisks directly above bars denote comparisons with fed rats, and # symbols denote comparisons with fasted rats. The same rats were compared under fed, fasted, and refed conditions using repeated-measures ANOVA with Bonferroni’s multiple-comparisons test, while groups were compared by ANOVA with Bonferroni’s multiple-comparisons test. In E and J, the 2 groups were compared using 2-tailed unpaired Student’s t test, and rats were compared before and after leptin injection with 2-tailed paired Student’s t test.
Obesity mitigates starvation-induced hypoleptinemia and prevents further postprandial changes in body temperature.
In order to examine the impact of obesity on the observed correlations among plasma leptin, catecholamines, and body temperature under fed and fasted conditions, we fed the same sham-operated and ADX rats a hypercaloric high-fat diet for 10 days. This intervention increased body weight and diminished fasting-induced reductions in plasma glucose, insulin, leptin, and catecholamines while abrogating fasting-induced increases in NEFA and corticosterone levels in sham-operated rats (Figure 1, F–H, and Supplemental Figure 1, H–L). Consistent with obesity’s effect of abrogating fasting-induced suppression of catecholamines and a further rise in catecholamines with refeeding, high-fat feeding abolished feeding-induced increases in body temperature (Figure 1I). This appeared to be due to an inability to further increase body temperature beyond the already high baseline in fasted obese rats in response to central leptin action: ICV leptin injection failed to generate a further increase in plasma catecholamines, glucose, insulin, NEFA concentrations, or body temperature beyond the already high fasting levels observed in these animals (Figure 1J and Supplemental Figure 2, H–K).
Fasting- and refeeding-induced changes in leptin, catecholamines, and body temperature are preserved at thermoneutrality.
Standard housing conditions (22°C) are subthermoneutral for rodents. To remove this potential confounder, we studied intact and ADX rats at thermoneutrality (30°C). The fasting- and refeeding-induced changes in plasma leptin concentrations, and the absence of appreciable plasma catecholamines, were similar in sham-operated and ADX rats housed at 30°C as compared with 22°C (Figure 1, K–M, and Supplemental Figure 3, A and B). As predicted by these data, body temperature was reduced with fasting and restored with refeeding in sham-operated rats, but was unchanged in ADX animals both at thermoneutrality and at room temperature (Figure 1N). Housing the rats at 30°C also did not alter the changes in plasma glucose, insulin, or NEFA concentrations observed with fasting and refeeding at 22°C (Supplemental Figure 3, C–E).
Normalizing body weight restores the leptin-catecholamine-temperature response to fasting and refeeding.
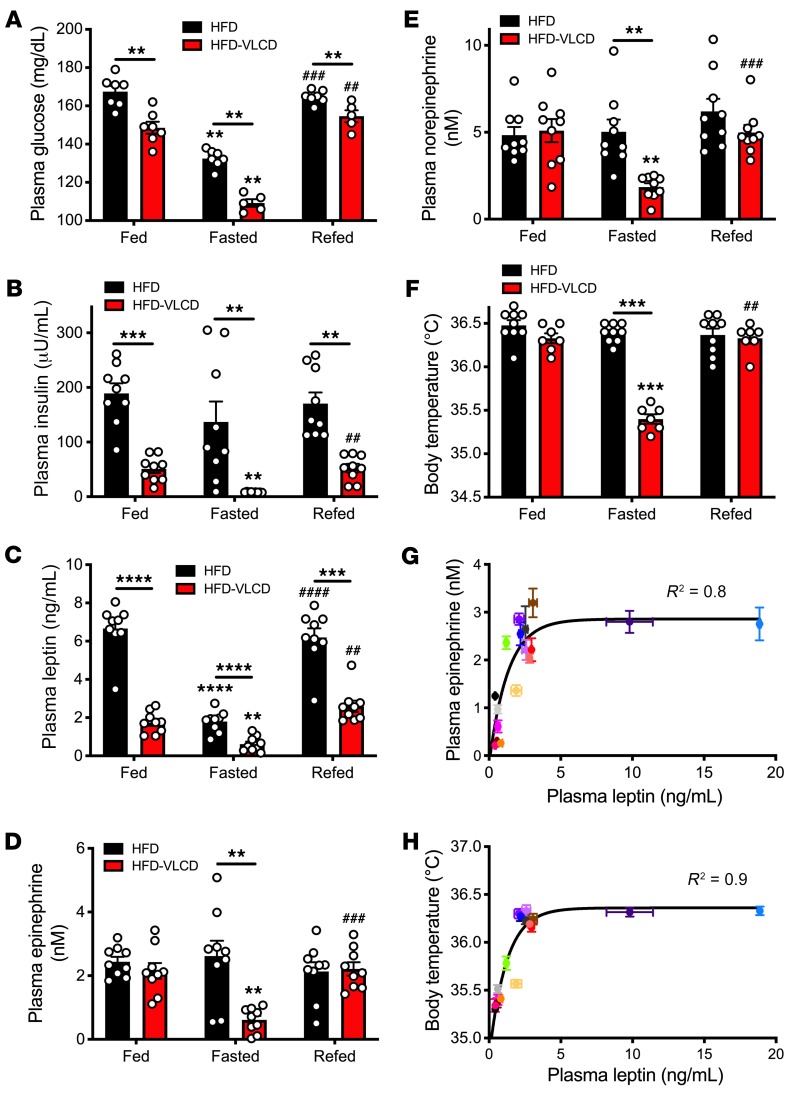
Having observed that obesity abrogates acute increases in plasma leptin, catecholamine concentrations, and body temperature upon refeeding in lean rats, we next sought to determine whether normalization of body weight, via a very-low-calorie diet (VLCD), would restore physiologic responsiveness to fasting and refeeding in rats. To that end, we studied obese rats prior to and following a 4-week hypocaloric feeding period to normalize body weight (Supplemental Figure 4). This intervention lowered fed and fasting plasma glucose, insulin, and leptin concentrations and restored the reduction in plasma catecholamine concentrations observed under fasting conditions in lean animals (Figure 2, A–E). Consistent with the idea that leptin-mediated alterations in plasma catecholamines regulate body temperature, body temperature fell upon fasting and increased upon refeeding only after weight normalization with a VLCD (Figure 2F). Plasma leptin concentrations correlated closely with both plasma epinephrine concentrations and body temperature under various conditions (Figure 2, G and H).
Figure 2. Normalizing body weight restores the leptin-catecholamine-thermogenic response to fasting and refeeding.
(A–E) Plasma glucose, insulin, leptin, epinephrine, and norepinephrine concentrations in the fed state, after a 48-hour fast, and 2 hours after refeeding. To avoid any confounding effects of the high-fat diet, all rats were given ad libitum access to regular chow for 24 hours prior to obtaining the fed samples and were refed ad libitum with regular chow. (F) Body temperature. **P < 0.01, ***P < 0.001, ****P < 0.0001, ##P < 0.01, ###P < 0.001, ####P < 0.0001. Asterisks directly above bars denote comparisons with fed rats, and # symbols denote comparisons with fasted rats. In A–F, data are presented as mean ± SEM, with the same rats studied before and after VLCD. If no symbol appears, groups and time points are not statistically different. Rats were compared in the fed, fasted, and refed states by paired ANOVA with Bonferroni’s multiple-comparisons test, and rats were compared before (HFD) and after VLCD (HFD-VLCD) by 2-tailed paired Student’s t test. (G) Correlation between plasma epinephrine and plasma leptin in all groups studied (data are presented as mean ± SEM for each group). Groups are defined by color in Supplemental Figure 14. (H) Correlation between body temperature and plasma leptin.
Leptin is required for postprandial increases in body temperature.
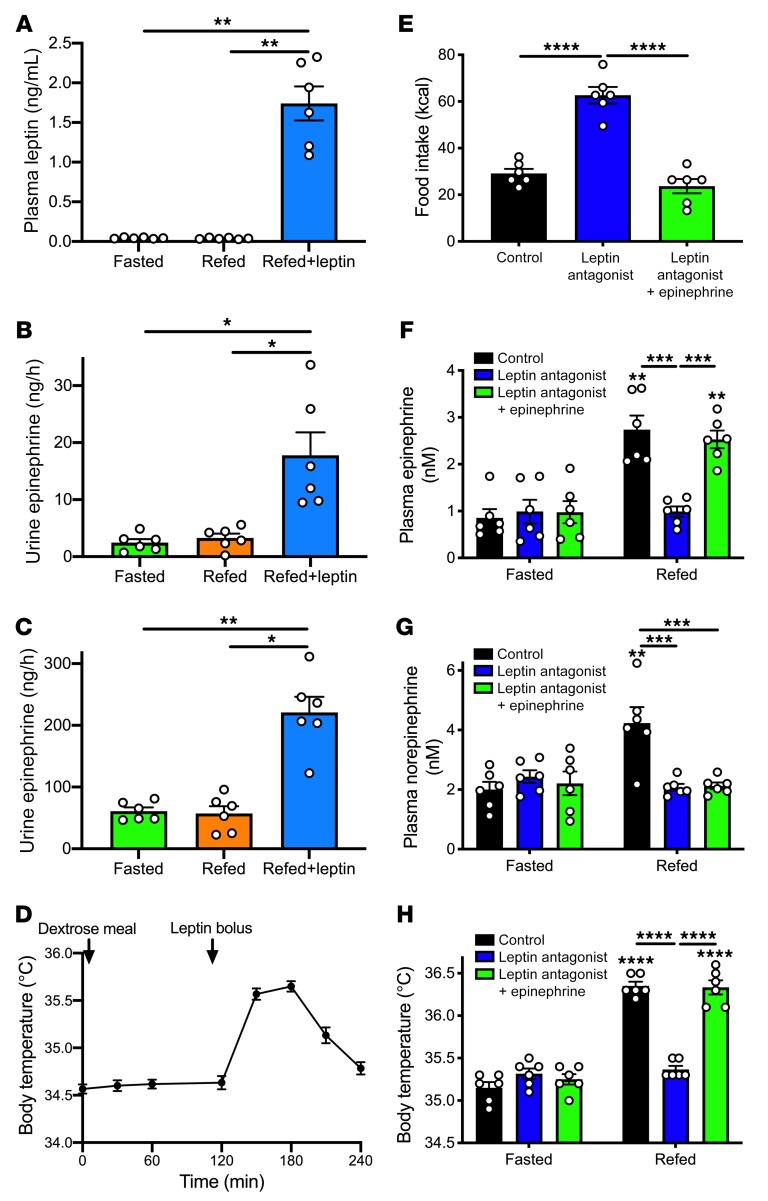
In order to examine whether increases in plasma leptin concentrations are required for increases in body temperature under hyperglycemic conditions, we first performed hyperglycemic clamp studies in conditional fat-specific O-linked β-d-N-acetylglucosamine–KO (OGT FKO) mice (Supplemental Figure 5A; original, uncut gels in Supplemental Figure 15). The hyperglycemic clamp allowed us to carefully control plasma glucose and insulin concentrations, avoiding the impact of differences in plasma glucose or insulin concentrations that would be expected to result from the apparent impairment in insulin sensitivity exhibited by the OGT FKO animals. In these mice, plasma leptin concentrations and body temperatures failed to increase in response to hyperglycemia, demonstrating a critical role for OGT in white adipose tissue (WAT) in mediating hyperglycemia-hyperinsulinemia–induced leptin secretion (Supplemental Figure 5, B–F). However, when mice were injected i.p. with leptin to increase plasma leptin concentrations approximately 5-fold, body temperatures were similarly increased in the two genotypes, demonstrating that the observed lack of meal- or hyperglycemia-hyperinsulinemia–induced increases in body temperature in OGT FKO mice was due to impaired leptin secretion and not to leptin unresponsiveness (Supplemental Figure 5, G–I). However, pretreatment with a β-adrenergic antagonist, atenolol, prevented the leptin-induced increase in body temperature in both genotypes, suggesting that leptin acts via stimulation of β-adrenergic activity to increase body temperature (Supplemental Figure 5I). We then studied a model completely deficient in leptin: ob/ob mice (Figure 3A). In these animals plasma leptin, urinary catecholamines, and body temperature failed to increase in response to refeeding following a 24-hour fast (Figure 3, B–D); however, injection with recombinant leptin to increase plasma leptin concentrations to what was observed in refed obese rats increased urinary catecholamine concentrations by 3- to 5-fold and rectal temperature by 1°C, demonstrating a critical role for leptin in mediating postprandial increases in body temperature.
