Abstract
Multiple myeloma (MM), a bone marrow–resident hematological malignancy of plasma cells, has remained largely incurable despite dramatic improvements in patient outcomes in the era of myeloma-targeted and immunomodulatory agents. It has recently become clear that T cells from MM patients are able to recognize and eliminate myeloma, although this is subverted in the majority of patients who eventually succumb to progressive disease. T cell exhaustion and a suppressive bone marrow microenvironment have been implicated in disease progression, and once these are established, immunotherapy appears largely ineffective. Autologous stem cell transplantation (ASCT) is a standard of care in eligible patients and results in immune effects beyond cytoreduction, including lymphodepletion, T cell priming via immunogenic cell death, and inflammation; all occur within the context of a disrupted bone marrow microenvironment. Recent studies suggest that ASCT reestablishes immune equilibrium and thus represents a logical platform in which to intervene to prevent immune escape. New immunotherapies based on checkpoint inhibition targeting the immune receptor TIGIT and the deletion of suppressive myeloid populations appear attractive, particularly after ASCT. Finally, the immunologically favorable environment created after ASCT may also represent an opportunity for approaches utilizing bispecific antibodies or chimeric antigen receptor T cells.
Introduction
Multiple myeloma (MM) is a hematological malignancy characterized by expansion of clonal plasma cells in the bone marrow (BM) that produce monoclonal immunoglobulin (M band) (1). MM typically causes end-organ damage consisting of anemia, renal impairment, lytic bony lesions, and hypercalcemia (1). Global incidence has increased by 126% since 1990 (2), and it typically occurs in the elderly, with 85% and 60% of diagnoses made in individuals over 55 and 65 years of age, respectively. With improved treatment regimens and the use of myeloablative chemotherapy with autologous stem cell transplantation (ASCT), median survival now exceeds 6 years, although this is highly variable depending on disease risk factors. Despite dramatic therapeutic evolution, myeloma remains largely incurable.
Interestingly, MM often progresses from a premalignant state, monoclonal gammopathy of undetermined significance (MGUS), that displays a lifelong rate of progression of 1% per year (3, 4). Smoldering multiple myeloma (SMM) is a second precursor state of active MM wherein patients have higher frequencies of BM clonal plasma cells than do MGUS patients, but have yet to develop symptoms of myeloma-related end-organ damage (5). Malignant transformation is a consequence of a combination of factors including both primary and secondary genetic events, genetic heterogeneity with subsequent clonal evolution, and changes in the BM microenvironment (6, 7). Additionally, immune dysfunction has been observed in myeloma patients (8–14), raising the question of whether immunological escape is an additional mechanism of disease progression.
In this Review we discuss potential immunological processes of myeloma control and immunological escape that manifests as disease progression. In this context, we will address the current status of immunotherapy in the clinical setting and in preclinical models that together provide a perspective on the future directions of immunotherapy for myeloma.
Evidence for immune-mediated myeloma control
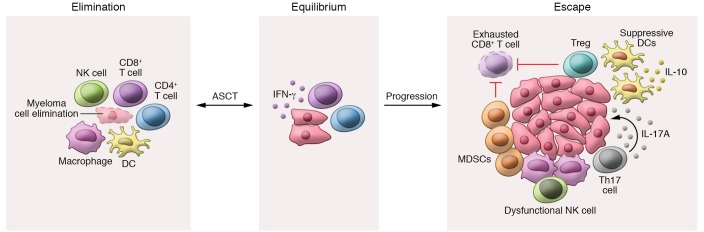
The role of immunosurveillance and the concept that tumors progress from a state of immune equilibrium to an escape phase are well described for solid tumors (Figure 1). Incomplete elimination of tumor cells results in an equilibrium whereby adaptive and innate immunity keeps remaining tumor cells in a state of functional dormancy (15). Escape occurs in the context of genetic changes leading to loss of antigen expression or presentation, induction of immunosuppressive microenvironment, and/or development of resistance to immune effector responses (15). Increasing evidence suggests that MGUS/SMM may represent a state of immune equilibrium that is subsequently disrupted during progression to active myeloma. Whole-exome sequencing of paired patient samples collected at diagnosis of MGUS/SMM and again at MM found that most somatic mutations preceded diagnosis of clinical MM (16, 17), suggesting that although genetic mutations are necessary for tumorigenesis, they are not sufficient for transformation of myeloma. Consistent with this, the mutational burden of MGUS/SMM patients who did not progress to MM was found to be equivalent to the mutational burden of progressors (18). Thus, extrinsic factors are likely an additional determinant of subclonal evolution and progression from premalignant states to clinical myeloma (17).
Figure 1. Potential immunoediting in multiple myeloma.
Cancer immunoediting involves three sequential phases: elimination, equilibrium, and escape (15). Elimination is mediated by collaboration of the adaptive and the innate immunity to eradicate malignant cells prior to the onset of clinical presentation. However, if elimination is incomplete and rare myeloma cell variants enter dormancy, equilibrium is established. After autologous stem cell transplantation (ASCT), equilibrium is mediated by effector T cells and is IFN-γ–dependent. Escape is associated with the accumulation of genetic mutations, resistance to immune effectors, CD8+ T cell exhaustion, and changes in the microenvironment. Regulatory T cells (Tregs), suppressive dendritic cells (DCs), T helper 17 (Th17) cells, tumor-associated macrophages, and myeloid-derived suppressor cells (MDSCs) all encourage escape and inhibit CD8+ T cell function. ASCT appears to restore a period of immune equilibrium but is usually followed by further escape and disease progression.
Two randomized phase III trials recently provided direct evidence of immune-mediated myeloma control, as patients with high-risk SMM (5) demonstrated longer time to progression with lenalidomide-based treatment compared with observation alone (SWOGS0120, NCT00480363, ref. 19; ECOG E3A06, NCT01169337, ref. 20). Further support for immune-mediated MM control lies in the ability to generate cytotoxic T cells against autologous tumors from myeloma patients ex vivo (21–24), even in the context of a low mutational burden (25). Indeed, freshly isolated T cells from MGUS patients’ BM produced IFN-γ in response to autologous preneoplastic cell–loaded DCs, while freshly isolated T cells from MM patients were unresponsive (21, 22). In a preclinical model, adding autologous or syngeneic T cells to the BM graft dramatically improved survival and reduced myeloma progression (26). In this model, myeloma-specific T cells could also be recovered from recipient BM of long-term survivors of ASCT and could transfer myeloma-specific immunity to secondary recipients. It is important to note that the Vk*MYC model of myeloma used in these preclinical studies generates similar disease to that in patients, with lytic lesions, renal impairment, clonal plasma cell expansion, and associated M bands (27). Moreover, this myeloma’s mutational burden is comparable to that reported in humans (26, 28–30). The importance of memory CD8+ T cells as mediators of MM progression was also demonstrated in patients, as a recent clinical study highlighted attrition of stem-like memory CD8+ T cells in MGUS patients’ BM as a potential catalyst for progression to MM (31).
γδ T cells and natural killer T (NKT) cells also play an important role in immunosurveillance either by directly lysing tumor cells or via activating other immune subsets (32–39). In myeloma, γδ T cells from patients’ BM or peripheral blood exhibited strong antitumor responses to autologous myeloma cells, but not benign cells (40). As myeloma cells express CD1d, they are also sensitive to lysis by NKT cells (41). Interestingly, antitumor NKT cells could be detected in patients with MGUS, nonprogressive disease, or progressive myeloma; however, freshly isolated NKT cells from both the blood and tumor bed of patients with progressive disease had markedly impaired IFN-γ production, although this phenotype was reversible (13). Together, these studies indicate that T cell–dependent myeloma immunity is present, albeit suppressed, in patients with myeloma.
NK cells play a key role in myeloma immunity, and NK dysfunction has been implicated in myeloma progression in nontransplanted myeloma-bearing mice (42). MGUS patients were found to have similar or increased numbers of NK cells compared with healthy donors, while patients with late-stage myeloma have significantly reduced NK cell numbers (43, 44). NK cells are particularly important in the context of treatment with immunomodulatory imide drugs (IMiDs), as IMiDs stimulate IL-2 production by T cells, resulting in NK cell activation and expansion (40, 45, 46). Furthermore, a recent study found that IMiDs prime myeloma for killing by daratumumab, a CD38-targeting mAb, by upregulating CD38 expression and sensitizing myeloma cells to NK cell–mediated antibody-dependent cell-mediated cytotoxicity (ADCC) (47). A second antibody, elotuzumab, binds SLAMF7 on MM cells and Fc receptors (CD16) on NK cells and macrophages to promote ADCC and antibody-dependent cellular phagocytosis (48). This mAb does not have single-agent activity but is active in combination with IMiDs. NK cell–mediated myeloma immunity was addressed in a murine model of ASCT, and surprisingly, NK cells were not required for myeloma control in this setting (26). These data suggest that alternative mechanisms may underpin responses after ASCT, although this has yet to be definitively investigated in a clinical setting.
Immunological escape facilitates myeloma progression
Immunological escape is attributed to a multitude of factors, including T cell exhaustion, tolerization by tumor-associated antigen-presenting cells, alterations in cytokine production, and accumulation of myeloid-derived suppressor cells (MDSCs) and suppressive tumor-associated macrophages (15, 49, 50).
