Abstract
Aldosterone, which plays a key role in the regulation of blood pressure, is produced by zona glomerulosa (ZG) cells of the adrenal cortex. Exaggerated overproduction of aldosterone from ZG cells causes primary hyperaldosteronism. In ZG cells, calcium entry through voltage-gated calcium channels plays a central role in the regulation of aldosterone secretion. Previous studies in animal adrenals and human adrenal adrenocortical cell lines suggest that the T-type but not the L-type calcium channel activity drives aldosterone production. However, recent clinical studies show that somatic mutations in L-type calcium channels are the second most prevalent cause of aldosterone-producing adenoma. Our objective was to define the roles of T and L-type calcium channels in regulating aldosterone secretion from human adrenals. We find that human adrenal ZG cells mainly express T-type CaV3.2 /3.3 and L-type CaV1.2/1.3 calcium channels. TTA-P2, a specific inhibitor of T-type calcium channel subtypes, reduced basal aldosterone secretion from acutely prepared slices of human adrenals. Surprisingly, nifedipine, the prototypic inhibitor of L-type calcium channels, also decreased basal aldosterone secretion, suggesting that L-type calcium channels are active under basal conditions. In addition, TTA-P2 or nifedipine also inhibited aldosterone secretion stimulated by angiotensin II- or elevations in extracellular K+. Remarkably, blockade of either L- or T-type calcium channels inhibits basal and stimulated aldosterone production to a similar extent. Low concentrations of TTA-P2 and nifedipine showed additive inhibitory effect on aldosterone secretion. We conclude that T- and L-type calcium channels play equally important roles in controlling aldosterone production from human adrenals.
Keywords: T-type calcium channels, L-type calcium channels, aldosterone secretion, primary aldosteronism
Introduction:
Aldosterone, a steroid hormone produced by zona glomerulosa (ZG) cells of the adrenal cortex, plays a key role in maintaining electrolyte and water balance, and thereby the control of blood pressure. Primary aldosteronism (PA), caused by autonomous overproduction of aldosterone, is the most common form of secondary endocrine hypertension (Stowasser 2001). Approximately 10% of hypertensive patients have PA (Hannemann and Wallaschofski 2012; Rossi 2011; Rossi, et al. 2006), compared to 20% of individuals with resistant hypertension(Calhoun, et al. 2002; Douma, et al. 2008; Hannemann and Wallaschofski 2012; Rossi 2011). Idiopathic hyperaldosteronism and aldosterone-producing adenoma are the two most common subtypes of PA (Funder, et al. 2008; Monticone, et al. 2017). Notably, somatic mutations of potassium and calcium channels have been found in more than half of aldosterone producing adenomas (Yang, et al. 2018).
Intracellular Ca2+ regulates key steps in aldosterone biosynthesis from ZG cells (Barrett, et al. 2016). Calcium controls the early rate-limiting step, the conversion of cholesterol to pregnenolone, by stimulating the transfer of cholesterol to CYP11A1 on the inner mitochondrial membrane (Cherradi, et al. 1996). Calcium controls the late rate-limiting step, the conversion of deoxycorticosterone to aldosterone, by stimulating the production of NADPH, a required cofactor for CYP11B2 activity (Lalevee, et al. 2003; Lifton, et al. 1992; Rossier, et al. 1996a; Wiederkehr, et al. 2011). In ZG cells, a rise of intracellular Ca2+ can be generated either by releasing Ca2+ from intracellular stores, or by increasing extracellular Ca2+ entry through voltage-gated calcium channels (Lotshaw 2001).
Extracellular Ca2+ entry through ZG voltage-gated calcium channels is necessary to maintain stimulated secretion induced by angiotensin II (Ang II) or K+, which are the two primary physiological aldosterone secretagogues in vivo (Barrett et al. 2016; Yang et al. 2018). ZG voltage-gated calcium channels are differentially expressed among mammalian species. Mainly two types of calcium currents have been identified in human, rat, mouse, and bovine ZG cells: high voltage-activated (L-type, including CaV1.1–1.4 subtypes) and low voltage-activated (T-type, including CaV3.1–3.3 subtypes) calcium channel currents (Cohen, et al. 1988; Hu, et al. 2012; Payet, et al. 1994; Rossier, et al. 1996b; Schrier, et al. 2001). However, previous studies indicate that the activity of the T-type, but not that of the L-type channel, correlates with aldosterone secretion in dispersed preparations of animal ZG cells and human adrenocortical cell lines (Akizuki, et al. 2008; Barrett, et al. 1995; Imagawa, et al. 2006; Rossier et al. 1996b). Thus, surprisingly recent clinical studies have found more than 25 different somatic CaV1.3 L-type calcium channels mutations in patients with PA (Yang et al. 2018), suggesting that in humans L-type calcium channels may also play an equally important role in controlling aldosterone production.
In this study, we assessed the contribution of T- and L-type calcium channels to the control of basal and stimulated aldosterone production from human adrenal slices. We find that both channel types participate in the control of aldosterone production from human ZG cells that remained within adrenal tissue slices.