Figure 3. Leptin is required for postprandial increases in body temperature.
(A) Plasma leptin in 24-hour-fasted, refed (dextrose gavage), and recombinant leptin–treated ob/ob mice. (B and C) Urine epinephrine and norepinephrine concentrations. (D) Body temperature (n = 6). (B and C) Plasma leptin concentrations and body temperature in the 24-hour-fasted and hyperglycemic clamp state. Mice were compared under each of the 3 conditions in A–C by repeated-measures ANOVA with Bonferroni’s multiple-comparisons test. (E) Food intake measured for a 2-hour period beginning 1 hour after treatment with a small molecule leptin antagonist. (F and G) Plasma epinephrine and norepinephrine concentrations. In F–H, symbols directly above bars denote comparisons to the same group in the fasted state. (H) Body temperature. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. In all panels, data are presented as mean ± SEM. If no symbol appears, groups and time points are not statistically different.
Finally, to more specifically interrogate the impact of leptin in mediating postprandial increases in body temperature, we treated rats prior to refeeding with a small molecule leptin antagonist. This agent inhibited leptin action, as demonstrated by a doubling in food intake upon refeeding in 48-hour-fasted/refed rats, and accordingly abolished the ability of refeeding to increase plasma catecholamine levels, lipolysis, and body temperature and to suppress hypercorticosteronemia (53, 54) (Figure 3, E–G, and Supplemental Figure 6, A–F).
β-Adrenergic activity mediates leptin’s effect of increasing body temperature after refeeding.
Next, we examined the mechanism by which leptin increases temperature after a meal. β-Adrenergic antagonism with atenolol fully abrogated the thermogenic responses to both refeeding and to leptin, despite further increases in plasma catecholamine concentrations, and suppressed sympathetic activity, as indicated by reversal of leptin- and refeeding-induced increases in heart rate (Supplemental Figure 7, A–H). To conclusively demonstrate the requirement for adrenal medulla–derived circulating catecholamines to promote leptin-induced increases in body temperature, we infused ADX rats acutely with leptin to mimic the postprandial condition. We observed no thermogenic or sympathetic effect of leptin in ADX rats, as demonstrated by the inability of leptin to increase plasma catecholamine, glucose, or insulin concentrations in ADX rats, despite an increase in T3 and T4 concentrations (Figure 4, A–D, and Supplemental Figure 8, A–F). However, infusing epinephrine replicated leptin’s effect of raising body temperature, mediated by β-adrenergic activity, as demonstrated by the reversal of the hyperthermic effect of epinephrine following atenolol injection.
Figure 4. β-Adrenergic activity in BAT mediates leptin’s effect of driving postprandial increases in body temperature.
(A and B) Plasma leptin and epinephrine concentrations in 24-hour-fasted sham-operated and ADX rats infused with low corticosterone by subcutaneous pump and given access to 2% sucrose drinking water. (C and D) Body temperature and heart rate. In A–D, ****P < 0.0001 between groups using 2-tailed unpaired Student’s t test. n = 8 per group. (E–G) Plasma leptin, epinephrine, and norepinephrine concentrations in 48-hour-fasted-refed and –leptin-infused sham-operated and BATectomized rats. (H) Body temperature. In E and H, comparisons with time 0 (asterisks above bars) were performed using 2-tailed paired Student’s t test and comparisons between groups performed using ANOVA with Bonferroni’s multiple-comparisons test: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. In all panels, data are presented as mean ± SEM. If no symbol appears, groups and time points are not statistically different.
Lipids generated by lipolysis are required for epinephrine-induced increases in body temperature.
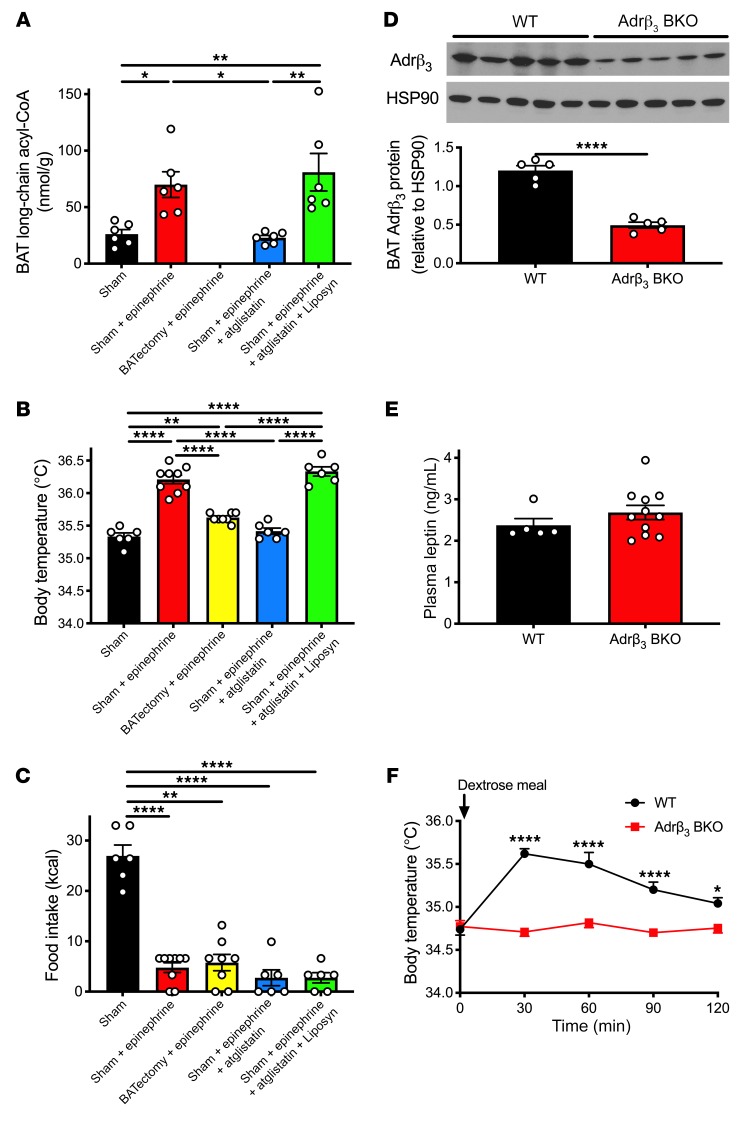
Having demonstrated that increases in β-adrenergic activity mediate leptin’s effect of increasing body temperature, we next sought to determine the primary tissue that accounts for leptin-mediated increases in body temperature and hypothesized that BAT may be at least in part responsible. To test this hypothesis, we studied 48-hour-fasted rats 1 week after surgery to remove interscapular BAT (BATectomy). Despite unchanged body weight and similar increases in plasma leptin, glucose, insulin, catecholamine concentrations, and heart rate after refeeding, the temperature response to both refeeding and leptin infusion was reduced by approximately 60% in BATectomized rats (Figure 4, E–H, and Supplemental Figure 9, A–D), suggesting that activation of interscapular BAT accounts for most, but not all, of the response to leptin. It is likely that increases in skeletal and/or cardiac muscle energy expenditure, beige WAT, and/or non-interscapular BAT depots may account for the leptin-induced increases in body temperature in these animals. Because branched-chain amino acids have recently been implicated in BAT thermogenesis under cold conditions (61), we measured plasma amino acid concentrations and found that both total and branched-chain (leucine, isoleucine) concentrations were reduced with both refeeding and leptin infusion in sham-operated but not BATectomized rats (Supplemental Figure 9, E–G).
Based on the fact that long-chain acyl-CoA concentrations increased by 70% in BAT following an infusion of epinephrine, we hypothesized that an increase in catecholamine-driven lipolysis is required for the temperature response to catecholamines. Consistent with this, inhibition of adipose triglyceride lipase (ATGL) with atglistatin fully abrogated the increase in body temperature observed after epinephrine infusion, whereas restoring plasma fatty acid concentrations in atglistatin-treated rats via an infusion of a lipid emulsion (Liposyn) and heparin restored the increase in body temperature observed with epinephrine infusion (Figure 5, A and B, Supplemental Figure 10, A and B, and Supplemental Table 1). Interestingly, epinephrine infusion also limited food intake following a 48-hour fast, but this effect was not dependent on lipolysis (as demonstrated by unchanged food intake in epinephrine-infused rats treated with atglistatin with or without Liposyn) or thermogenesis (as demonstrated by suppression of food intake despite a reduction in the thermogenic response in BATectomized rats) (Figure 5C). To specifically delineate the role of catecholamine activation of BAT, we studied mice with a BAT-specific inducible KO of the β3-adrenergic receptor, which is the primary β-adrenergic receptor found in adipose tissue (Adrβ3 BKO mice) (Figure 5D; original, uncut gels in Supplemental Figure 15). In these mice body temperature failed to increase after a dextrose meal despite plasma glucose, insulin, and leptin concentrations that were unchanged from those of their WT littermates (Figure 5, E and F, and Supplemental Figure 10, A–D).
Figure 5. Lipolysis is required for epinephrine-induced increases in body temperature resulting from β-adrenergic activity in BAT.
(A) BAT long-chain acyl-CoA concentrations in 48-hour-fasted rats infused with epinephrine ± atglistatin ± Liposyn. (B) Body temperature prior to refeeding. (C) Food intake within 2 hours of refeeding. In A–C, *P < 0.05, **P < 0.01, ****P < 0.0001 by ANOVA with Bonferroni’s multiple-comparisons test. (D) WAT and BAT β3-adrenergic receptor expression in Adrβ3 BAT-specific KO mice. Full, uncut gels are shown in Supplemental Figure 15. (E) Plasma leptin concentrations 60 minutes after dextrose gavage. (F) Body temperature. In D–F, *P < 0.05, ****P < 0.0001 by 2-tailed unpaired Student’s t test. n = 5 (WT) and n = 11 (Adrβ3 BKO). In all panels, data are presented as mean ± SEM. If no symbol appears, groups are not statistically different.