T cell dysfunction can be due to senescence, characterized by maintained functionality but limited proliferative capacity, or exhaustion (51). The exhaustion phenotype is a continuum whereby early exhaustion is associated with inflammatory cytokine production and self-renewal capabilities, which are progressively lost in the context of repeated antigen exposure (52). The presence of exhausted T cells in myeloma patients is somewhat controversial, with some studies suggesting that CD8+ T cells are senescent (8, 10, 11). Interestingly, senescence was telomere-independent (8), and PD-1 expression, which is more traditionally associated with exhaustion (53), was observed on CD57+ CD8+ T cells. These cells displayed markers for both exhaustion and senescence, possibly representing a composite state of dysfunction. Nonetheless, it is widely reported that CD8+ T cells from myeloma patients express multiple immune checkpoint receptors, including PD-1, CTLA-4, TIM-3, LAG-3, and, recently, TIGIT (9–12, 54). Chung et al. also found that these inhibitory receptors are expressed both before and after ASCT (10). Terminal T cell exhaustion is associated with loss of cytotoxicity by subsets of CD4+ and CD8+ T cells that produce IFN-γ, a cytokine critical to tumor immunity (49, 50). Early in vitro studies indicate that IFN-γ directly inhibits myeloma cell growth (55), and preclinical in vivo studies showed enhanced myeloma mortality when IFN-γ was absent in both transplant and nontransplant settings (26, 42). Importantly, studies of BM CD8+ T cells in patients with myeloma revealed decreased IFN-γ secretion and reduced degranulation, indicative of terminal T cell exhaustion (11). Preclinical models also support an active role for CD8+ T cell exhaustion in myeloma progression, as immune checkpoint receptor expression correlates with disease progression in both transplant and nontransplant models (9, 14). Furthermore, both IFN-γ production and CD107a production are decreased in mice with high myeloma burdens, and loss of effector function correlated with disease progression (14, 26). While T cell exhaustion occurs in response to chronic antigen stimulation (53), myeloma cells can also express PD-L1 (56) and CD155 (57) (ligands for the T cell immune receptors PD-1 and TIGIT, respectively) and may thus contribute to exhaustion directly. Indeed, PD-L1 expression on myeloma is associated with drug resistance, and serum levels of PD-L1 predict progression-free survival in myeloma patients (56, 58–60). NK cells from patients with MM were also reported to have reduced expression of activating receptors and upregulation of PD-1, which allows inhibition of NK cytotoxicity by PD-L1–expressing MM cells (61, 62).
Several studies suggest that DCs from patients with myeloma are not only dysfunctional, but also promote myeloma cell survival and may be key determinants of the progression from MGUS to active myeloma (63–66). In preclinical models of ASCT, DC-derived IL-10 is pathogenic, and there is accumulation of IL-10+MHC-IIlo DCs in the BM of myeloma-relapsed mice (14), a finding concurring with reports of DC accumulation in myeloma patients’ BM (67). In these preclinical systems, myeloma control is improved when donor DCs are specifically unable to produce IL-10 (14). Several studies have demonstrated that DCs from patients with myeloma can elicit strong myeloma-specific T cell responses ex vivo (22, 68), indicating that the tumor microenvironment (TME) may also influence DCs’ ability to prime effective antimyeloma immunity. Consistent with this, aberrant IL-6 levels in the myeloma milieu have been associated with dysfunctional antigen presentation (63, 69).
Additionally, changes in the TME cytokine milieu can influence tumor escape by driving noncytolytic T cell differentiation paradigms that in turn are permissive of tumor growth. IL-6, a cytokine known to be dysregulated in patients with myeloma (70), plays a role in myeloma progression (71) and, together with TGF-β, IL-21, and IL-23 (72), promotes the expansion of IL-17A–producing Th17 cells (73, 74). Clinical studies have linked angiogenesis with elevated IL-17A levels in the sera of myeloma patients, and IL-17A broadly promotes myeloma growth (75–78). Importantly, IL-17A deletion in donor grafts, or IL-17A mAb blockade, was sufficient to promote long-term myeloma control in mice after transplantation (26); IL-17A inhibition was also able to delay disease progression in the nontransplant setting (79).
In the TME, macrophages are either antitumorigenic (M1) or differentiate into tumor-associated macrophages with an immunosuppressive M2-like phenotype. This differentiation occurs in response to cytokines, chemokines, and growth factors in the TME (80). CSF-1 is an important mediator of macrophage survival, differentiation, and function, and CSF-1 overexpression has been associated with tumor development and progression (81–83). Accordingly, CSF-1 receptor (CSF-1R) blockade has been shown to promote antitumor immunity (83, 84). Importantly, myeloma progression has been associated with accumulation of CSF-1R–expressing macrophages in preclinical studies, and targeting these populations using CSF-1R–blocking antibodies has proven effective, particularly after ASCT (14, 82, 85). Additionally, IL-18–dependent MDSCs have been implicated in myeloma progression, and IL-18 blockade improved survival in a murine model (86). Importantly, this finding was supported by clinical data: IL-18 and polymorphonuclear MDSC signature genes correlated with myeloma outcome, and high IL-18 levels in BM were associated with poor prognosis (86).
Thus, increasing evidence suggests that myeloma progression is associated with loss of immune control that is reflective of changes in T cell differentiation, T cell exhaustion, and a suppressive BM microenvironment. Importantly, these changes can now be targeted with rational immunotherapeutic combinations.
Clinical status of myeloma immunotherapy and preclinical lessons
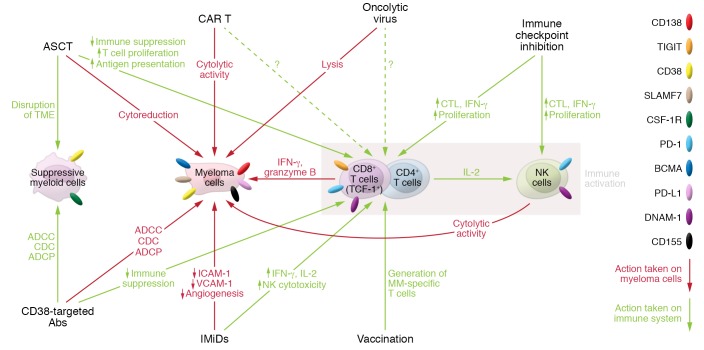
We have now entered a new era of therapy for myeloma firmly centered on immunotherapy, with a clear expansion in the number of clinical trials exploring various immune-based therapies. Most prominently, these therapeutics include agents targeting myeloma-specific antigens, including daratumumab (CD38), oncolytic viruses, and chimeric antigen receptor (CAR) T cells (targeting B cell maturation antigen [BCMA]), and T cell–targeted therapies, including checkpoint inhibition and tumor vaccination, typically in combination with current standard-of-care drugs (Figure 2).
Figure 2. Immunotherapies for myeloma.
Immunotherapies for myeloma target the tumor itself, suppressive myeloid populations, and/or immune cells. CD38-targeted mAbs target CD38-expressing myeloma cells and suppressive myeloid cells by antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP), which also facilitates immune cell activation. Autologous stem cell transplantation (ASCT) disrupts the tumor microenvironment (TME), directly eliminates myeloma cells, and promotes T cell–mediated antimyeloma responses. Chimeric antigen receptor T cells (CAR T) and oncolytic viruses directly promote lysis of myeloma cells. Immune checkpoint inhibitors and cancer vaccination approaches directly enhance T and NK cell–mediated antimyeloma responses. Immunomodulatory imide drugs (IMiDs) directly inhibit myeloma cell growth, reduce angiogenesis, and promote immune cell activation.
Immune checkpoint inhibition.
Use of immunotherapy in myeloma patients has had a somewhat tumultuous start, with early clinical studies reporting a lack of efficacy of nivolumab monotherapy (87). Furthermore, in preclinical studies to date, anti–PD-1 monotherapy has only been described as effective in myeloma when administered after stem cell transplantation (14, 42, 88–90), and at an early time point (26). Interestingly, a clinical trial using pembrolizumab early after ASCT, followed by lenalidomide, reported a complete response (CR) in 7 of 23 (31%) patients (91). This represents an improvement from the initial trial in relapsed/refractory MM (RRMM) patients, in which CR was observed only in one patient who underwent radiotherapy (87). Notably, this small post-transplant study was terminated early for failure to meet its interim analysis endpoint; however, the treatment was associated with minimal residual disease (MRD) negativity in 75% of patients at 180 days after ASCT (91). Nonetheless, although preclinical studies suggest that current immunotherapies may be most effective when implemented early after ASCT (a concept supported by clinical observations in patients) (10), further investigation in large randomized clinical trial cohorts is required.