Materials and methods
Cell culture and chemicals
Human adrenocortical cell line, H295R (ATCC#: CRL-2128), was purchased from American Type Culture Collection. Cells were cultured in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 medium (GIBCO, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (GIBCO), 1% antibiotic solution, and 0.1% insulin, transferrin and selenium premix (BD Biosciences). Human embryonic kidney cell line, HEK293, was purchased from The Cell Bank of Chinese Academy of Science. Cells were cultured in DMEM (Gibco) supplemented with 10% fetal bovine serum and 1% antibiotic antimycotic solution. TTA-P2 was purchased from Alomone Laboratories; Angiotensin II and nifedipine were purchased from Sigma-Aldrich (Munich, Germany).
Aldosterone secretion of human adrenal gland
Adrenal gland samples were obtained from 28 patients (16 men and 12 women, age 36–70 years old) undergoing radical nephrectomy to remove kidney cancer and adjacent ipsilateral adrenal. The protocol for obtaining and using human adrenal tissues in this work was approved by the Ethics Committee of Shanghai Cancer Center (050434-4-1212B) and School of Life Sciences (2017–601), Fudan University. Written informed consent from all patients was obtained. All protocols were in accordance with institutional guidelines.
Human adrenal samples were prepared as previously described (Yang, et al. 2016). Briefly, human adrenal samples were transported in cold PBS, and then kept in ice-cold bicarbonate-buffered saline containing (in mmol/L): 140 NaCl, 2 KCl, 0.1 CaCl2, 5 MgCl2, 26 NaHCO3, and 10 Glucose, bubbled with 95% O2 and 5% CO2. Fat tissues around adrenal glands were removed. Tissues were embedded in 2.5% low-melting temperature agarose (2-Hydroxyethylagarose, Sigma-Aldrich, Munich, Germany) and sectioned into 300 μm slices using DSK microslicer (DOSAKA). The slices were placed on Millicell-CM membranes (pore size 0.4 μm, Millipore, Burlington, MA, USA) and incubated in Hanks MEM (Gibco) containing (in mmol / L) 1 L-Glutamine, 1 CaCl2, 2 MgCl2, 30 HEPES, 12.8 Glucose, 5.24 NaHCO3, 12-mg / L bovine insulin, 0.12% ascorbic acid, and 20% horse serum (Gibco) at 37℃ with 5% CO2. Because the steroid synthesis profile of ZG cells may change during primary culture (Williams, et al. 2014), in this study we conducted relatively short time course secretion experiments. Slices were incubated for 2 hour to determine basal production (control), and then treated with the indicated agents for 6 hours to evaluate stimulated production. Slice culture supernatants were collected before and after treatment. Supernatant aldosterone concentrations were measured by an Aldosterone ELISA Kit (ENZO Life Science) as previous reported (Yang et al. 2016). For 62.5pg/ml and 125pg/ml aldosterone, the intra-assay co-efficients of variation were 5.2 and 6.2, and the inter-assay co-efficients of variation were 11.7 and 7.9. A standard curve (ranging from 3.9 pg/ml to 500pg/ml) was conducted along with samples for every measurement. 10uL supernatant (1:10 dilution in assay buffer) was used for aldosterone ELISA assay. In this condition, the mean percent recovery for 150 pg/ml and 50pg/ml aldosterone were 93% and 98% respectively. Data were presented as fold over baseline to avoid ZG mass differences among the adrenal slices.
Immunofluorescence
Human adrenal glands were fixed in 4% paraformaldehyde at 4℃ overnight, and transferred sequentially to 10%, 20%, and 30% sucrose / PBS. Tissues were embedded by OCT, and placed at −80℃. Tissues were sectioned (Leica CM1950 cryostat) into 20 μm slices and dried at room temperature. Slices were post-fixed in 4% paraformaldehyde for 15 min, washed (cold PBS), incubated in 0.5% PBST for 10 min, and blocked (10% horse serum, 0.3% PBST) for 2 hours at room temperature. Slices were then incubated in primary antibody solution [primary antibody (mouse anti-CaV1.2: 1:200, AB11001554, Neuromab; mouse anti-CaV1.3: 1:200, AB10673964, Neuromab; mouse anti-CaV3.1: 1:200, AB2069421 Neuromab; rabbit anti-CaV3.2: 1:200, #ACC-025, Alomone; rabbit anti-CaV3.3: 1:200, #ACC-009, Alomone; rabbit anti-CYP11B2: 1:200, #ab168388, Abcam), 1% horse serum, and 0.3% PBST] for 1 day at 4℃. Slices were washed with PBS, and incubated in secondary antibody solution [secondary antibody (Cy3-labled goat anti-rabbit IgG or FITC-labeled goat anti-mouse IgG, 1:500, Beyotime), containing 1% horse serum, and 0.3% PBST] at 4℃ overnight. After washing with PBS, slices were treated with DAPI. Confocal images were obtained using a Leica SP2 confocal microscope.