Increases in postprandial body temperature, but not energy expenditure, depend on meal composition.
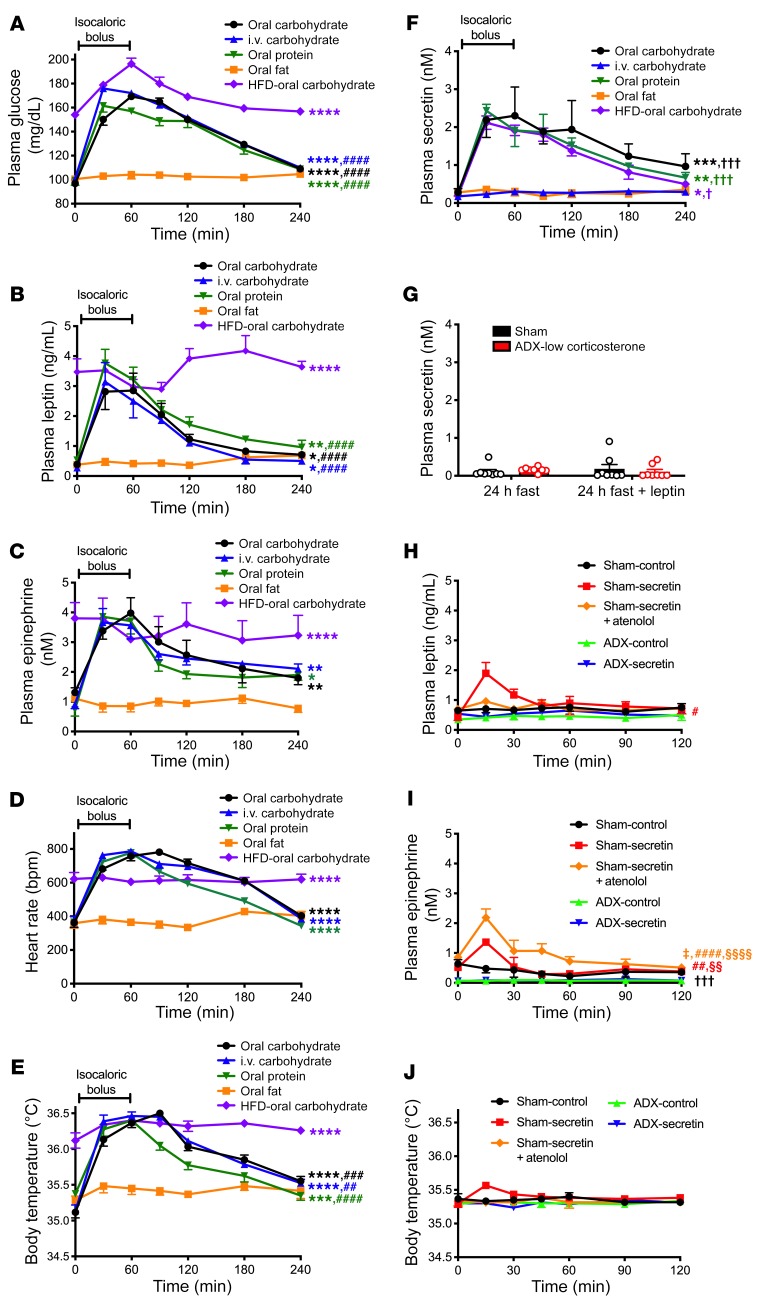
To determine whether meal-induced increases in plasma leptin, catecholamines, and body temperature are a function of a specific nutrient(s) or of meal ingestion per se, we next examined the impact of isocaloric carbohydrate, fat, or protein meals on these parameters. Although glucose and protein meals had a similar effect of increasing plasma glucose, insulin, leptin, catecholamines, heart rate, and body temperature in rats, an isocaloric fat bolus had no effect on any of these parameters (Figure 6, A–E, and Supplemental Figure 11, A and B). Similarly, in mice, isocaloric boluses of glucose and protein both increased body temperature, whereas fat did not (Supplemental Figure 11C). In contrast, all 3 substrates had a similar effect of increasing energy expenditure in mice (Supplemental Figure 11D). These data demonstrate that under certain conditions, meal-induced increases in body temperature may be dissociated from increases in energy expenditure, as assessed by indirect calorimetry, in response to feeding.
Figure 6. Increases in postprandial body temperature, but not energy expenditure, depend on meal composition; secretin has a modest, catecholamine-dependent effect of increasing temperature.
(A–C) Plasma glucose, leptin, and epinephrine concentrations after an isocaloric bolus of carbohydrate (dextrose), fat (canola oil), or protein (casein). (D and E) Heart rate and body temperature. (F) Plasma secretin. n = 8 per group. In A–F, n = 4 (oral protein), n = 5 (HFD–oral carbohydrate), n = 6 (oral carbohydrate, oral fat), and n = 8 (i.v. carbohydrate). AUC was compared by ANOVA with Bonferroni’s multiple-comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. oral fat; ##P < 0.01, ###P < 0.001, ####P < 0.0001 vs. HFD–oral carbohydrate; †P < 0.05, †††P <0.01 vs. oral fat. (G) Plasma secretin in 24-hour-fasted sham-operated and ADX rats given 2% sucrose water. In G–J, n = 6–8 per group. (H and I) Plasma leptin and epinephrine concentrations in rats given an i.p. injection of secretin at time 0. #P < 0.05, ##P < 0.01, ####P < 0.0001 vs. ADX-control; §§P < 0.01, §§§§P < 0.0001 vs. ADX-secretin; †††P < 0.001 vs. sham-secretin+atenolol; ‡P < 0.05 vs. sham-secretin by ANOVA with Bonferroni’s multiple-comparisons test (comparison of AUC). (J) Body temperature. In all panels, data are presented as mean ± SEM. If no symbol appears, groups are not statistically different.
Secretin has a modest, adrenal medulla–dependent effect of promoting thermogenesis.
Secretin has recently been reported to promote meal thermogenesis and satiety (37); thus, we were interested in determining whether secretin had a dependence similar to that of leptin on the hypothalamus–adrenal medulla–adipose tissue axis in mediating this effect. We observed a nutrient-dependent increase in secretin with refeeding: both oral carbohydrate and protein meals increased plasma secretin, whereas oral fat did not (Figure 6F). We were also able to dissociate postprandial increases in body temperature from increases in plasma secretin concentrations, as reflected by the fact that an intravenous infusion of glucose had an effect, similar to that of oral glucose, of increasing body temperature, whereas only oral glucose increased plasma secretin concentrations (Figure 6, E and F). Postprandial increases in plasma secretin concentrations are upstream of meal-induced increases in plasma leptin and catecholamine concentrations, as demonstrated by the fact that leptin infusion in intact and ADX rats did not increase plasma secretin concentrations (Figure 6G). Next, to directly examine the physiologic impact of the observed increase in secretin with feeding, we treated 48-hour-fasted rats with an i.p. injection of secretin and observed a modest, transient increase in plasma glucose, insulin, and catecholamine concentrations as well as body temperature at 15 minutes. These effects were all dependent on adrenomedullary secretion of catecholamines and β-adrenergic activity, as demonstrated by the absence of an effect of secretin on body temperature in ADX or atenolol-treated rats (Figure 6, H–J, and Supplemental Figure 12, A–D).
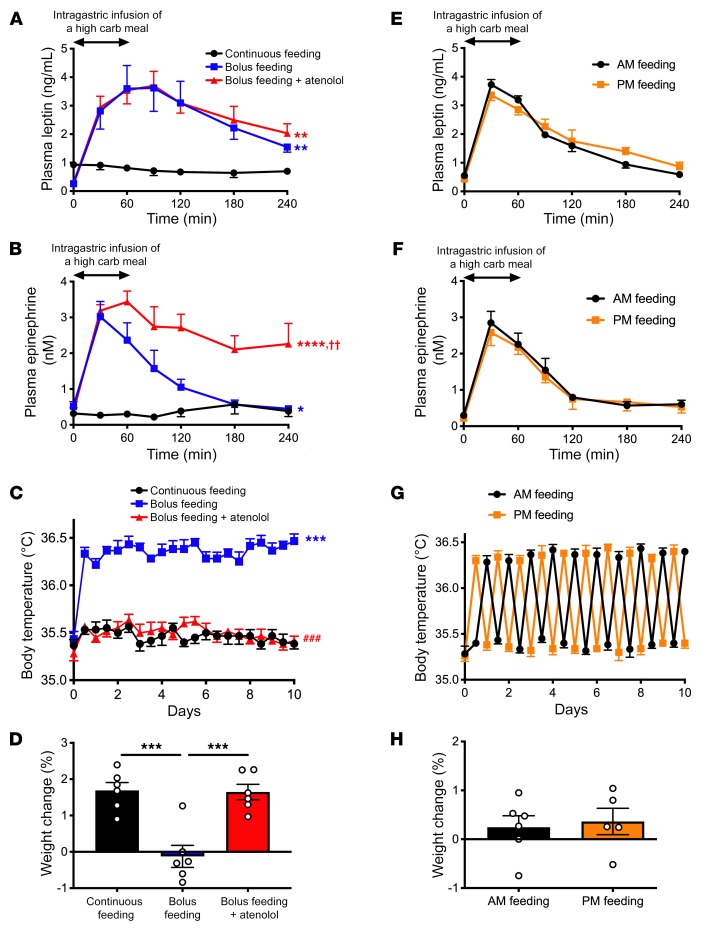
Bolus feeding may minimize weight gain through a β-adrenergic effect to promote meal-induced increases in body temperature.
To examine the physiologic impact of alterations in meal timing and changes in leptin, catecholamines, thermogenesis, and body weight gain, we compared isocaloric portions of a high-carbohydrate meal replacement administered either via twice-daily intragastric boluses or by continuous infusion in rats with chronically implanted gastric catheters. Bolus feeding increased plasma leptin, catecholamines, and body temperature and suppressed weight gain due to increased β-adrenergic activity compared with continuous infusion feeding (Figure 7, A–D, and Supplemental Figure 13, A–D). In contrast, meal timing had no impact on any of these parameters (Figure 7, E–H, and Supplemental Figure 13, E–H). Taken together these results indicate that meal timing affects weight gain to the extent that meal size affects plasma catecholamines and postprandial thermogenesis, but the timing of meals exactly matched in calorie content does not affect weight change in rats.
Figure 7. Bolus feeding minimizes weight gain through a β-adrenergic effect to increase body temperature.