Preclinical models also suggest that a combination approach targeting multiple checkpoint inhibitors may promote synergistic tumor control (88). A corroborating, phase I/II trial (NCT02681302, ClinicalTrials.gov) investigating ipilimumab in combination with nivolumab early after ASCT, in high-risk transplant-naive or recurrent MM patients, reported promising preliminary results with 71% and 67% of patients, respectively, achieving progression-free survival at 18 months follow-up. This promising rate of progression-free survival may be, in part, due to Treg depletion, which is at least one mechanism of action of ipilimumab in vivo (92, 93). This trial provides further evidence for both the implementation of immunotherapy early after ASCT and the synergistic potential of combination approaches. Unsurprisingly, with this combination of checkpoint inhibitors, 65% of patients developed immune-related adverse events grade 2 or higher and required treatment with systemic steroids. An alternative strategy to checkpoint blockade is treatment with agonist antibodies against the costimulatory receptor CD137 (4-1BB), which promotes CD8+ T cell effector function and proliferation (94). Accordingly, CD137 agonists have been shown to prolong myeloma control in preclinical models by promoting CD8+ T cell effector function in BM in both transplant and nontransplant settings (26, 42, 95). A phase I clinical trial (NCT02252263) investigating the combination of elotuzumab and the CD137 agonist urelumab in patients with myeloma has been completed; however, results have not been reported. It should be noted that, in a preclinical model, treatment with a CD137 agonist antibody early after ASCT also upregulated PD-1 and TIM-3 expression on CD8+ T cells, and the staged addition of an anti–PD-1 blocking antibody further enhanced myeloma control (26). Delayed, or staged, anti–PD-1 treatment is particularly important, as simultaneous PD-1 blockade abrogated the effects of a CD137 agonist in a preclinical model (96). This effect, although yet to be confirmed in a clinical setting, will need to be considered in the design of clinical trials in an era of combination approaches. Furthermore, the potential toxicities of agonist CD137 with anti–PD-1 after ASCT are yet to be assessed and will likely be specific to the particular CD137 agonist mAb used.
IMiDs.
Myeloma-targeted therapies provide another avenue to promote disease control and are particularly attractive in combination with immune-targeted therapies. An additional strategy involves the use of agents with both myeloma on-target effects and immunologically favorable off-target effects, such as IMiDs (e.g., thalidomide, lenalidomide, and pomalidomide). IMiDs act through cereblon-dependent degradation of the transcription factors Ikaros (IKZF1) and Aiolos (IKZF3), which induce myeloma cell apoptosis but also stimulate T and NK cells (97, 98). Importantly, studying these drugs in preclinical models is now possible with the generation of genetically modified mice that metabolize thalidomide derivatives with demonstrable degradation of Ikaros and Aiolos (99). Specifically, IMiDs boost proliferation, enhance IL-2 and IFN-γ production, and reduce IL-10 production in both CD4+ and CD8+ T cells, which subsequently enhance NK cell activation (100, 101). Treg expansion is also suppressed in vitro (102). To this end, lenalidomide is being explored in combination with DC/myeloma hybridoma vaccines and was shown to increase this therapy’s immunogenicity, with enhanced cytolytic capacity observed against myeloma cells (101). In patients with RRMM, the combination of elotuzumab with pomalidomide and dexamethasone produced superior outcomes and reduced risk of progression compared with pomalidomide and dexamethasone alone (NCT02654132) (103).
In accordance with the concept of improving responses using combinatory approaches, several studies suggested that combining IMiDs with anti–PD-1/PD-L1 antibodies may produce superior responses compared with either agent alone (104). Unfortunately, the clinical implementation of anti–PD-1/PD-L1 agents with IMiDs has resulted in substantial toxicity and no improvement in objective response rates (ORRs; NCT02289222, NCT02036502) (105–107), such that a number of trials were placed on a clinical hold by the FDA and subsequently terminated. With the recent development of a murine model that is sensitive to thalidomide and its derivatives (99), it may be prudent to assess the toxicity of combinations in preclinical myeloma models before taking any new IMiD-containing combinatory approaches to the clinic.
Monoclonal antibodies.
Elotuzumab, described above, showed clinical efficacy in combination with IMiDs and dexamethasone and is FDA-approved for use in previously treated myeloma patients (103). A preclinical mouse study suggests that elotuzumab in combination with anti–PD-1 may further improve response rates, and a phase III clinical trial in RRMM patients is currently under way (NCT02726581) (108).
Daratumumab, a fully human mAb that binds to CD38, also has FDA approval for previously treated myeloma patients after several phase III trials demonstrated strikingly improved outcomes for RRMM patients in the daratumumab arms when it was administered in combination with dexamethasone and lenalidomide (NCT02076009) (109) or bortezomib (NCT02136134) (110). Interestingly, although daratumumab targets CD38+ myeloma cells, it also depletes suppressive CD38+ Tregs and myeloid populations. Subsequently, T cells from patients treated with daratumumab had oligoclonal expansion and enhanced capacity to secrete IFN-γ (111). This represents a mechanism beyond the direct killing of CD38-expressing myeloma cells by ADCC and complement-mediated cytotoxicity (111). Preliminary results from preclinical solid tumor models suggest that combination therapy with daratumumab and anti–PD-1 may prove synergistic (112). Clinical trials are currently ongoing of daratumumab and nivolumab/pembrolizumab in RRMM patients, and results are eagerly awaited (NCT02431208, NCT01592370, NCT03357952).
IL-17A’s role in promoting myeloma progression is now well established, and treatment with anti–IL-17A after stem cell transplantation prolonged myeloma control in preclinical models (26, 76). A phase I clinical trial combining anti–IL-17A with PDR001 (anti–PD-1) is currently recruiting RRMM patients (NCT03111992). This combination’s activity in RRMM remains to be seen, and negative data would not preclude activity when it is used early after ASCT.
Oncolytic viruses.
Oncolytic viruses are used to promote specific lysis of myeloma cells; however, early clinical trials showed limited long-term tumor control, and this therapy is currently being investigated in various combinations. The most common combinations are with standard myeloma therapies including IMiDs (reovirus: NCT03015922), proteasome inhibitors plus dexamethasone (reovirus: NCT02514382, NCT02101944), or cyclophosphamide to limit natural antiviral immunity (measles: NCT00450814, NCT02192775; vesicular stomatitis virus: NCT03017820, NCT00450814). A preclinical study using a bortezomib-resistant Vk*MYC myeloma clone demonstrated a synergistic antitumor effect with coadministration of bortezomib and reovirus (113). This synergism was due to augmented reovirus replication in target cells, which stimulated T and NK cell responses and reduced Treg accumulation. Another study, using the 5TGM1 murine model and human myeloma cell lines, demonstrated enhanced immune-mediated antimyeloma effects after treatment with a reovirus (114), which was augmented by lenalidomide in vitro (115). Oncolytics remain in early stages of clinical investigation, and more information is needed about how these viruses promote immune-mediated antimyeloma effects. Results from clinical trials combining oncolytic viruses with IMiDs may provide some further insights into this possible mechanism of action.
Cellular-based therapies.
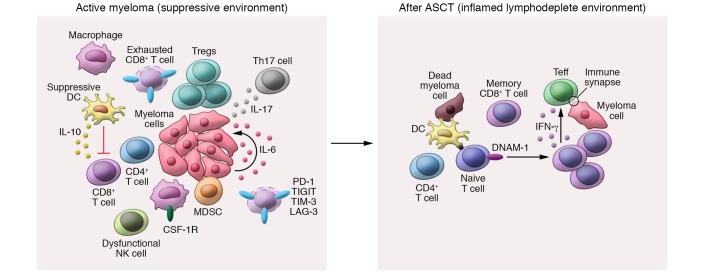
ASCT remains an effective therapy for eligible patients and provides a survival benefit beyond novel agents alone (116–119). Currently, the prolongation of plateau phase induced by ASCT is largely assumed to be the result of myeloablative chemotherapy and cytoreduction therein (120). However, a subset of patients entering ASCT in complete remission demonstrate a survival plateau similar to that seen with immune-mediated graft-versus-leukemia effects after allogeneic stem cell transplantation (121). Indeed, several key immunological changes associated with ASCT suggest that disease plateau after transplant may arise from more than just cytoreduction. Firstly, melphalan, the cytotoxic agent routinely used during conditioning, has been shown to induce immunogenic cell death, a rapid burst of inflammatory cytokines, and enhanced tumor antigen uptake by DCs (122). Secondly, the reconstituting CD4+/CD8+ T cell ratio is inverted following ASCT and provides a favorable effector T cell/Treg ratio (10, 123). Finally, ASCT conditioning ablates BM, disrupting the suppressive TME that is established in myeloma patients. Indeed, given that ASCT generates an inflammatory environment, in the context of lymphodepletion, antigen presentation, and BM microenvironment disruption, it can be postulated that ASCT reestablishes a state of myeloma-immune equilibrium, perhaps even elimination, in patients who achieve long-term control of disease (Figure 3).
Figure 3. Induction of a favorable immunological environment after ASCT.
Active myeloma is associated with an immunosuppressive bone marrow (BM) microenvironment that is characterized by an expansion of suppressive dendritic cells (DCs), myeloid-derived suppressor cells (MDSCs), CSF-1R–expressing macrophages, regulatory T cells (Tregs), and exhausted CD8+ T cells. T cell exhaustion occurs in response to chronic antigen exposure and IL-10 derived from suppressive DCs. Furthermore, myeloma cell growth is supported by IL-17A from Th17 cells and paracrine IL-6 production. After ASCT, a lymphodepleted and inflammatory environment is created that promotes myeloma-specific memory T cell expansion and the priming of naive T cells by functional dendritic cells. Myeloma-specific CD8+ effector T cells (Teff) mediate IFN-γ–dependent myeloma-specific immunity in the context of CD4+ T cell help.