ZG cell isolation, RNA extraction and RT-PCR
The ZG layer was isolated from human adrenal glands using laser capture microdissection (Arcturus XT system, Life Technologies). Before laser capture microdissection, adrenal glands were incubated in RNAlater Solution (Life Technologies) at 4℃ overnight, embedded with OCT, sectioned into 20 μm slices, and stained with HistoGene Staining Solution (Life Technologies). RNA was extracted (RNeasy Micro Kit, QIAGEN), reverse transcribed (SMART-Seq v4 Ultra Low Input RNA Kit, Clontech Laboratories). PCR was performed in 20 μL reactions containing: 2 μL of template, 0.4 μmol/L of each paired primer, and iQ SYBR Green Supermix (Bio-Rad). The thermocycling conditions were 95℃, 3 min; 40 cycles of 95℃, 15 s; 58℃, 30 s; 72℃, 30 s; and 72℃, 10 min. Primers used in PCR: CaV1.1: forward 5’ CAGCAC TACAAC CAGTCG GA, reverse 5’ CTCCAC CCAGGC AATACA GTC; CaV1.2: forward 5’ CCTTTC TGGTTT AGCTGT GGG AAG ATCT, reverse 5’ AATGCA AAGAGT TACTGAT TCCCGT TTCAG; CaV1.3: forward 5’ GCACTA CGA GCA GTCCAA GA, reverse 5’ GCTGCC GATTAC GATGAG GG; CaV1.4: forward 5’ ACGGTG GAGATG CTTCTC AAATTG TACG, reverse 5’ GGTGAC CTTAAA GATCCT GAGGAG GC; CaV3.1: forward 5’ GATCCT GACCCA GGAGGA CT, reverse 5’ AAGAGC ACGTAG TTGCCGAA; CaV3.2: forward 5’ CCTGGA GGAGAG CAACAAGG, reverse 5’ GGTCTC CATCTC CACCTCCT; CaV3.3: forward 5’ ACGCAT CTTTCT CACCGTGT, reverse 5’ ATGGGC TTGAGG GAGGAGAT;
Cell transfection and electrophysiology
Plasmids for human CaV3.1, CaV3.2 and CaV3.3 channels (as previously reported (Cui, et al. 2014) were transiently transfected in H295R or HEK293 cells using jetPRIME (Polyplus) according to the manufacturer’s instruction. For electrophysiological experiments, cells were used 24 hours after transfection. CaV1.2 (Plasmid # 26572, Addgene)(Helton, et al. 2005) or CaV1.3 (Plasmid # 26576, Addgene)(Xu and Lipscombe 2001) channels from Addgene (Cambridge, MA, USA) were transfected together with β2 and α2δ1 subunits (ratio 1:1:1) in H295R cells for 48–72 hours before electrophysiological measurements. Whole-cell currents in the H295R cells were recorded using an Axopatch 200B amplifier (Axon Instruments). The bath solution contained (in mmol/L): 143 TEACl, 10 CaCl2 (for CaV3 channel) or BaCl2 (for CaV1 channel), 2 MgCl2, 10 HEPES, and 10 Glucose, pH 7.4 (adjusted with TEAOH). The internal solution contained (in mmol/L): 125 CsCl, 10 HEPES, 10 EGTA, 1 MgCl2, 1 CaCl2, 5 Mg-ATP, and 0.3 Tris-GTP (pH adjusted to 7.2 using CsOH). Recording pipettes (capillary tubing, BRAND) had resistances of 4–6 MΩ. All of the recordings were performed at room temperature. Currents were sampled at 10 kHz and filtered at 2 kHz, and corrected online for leak and residual capacitance transients using a P/4 protocol.
Data presentation and statistical analysis
Data analysis was performed with Clampfit 10.2 (Axon Instruments) and Origin 8.0 (OriginLab). The normal distribution of the data was checked by the Shapiro–Wilk test. Statistical analysis was performed using paired Student t tests unless indicated otherwise. The nonparametric Kruskal-Wallis test was used to compare groups that did not meet the assumption of normality. Values are given as means ± S.E.M, n indicated the number of tested cells, patient samples or independent tests. P < 0.05 was considered statistically significant.
Results
L- and T-type calcium channels regulate basal aldosterone production from human adrenal slices.