(A and B) Plasma leptin and epinephrine concentrations during and after the last meal (day 10) or during continuous feeding (day 10). *P < 0.05, **P < 0.01, ****P < 0.0001 vs. continuous feeding; ††P < 0.01 vs. bolus feeding, comparing the AUC at 240 minutes by ANOVA with Bonferroni’s multiple-comparisons test. carb, carbohydrate. (C) Body temperature. ***P < 0.001 vs. continuous feeding; ###P < 0.001 vs. bolus feeding by ANOVA with Bonferroni’s multiple-comparisons test. (D) Weight change after 10 days. ***P < 0.001 by ANOVA with Bonferroni’s multiple-comparisons test. (E and F) Plasma leptin and epinephrine concentrations after the last meal on day 10 in rats fed once daily, either in the morning (AM) or evening (PM). In E–H, groups were compared by 2-tailed unpaired Student’s t test. n = 5 (PM feeding) and n = 6 (AM feeding). (G) Body temperature. (H) Weight change after 10 days. Data are presented as mean ± SEM. If no symbol appears, groups are not statistically different.
Discussion
Postprandial thermogenesis has been proposed to be an important contributor to body weight regulation, particularly in light of the fact that increases in energy expenditure induced by a meal are blunted in obesity (12, 14–28); however, the mechanism by which meal-induced thermogenesis occurs in lean individuals but is blunted in obese subjects remains poorly understood.
Sympathetic activation has been correlated with increases in body temperature in numerous prior studies (6, 9, 41, 62–64), but the mechanism by which sympathetic activity is stimulated postprandially and the mechanism by which it may alter energy expenditure remain under debate. Our study establishes that increases in body temperature following meal ingestion, which mostly reflect increases in energy expenditure — due to increased mitochondrial uncoupling by UCP1 activation in brown and beige adipose tissue (65–71) and possibly sarcolipin induction in skeletal muscle (69–71) — can be dissociated under certain conditions from energy expenditure. It is likely that the two processes, postprandial increases in body temperature and postprandial increases in energy expenditure, are regulated independently and that their dissociation is not the result of a specific regulatory action, but rather the divergence of two independent regulatory mechanisms. The current study demonstrates that meals comprising carbohydrate or protein, but not fat, increase plasma leptin, catecholamines, and body temperature; however, all meals increase energy expenditure, as assessed by indirect calorimetry. These data therefore argue against the hypothesis that meal thermogenesis and postprandial energy expenditure are components of the same phenomenon (33).
In order to elucidate the mechanism of feeding-induced increases in body temperature, we first hypothesized that a postprandial rise in temperature would be initiated by a physiologic change that signals to the body that substrate has been provided. We hypothesized that the increase in plasma leptin concentrations that has been shown in numerous studies to occur upon refeeding, reversing the starvation-induced hypoleptinemia that occurs in lean animals and humans (72, 73), may provide this signal. Consistent with this hypothesis, an increase in plasma leptin concentrations was associated with increased body temperature in refed rats (Figure 1D); and an infusion of recombinant leptin to increase plasma leptin concentrations to physiologic levels observed in refed rats increased body temperature to refed levels (Figure 4C), while pretreatment with a leptin antagonist abrogated feeding-induced increases in body temperature (Figure 3H), demonstrating a causal link between leptin and postprandial increases in body temperature. To confirm this hypothesis, we employed inducible fat-specific OGT KO mice. Mice with lifelong deletion of this gene in adipose tissue showed reductions in white adipose leptin mRNA and plasma leptin concentrations under conditions of high-fat diet but not chow feeding after a 6-hour fast (74); however, postprandial leptin secretion was not studied in this report. In addition, since it is likely that compensatory mechanisms develop in the setting of lifelong deletion of OGT in adipose tissue, the inducible model offers certain advantages in testing the physiologic impact of postprandial leptin secretion. We show here that OGT FKO mice failed to secrete leptin under hyperglycemic-hyperinsulinemic conditions, and that this lack of glucose/insulin-induced hyperleptinemia was correlated with an absent thermogenic response to glucose. In this model, we show that leptin mediated hyperglycemia-hyperinsulinemia–induced hyperthermia: after a leptin bolus, OGT FKO mice demonstrated a normal increase in body temperature (Supplemental Figure 5, E–I). Similarly, ob/ob mice, which lack the ability to secrete leptin, showed no catecholamine or temperature response to refeeding, but both increased in response to injection with recombinant leptin, again directly implicating leptin in postprandial increases in body temperature (Figure 3, B–D). However, blocking leptin action with a small molecule antagonist reversed the feeding-induced increases in catecholamines and body temperature while increasing food intake and preventing the reversal of hypercorticosteronemia with refeeding (Figure 3, F–H) (54). Taken together, these data indicate that an increase in plasma leptin concentration is both necessary and sufficient to cause increases in body temperature under postprandial conditions and that OGT plays an important role in mediating hyperglycemia-hyperinsulinemia stimulation of WAT leptin secretion. In addition, the similar effect of leptin on body temperature in rats and mice bolsters the potential translatability of our findings.
Next, we investigated the mechanism by which an increase in plasma leptin concentration mediates postprandial increases in body temperature. Feeding-induced increases in plasma leptin concentrations were correlated with increases in plasma catecholamine concentrations: refeeding and leptin infusion increased plasma catecholamine concentrations in sham-operated but not ADX rats (Figure 1, B, C, L and M, Figure 4B, and Supplemental Figure 8E). β3-Adrenergic activity in BAT was necessary for increases in body temperature: body temperature of Adrβ3 BKO mice failed to increase in response to a glucose meal, in contrast to that of their WT littermates (Figure 5F). These data are consistent with previous studies in which β-adrenergic blockade was found to abrogate the postprandial thermogenic response in humans and animals (43, 46, 47, 49, 50, 75–77) but in contrast to similar studies showing no impact of beta blockers on meal thermogenesis (48, 51, 52, 78, 79). The central mechanism of leptin stimulation of β-adrenergic activity was confirmed by ICV injection of leptin: leptin (10 μg) injected into the ICV space did not alter jugular venous plasma leptin concentrations, but it increased plasma catecholamine concentrations and body temperature to postprandial levels (Figure 1E and Supplemental Figure 2, A and F). Although both ICV and systemic leptin infusion, as well as refeeding, increased plasma thyroid hormone concentrations, these effects were dissociated from body temperature: both refeeding and leptin infusion increased T3 and T4 concentrations in ADX rats but failed to increase body temperature. These results support the hypothesis that basal body temperature and energy expenditure are regulated differently from postprandial body temperature and energy expenditure: whereas thyroid function is well established to play an important role in maintenance of basal body temperature and energy expenditure, it appears to play a less important role in the increases in both parameters that occur following a meal. However, it does remain possible that leptin may alter tissue deiodinase activity and therefore conversion of T4 to T3; further studies will be required to address this point. Taken together, these data demonstrate that leptin stimulates adrenomedullary secretion of catecholamines through a CNS-mediated process, likely through signaling in the arcuate nucleus, and that β-adrenergic activity is required for mediation of leptin-induced postprandial increases in body temperature. Surprisingly, both rats treated with an antagonist primarily suppressing β1-adrenergic activity and mice lacking the β3-adrenergic receptor in BAT showed a similar lack of a temperature response to refeeding, potentially due to β1-β3 crosstalk — particularly in rodents living at subthermoneutrality throughout their lives, which may have beiged WAT sensitive to β1 — and/or an effect of atenolol to suppress β3 in addition to β1 activity (80).
Having established that leptin causes postprandial thermogenesis via increases in β-adrenergic activity, we next asked which tissue(s) are primarily responsible for catecholamines’ effect of stimulating thermogenesis and hypothesized that BAT may be a key mediator of the thermogenic effect of leptin. Consistent with a role for BAT thermogenesis, interscapular BATectomy reduced the thermogenic effect of food and of leptin by approximately 60% (Figure 5B), indicating that interscapular BAT thermogenesis accounts for the majority of the postprandial thermogenic response. Next, we asked whether the stimulation of thermogenesis by catecholamines requires increased adipose tissue lipolysis to provide a substrate for catecholamine-induced BAT thermogenesis. Consistent with a requirement for catecholamine-driven lipolysis to mediate postprandial increases in body temperature, treatment with a small molecule inhibitor of ATGL, atglistatin, abrogated the thermogenic effect of leptin, while infusion of Liposyn/heparin to restore BAT long-chain acyl-CoA concentrations in epinephrine- and atglistatin-treated rats replicated the thermogenic effect of epinephrine (Figure 5E). Taken together, these studies demonstrate that β-adrenergic stimulation of lipolysis is required for leptin-induced, BAT-mediated increases in body temperature. Furthermore, they demonstrate the necessity for leptin-induced stimulation of adrenomedullary secretion of catecholamines in leptin-induced adipose tissue lipolysis as opposed to leptin stimulation of lipolysis through direct innervation of WAT; this was demonstrated by the lower plasma NEFA concentrations observed in ADX-high corticosterone treated rats after refeeding despite identical plasma insulin and corticosterone concentrations but the absence of circulating catecholamines. These data would suggest that direct sympathetic innervation of WAT may be required for tonic, low rates of lipolysis, but not for leptin’s promotion of lipolysis after refeeding. It is also possible that branched-chain amino acids contribute to BAT-mediated increases in temperature, as demonstrated by a recent study (61) and consistent with the reduction in plasma leucine and isoleucine concentrations in leptin-treated and refed rats; however, since atglistatin treatment abrogated the temperature response to epinephrine, it is likely that under fasted-refed conditions, fatty acids derived from WAT lipolysis are the predominant energy source fueling BAT thermogenesis.
As meal thermogenesis itself has been proposed to inhibit food intake (37, 40, 41, 81), we measured refed food intake in rats treated with epinephrine and found that after a 48-hour fast it was markedly reduced in epinephrine-infused rats; however, this effect was not dependent upon increases in body temperature, as demonstrated by the fact that neither BATectomy nor atglistatin treatment with or without fatty acid replacement altered food intake in epinephrine-infused animals (Figure 5F). Taken together, these data demonstrate that catecholamine-induced increases in body temperature, which occur with meal ingestion, can be dissociated from reduction of food intake and that increased circulating catecholamine concentrations per se, but not the resulting increase in body temperature, may mediate the impact of β-adrenergic activity of suppressing food intake. These results also highlight the yin-yang nature of the adrenal gland in the regulation of food intake by leptin: the transition from high (>2.4 ng/mL) to low (<1 ng/mL) plasma concentrations of leptin stimulates the adrenal cortex to secrete corticosterone, which in turn stimulates food intake (54), whereas the transition from low to high plasma concentrations of leptin concentrations stimulates the adrenal medulla to secrete catecholamines, which in turn inhibit food intake. This logic would allow circulating leptin levels to serve as both an “on” signal (with low leptin increasing appetite due to hypercorticosteronemia) and an “off” signal (as high leptin suppresses appetite by increasing catecholamine concentrations) for food intake.