Allogeneic stem cell transplantation (allo-SCT) remains the only curative treatment option for many hematological malignancies, particularly leukemias (124). The curative potential of allo-SCT is largely mediated by alloreactive T cells, referred to as the graft-versus-leukemia effect (125). However, allo-SCT is limited by transplant-related complications, particularly graft-versus-host disease (GVHD), and relapse remains the major cause of failure (120). Surprisingly, in patients with myeloma, alloreactive T cells are limited in their ability to generate a potent graft-versus-myeloma (GVM) response, and allo-SCT is not used in this patient cohort outside of clinical trials. Clinical evidence of GVM effects was observed in some patients, who relapsed after allo-SCT and subsequently responded to donor lymphocyte infusions (DLI), often in association with GVHD (124, 125). However, the comparison of response rates to DLI highlighted less potent graft-versus-tumor effects in patients with myeloma compared with other hematological malignancies (126, 127). Furthermore, a large prospective study found that allografting patients with myeloma did not provide a survival advantage above ASCT, and relapse remained the major cause of death (48%) (128). Therefore, it appears that alloreactive T cell responses are specifically subverted in patients with myeloma, and the potential mechanisms governing this immune escape remain unclear.
Another cellular-based therapy still under evaluation is the use of marrow-infiltrating lymphocytes (MILs) as a source of T cells for adoptive cell therapy. MILs have been shown to be a particularly rich source of myeloma-specific cytotoxic and memory T cells owing to exposure to malignant plasma cells in BM (129). In a murine myeloma model, adoptive transfer of MILs resulted in superior survival compared with peripheral blood lymphocytes (130). Furthermore, in a small clinical trial with 25 patients, an approximately 30% CR rate was observed in patients receiving MILs early after ASCT, and median overall survival had not been reached at 7 years (130). A randomized phase II trial assessing the efficacy of MILs administered early after ASCT with lenalidomide is ongoing (NCT01858558).
The future of immunotherapy in myeloma
Novel immune checkpoint inhibitors.
The upregulation of TIGIT on T cells from both mice and patients with myeloma, in both transplant and nontransplant settings, has revealed a novel therapeutic target that may prove more attractive than current PD-1–targeted therapies (9, 14). TIGIT mAb blockade significantly enhanced effector CD8+ T cell function and improved survival when administered early after ASCT in mice (14). Surprisingly, TIGIT blockade was also effective at preventing myeloma progression when administered in a nontransplant, preclinical setting prior to myeloma progression (9). Furthermore, TIGIT blockade effectively targets both T cell exhaustion and DC-driven immunosuppression, as this therapy also reduced DC-derived IL-10 (14), another described mechanism of immune evasion (131). A preliminary report (132) suggests that specific Fc-binding anti-TIGIT antibodies may also deplete Tregs in vivo. Therefore, targeting of TIGIT holds considerable promise, and it would be particularly interesting to explore TIGIT blockade in combination with immunotherapies targeting the BM microenvironment, including CSF-1R–dependent (14, 84) or IL-18–dependent (86) myeloid cells. The combination approach of stimulating T cells with a CD137 agonist followed by PD-1 blockade after ASCT (to allow expansion of myeloma-specific clones in the absence of exhaustion) is also attractive (26).
Vaccination approaches.
Several vaccination approaches have been tested in myeloma, including idiotype-based, DC-based, cancer testis antigen–based (MAGE-A3, NY-ESO-1, etc.), and GM-CSF cellular-based vaccines (133). Unfortunately, despite induction of immunogenicity, many of these formulations have proven largely ineffective as monotherapies (54, 134–138). This has been attributed to a range of factors, including impaired antigen presentation (63, 67, 139), an immunosuppressive TME (84–86), and low immunogenicity or lack of activity with single peptide targets (138) in patients with myeloma. To provide meaningful clinical responses, recent efforts have sought to combine immunomodulating therapies with myeloma vaccination approaches. The most successful combination to date is the administration of a DC/myeloma fusion vaccination after ASCT (140). In this trial with a cohort of 24 patients, 78% achieved a CR or a very good partial response (VGPR), and all evaluable patients showed at least a two-fold expansion of myeloma-specific CD4+/CD8+ T cells. Notably, the rate of CRs increased dramatically between day 100 and 1 year after vaccination, in the absence of other maintenance therapy. These data suggest that the clinical effect is largely due to the vaccination, such that a randomized, multicenter trial using DC/myeloma fusion vaccination is being conducted through a cooperative consortium (BMT CTN 1401, NCT02728102). In RRMM patients without ASCT, the same DC/myeloma fusion vaccination approach resulted only in disease stabilization in 69% of patients (141). Therefore, the rationale to incorporate vaccination early in the course of disease, particularly in combination with ASCT, is strong (142).
Idiotype vaccination has, historically, proven unsuccessful in patients with myeloma (143, 144). However, a recent study incorporating idiotype-pulsed DC vaccination after ASCT showed improved survival in treated patients compared with historical controls who underwent ASCT without vaccination at the same center during the same time period (median overall survival 5.3 vs. 3.4 years) (145). Although promising, this therapy does need to be investigated in a controlled clinical trial before these results can be clearly interpreted.
Bispecific antibodies.
Bispecific antibodies that bridge T cells (typically via CD3) and tumor-specific antigens (typically BCMA in MM) are now entering clinical trials (146). The most common formulations are bispecific T cell engagers (BiTEs), which only comprise the variable heavy and light chain regions. This allows for T cell engagement and activation after tumor antigen recognition that is independent of TCR specificity (147). The BCMA BiTE AMG-420 was tested in a heavily pretreated patient cohort and showed a 70% response rate, including 5 MRD-negative stringent CRs, 1 VGPR, and 1 partial response (148). An additional BCMA BiTE with an extended half-life is also being tested (149) and showed increased efficacy in vitro in combination with lenalidomide or pomalidomide (150). It is important to note that BiTEs rely on the presence of a functional T cell response, and this therapy is likely to be most efficacious after ASCT or in newly diagnosed patients. Nonetheless, an early-phase trial demonstrates promising efficacy of a BCMA-CD3 bispecific antibody (CC-93269) in heavily pretreated patients, with an ORR of 88.9% after treatment at the highest dose bracket (151). However, longer follow-up and larger cohorts are necessary to determine the durability of these responses.
CAR T cells.
Chimeric antigen receptor (CAR) engineered T cells have been revolutionary in the treatment of patients with B cell malignancies, in which CD19 serves as an ideal target. The use of CAR T cells in hematological malignancies is comprehensively reviewed by Frigault and Maus in this Review series (152). In patients with myeloma, there are several available targets, but BCMA is the most widely studied (153). BCMA is expressed only in late memory B cells and plasma cells, is more highly expressed on malignant plasma cells compared with healthy cells (154), and is widely expressed in myeloma patients (155). BCMA-targeted CAR T cells have produced very promising results in phase I clinical trials in RRMM patients, with many reporting ORRs of 64%–88% in this historically difficult-to-treat patient cohort (NCT03090659, NCT03093168, NCT03288493, NCT03318861, NCT02658929, NCT02215967) (156–158). Other potential CAR T cell targets in myeloma include CD138 (NCT03672318) (159), SLAMF7 (NCT03958656) (160), and GPRC5D (161). These targets are all in early stages of testing, but preclinical and in vitro studies show promising activity against tumor cell lines and primary myeloma cells.
Disappointingly, despite impressive responses early after CAR T cell infusion, lack of persistence and durability of current CAR T cells has precluded long-term disease control in many patients (153), likely owing to CAR T cell–intrinsic factors (162, 163), rejection, loss of target antigen (156, 164), and the immunosuppressive BM TME (165). Early clinical formulations of CAR T cells were generated without regard for phenotype or functional heterogeneity in leukapheresis products. However, a clinical study found that higher frequencies of CD8+ T cells with a naive or stem memory phenotype in the leukapheresis product correlated with a better outcome (158). Importantly, the frequency of early memory T cells was reduced in T cell products from heavily pretreated patients, which suggests that intervention at earlier stages of disease might prove beneficial (166). Interestingly, a clinical study (NCT03455972) exploring combination of CD19 and BMCA CAR T cells administered between days 14 and 20 after ASCT reported dramatic in vivo CAR T cell expansion, 100-fold greater than that observed in the group’s previous study using RRMM patients (167); however, the presence of the CD19 CAR T cells is potentially confounding (168). Nonetheless, the degree of CAR T cell expansion was the most robust marker correlating with response across MM trials, and this expansion was most prominent in lymphodepleted patients (156, 158, 169). Therefore, it is plausible that CAR T cells will also be most efficacious when used in combination with ASCT. Additional approaches to improve CAR T cell quality include selecting for naive or stem/memory T cells (158), engineering of CAR T cells for exhaustion resistance (170), administration of defined compositions of CD4+/CD8+ T cells (164), and informed selection of costimulatory domains for CD28 (brisk T cell proliferation but limited T cell persistence) and/or 4-1BB (less potent effector function but increased persistence) (171–173). Recently, BCMA expression by MM has been dramatically enhanced by prevention of cleavage of BCMA from the cell surface with a γ-secretase inhibitor, and a phase I clinical trial (NCT03502577) is ongoing (174). Finally, the BM TME could be targeted with therapies including daratumumab, other suppressive myeloid cell–targeted mAbs, or IMiDs (14, 86, 111, 175) to limit CAR T cell exhaustion. Ideally, these combination approaches should be studied in preclinical models to examine the potential for additive or synergistic toxicity.