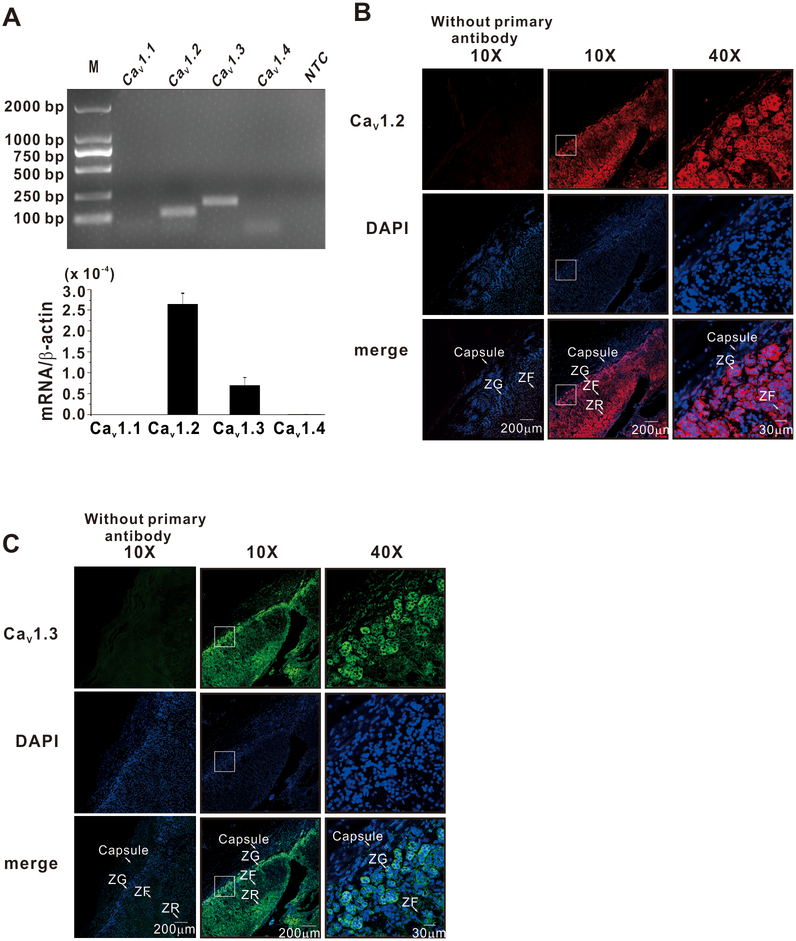
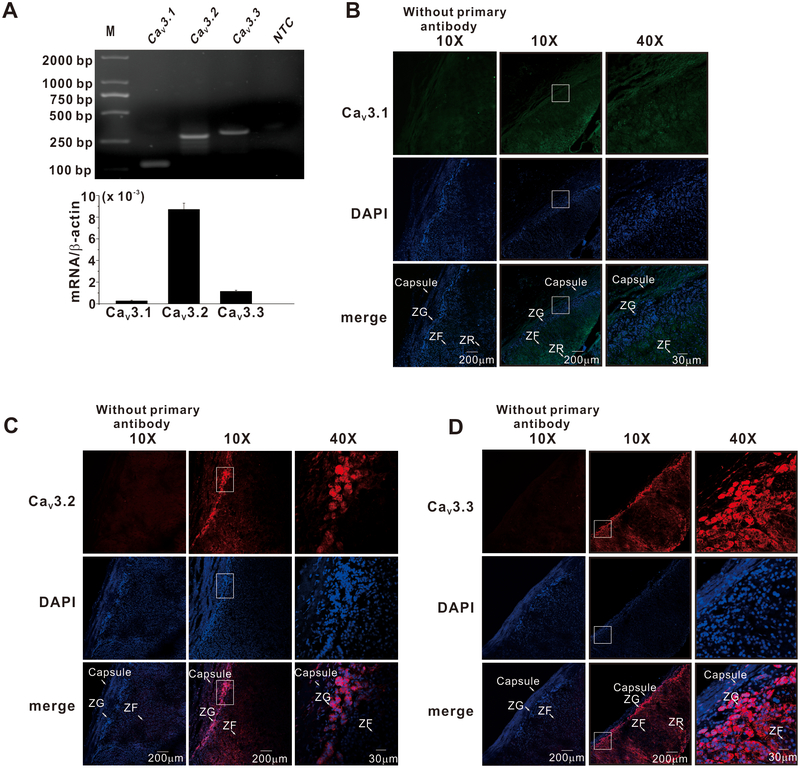
To determine which L-type calcium channel subtypes were expressed in human ZG cells, we isolated ZG cells by laser capture microdissection and tested for mRNA expression. As shown in Figure 1A, mRNA for CaV1.2 and CaV1.3 but not CaV1.1 and CaV1.4 was detected in ZG cells. All PCR products were confirmed by sequencing. To corroborate protein expression of CaV1.2 and CaV1.3 channels we used immunofluorescence. Immunodetection showed high expression of both CaV1.2 and CaV1.3 channel protein in the ZG layer of the human adrenal (Fig. 1B). By contrast, although the mRNA for all three T-type calcium channel subtypes was detected in ZG cells (Fig. 2A), the expression of T-type calcium channel protein, as reported by immunofluorescence, showed only strong expression for CaV3.2 and CaV3.3 channel protein. Signals for CaV3.1 channels were not detected (Fig. 2B–D) in the ZG layer of the human adrenal cortex, indicating a discrepancy between mRNA and protein expression. To assess the selectivity of these antibodies, and thus verify our putative expression findings, HEK293 cells were transfected with a vector encoding one of five channel subtypes to provide both positive and negative immunodetection controls. HEK293 cells expressing CaV3.1, CaV3.2, CaV3,3, CaV1.2 and CaV1.3 channels showed strong immunofluorescence signals only with antibodies against their own antigenic target; no signals were detected in either un-transfected HEK293 cells or in cells transfected with other channel family members (Fig. S1 and S2). Thus, the normal human adrenal cortex, indeed expresses proteins for both Cav1x and Cav3x channels.