The gut hormone secretin has recently been proposed to mediate both thermogenesis and postprandial satiety (37). In this study, we sought to determine whether secretin’s effects of promoting postprandial thermogenesis might also be dependent on the adrenal medulla–adipose tissue axis. Consistent with previous findings (37, 82–84), oral carbohydrate and protein meals caused a transient increase in plasma secretin concentrations. However, we found that these effects could be dissociated from the thermogenic response: an isocaloric intravenous glucose infusion caused a similar thermogenic response without any increase in plasma secretin concentrations (Figure 6F). To further explore the physiologic effect of secretin on body temperature, we treated 48-hour-fasted lean rats with an i.p. injection of secretin to mimic postprandial secretin concentrations and found that this intervention caused a transient, modest increase in plasma glucose, insulin, leptin, and catecholamine concentrations in sham-operated rats at 15 minutes but not ADX or atenolol-treated rats (Figure 6), demonstrating that secretin’s effect of causing a small increase in body temperature was mediated by adrenomedullary secretion of plasma catecholamine concentrations. These data are in contrast to the findings of Li et al. (37), who demonstrated that pretreatment with propranolol did not affect BAT temperature in secretin-treated mice. It is possible that these divergent results may be explained by the short duration of the thermogenic response to secretin (Figure 6J): the previous study reported temperature AUC over 2.5 hours, a timescale over which the impact of secretin is expected to be minimal. Most importantly, the thermogenic effect of secretin is minor compared with the effect of leptin and thus may contribute modestly to meal thermogenesis but cannot explain the majority of the temperature response to refeeding in awake rats.
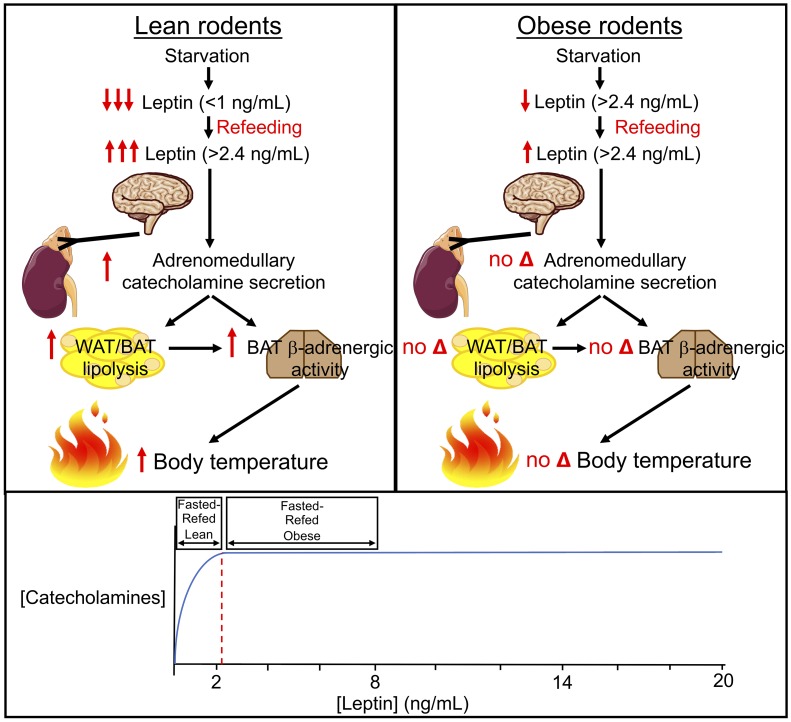
Next, we aimed to examine why the thermogenic response to refeeding is blunted in obesity. To that end, we studied rats both before and after high-fat feeding, which increased body weight by approximately 25%. This increase in body weight caused hyperleptinemia such that fasting plasma leptin concentrations were increased 6-fold and fasting plasma catecholamine concentrations were increased 3-fold from those measured in the same animals prior to the induction of obesity. In obese animals, neither refeeding nor ICV leptin infusion was associated with a thermogenic response beyond the already high baseline body temperatures (Figure 1, I and J). These data suggest that the lack of a temperature response to refeeding may be attributable to the failure of leptin to drop below the threshold at which catecholamines and temperature are reduced in a physiologically meaningful way. The correlation between plasma epinephrine and plasma leptin concentrations showed a 95% maximal epinephrine response to leptin and a 95% maximal temperature response to epinephrine at ~2.4 ng/mL (Supplemental Figure 11, A–C), approximately the plasma leptin concentrations measured in fasting obese rodents. These data demonstrate that there is a relatively low threshold (~2.4 ng/mL in rats) for plasma leptin concentration at which the response to leptin on adrenomedullary secretion of catecholamines is maximized such that there is no physiologic response to reductions in plasma leptin that still exceed this threshold. Taken together, these data suggest that “leptin resistance,” i.e., an inability to increase energy expenditure and/or inhibit food intake in response to leptin, may not be a real phenomenon but may simply reflect the maximal effects of leptin to stimulate adrenomedullary catecholamine secretion and suppress adrenocortical corticosterone secretion, which both plateau at plasma leptin concentrations of approximately 2.4 ng/mL.
Having demonstrated that obese rats exhibit impaired meal-induced increases in body temperature, because fasting does not decrease plasma leptin concentrations below the threshold at which leptin responsiveness occurs, we next asked whether feeding-induced increases in body temperature could be restored following weight normalization on a VLCD. Associated with normalization of plasma leptin concentrations to those measured in lean rats, the VLCD lowered fasting plasma catecholamine concentrations and body temperature, restoring the thermogenic effect of refeeding in formerly obese rats (Figure 2, C–F). These results demonstrate that obesity increases total energy expenditure, while normalization of body weight and body fat content with hypocaloric feeding restores the normal diurnal variation of energy expenditure with feeding and fasting.
Results of the current study as well as previous studies linking alterations in meal thermogenesis to obesity raise the question of whether chronic alterations in postprandial body temperature may alter body weight gain over time. To answer this question, we compared weight change in rats given their total daily calories in 2 carbohydrate-rich boluses through a chronically implanted intragastric catheter, so that food administration could be precisely controlled, as compared with the same amount of total daily calories continuously infused over 24 hours. Bolus feeding increased prandial leptin and catecholamine spikes, thereby increasing body temperature and limiting weight gain over the 10-day infusion period (Figure 7, A–D). The alterations in weight gain with bolus feeding were mediated by increases in plasma catecholamines in bolus-fed rats: treatment of bolus-fed rats with atenolol abrogated the protective effect of bolus feeding. Thus, these data provide a mechanism by which time-restricted feeding/intermittent fasting may improve metabolic health (85–87). In contrast, the timing of intragastric bolus feeding (once daily in the morning or evening) had no impact on any of these parameters. Based on these data, it is possible that previous studies reporting differences in weight gain observed with alteration of feeding time in rodents allowed to consume food per orem may reflect small differences in food consumption when meals are given ad libitum, and/or alterations in energy expenditure induced by awakening the rodents at times when they may not typically be active; in the absence of these factors, as in our study, feeding time does not alter postprandial body temperature and has no impact on weight gain. Further studies will be required to determine to what extent these findings translate to humans, in whom the role of BAT in whole-body energetics remains the subject of active investigation.
In summary, our data establish a mechanism by which body temperature increases postprandially through a leptin–brain–adrenal medulla–adipose tissue axis. Specifically, we show the following: (a) Through a central mechanism, meal-stimulated increases in leptin promote adrenomedullary secretion of catecholamines, which in turn are necessary and sufficient for meal-induced increases in body temperature through β-adrenergic agonism. (b) The dose-response curve for leptin stimulation of adrenomedullary secretion of catecholamines and thermogenesis plateaus at approximately 2.4 ng/mL. (c) Adipose O-linked β-d-N-acetylglucosamine is required for glucose-induced hyperleptinemia and postprandial increases in body temperature. (d) Upon weight normalization, formerly obese rodents regain normal diurnal feeding/fasting variations in plasma leptin, catecholamines, and body temperature. (e) Both increases in plasma epinephrine and stimulation of adipose tissue lipolysis leading to increases in plasma fatty acid concentrations and increased BAT acyl-CoA concentrations — derived mostly from WAT lipolysis — are required for postprandial increases in body temperature. Branched-chain amino acids may also contribute to BAT thermogenesis. (f) Alterations in thyroid hormone may play some role in the regulation of body temperature by central leptin administration, but can be dissociated from temperature regulation under postprandial conditions in ADX animals. (g) Secretin is not required for meal-induced thermogenesis. (h) Meals comprising carbohydrate or protein, but not fat, increase plasma leptin, catecholamines, and body temperature; however, all meals increase energy expenditure, as assessed by indirect calorimetry. These data demonstrate that meal thermogenesis and postprandial energy expenditure are not one phenomenon. (i) Epinephrine suppresses food intake, independently of changes in adipose tissue lipolysis and thermogenesis. (j) Rats fed with high-carbohydrate meal boluses are protected from weight gain relative to those fed continuously, due to increased β-adrenergic activity–induced increases in body temperature; however, meal timing has no impact on weight change.
Taken together, these data provide insights into leptin biology and demonstrate that activation of the leptin–adrenal medulla–adipose tissue axis contributes to the maintenance of metabolic homeostasis by regulating postprandial thermogenesis. These results also provide a potential mechanism by which time-restricted feeding may improve metabolic health.
Methods
Animals.
All rat studies were performed in awake, unrestrained animals. Male Sprague-Dawley rats were purchased from Charles River at approximately 250 g and were group housed (2 per cage) for 1–2 weeks until they underwent surgery under general isoflurane anesthesia for placement of polyethylene catheters in the common carotid artery, advanced into the aortic arch (PE50 tubing, Instech Solomon), and in the external jugular vein, advanced into the right atrium (silicone tubing, Instech). After the surgical procedure, all mice were singly housed until sacrifice. In BATectomized rats, interscapular BAT was visualized through an incision in the midline, then the Sulzer vein was tied off with a suture and cut. The entire interscapular BAT pad was then cut and removed. For the ICV infusion studies, 250- to 300-g male rats with catheters placed in the third ventricle of the brain were obtained from Charles River and singly housed upon arrival. Five to 7 days after arrival, they underwent the same catheterization surgery as described above. ADX rats were purchased from Charles River and maintained on drinking water containing 0.9% NaCl and 2% sucrose. Five to 7 days after arrival, they underwent surgery to place an Alzet pump to deliver low (5 mg/d) or high corticosterone (20 mg/d) doses continuously, as well as venous and arterial catheters as described above. All rats were fasted for the time period specified in the figure legends (24–48 hours) prior to study. During the fast, ADX rats were given 2% sucrose/0.9% drinking water, and the sham-operated controls for those experiments were also given drinking water containing 2% sucrose. Food intake was monitored in a subset of rats by weighing food pellets provided and food remaining after 120 minutes.
Male C57BL/6J mice and ob/ob mice were obtained at 7–8 weeks of age from the Jackson Laboratory and group housed (3–5 per cage). Whole-body energetics was measured before and after a carbohydrate, fat, or protein meal (described below) using Columbus Lab Animal Monitoring System metabolic cages. Inducible OGT FKO mice were generated by breeding OGTfl/fl female mice with Adiponectin-CreER male mice. OGTfl/Y Adiponectin-CreER (OGT FKO) male mice and their WT male littermates (OGTfl/Y) were used for studies. Inducible β3-adrenergic receptor–KO mice (Adrβ3 BKO mice) were generated by breeding Adrβ3fl/fl to UCP1-CreER mice (88, 89). The mice used for hyperglycemic clamp studies underwent surgery under isoflurane anesthesia to place catheters in the jugular vein, and they were studied 5–7 days later after regaining their presurgical body weight, in the awake state, 2 hours after acclimatization to restrainers. To induce knockdown of adipose tissue OGT, we injected mice with tamoxifen (2.0 mg; MilliporeSigma) in 100 μL vegetable oil once daily for 5 days, beginning 12 days prior to the hyperglycemic clamp studies, and they were then refed and allowed to recover for 3 days before a second study (bolus injection of leptin, as described below) also performed after a 48-hour fast. The β3-adrenergic receptor was conditionally deleted in BAT by injection of tamoxifen (2.0 mg) dissolved in peanut oil i.p. once daily for 5 days, beginning 12 days prior to a terminal refeeding study. Cre-negative littermate controls were also injected with tamoxifen.