Conclusions
Immunotherapy is now a cornerstone therapeutic approach for the treatment of myeloma but at present is limited by the ability to generate durable functional antimyeloma T cell responses. While preclinical models of myeloma are important in generating rational therapeutic paradigms, these must be tested in rigorous and well-designed clinical trials. We now have a wide range of active agents to choose from for the treatment of myeloma, and while many of these are immunostimulatory (e.g., IMiDs, elotuzumab), many are immunosuppressive (e.g., dexamethasone, proteasome inhibitors). To date, the field has combined agents without a clear regard for the immunological consequences and has largely taken a “more agents is better” approach. Given our increasing understanding of the importance of myeloma-specific immunity, now would seem an appropriate time to consider combining agents in a more strategic fashion and including exploratory immunological endpoints in studies. Furthermore, initiating immunotherapy earlier in the course of disease at a time of superior T cell fitness may also improve the quality of responses. Additionally, there is strong preclinical and preliminary clinical evidence to suggest that ASCT generates a state of T cell–dependent myeloma control and thus represents a rational MRD-low platform for immunotherapy approaches. Finally, combining immunotherapies that target myeloma cells and the BM TME with ASCT, CAR T cell, and/or vaccination approaches may be the key to reestablishing immune equilibrium and generating durable immunological control of disease.
Acknowledgments
The authors thank Madeleine Flynn of QIMR Berghofer for generation of the graphics. This work was supported by a research grant from the National Cancer Institute of the NIH (U01 CA244291), USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. GRH is an Andy Hill CARE Distinguished Researcher.
Version 1. 03/09/2020
Electronic publication
Version 2. 04/01/2020
Print issue publication
Footnotes
Conflict of interest: GRH has received research funding from Compass Therapeutics and Roche.
Copyright: © 2020, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2020;130(4):1565–1575.https://doi.org/10.1172/JCI129205.
References
- 1.Kumar SK, et al. Multiple myeloma. Nat Rev Dis Primers. 2017;3:17046. doi: 10.1038/nrdp.2017.46. [DOI] [PubMed] [Google Scholar]
- 2.Cowan AJ, et al. Global burden of multiple myeloma: a systematic analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 2018;4(9):1221–1227. doi: 10.1001/jamaoncol.2018.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landgren O, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113(22):5412–5417. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyle RA, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354(13):1362–1369. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 5.Kyle RA, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582–2590. doi: 10.1056/NEJMoa070389. [DOI] [PubMed] [Google Scholar]
- 6.Manier S, Salem KZ, Park J, Landau DA, Getz G, Ghobrial IM. Genomic complexity of multiple myeloma and its clinical implications. Nat Rev Clin Oncol. 2017;14(2):100–113. doi: 10.1038/nrclinonc.2016.122. [DOI] [PubMed] [Google Scholar]
- 7.Kuehl WM, Bergsagel PL. Molecular pathogenesis of multiple myeloma and its premalignant precursor. J Clin Invest. 2012;122(10):3456–3463. doi: 10.1172/JCI61188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suen H, et al. Multiple myeloma causes clonal T-cell immunosenescence: identification of potential novel targets for promoting tumour immunity and implications for checkpoint blockade. Leukemia. 2016;30(8):1716–1724. doi: 10.1038/leu.2016.84. [DOI] [PubMed] [Google Scholar]
- 9.Guillerey C, et al. TIGIT immune checkpoint blockade restores CD8+ T-cell immunity against multiple myeloma. Blood. 2018;132(16):1689–1694. doi: 10.1182/blood-2018-01-825265. [DOI] [PubMed] [Google Scholar]
- 10.Chung DJ, et al. T-cell exhaustion in multiple myeloma relapse after autotransplant: optimal timing of immunotherapy. Cancer Immunol Res. 2016;4(1):61–71. doi: 10.1158/2326-6066.CIR-15-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zelle-Rieser C, et al. T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. J Hematol Oncol. 2016;9(1):116. doi: 10.1186/s13045-016-0345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yadav M, et al. Tigit, CD226 and PD-L1/PD-1 are highly expressed by marrow-infiltrating T cells in patients with multiple myeloma. Blood. 2016;128(22):2102. doi: 10.1182/blood.V128.22.2102.2102. [DOI] [Google Scholar]
- 13.Dhodapkar MV, et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003;197(12):1667–1676. doi: 10.1084/jem.20021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minnie SA, et al. Myeloma escape after stem cell transplantation is a consequence of T-cell exhaustion and is prevented by TIGIT blockade. Blood. 2018;132(16):1675–1688. doi: 10.1182/blood-2018-01-825240. [DOI] [PubMed] [Google Scholar]
- 15.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 16.Walker BA, et al. Intraclonal heterogeneity is a critical early event in the development of myeloma and precedes the development of clinical symptoms. Leukemia. 2014;28(2):384–390. doi: 10.1038/leu.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutta AK, et al. Subclonal evolution in disease progression from MGUS/SMM to multiple myeloma is characterised by clonal stability. Leukemia. 2019;33(2):457–468. doi: 10.1038/s41375-018-0206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao S, et al. Serial exome analysis of disease progression in premalignant gammopathies. Leukemia. 2014;28(7):1548–1552. doi: 10.1038/leu.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mateos MV, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369(5):438–447. doi: 10.1056/NEJMoa1300439. [DOI] [PubMed] [Google Scholar]
- 20. doi: 10.1200/JCO.19.01740. Lonial S, et al. Randomized trial of lenalidomide versus observation in smoldering multiple myeloma [published online October 25, 2019]. J Clin Oncol. https://doi.org/10.1200/JCO.19.01740. [DOI] [PMC free article] [PubMed]
- 21.Dhodapkar MV, Krasovsky J, Osman K, Geller MD. Vigorous premalignancy-specific effector T cell response in the bone marrow of patients with monoclonal gammopathy. J Exp Med. 2003;198(11):1753–1757. doi: 10.1084/jem.20031030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhodapkar MV, Krasovsky J, Olson K. T cells from the tumor microenvironment of patients with progressive myeloma can generate strong, tumor-specific cytolytic responses to autologous, tumor-loaded dendritic cells. Proc Natl Acad Sci U S A. 2002;99(20):13009–13013. doi: 10.1073/pnas.202491499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi T, et al. Ex vivo induction of multiple myeloma-specific cytotoxic T lymphocytes. Blood. 2003;102(4):1435–1442. doi: 10.1182/blood-2002-09-2828. [DOI] [PubMed] [Google Scholar]
- 24.Wen YJ, Min R, Tricot G, Barlogie B, Yi Q. Tumor lysate-specific cytotoxic T lymphocytes in multiple myeloma: promising effector cells for immunotherapy. Blood. 2002;99(9):3280–3285. doi: 10.1182/blood.V99.9.3280. [DOI] [PubMed] [Google Scholar]
- 25.Alexandrov LB, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vuckovic S, et al. Bone marrow transplantation generates T cell-dependent control of myeloma in mice. J Clin Invest. 2019;129(1):106–121. doi: 10.1172/JCI98888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chesi M, et al. AID-dependent activation of a MYC transgene induces multiple myeloma in a conditional mouse model of post-germinal center malignancies. Cancer Cell. 2008;13(2):167–180. doi: 10.1016/j.ccr.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gatza E, et al. Etanercept plus topical corticosteroids as initial therapy for grade one acute graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20(9):1426–1434. doi: 10.1016/j.bbmt.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller A, et al. High somatic mutation and neoantigen burden are correlated with decreased progression-free survival in multiple myeloma. Blood Cancer J. 2017;7(9):e612. doi: 10.1038/bcj.2017.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lohr JG, et al. Widespread genetic heterogeneity in multiple myeloma: implications for targeted therapy. Cancer Cell. 2014;25(1):91–101. doi: 10.1016/j.ccr.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bailur JK, et al. Early alterations in stem-like/resident T cells, innate and myeloid cells in the bone marrow in preneoplastic gammopathy. JCI Insight. 2019;5:127807. doi: 10.1172/jci.insight.127807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carnaud C, et al. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163(9):4647–4650. [PubMed] [Google Scholar]
- 33.Eberl G, MacDonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur J Immunol. 2000;30(4):985–992. doi: 10.1002/(SICI)1521-4141(200004)30:4<985::AID-IMMU985>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 34.Silk JD, et al. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J Clin Invest. 2004;114(12):1800–1811. doi: 10.1172/JCI22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viey E, Laplace C, Escudier B. Peripheral γδ T-lymphocytes as an innovative tool in immunotherapy for metastatic renal cell carcinoma. Expert Rev Anticancer Ther. 2005;5(6):973–986. doi: 10.1586/14737140.5.6.973. [DOI] [PubMed] [Google Scholar]
- 36.Corvaisier M, et al. Vγ9Vδ2 T cell response to colon carcinoma cells. J Immunol. 2005;175(8):5481–5488. doi: 10.4049/jimmunol.175.8.5481. [DOI] [PubMed] [Google Scholar]