Figure 1. L-type calcium channel expression in the zona glomerulosa of human adrenals.
A, Human ZG cells express mRNA for L-type calcium channels. Zona glomerulosa was isolated using laser capture microdissection. Real-time polymerase chain reaction revealed message for L-type Ca2+ channels (CaV1.2 and CaV1.3, but not CaV1.1 and CaV1.4). B, Representative examples of immunofluorescence images showing high expression of CaV1.2 and CaV1.3 channels in zona glomerulosa of the human adrenal cortex. Zona fasciculata (ZF). zona reticularis (ZR).
Figure 2. T-type calcium channel expression in the zona glomerulosa of human adrenals.
A, Human ZG cells express mRNA for T-type calcium channels. Zona glomerulosa was isolated using laser capture microdissection. Real-time polymerase chain reaction revealed message for T-type Ca2+ channels (CaV3.1, CaV3.2 and CaV3.3). B-D, Representative examples of immunofluorescence images showing high expression of CaV3.2 and CaV3.3 but not CaV3.1 channels in zona glomerulosa of the human adrenal cortex.
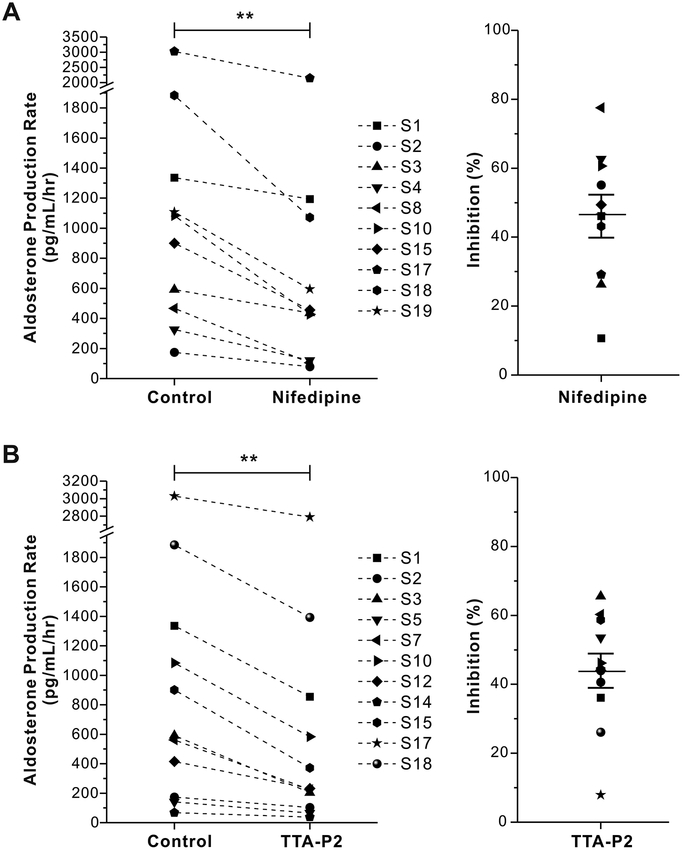
Next, we used the L-type calcium channel specific inhibitor, nifedipine and the T-type calcium channel specific inhibitor, TTA-P2, to test the contribution of each channel class to basal aldosterone secretion from human adrenal slices. 500 nmol/L nifedipine significantly and consistently reduced aldosterone secretion from adrenal tissue slices prepared from 10 individual patient adrenals (percent inhibition: 46±6%, n = 10, p < 0.01 compared with control by student t tests, Fig. 3A). 2 μmol/L TTA-P2 also significantly inhibited aldosterone secretion from adrenal tissue slices prepared from 11 individual patient adrenals (percent inhibition: 44±5%, n = 11, p < 0.01, Fig. 3B).
Figure 3. Effects of L - and T -type calcium channels on basal aldosterone secretion from human adrenal slices.
A, Specific L-type channel blocker, nifedipine (500 nmol/L), inhibits basal aldosterone production from human adrenal slices (left); Statistical analysis of nifedipine induced inhibition of aldosterone secretion from human adrenal slices (right). Data expressed as fold over control. **P < 0.01 compared with control (n = 10). B, Specific T-type channel blocker TTA-P2 (2 μmol/L) reduced basal aldosterone production from human adrenal slices (left); Statistical analysis of TTA-P2 induced inhibition of aldosterone secretion from human adrenal slices (right). **p < 0.01 compared with control (n = 11). (S1–19 = samples from different individual patients).