All animals were housed at 22°C for the duration of the study, with the exception of the thermoneutrality studies, in which rats previously housed at 22°C were moved to 30°C chambers and allowed to acclimate for 5 days prior to the initiation of a feeding-fasting-refeeding study. Unless otherwise specified, both rats and mice were fed a regular chow diet (Harlan Teklad 2018) throughout, and fasted as described below. High-fat diet–fed rats were given ad libitum access to a safflower oil–based high-fat diet containing 60% calories from fat (Dyets Inc. 112245), and high-fat diet–fed mice were given a lard-based high-fat diet containing 60% calories from fat (Research Diets 12492). The duration of the high-fat feeding period was 10 days or 4 weeks, as specified in the text. In the VLCD studies, following a 6-week ad libitum feeding period, rats were placed on a VLCD for up to 6 weeks, during which time they were given 2 g of the safflower oil HFD daily, and body weight was monitored twice weekly. Rats were studied once their fed body weight was lower than 350 g.
Pharmacologic interventions.
Rats underwent infusions of leptin (MilliporeSigma; 60 pmol/kg/min for 60–150 minutes, as shown in the figures) into an arterial catheter advanced into the aortic arch. Epinephrine (MilliporeSigma) was infused into the arterial catheter at a rate of 4 μg/kg/min (low dose), except in rats noted as receiving high-dose epinephrine: 20 μg/kg/min. Unless otherwise stated in the figures, epinephrine was infused for 120 minutes. When noted, rats were given an i.p. injection of atenolol (MilliporeSigma; 10 mg/kg) or atglistatin (MilliporeSigma; 200 μmol/kg), which was solubilized with HCl, and pH was adjusted to 6.4 using Tris base immediately before injection. A small molecule superactive leptin antagonist was obtained as a gift from Protein Laboratories Rehovot Ltd. and was subsequently obtained from MyBioSource. The inhibitor was dissolved in PBS, and 25 μg was injected i.p. in 48-hour-fasted rats 60 minutes prior to refeeding. To restore BAT long-chain acyl-CoA concentrations, Liposyn (MilliporeSigma)/heparin (5 U/mL) was infused at a rate of 83.3 μL/kg/min for 120 minutes. Secretin (MilliporeSigma; 5 nmol in PBS) was administered as a single i.p. injection.
In the ICV leptin infusion studies, somatostatin (1:1 somatostatin-14 and -28; Bachem) was infused into an arterial catheter for 30 minutes at a rate of 4 ng/kg/min, after which 0.625 nmol leptin in PBS, or PBS vehicle, was infused through the ICV catheter (total volume 10 μL), with the somatostatin infusion continuing. Thirty minutes after the initiation of the leptin infusion, blood samples were obtained and rectal temperature was measured. At the conclusion of this study, a heparin/glycerol solution (500 U heparin/mL, 50% glycerol) was injected into the catheters, which were then tied off. Rats were fed a high-fat diet for 10 days and studied again using the same protocol.
OGT FKO mice and their WT littermates undergoing a hyperglycemic clamp were treated with a bolus injection of leptin (5 nmol/kg) following a 48-hour fast. Fifteen minutes thereafter, body temperature was measured, and the mice were treated with a bolus injection of atenolol (10 mg/kg), with body temperature measured 15 minutes later.
Tamoxifen-treated Adrβ3 BKO mice and their Cre-WT littermate controls were fasted for 48 hours and gavaged with dextrose (0.58 kcal). Body temperature was measured every 30 minutes thereafter. Blood was obtained from the tail vein in the awake state 30 minutes after dextrose gavage.
Body temperature and heart rate.
Unless otherwise specified, body temperature was measured in the awake state using a probe inserted 2 cm (rats) or 0.5 cm (mice) into the rectum while the rodent was briefly restrained lightly by hand. Tape was placed on the probe to ensure accurate distance. During the colonic temperature validation studies, rats were lightly anesthetized under isoflurane anesthesia such that they would respond to a firm toe pinch but did not flinch during the insertion of the rectal probe. Body temperature was measured at 1-cm increments 0–6 cm into the rectum, based on distance markings placed beforehand on the probe. Heart rate measurements were obtained using the carotid arterial catheter using BDAS Basic Data Acquisition Software from Harvard Apparatus.
Hyperglycemic clamps.
After a 48-hour fast, WT and OGT FKO mice underwent a 120-minute hyperglycemic clamp. Dextrose (20%) was infused at a variable rate through the jugular venous catheter, with plasma glucose concentrations measured every 10–15 minutes and the glucose infusion rate adjusted to achieve a plasma glucose concentration of approximately 200 mg/dL.
Acute feeding studies.
In the acute meal tests, mice were gavaged with an isocaloric (0.58 kcal) bolus of 50% dextrose (carbohydrate), canola oil (fat), or casein suspension (protein). Rats were administered an isocaloric (9 kcal) bolus of 50% dextrose, canola oil, or casein through a catheter in the stomach (oral administration) or through the jugular venous catheter (i.v. administration) over 60 minutes.
Urine collection.
Urine was collected into tubes precoated with EDTA using Harvard Apparatus metabolic cages over a duration of 12 hours under fasted (12- to 24-hours-fasted) and refed (0–12 hours following refeeding) conditions, and for 4 hours after an injection of recombinant mouse leptin (5 nmol/kg).
Chronic feeding studies.
In the basal versus bolus feeding studies, rats were administered their total daily calories in 2 boluses of Ensure Plus enriched with 25% dextrose (11.2 kcal per bolus) through an intragastric catheter, or continuously through the intragastric catheter. The rats also treated with atenolol were given the same Ensure Plus, with atenolol added (total daily dose: 3.5 mg per rat per day). Body temperature was taken daily at the end of the 60-minute infusion period for bolus-fed rats, while the continuous infusion continued.
In the timed feeding studies, rats were given their total daily calories (22.4 kcal per day) in a single intragastric bolus lasting 60 minutes, either between 7:00 and 9:00 am, or between 6 :00and 8:00 pm. Body temperature was measured in all animals at the conclusion of the bolus (and at the same time in rats whose feeding time was offset from the recently fed group).
Biochemical analysis.
Plasma glucose was measured enzymatically using the YSI Glucose Analyzer and NEFAs using the Wako Diagnostics HR(2) kit. Plasma insulin, leptin, corticosterone, catecholamines, secretin, T3, and T4 concentrations were measured by ELISA (Mercodia, R&D Systems, Alpco, Abnova, RayBioTech, MyBioSource, and MyBioSource, respectively) with the exception of the somatostatin/ICV leptin infusion study, in which plasma insulin concentrations were measured by radioimmunoassay by the Yale Diabetes Research RIA Core. Plasma amino acid concentrations were measured by gas chromatography/mass spectrometry (GC/MS) (53).
OGT and β3-adrenergic receptor expression were measured by Western blot analysis using antibodies from Cell Signaling Technology (clone D1D8Q; catalog 24083) and Abcam (catalog ab94506). BAT long-chain acyl-CoA concentrations were measured by liquid chromatography–MS/MS (LC-MS/MS) (90).
Statistics.
In all figures, data are presented as mean ± SEM. Comparisons of 3 or more groups were performed using 1-way ANOVA with Bonferroni’s multiple-comparisons test, and 2 groups were compared by 2-tailed Student’s t test (paired or unpaired as reported in the figure legends). P values less than 0.05 were considered significant. Statistical analysis was performed using GraphPad Prism version 7.0a.
Study approval.
All rodent studies were approved by the Yale University Institutional Animal Care and Use Committee.
Author contributions
This study was designed by RJP and GIS. Data were collected and analyzed by RJP, KL, ARC, and XL. OGT FKO mice were generated by YY and XY, and Adrβ3 BKO mice by HQ and AW. The BATectomy surgical procedure was developed and all rat surgeries performed by JD. The manuscript was written by RJP and GIS with contributions from all authors.
Supplementary Material
Acknowledgments
The authors thank Elena Gracheva and Slav Bagriantsev for helpful discussions, and Ali Nasiri, Wanling Zhu, Xiaoxian Ma, Dongyan Zhang, and Mario Kahn for expert technical assistance. The UCP1-CreER mice were a gift from Christian Wolfrum (ETH Zurich). The Adrβ3fl/fl mice were a gift from Jean-Luc Balligand (UC Louvain). This study was funded by grants from the US Public Health Service: R01 DK116774, R01 DK11968, R01 DK113984, and P30 DK045735 (to GIS); K99/R00 CA215315 (to RJP); R01 DK089098 and R01 DK102648 (to XY); K08AI128745 (to AW); and T32 DK101019, R01 NS087568, UL1TR000142, T32 DK007058.
Version 1. 03/09/2020
Electronic publication
Version 2. 04/01/2020
Print issue publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2020, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2020;130(4):2001–2016.https://doi.org/10.1172/JCI134699.
Contributor Information
Rachel J. Perry, Email: rachel.perry@yale.edu.
Kun Lyu, Email: kun.lyu@yale.edu.
Aviva Rabin-Court, Email: aviva.rabin-court@yale.edu.
Jianying Dong, Email: jianying.dong@yale.edu.
Xiruo Li, Email: xiruo.li@yale.edu.
Yunfan Yang, Email: yunfan.yang@yale.edu.
Hua Qing, Email: hua.qing@yale.edu.
Andrew Wang, Email: andrew.wang@yale.edu.
Xiaoyong Yang, Email: xiaoyong.yang@yale.edu.
Gerald I. Shulman, Email: gerald.shulman@yale.edu.