- 37.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived γδ T cells of MICA and MICB. Proc Natl Acad Sci U S A. 1999;96(12):6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fournié JJ, et al. What lessons can be learned from γδ T cell-based cancer immunotherapy trials? Cell Mol Immunol. 2013;10(1):35–41. doi: 10.1038/cmi.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zocchi MR, Poggi A. Role of γδ T lymphocytes in tumor defense. Front Biosci. 2004;9:2588–2604. doi: 10.2741/1419. [DOI] [PubMed] [Google Scholar]
- 40.Carbone E, et al. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood. 2005;105(1):251–258. doi: 10.1182/blood-2004-04-1422. [DOI] [PubMed] [Google Scholar]
- 41.Dhodapkar MV, Richter J. Harnessing natural killer T (NKT) cells in human myeloma: progress and challenges. Clin Immunol. 2011;140(2):160–166. doi: 10.1016/j.clim.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guillerey C, et al. Immunosurveillance and therapy of multiple myeloma are CD226 dependent. J Clin Invest. 2015;125(5):2077–2089. doi: 10.1172/JCI77181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osterborg A, Nilsson B, Björkholm M, Holm G, Mellstedt H. Natural killer cell activity in monoclonal gammopathies: relation to disease activity. Eur J Haematol. 1990;45(3):153–157. doi: 10.1111/j.1600-0609.1990.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 44.Paiva B, et al. Immune status of high-risk smoldering multiple myeloma patients and its therapeutic modulation under LenDex: a longitudinal analysis. Blood. 2016;127(9):1151–1162. doi: 10.1182/blood-2015-10-662320. [DOI] [PubMed] [Google Scholar]
- 45.Davies FE, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98(1):210–216. doi: 10.1182/blood.V98.1.210. [DOI] [PubMed] [Google Scholar]
- 46.Hayashi T, et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. Br J Haematol. 2005;128(2):192–203. doi: 10.1111/j.1365-2141.2004.05286.x. [DOI] [PubMed] [Google Scholar]
- 47.Fedele PL, et al. IMiDs prime myeloma cells for daratumumab-mediated cytotoxicity through loss of Ikaros and Aiolos. Blood. 2018;132(20):2166–2178. doi: 10.1182/blood-2018-05-850727. [DOI] [PubMed] [Google Scholar]
- 48.Campbell KS, Cohen AD, Pazina T. Mechanisms of NK cell activation and clinical activity of the therapeutic SLAMF7 antibody, elotuzumab in multiple myeloma. Front Immunol. 2018;9:2551. doi: 10.3389/fimmu.2018.02551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases — elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guillerey C, Nakamura K, Vuckovic S, Hill GR, Smyth MJ. Immune responses in multiple myeloma: role of the natural immune surveillance and potential of immunotherapies. Cell Mol Life Sci. 2016;73(8):1569–1589. doi: 10.1007/s00018-016-2135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013;25(2):214–221. doi: 10.1016/j.coi.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blank CU, et al. Defining ‘T cell exhaustion’. Nat Rev Immunol. 2019;19(11):665–674. doi: 10.1038/s41577-019-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosenblatt J, et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J Immunother. 2011;34(5):409–418. doi: 10.1097/CJI.0b013e31821ca6ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palumbo A, Bruno B, Boccadoro M, Pileri A. Interferon-gamma in multiple myeloma. Leuk Lymphoma. 1995;18(3–4):215–219. doi: 10.3109/10428199509059610. [DOI] [PubMed] [Google Scholar]
- 56.Yousef S, et al. Immunomodulatory molecule PD-L1 is expressed on malignant plasma cells and myeloma-propagating pre-plasma cells in the bone marrow of multiple myeloma patients. Blood Cancer J. 2015;5:e285. doi: 10.1038/bcj.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.El-Sherbiny YM, et al. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 2007;67(18):8444–8449. doi: 10.1158/0008-5472.CAN-06-4230. [DOI] [PubMed] [Google Scholar]
- 58.Bahlis NJ, et al. CD28-mediated regulation of multiple myeloma cell proliferation and survival. Blood. 2007;109(11):5002–5010. doi: 10.1182/blood-2006-03-012542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ishibashi M, et al. Myeloma drug resistance induced by binding of myeloma B7-H1 (PD-L1) to PD-1. Cancer Immunol Res. 2016;4(9):779–788. doi: 10.1158/2326-6066.CIR-15-0296. [DOI] [PubMed] [Google Scholar]
- 60.Wang L, et al. Serum levels of soluble programmed death ligand 1 predict treatment response and progression free survival in multiple myeloma. Oncotarget. 2015;6(38):41228–41236. doi: 10.18632/oncotarget.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fauriat C, Mallet F, Olive D, Costello RT. Impaired activating receptor expression pattern in natural killer cells from patients with multiple myeloma. Leukemia. 2006;20(4):732–733. doi: 10.1038/sj.leu.2404096. [DOI] [PubMed] [Google Scholar]
- 62.Benson DM, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116(13):2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ratta M, et al. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100(1):230–237. doi: 10.1182/blood.V100.1.230. [DOI] [PubMed] [Google Scholar]
- 64.Bahlis NJ, et al. CD28-mediated regulation of multiple myeloma cell proliferation and survival. Blood. 2007;109(11):5002–5010. doi: 10.1182/blood-2006-03-012542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murray ME, et al. CD28-mediated pro-survival signaling induces chemotherapeutic resistance in multiple myeloma. Blood. 2014;123(24):3770–3779. doi: 10.1182/blood-2013-10-530964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kukreja A, et al. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J Exp Med. 2006;203(8):1859–1865. doi: 10.1084/jem.20052136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leone P, et al. Dendritic cells accumulate in the bone marrow of myeloma patients where they protect tumor plasma cells from CD8+ T-cell killing. Blood. 2015;126(12):1443–1451. doi: 10.1182/blood-2015-01-623975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Racanelli V, et al. Alterations in the antigen processing-presenting machinery of transformed plasma cells are associated with reduced recognition by CD8+ T cells and characterize the progression of MGUS to multiple myeloma. Blood. 2010;115(6):1185–1193. doi: 10.1182/blood-2009-06-228676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1(6):510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 70.van Zaanen HC, et al. Endogenous interleukin 6 production in multiple myeloma patients treated with chimeric monoclonal anti-IL6 antibodies indicates the existence of a positive feed-back loop. J Clin Invest. 1996;98(6):1441–1448. doi: 10.1172/JCI118932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hilbert DM, Kopf M, Mock BA, Köhler G, Rudikoff S. Interleukin 6 is essential for in vivo development of B lineage neoplasms. J Exp Med. 1995;182(1):243–248. doi: 10.1084/jem.182.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Serody JS, Hill GR. The IL-17 differentiation pathway and its role in transplant outcome. Biol Blood Marrow Transplant. 2012;18(1 suppl):S56–S61. doi: 10.1016/j.bbmt.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 74.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGF-β in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 75.Prabhala RH, et al. Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood. 2010;115(26):5385–5392. doi: 10.1182/blood-2009-10-246660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prabhala RH, et al. Targeting IL-17A in multiple myeloma: a potential novel therapeutic approach in myeloma. Leukemia. 2016;30(2):379–389. doi: 10.1038/leu.2015.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y, et al. Potential relationship and clinical significance of miRNAs and Th17 cytokines in patients with multiple myeloma. Leuk Res. 2014;38(9):1130–1135. doi: 10.1016/j.leukres.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 78.Alexandrakis MG, et al. Serum interleukin-17 and its relationship to angiogenic factors in multiple myeloma. Eur J Intern Med. 2006;17(6):412–416. doi: 10.1016/j.ejim.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 79.Calcinotto A, et al. Microbiota-driven interleukin-17-producing cells and eosinophils synergize to accelerate multiple myeloma progression. Nat Commun. 2018;9(1):4832. doi: 10.1038/s41467-018-07305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aras S, Zaidi MR. TAMeless traitors: macrophages in cancer progression and metastasis. Br J Cancer. 2017;117(11):1583–1591. doi: 10.1038/bjc.2017.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193(6):727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ries CH, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25(6):846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 83.Ao JY, et al. Colony-stimulating factor 1 receptor blockade inhibits tumor growth by altering the polarization of tumor-associated macrophages in hepatocellular carcinoma. Mol Cancer Ther. 2017;16(8):1544–1554. doi: 10.1158/1535-7163.MCT-16-0866. [DOI] [PubMed] [Google Scholar]