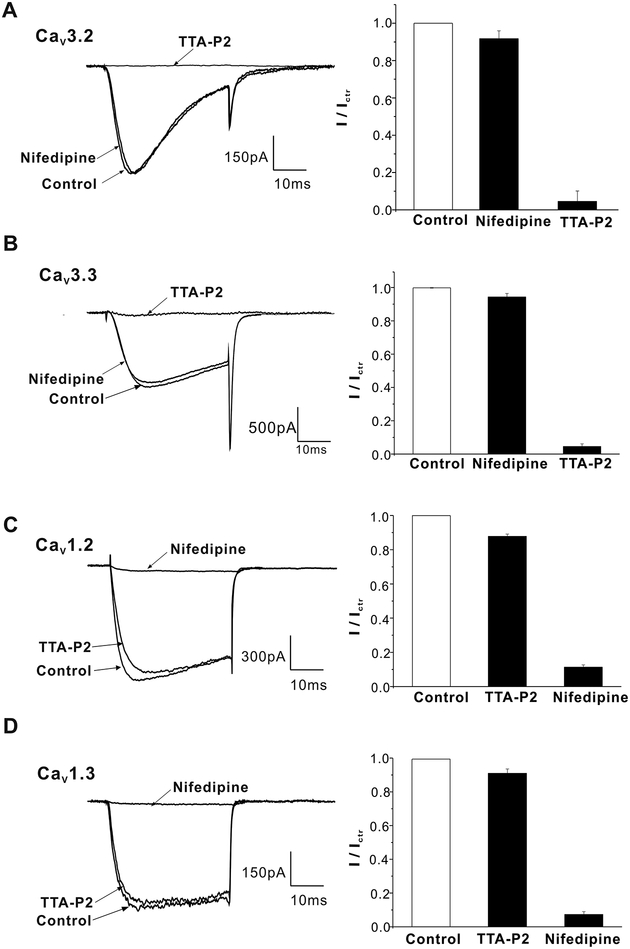
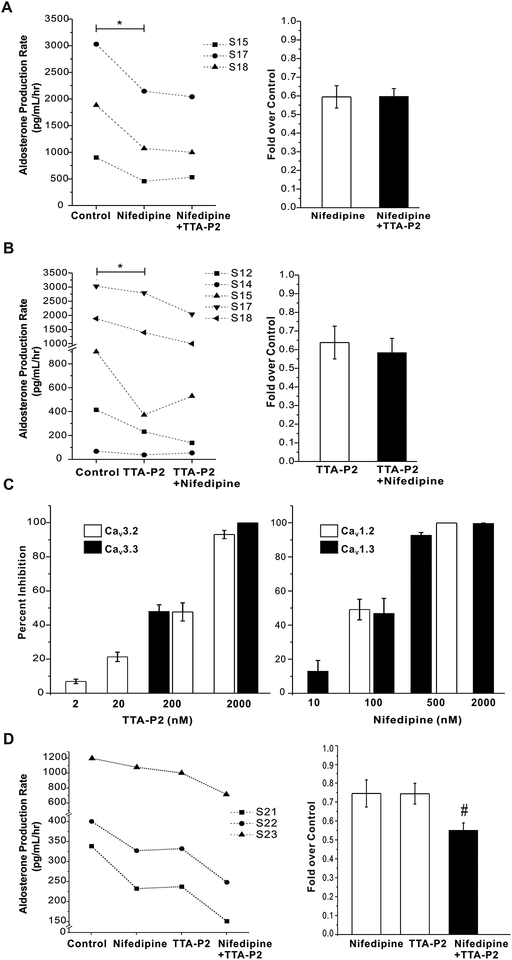
Although nifedipine is considered to be a highly selective L-type calcium channels blocker (Stengel, et al. 1998), we tested if nifedipine could also partially inhibit T-type calcium channels at high nanomolar concentration. Because human adrenal ZG cells express both CaV3.2 and CaV3.3 channels, we evaluated inhibition of CaV3.2 and CaV3.3 currents in H295R cells by 500 nmol/L nifedipine. Calcium currents were elicited by a 50-ms depolarizing pulse to −20 mV from a holding potential of −90 mV at 10 s intervals. 500 nmol/L nifedipine did not inhibit either CaV3.2 or CaV3.3 channel currents, whereas 2 μmol/L TTA-P2 fully inhibited the currents. (Fig. 4A and B, n = 5–6). To confirm the efficacy of nitrendipine, we tested its effects on CaV1.2 and CaV1.3 channel currents at 500 nmol/L. As shown in Fig. 4C and D, 500 nmol/L nifedipine almost fully inhibited CaV1.2 and CaV1.3 channel currents in H295R cells, in contrast to 2 μmol/L TTA-P2 which had little effect (n = 5). To rule out a nonselective inhibitory effect of these drugs at voltages other than −20 mV, we recorded the current-voltage relationship for each channel (Cav1.2, 1.3, 3.2, 3.3) and tested for drug-induced changes. At all voltages, 500 nmol/L nifedipine or 2 μmol/L TTA-P2 remained selective for their channel targets (Fig.S3). The data above indicate that the inhibition of L- or T-type calcium channel activity should account for the induced effects of 500 nmol/L nifedipine or 2 μmol/L TTA-P2 reported here.
Figure 4. Effects of nifedipine (500 nmol/L) or TTA-P2(2 μmol/L) on CaV3.2/3 and CaV1.2 /3 channel currents in H295R cells.