References
- 1.Welle SL, Seaton TB, Campbell RG. Some metabolic effects of overeating in man. Am J Clin Nutr. 1986;44(6):718–724. doi: 10.1093/ajcn/44.6.718. [DOI] [PubMed] [Google Scholar]
- 2.Dulloo AG, Girardier L. Energy expenditure and diet-induced thermogenesis in presence and absence of hyperphagia induced by insulin. Am J Physiol. 1989;257(4 Pt 2):R717–R725. doi: 10.1152/ajpregu.1989.257.4.R717. [DOI] [PubMed] [Google Scholar]
- 3.Kassis A, et al. Effects of protein quantity type on diet induced thermogenesis in overweight adults: a randomized controlled trial. Clin Nutr. 2019;38(4):1570–1580. doi: 10.1016/j.clnu.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Tentolouris N, et al. Differential effects of high-fat and high-carbohydrate isoenergetic meals on cardiac autonomic nervous system activity in lean and obese women. Metabolism. 2003;52(11):1426–1432. doi: 10.1016/S0026-0495(03)00322-6. [DOI] [PubMed] [Google Scholar]
- 5.Robinson SM, Jaccard C, Persaud C, Jackson AA, Jequier E, Schutz Y. Protein turnover and thermogenesis in response to high-protein and high-carbohydrate feeding in men. Am J Clin Nutr. 1990;52(1):72–80. doi: 10.1093/ajcn/52.1.72. [DOI] [PubMed] [Google Scholar]
- 6.Nagai N, Sakane N, Moritani T. Metabolic responses to high-fat or low-fat meals and association with sympathetic nervous system activity in healthy young men. J Nutr Sci Vitaminol (Tokyo) 2005;51(5):355–360. doi: 10.3177/jnsv.51.355. [DOI] [PubMed] [Google Scholar]
- 7.Welle S, Lilavivat U, Campbell RG. Thermic effect of feeding in man: increased plasma norepinephrine levels following glucose but not protein or fat consumption. Metabolism. 1981;30(10):953–958. doi: 10.1016/0026-0495(81)90092-5. [DOI] [PubMed] [Google Scholar]
- 8.Ingves S, Vilhelmsson N, Ström E, Fredrikson M, Guldbrand H, Nystrom FH. A randomized cross-over study of the effects of macronutrient composition and meal frequency on GLP-1, ghrelin and energy expenditure in humans. Peptides. 2017;93:20–26. doi: 10.1016/j.peptides.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Marques-Lopes I, Forga L, Martínez JA. Thermogenesis induced by a high-carbohydrate meal in fasted lean and overweight young men: insulin, body fat, and sympathetic nervous system involvement. Nutrition. 2003;19(1):25–29. doi: 10.1016/S0899-9007(02)00950-4. [DOI] [PubMed] [Google Scholar]
- 10.Markovic TP, Furler SM, Jenkins AB, Campbell LV, Kraegen EW, Chisholm DJ. Importance of early insulin levels on prandial glycaemic responses and thermogenesis in non-insulin-dependent diabetes mellitus. Diabet Med. 1995;12(6):523–530. doi: 10.1111/j.1464-5491.1995.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 11.Heijnen ML, Deurenberg P, van Amelsvoort JM, Beynen AC. Replacement of digestible by resistant starch lowers diet-induced thermogenesis in healthy men. Br J Nutr. 1995;73(3):423–432. doi: 10.1079/BJN19950044. [DOI] [PubMed] [Google Scholar]
- 12.Rothwell NJ, Saville ME, Stock MJ. Role of insulin in thermogenic responses to refeeding in 3-day-fasted rats. Am J Physiol. 1983;245(2):E160–E165. doi: 10.1152/ajpendo.1983.245.2.E160. [DOI] [PubMed] [Google Scholar]
- 13.Diamond P, LeBlanc J. A role for insulin in cephalic phase of postprandial thermogenesis in dogs. Am J Physiol. 1988;254(5 pt 1):E625–E632. doi: 10.1152/ajpendo.1988.254.5.E625. [DOI] [PubMed] [Google Scholar]
- 14.Shetty PS, Jung RT, James WP, Barrand MA, Callingham BA. Postprandial thermogenesis in obesity. Clin Sci. 1981;60(5):519–525. doi: 10.1042/cs0600519. [DOI] [PubMed] [Google Scholar]
- 15.Blaak EE, et al. Impaired fat-induced thermogenesis in obese subjects: the NUGENOB study. Obesity (Silver Spring) 2007;15(3):653–663. doi: 10.1038/oby.2007.606. [DOI] [PubMed] [Google Scholar]
- 16.Marrades MP, Martínez JA, Moreno-Aliaga MJ. Differences in short-term metabolic responses to a lipid load in lean (resistant) vs obese (susceptible) young male subjects with habitual high-fat consumption. Eur J Clin Nutr. 2007;61(2):166–174. doi: 10.1038/sj.ejcn.1602500. [DOI] [PubMed] [Google Scholar]
- 17.Nagai N, Sakane N, Hamada T, Kimura T, Moritani T. The effect of a high-carbohydrate meal on postprandial thermogenesis and sympathetic nervous system activity in boys with a recent onset of obesity. Metab Clin Exp. 2005;54(4):430–438. doi: 10.1016/j.metabol.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 18.De Palo C, Macor C, Sicolo N, Vettor R, Scandellari C, Federspil G. Dietary-induced thermogenesis in obesity. Response to mixed and carbohydrate meals. Acta Diabetol Lat. 1989;26(2):155–162. doi: 10.1007/BF02581367. [DOI] [PubMed] [Google Scholar]
- 19.Segal KR, Gutin B, Albu J, Pi-Sunyer FX. Thermic effects of food and exercise in lean and obese men of similar lean body mass. Am J Physiol. 1987;252(1 pt 1):E110–E117. doi: 10.1152/ajpendo.1987.252.1.E110. [DOI] [PubMed] [Google Scholar]
- 20.Segal KR, Lacayanga I, Dunaif A, Gutin B, Pi-Sunyer FX. Impact of body fat mass and percent fat on metabolic rate and thermogenesis in men. Am J Physiol. 1989;256(5 pt 1):E573–E579. doi: 10.1152/ajpendo.1989.256.5.E573. [DOI] [PubMed] [Google Scholar]
- 21.Brundin T, Thörne A, Wahren J. Heat leakage across the abdominal wall and meal-induced thermogenesis in normal-weight and obese subjects. Metab Clin Exp. 1992;41(1):49–55. doi: 10.1016/0026-0495(92)90190-L. [DOI] [PubMed] [Google Scholar]
- 22.Salas-Salvadó J, Barenys-Manent M, Recasens Gracia MA, Martí-Henneberg C. Influence of adiposity on the thermic effect of food and exercise in lean and obese adolescents. Int J Obes Relat Metab Disord. 1993;17(12):717–722. [PubMed] [Google Scholar]
- 23.Steiniger J, Karst H, Noack R, Steglich HD. Diet-induced thermogenesis in man: thermic effects of single protein and carbohydrate test meals in lean and obese subjects. Ann Nutr Metab. 1987;31(2):117–125. doi: 10.1159/000177258. [DOI] [PubMed] [Google Scholar]
- 24.Maffeis C, Schutz Y, Zoccante L, Micciolo R, Pinelli L. Meal-induced thermogenesis in lean and obese prepubertal children. Am J Clin Nutr. 1993;57(4):481–485. doi: 10.1093/ajcn/57.4.481. [DOI] [PubMed] [Google Scholar]
- 25.Schutz Y, Bessard T, Jequier E. Diet-induced thermogenesis measured over a whole day in obese and nonobese women. Am J Clin Nutr. 1984;40(3):542–552. doi: 10.1093/ajcn/40.3.542. [DOI] [PubMed] [Google Scholar]
- 26.Katzeff HL, O’Connell M, Horton ES, Danforth E, Young JB, Landsberg L. Metabolic studies in human obesity during overnutrition and undernutrition: thermogenic and hormonal responses to norepinephrine. Metab Clin Exp. 1986;35(2):166–175. doi: 10.1016/0026-0495(86)90119-8. [DOI] [PubMed] [Google Scholar]
- 27.Thörne A, Hallberg D, Wahren J. Meal-induced thermogenesis in obese patients before and after weight reduction. Clin Physiol. 1989;9(5):481–498. doi: 10.1111/j.1475-097X.1989.tb01002.x. [DOI] [PubMed] [Google Scholar]
- 28.Maffeis C, Schutz Y, Pinelli L. Postprandial thermogenesis in obese children before and after weight reduction. Eur J Clin Nutr. 1992;46(8):577–583. [PubMed] [Google Scholar]
- 29.Tentolouris N, Pavlatos S, Kokkinos A, Perrea D, Pagoni S, Katsilambros N. Diet-induced thermogenesis and substrate oxidation are not different between lean and obese women after two different isocaloric meals, one rich in protein and one rich in fat. Metab Clin Exp. 2008;57(3):313–320. doi: 10.1016/j.metabol.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Felig P, Cunningham J, Levitt M, Hendler R, Nadel E. Energy expenditure in obesity in fasting and postprandial state. Am J Physiol. 1983;244(1):E45–E51. doi: 10.1152/ajpendo.1983.244.1.E45. [DOI] [PubMed] [Google Scholar]
- 31.Nair KS, Halliday D, Garrow JS. Thermic response to isoenergetic protein, carbohydrate or fat meals in lean and obese subjects. Clin Sci (Lond) 1983;65(3):307–312. doi: 10.1042/cs0650307. [DOI] [PubMed] [Google Scholar]
- 32.Glick Z, Wickler SJ, Stern JS, Horwitz BA. Regional blood flow in rats after a single low-protein, high-carbohydrate test meal. Am J Physiol. 1984;247(1 Pt 2):R160–R166. doi: 10.1152/ajpregu.1984.247.1.R160. [DOI] [PubMed] [Google Scholar]
- 33.Glick Z, Teague RJ, Bray GA. Brown adipose tissue: thermic response increased by a single low protein, high carbohydrate meal. Science. 1981;213(4512):1125–1127. doi: 10.1126/science.7268419. [DOI] [PubMed] [Google Scholar]
- 34.U Din M, et al. Postprandial oxidative metabolism of human brown fat indicates thermogenesis. Cell Metab. 2018;28(2):207–216.e3. doi: 10.1016/j.cmet.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 35.Vosselman MJ, et al. Brown adipose tissue activity after a high-calorie meal in humans. Am J Clin Nutr. 2013;98(1):57–64. doi: 10.3945/ajcn.113.059022. [DOI] [PubMed] [Google Scholar]
- 36.Glick Z, Teague RJ, Bray GA, Lee M. Compositional and metabolic changes in brown adipose tissue following a single test meal. Metab Clin Exp. 1983;32(12):1146–1150. doi: 10.1016/0026-0495(83)90062-8. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, et al. Secretin-activated brown fat mediates prandial thermogenesis to induce satiation. Cell. 2018;175(6):1561–1574.e12. doi: 10.1016/j.cell.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Blessing W, Mohammed M, Ootsuka Y. Heating and eating: brown adipose tissue thermogenesis precedes food ingestion as part of the ultradian basic rest-activity cycle in rats. Physiol Behav. 2012;105(4):966–974. doi: 10.1016/j.physbeh.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Kontos A, de Menezes RC, Ootsuka Y, Blessing W. Brown adipose tissue thermogenesis precedes food intake in genetically obese Zucker (fa/fa) rats. Physiol Behav. 2013;118:129–137. doi: 10.1016/j.physbeh.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 40.Blessing W, Mohammed M, Ootsuka Y. Brown adipose tissue thermogenesis, the basic rest-activity cycle, meal initiation, and bodily homeostasis in rats. Physiol Behav. 2013;121:61–69. doi: 10.1016/j.physbeh.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 41.Himms-Hagen J. Role of brown adipose tissue thermogenesis in control of thermoregulatory feeding in rats: a new hypothesis that links thermostatic and glucostatic hypotheses for control of food intake. Proc Soc Exp Biol Med. 1995;208(2):159–169. doi: 10.3181/00379727-208-43847A. [DOI] [PubMed] [Google Scholar]
- 42.Mansell PI, MacDonald IA. The effect of underfeeding on the physiological response to food ingestion in normal weight women. Br J Nutr. 1988;60(1):39–48. doi: 10.1079/BJN19880074. [DOI] [PubMed] [Google Scholar]