- 84.Wang Q, et al. Therapeutic effects of CSF1R-blocking antibodies in multiple myeloma. Leukemia. 2018;32(1):176–183. doi: 10.1038/leu.2017.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zheng Y, et al. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009;114(17):3625–3628. doi: 10.1182/blood-2009-05-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakamura K, et al. Dysregulated IL-18 is a key driver of immunosuppression and a possible therapeutic target in the multiple myeloma microenvironment. Cancer Cell. 2018;33(4):634–648.e5. doi: 10.1016/j.ccell.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 87.Lesokhin AM, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34(23):2698–2704. doi: 10.1200/JCO.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jing W, et al. Combined immune checkpoint protein blockade and low dose whole body irradiation as immunotherapy for myeloma. J Immunother Cancer. 2015;3(1):2. doi: 10.1186/s40425-014-0043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hallett WH, Jing W, Drobyski WR, Johnson BD. Immunosuppressive effects of multiple myeloma are overcome by PD-L1 blockade. Biol Blood Marrow Transplant. 2011;17(8):1133–1145. doi: 10.1016/j.bbmt.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 90.Kearl TJ, Jing W, Gershan JA, Johnson BD. Programmed death receptor-1/programmed death receptor ligand-1 blockade after transient lymphodepletion to treat myeloma. J Immunol. 2013;190(11):5620–5628. doi: 10.4049/jimmunol.1202005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.D’Souza A, et al. A phase 2 study of pembrolizumab during lymphodepletion after autologous hematopoietic cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2019;25(8):1492–1497. doi: 10.1016/j.bbmt.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 92.Du X, et al. A reappraisal of CTLA-4 checkpoint blockade in cancer immunotherapy. Cell Res. 2018;28(4):416–432. doi: 10.1038/s41422-018-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arce Vargas F, et al. Fc Effector function contributes to the activity of human anti-CTLA-4 antibodies. Cancer Cell. 2018;33(4):649–663.e4. doi: 10.1016/j.ccell.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yonezawa A, Dutt S, Chester C, Kim J, Kohrt HE. Boosting cancer immunotherapy with anti-CD137 antibody therapy. Clin Cancer Res. 2015;21(14):3113–3120. doi: 10.1158/1078-0432.CCR-15-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Murillo O, et al. Therapeutic antitumor efficacy of anti-CD137 agonistic monoclonal antibody in mouse models of myeloma. Clin Cancer Res. 2008;14(21):6895–6906. doi: 10.1158/1078-0432.CCR-08-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McKee SJ, Doff BL, Soon MS, Mattarollo SR. Therapeutic efficacy of 4-1BB costimulation is abrogated by PD-1 blockade in a model of spontaneous B-cell lymphoma. Cancer Immunol Res. 2017;5(3):191–197. doi: 10.1158/2326-6066.CIR-16-0249. [DOI] [PubMed] [Google Scholar]
- 97.Krönke J, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343(6168):301–305. doi: 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu G, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343(6168):305–309. doi: 10.1126/science.1244917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fink EC, et al. CrbnI391V is sufficient to confer in vivo sensitivity to thalidomide and its derivatives in mice. Blood. 2018;132(14):1535–1544. doi: 10.1182/blood-2018-05-852798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Quach H, et al. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010;24(1):22–32. doi: 10.1038/leu.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Luptakova K, et al. Lenalidomide enhances anti-myeloma cellular immunity. Cancer Immunol Immunother. 2013;62(1):39–49. doi: 10.1007/s00262-012-1308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Galustian C, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009;58(7):1033–1045. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dimopoulos MA, et al. Elotuzumab plus pomalidomide and dexamethasone for multiple myeloma. N Engl J Med. 2018;379(19):1811–1822. doi: 10.1056/NEJMoa1805762. [DOI] [PubMed] [Google Scholar]
- 104.Görgün G, et al. Lenalidomide enhances immune checkpoint blockade-induced immune response in multiple myeloma. Clin Cancer Res. 2015;21(20):4607–4618. doi: 10.1158/1078-0432.CCR-15-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Badros A, et al. Pembrolizumab, pomalidomide, and low-dose dexamethasone for relapsed/refractory multiple myeloma. Blood. 2017;130(10):1189–1197. doi: 10.1182/blood-2017-03-775122. [DOI] [PubMed] [Google Scholar]
- 106.Ocio EM, et al. Pembrolizumab (Pembro) plus lenalidomide (Len) and low-dose dexamethasone (Dex) for relapsed/refractory multiple myeloma (RRMM) efficacy and biomarker analyses. J Clin Oncol. 2017;35(15 suppl):8015. doi: 10.1200/JCO.2017.35.15_suppl.8015. [DOI] [Google Scholar]
- 107.Gormley NJ, Pazdur R. Immunotherapy combinations in multiple myeloma — known unknowns. N Engl J Med. 2018;379(19):1791–1795. doi: 10.1056/NEJMp1803602. [DOI] [PubMed] [Google Scholar]
- 108.Bezman NA, et al. PD-1 blockade enhances elotuzumab efficacy in mouse tumor models. Blood Adv. 2017;1(12):753–765. doi: 10.1182/bloodadvances.2017004382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dimopoulos MA, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319–1331. doi: 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]
- 110.Palumbo A, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–766. doi: 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]
- 111.Krejcik J, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–394. doi: 10.1182/blood-2015-12-687749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bezman NA, et al. Abstract 1727: Antitumor activity associated with dual targeting of CD38 and programmed death-1 (PD-1) pathways in preclinical models. Cancer Res. 2018;78(suppl 13):1727. doi: 10.1158/1538-7445.AM2018-1727. [DOI] [Google Scholar]
- 113.Thirukkumaran CM, et al. Oncolytic immunotherapy and bortezomib synergy improves survival of refractory multiple myeloma in a preclinical model. Blood Adv. 2019;3(5):797–812. doi: 10.1182/bloodadvances.2018025593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Müller LME, et al. Abstract 18: Evaluating the contribution of anti-myeloma immunity for the efficacy of oncolytic reovirus therapy. Clin Cancer Res. 2017;23(suppl 24):18. doi: 10.1158/1557-3265.HEMMAL17-18. [DOI] [Google Scholar]
- 115.Parrish C, Scott GB, Coffey M, Melcher A, Errington-Mais F, Cook G. Combination therapy with reovirus and immunomodulatory drugs induces direct oncolytic and immune-mediated killing of multiple myeloma cells and overcomes stromal-mediated microenvironmental protection. Blood. 2014;124(21):4778. doi: 10.1182/blood.V124.21.4778.4778. [DOI] [Google Scholar]
- 116.Attal M, et al. Autologous transplantation for multiple myeloma in the era of new drugs: a phase III study of the Intergroupe Francophone Du Myelome (IFM/DFCI 2009 Trial) Blood. 2015;126(23):391. doi: 10.1182/blood.V126.23.391.391. [DOI] [Google Scholar]
- 117.Cavo M, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376(9758):2075–2085. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 118.Attal M, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996;335(2):91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 119.Child JA, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 120.Bensinger WI. Role of autologous and allogeneic stem cell transplantation in myeloma. Leukemia. 2009;23(3):442–448. doi: 10.1038/leu.2008.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martinez-Lopez J, et al. Long-term prognostic significance of response in multiple myeloma after stem cell transplantation. Blood. 2011;118(3):529–534. doi: 10.1182/blood-2011-01-332320. [DOI] [PubMed] [Google Scholar]
- 122.Lu X, et al. Alkylating agent melphalan augments the efficacy of adoptive immunotherapy using tumor-specific CD4+ T cells. J Immunol. 2015;194(4):2011–2021. doi: 10.4049/jimmunol.1401894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Olsen GA, Gockerman JP, Bast RC, Borowitz M, Peters WP. Altered immunologic reconstitution after standard-dose chemotherapy or high-dose chemotherapy with autologous bone marrow support. Transplantation. 1988;46(1):57–60. doi: 10.1097/00007890-198807000-00009. [DOI] [PubMed] [Google Scholar]
- 124.Aschan J, Lönnqvist B, Ringdén O, Kumlien G, Gahrton G. Graft-versus-myeloma effect. Lancet. 1996;348(9023):346. doi: 10.1016/s0140-6736(05)64525-4. [DOI] [PubMed] [Google Scholar]
- 125.Tricot G, Vesole DH, Jagannath S, Hilton J, Munshi N, Barlogie B. Graft-versus-myeloma effect: proof of principle. Blood. 1996;87(3):1196–1198. doi: 10.1182/blood.V87.3.1196.bloodjournal8731196. [DOI] [PubMed] [Google Scholar]
- 126.Halapi E, et al. T cell repertoire in patients with multiple myeloma and monoclonal gammopathy of undetermined significance: clonal CD8+ T cell expansions are found preferentially in patients with a low tumor burden. Eur J Immunol. 1997;27(9):2245–2252. doi: 10.1002/eji.1830270919. [DOI] [PubMed] [Google Scholar]
- 127.Helg C, Starobinski M, Jeannet M, Chapuis B. Donor lymphocyte infusion for the treatment of relapse after allogeneic hematopoietic stem cell transplantation. Leuk Lymphoma. 1998;29(3–4):301–313. doi: 10.3109/10428199809068567. [DOI] [PubMed] [Google Scholar]
- 128.Yin X, Tang L, Fan F, Jiang Q, Sun C, Hu Y. Allogeneic stem-cell transplantation for multiple myeloma: a systematic review and meta-analysis from 2007 to 2017. Cancer Cell Int. 2018;18:62. doi: 10.1186/s12935-018-0553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Noonan KA, Borrello IM. Marrow infiltrating lymphocytes: their role in adoptive immunotherapy. Cancer J. 2015;21(6):501–505. doi: 10.1097/PPO.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 130.Noonan KA, et al. Adoptive transfer of activated marrow-infiltrating lymphocytes induces measurable antitumor immunity in the bone marrow in multiple myeloma. Sci Transl Med. 2015;7(288):288ra78. doi: 10.1126/scitranslmed.aaa7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yu X, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10(1):48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 132.Leroy X, et al. Abstract LB-114: a-TIGIT antagonist antibody EOS884448 shows dual mechanism of action by restoration of T cell effector functions and preferential depletion of Treg. Cancer Res. 2018;78(suppl 13):LB-114-LB-114. doi: 10.1158/1538-7445.AM2018-LB-114. [DOI] [Google Scholar]