A, Effects of L-type Ca2+ channel inhibitor nifedipine and T-type Ca2+ channel inhibitor TTA-P2 on CaV3.2 channel currents (n = 6). B, Effects of 500 nmol/L nifedipine and 2 μmol/L TTA-P2 on CaV3.3 channel currents(n = 5). C, Effects of 500 nmol/L nifedipine and 2 μmol/L TTA-P2 on CaV1.2 channel currents(n = 5). D, Effects of 500 nmol/L nifedipine and 2 μmol/L TTA-P2 on CaV1.3 channel currents(n = 5). Calcium currents were elicited by a 50-ms depolarizing pulse to −20 mV from a holding potential of −90 mV at 10 s intervals.
Effect of nifedipine and TTA-P2 on Ang II- and K+-induced aldosterone secretion from humane adrenal slices.
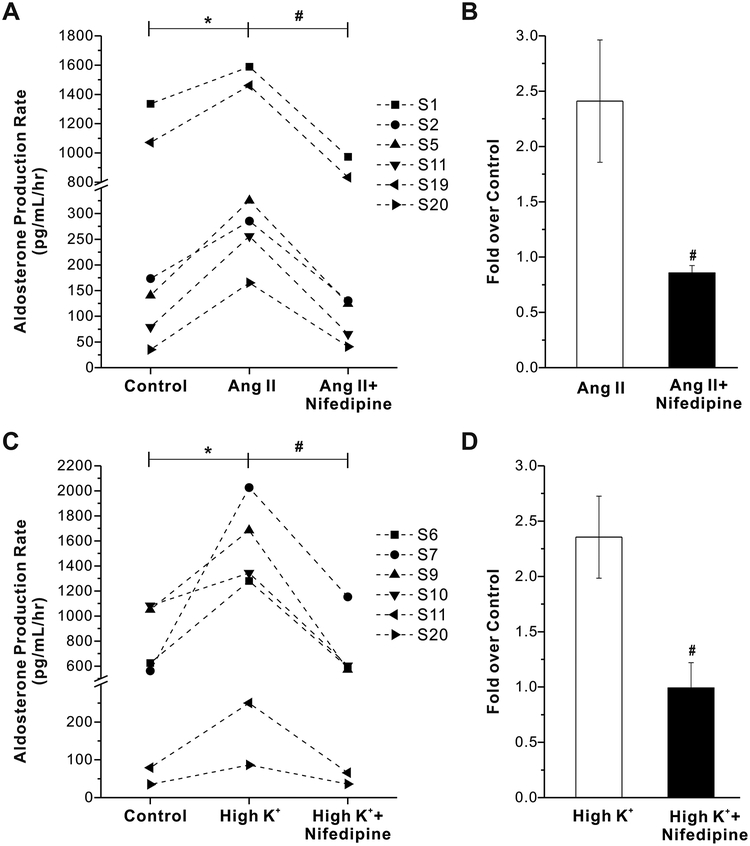
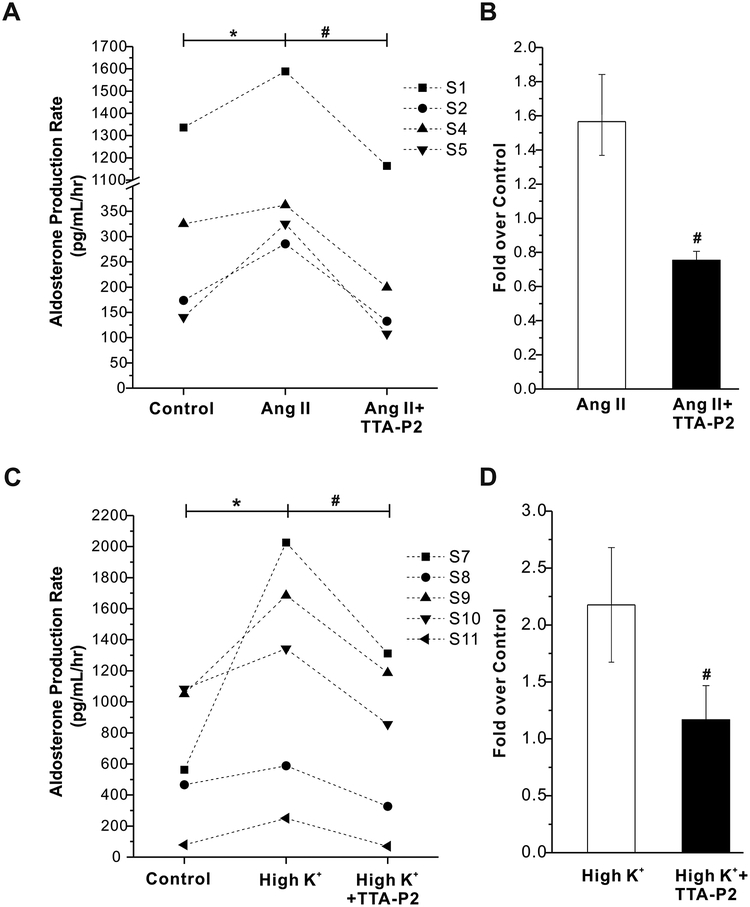
To assess the overall importance of L-type and T-type calcium channels to the production of aldosterone in human adrenals, we tested for nifedipine or TTA-P2 evoked modulation of Ang II- or K+-induced aldosterone secretion. As shown in Fig. 5A, 500 nmol/L nifedipine abrogated the stimulation of aldosterone production by 10 nmol/L Ang II (fold increase: Ang II, 2.4±0.6; Ang II + nifedipine, 0.9±0.1, n = 6, p < 0.05 compared with Ang II alone, Fig. 5B). 500 nmol/L nifedipine also abolished aldosterone production induced by High K+ (15 mmol/L) (fold increase: High K+, 2.4±0.4; High K+ + Nifedipine, 1.0±0.2, n = 6, p < 0.05 compared with High K+ alone, Fig. 5C and D). Interestingly, 2 μmol/L TTA-P2 also abrogated the stimulation of aldosterone production by Ang II or High K+ from acutely prepared slices of human adrenals (p < 0.05, Fig. 6). Together, these data indicate that in human adrenals both T- and L-type calcium channel activity is required for stimulated aldosterone synthesis.