- 43.Welle S. Evidence that the sympathetic nervous system does not regulate dietary thermogenesis in humans. Int J Obes. 1985;9(suppl 2):115–121. [PubMed] [Google Scholar]
- 44.Bo S, et al. Effects of meal timing on changes in circulating epinephrine, norepinephrine, and acylated ghrelin concentrations: a pilot study. Nutr Diabetes. 2017;7(12):303. doi: 10.1038/s41387-017-0010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ravussin E, Bogardus C. Thermogenic response to insulin and glucose infusions in man: a model to evaluate the different components of the thermic effect of carbohydrate. Life Sci. 1982;31(18):2011–2018. doi: 10.1016/0024-3205(82)90040-6. [DOI] [PubMed] [Google Scholar]
- 46.Limberg JK, et al. Resting sympathetic activity is associated with the sympathetically mediated component of energy expenditure following a meal. Physiol Rep. 2017;5(16):e13389. doi: 10.14814/phy2.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diamond P, LeBlanc J. Role of autonomic nervous system in postprandial thermogenesis in dogs. Am J Physiol. 1987;252(6 Pt 1):E719–E726. doi: 10.1152/ajpendo.1987.252.6.E719. [DOI] [PubMed] [Google Scholar]
- 48.Zed C, James WP. Dietary thermogenesis in obesity. Response to carbohydrate and protein meals: the effect of beta-adrenergic blockade and semistarvation. Int J Obes. 1986;10(5):391–405. [PubMed] [Google Scholar]
- 49.Welle S, Campbell RG. Stimulation of thermogenesis by carbohydrate overfeeding. Evidence against sympathetic nervous system mediation. J Clin Invest. 1983;71(4):916–925. doi: 10.1172/JCI110846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeFronzo RA, et al. Effect of beta and alpha adrenergic blockade on glucose-induced thermogenesis in man. J Clin Invest. 1984;73(3):633–639. doi: 10.1172/JCI111253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thörne A, Wahren J. Beta-adrenergic blockade does not influence the thermogenic response to a mixed meal in man. Clin Physiol. 1989;9(4):321–332. doi: 10.1111/j.1475-097X.1989.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 52.Menozzi R, Bondi M, Baldini A, Venneri MG, Velardo A, Del Rio G. Resting metabolic rate, fat-free mass and catecholamine excretion during weight loss in female obese patients. Br J Nutr. 2000;84(4):515–520. doi: 10.1017/S0007114500001823. [DOI] [PubMed] [Google Scholar]
- 53.Perry RJ, et al. Leptin mediates a glucose-fatty acid cycle to maintain glucose homeostasis in starvation. Cell. 2018;172(1–2):234–248.e17. doi: 10.1016/j.cell.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perry RJ, et al. Leptin’s hunger-suppressing effects are mediated by the hypothalamic-pituitary-adrenocortical axis in rodents. Proc Natl Acad Sci U S A. 2019;116(27):13670–13679. doi: 10.1073/pnas.1901795116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahima RS, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 56.Vujovic P, et al. Time-dependent effects of starvation on serum, pituitary and hypothalamic leptin levels in rats. Physiol Res. 2011;60(suppl 1):S165–S170. doi: 10.33549/physiolres.932174. [DOI] [PubMed] [Google Scholar]
- 57.Frederich RC, et al. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest. 1995;96(3):1658–1663. doi: 10.1172/JCI118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394(6696):897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 59.Ahima RS, Bjorbaek C, Osei S, Flier JS. Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology. 1999;140(6):2755–2762. doi: 10.1210/endo.140.6.6774. [DOI] [PubMed] [Google Scholar]
- 60.Kmiec Z, Pokrywka L, Kotlarz G, Kubasik J, Szutowicz A, Mysliwski A. Effects of fasting and refeeding on serum leptin, adiponectin and free fatty acid concentrations in young and old male rats. Gerontology. 2005;51(6):357–362. doi: 10.1159/000088698. [DOI] [PubMed] [Google Scholar]
- 61.Yoneshiro T, et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature. 2019;572(7771):614–619. doi: 10.1038/s41586-019-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barton C, York DA, Bray GA. Bombesin-induced hypothermia in rats tested at normal ambient temperatures: contribution of the sympathetic nervous system. Brain Res Bull. 1995;37(2):163–168. doi: 10.1016/0361-9230(94)00272-3. [DOI] [PubMed] [Google Scholar]
- 63.Matsumoto T, Miyawaki C, Ue H, Kanda T, Yoshitake Y, Moritani T. Comparison of thermogenic sympathetic response to food intake between obese and non-obese young women. Obes Res. 2001;9(2):78–85. doi: 10.1038/oby.2001.10. [DOI] [PubMed] [Google Scholar]
- 64.Vaz M, et al. Postprandial sympatho-adrenal activity: its relation to metabolic and cardiovascular events and to changes in meal frequency. Clin Sci. 1995;89(4):349–357. doi: 10.1042/cs0890349. [DOI] [PubMed] [Google Scholar]
- 65.Ikeda K, et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med. 2017;23(12):1454–1465. doi: 10.1038/nm.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li B, et al. Microbiota depletion impairs thermogenesis of brown adipose tissue and browning of white adipose tissue. Cell Rep. 2019;26(10):2720–2737.e5. doi: 10.1016/j.celrep.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 67.Presby DM, et al. Compensation for cold-induced thermogenesis during weight loss maintenance regain. Am J Physiol Endocrinol Metab. 2019;316(5):E977–E986. doi: 10.1152/ajpendo.00543.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lemecha M, et al. MiR-494-3p regulates mitochondrial biogenesis and thermogenesis through PGC1-α signalling in beige adipocytes. Sci Rep. 2018;8(1):15096. doi: 10.1038/s41598-018-33438-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bal NC, et al. Both brown adipose tissue and skeletal muscle thermogenesis processes are activated during mild to severe cold adaptation in mice. J Biol Chem. 2017;292(40):16616–16625. doi: 10.1074/jbc.M117.790451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rowland LA, Maurya SK, Bal NC, Kozak L, Periasamy M. Sarcolipin and uncoupling protein 1 play distinct roles in diet-induced thermogenesis and do not compensate for one another. Obesity (Silver Spring) 2016;24(7):1430–1433. doi: 10.1002/oby.21542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rowland LA, Bal NC, Kozak LP, Periasamy M. Uncoupling protein 1 and sarcolipin are required to maintain optimal thermogenesis, and loss of both systems compromises survival of mice under cold stress. J Biol Chem. 2015;290(19):12282–12289. doi: 10.1074/jbc.M115.637603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Friedman J. The long road to leptin. J Clin Invest. 2016;126(12):4727–4734. doi: 10.1172/JCI91578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Flier JS, Maratos-Flier E. Leptin’s physiologic role: does the emperor of energy balance have no clothes? Cell Metab. 2017;26(1):24–26. doi: 10.1016/j.cmet.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 74.Li MD, et al. Adipocyte OGT governs diet-induced hyperphagia and obesity. Nat Commun. 2018;9(1):5103. doi: 10.1038/s41467-018-07461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ingram DL, Dauncey MJ. Role of thyroid hormones in the modification of diet-induced thermogenesis by propranolol. Horm Metab Res. 1986;18(10):677–679. doi: 10.1055/s-2007-1012405. [DOI] [PubMed] [Google Scholar]
- 76.Rothwell NJ, Stock MJ, Tedstone AE. Effects of ciglitazone on energy balance, thermogenesis and brown fat activity in the rat. Mol Cell Endocrinol. 1987;51(3):253–257. doi: 10.1016/0303-7207(87)90035-9. [DOI] [PubMed] [Google Scholar]
- 77.Dauncey MJ, Ingram DL. Effect of dietary composition and cold exposure on non-shivering thermogenesis in young pigs and its alteration by the beta-blocker propranolol. Br J Nutr. 1979;41(2):361–370. doi: 10.1079/BJN19790045. [DOI] [PubMed] [Google Scholar]
- 78.Thörne A. Diet-induced thermogenesis. An experimental study in healthy and obese individuals. Acta Chir Scand Suppl. 1990;558:6–59. [PubMed] [Google Scholar]
- 79.Astrup A, Simonsen L, Bülow J, Madsen J, Christensen NJ. Epinephrine mediates facultative carbohydrate-induced thermogenesis in human skeletal muscle. Am J Physiol. 1989;257(3 pt 1):E340–E345. doi: 10.1152/ajpendo.1989.257.3.E340. [DOI] [PubMed] [Google Scholar]
- 80.Schnabel P, Maack C, Mies F, Tyroller S, Scheer A, Böhm M. Binding properties of beta-blockers at recombinant beta1-, beta2-, and beta3-adrenoceptors. J Cardiovasc Pharmacol. 2000;36(4):466–471. doi: 10.1097/00005344-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 81.Glick Z. Inverse relationship between brown fat thermogenesis and meal size: the thermostatic control of food intake revisited. Physiol Behav. 1982;29(6):1137–1140. doi: 10.1016/0031-9384(82)90310-9. [DOI] [PubMed] [Google Scholar]
- 82.Sundaresan S, et al. CD36-dependent signaling mediates fatty acid-induced gut release of secretin and cholecystokinin. FASEB J. 2013;27(3):1191–1202. doi: 10.1096/fj.12-217703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee KY, Tai HH, Chey WY. Plasma secretin and gastrin responses to a meat meal and duodenal acidification in dogs. Am J Physiol. 1976;230(3):784–789. doi: 10.1152/ajplegacy.1976.230.3.784. [DOI] [PubMed] [Google Scholar]
- 84.Rhodes RA, Tai HH, Chey WY. Observations of plasma secretin levels by radioimmunoassay in response to duodenal acidification and to a meat meal in humans. Am J Dig Dis. 1976;21(10):873–879. doi: 10.1007/BF01072080. [DOI] [PubMed] [Google Scholar]
- 85.Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23(6):1048–1059. doi: 10.1016/j.cmet.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rothschild J, Hoddy KK, Jambazian P, Varady KA. Time-restricted feeding and risk of metabolic disease: a review of human and animal studies. Nutr Rev. 2014;72(5):308–318. doi: 10.1111/nure.12104. [DOI] [PubMed] [Google Scholar]
- 87.Patterson RE, Sears DD. Metabolic effects of intermittent fasting. Annu Rev Nutr. 2017;37:371–393. doi: 10.1146/annurev-nutr-071816-064634. [DOI] [PubMed] [Google Scholar]
- 88.Rosenwald M, Perdikari A, Rülicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15(6):659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- 89.Hermida N, et al. Cardiac myocyte β3-adrenergic receptors prevent myocardial fibrosis by modulating oxidant stress-dependent paracrine signaling. Eur Heart J. 2018;39(10):888–898. doi: 10.1093/eurheartj/ehx366. [DOI] [PubMed] [Google Scholar]
- 90.Yu C, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277(52):50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.