- 133.Hoyos V, Borrello I. The immunotherapy era of myeloma: monoclonal antibodies, vaccines, and adoptive T-cell therapies. Blood. 2016;128(13):1679–1687. doi: 10.1182/blood-2016-05-636357. [DOI] [PubMed] [Google Scholar]
- 134.Yi Q, Desikan R, Barlogie B, Munshi N. Optimizing dendritic cell-based immunotherapy in multiple myeloma. Br J Haematol. 2002;117(2):297–305. doi: 10.1046/j.1365-2141.2002.03411.x. [DOI] [PubMed] [Google Scholar]
- 135.Curti A, et al. Phase I/II clinical trial of sequential subcutaneous and intravenous delivery of dendritic cell vaccination for refractory multiple myeloma using patient-specific tumour idiotype protein or idiotype (VDJ)-derived class I-restricted peptides. Br J Haematol. 2007;139(3):415–424. doi: 10.1111/j.1365-2141.2007.06832.x. [DOI] [PubMed] [Google Scholar]
- 136.Reichardt VL, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma — a feasibility study. Blood. 1999;93(7):2411–2419. [PubMed] [Google Scholar]
- 137.Rapoport AP, et al. Combination immunotherapy after ASCT for multiple myeloma using MAGE-A3/Poly-ICLC immunizations followed by adoptive transfer of vaccine-primed and costimulated autologous T cells. Clin Cancer Res. 2014;20(5):1355–1365. doi: 10.1158/1078-0432.CCR-13-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nahas MR, Rosenblatt J, Lazarus HM, Avigan D. Anti-cancer vaccine therapy for hematologic malignancies: an evolving era. Blood Rev. 2018;32(4):312–325. doi: 10.1016/j.blre.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 139.Brimnes MK, et al. Increased level of both CD4+FOXP3+ regulatory T cells and CD14+HLA-DR–/low myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand J Immunol. 2010;72(6):540–547. doi: 10.1111/j.1365-3083.2010.02463.x. [DOI] [PubMed] [Google Scholar]
- 140.Rosenblatt J, et al. Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin Cancer Res. 2013;19(13):3640–3648. doi: 10.1158/1078-0432.CCR-13-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rosenblatt J, et al. Vaccination with dendritic cell/tumor fusion cells results in cellular and humoral antitumor immune responses in patients with multiple myeloma. Blood. 2011;117(2):393–402. doi: 10.1182/blood-2010-04-277137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Avigan D, Rosenblatt J. Vaccine therapy in hematologic malignancies. Blood. 2018;131(24):2640–2650. doi: 10.1182/blood-2017-11-785873. [DOI] [PubMed] [Google Scholar]
- 143.Reichardt VL, Milazzo C, Brugger W, Einsele H, Kanz L, Brossart P. Idiotype vaccination of multiple myeloma patients using monocyte-derived dendritic cells. Haematologica. 2003;88(10):1139–1149. [PubMed] [Google Scholar]
- 144.Rhee Fv Idiotype vaccination strategies in myeloma: how to overcome a dysfunctional immune system. Clin Cancer Res. 2007;13(5):1353–1355. doi: 10.1158/1078-0432.CCR-06-2650. [DOI] [PubMed] [Google Scholar]
- 145.Lacy MQ, et al. Idiotype-pulsed antigen-presenting cells following autologous transplantation for multiple myeloma may be associated with prolonged survival. Am J Hematol. 2009;84(12):799–802. doi: 10.1002/ajh.21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zuch de Zafra CL, et al. Targeting multiple myeloma with AMG 424, a novel anti-CD38/CD3 bispecific T-cell-recruiting antibody optimized for cytotoxicity and cytokine release. Clin Cancer Res. 2019;25(13):3921–3933. doi: 10.1158/1078-0432.CCR-18-2752. [DOI] [PubMed] [Google Scholar]
- 147.Demichelis-Gómez R, Pérez-Sámano D, Bourlon C. Bispecific antibodies in hematologic malignancies: when, to whom, and how should be best used? Curr Oncol Rep. 2019;21(2):17. doi: 10.1007/s11912-019-0759-5. [DOI] [PubMed] [Google Scholar]
- 148.Topp MS, et al. Evaluation of AMG 420, an anti-BCMA bispecific T-cell engager (BiTE) immunotherapy, in R/R multiple myeloma (MM) patients: updated results of a first-in-human (FIH) phase I dose escalation study. J Clin Oncol. 2019;37(suppl 15):8007. doi: 10.1200/JCO.2019.37.15_suppl.8007. [DOI] [Google Scholar]
- 149.Cho S-F, et al. Anti-BCMA BiTE AMG 701 potently induces specific T cell lysis of human multiple myeloma (MM) cells and immunomodulation in the bone marrow microenvironment. Blood. 2018;132(suppl 1):592. doi: 10.1182/blood-2018-99-118425. [DOI] [Google Scholar]
- 150.Cho SF, et al. AMG 701 potently induces anti-multiple myeloma (MM) functions of T cells and IMiDs further enhance its efficacy to prevent MM relapse in vivo. Blood. 2019;134(suppl 1):135. doi: 10.1182/blood-2019-128528. [DOI] [Google Scholar]
- 151.Costa LJ, et al. First clinical study of the B-cell maturation antigen (BCMA) 2+1 T cell engager (TCE) CC-93269 in patients (Pts) with relapsed/refractory multiple myeloma (RRMM): interim results of a phase 1 multicenter trial. Blood. 2019;134(suppl 1):143. doi: 10.1182/blood-2019-122895. [DOI] [Google Scholar]
- 152.Frigault MJ, Maus MV. State of the art in CAR T cell therapy for CD19+ B cell malignancies. J Clin Invest. 2020;130(4):1586–1594. doi: 10.1172/JCI129208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sidana S, Shah N. CAR T-cell therapy: is it prime time in myeloma? Blood Adv. 2019;3(21):3473–3480. doi: 10.1182/bloodadvances.2019000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.O’Connor BP, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199(1):91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Friedman KM, et al. Effective targeting of multiple B-cell maturation antigen-expressing hematological malignances by anti-B-cell maturation antigen chimeric antigen receptor T cells. Hum Gene Ther. 2018;29(5):585–601. doi: 10.1089/hum.2018.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brudno JN, et al. T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol. 2018;36(22):2267–2280. doi: 10.1200/JCO.2018.77.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Raje N, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380(18):1726–1737. doi: 10.1056/NEJMoa1817226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cohen AD, et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest. 2019;129(6):2210–2221. doi: 10.1172/JCI126397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sun C, et al. Safety and efficacy of targeting CD138 with a chimeric antigen receptor for the treatment of multiple myeloma. Oncotarget. 2019;10(24):2369–2383. doi: 10.18632/oncotarget.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gogishvili T, et al. SLAMF7-CAR T cells eliminate myeloma and confer selective fratricide of SLAMF7+ normal lymphocytes. Blood. 2017;130(26):2838–2847. doi: 10.1182/blood-2017-04-778423. [DOI] [PubMed] [Google Scholar]
- 161.Smith EL, et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med. 2019;11(485):eaau7746. doi: 10.1126/scitranslmed.aau7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.McLellan AD, Ali Hosseini Rad SM. Chimeric antigen receptor T cell persistence and memory cell formation. Immunol Cell Biol. 2019;97(7):664–674. doi: 10.1111/imcb.12254. [DOI] [PubMed] [Google Scholar]
- 163.Fraietta JA, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24(5):563–571. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Green DJ, et al. Fully human Bcma targeted chimeric antigen receptor T cells administered in a defined composition demonstrate potency at low doses in advanced stage high risk multiple myeloma. Blood. 2018;132(suppl 1):1011. doi: 10.1182/blood-2018-99-117729. [DOI] [Google Scholar]
- 165.Cohen AD, Raje N, Fowler JA, Mezzi K, Scott EC, Dhodapkar MV. How to train your T cells: overcoming immune dysfunction in multiple myeloma. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-19-2111. doi: 10.1158/1078-0432.CCR-19-2111. [published online ahead of print October 31, 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Garfall AL, et al. T-cell phenotypes associated with effective CAR T-cell therapy in postinduction vs relapsed multiple myeloma. Blood Adv. 2019;3(19):2812–2815. doi: 10.1182/bloodadvances.2019000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shi X, et al. Tandom autologous transplantation and combined infusion of CD19 and Bcma-specific chimeric antigen receptor T cells for high risk MM: initial safety and efficacy report from a clinical pilot study. Blood. 2018;132(suppl 1):1009. doi: 10.1182/blood-2018-99-117964. [DOI] [Google Scholar]
- 168.Garfall AL, et al. Anti-CD19 CAR T cells with high-dose melphalan and autologous stem cell transplantation for refractory multiple myeloma. JCI Insight. 2018;3(8):120505. doi: 10.1172/jci.insight.120505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16(6):372–385. doi: 10.1038/s41571-019-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lynn RC, et al. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature. 2019;576(7786):293–300. doi: 10.1038/s41586-019-1805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.van der Stegen SJ, Hamieh M, Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov. 2015;14(7):499–509. doi: 10.1038/nrd4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kawalekar OU, et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity. 2016;44(2):380–390. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 173.Zhao Z, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015;28(4):415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pont MJ, et al. γ-Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood. 2019;134(19):1585–1597. doi: 10.1182/blood.2019000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jiao S, et al. Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell. 2019;179(5):1177–1190.e13. doi: 10.1016/j.cell.2019.10.029. [DOI] [PubMed] [Google Scholar]