Figure 5. Effects of nifedipine on Angiotensin II (Ang II) - or high K+- stimulated aldosterone secretion from human adrenal slices.
A, Nifedipine abrogates Ang II–stimulated aldosterone secretion from human adrenal slices. B, Statistical analysis of effect of nifedipine-induced inhibition of Ang II-stimulated aldosterone secretion. Data are presented as fold over control. *, p < 0.05 compared with control (n = 6). #, p < 0.05 compared with Ang II alone (n = 6). C, Nifedipine abrogated high K+-stimulated aldosterone secretion from human adrenal slices. D, Statistical analysis of nifedipine-induced inhibition of high K+-stimulated aldosterone secretion. Data are presented as fold over control. *, p < 0.05 compared with control (n = 6). #, p < 0.05 compared with high K+ alone (n = 6).
Figure 6. Effect of TTA-P2 on Angiotensin II (Ang II) - or high K+- stimulated aldosterone secretion from human adrenal slices.
A, TTA-P2 abrogates Ang II-stimulated aldosterone secretion from human adrenal slices. B, Statistical analysis of TTA-P2 induced inhibition of Ang II-stimulated aldosterone secretion. Data are presented as fold over control. *, P < 0.05 compared with control. #, p < 0.05 compared with Ang II alone (n = 4). C, TTA-P2 abrogates high K+-stimulated aldosterone secretion from human adrenal slices. D, Statistical analysis of TTA-P2 induced inhibition of high K+-stimulated aldosterone secretion. *, p < 0.05 compared with control.#, p < 0.05 compared with high K+ alone (n = 5).
Additivity of effects of nifedipine and TTA-P2 on aldosterone production from human adrenal slices.
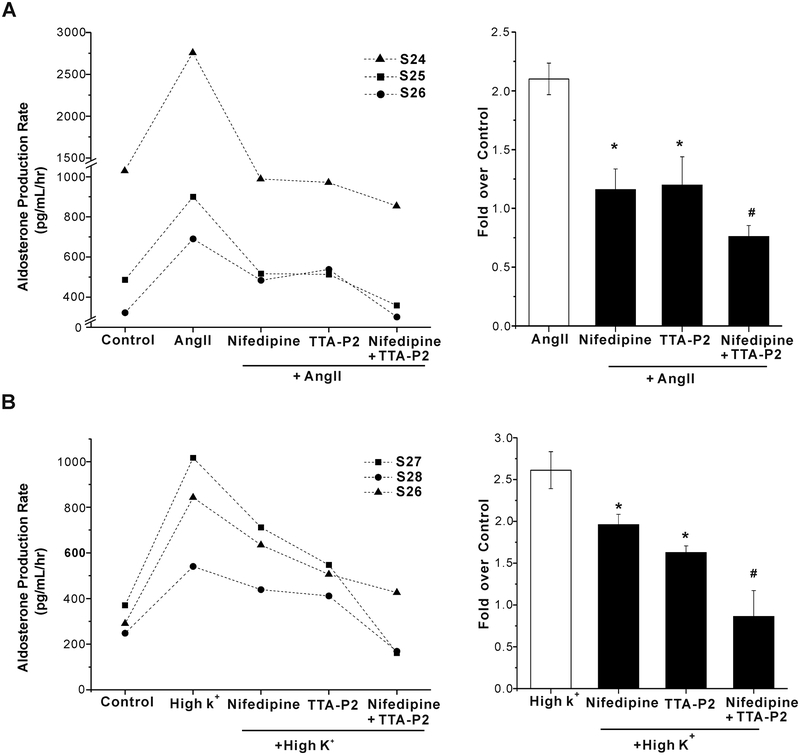
Finally, we tested whether the combined application of nifedipine and TTA-P2 could reduce aldosterone secretion from human adrenal slices further than the level of inhibition induced by either drug alone. As shown in Fig. 7A, 2 μmol/L TTA-P2 did not augment the degree of inhibition induced by nifedipine (P > 0.05 compared with nifedipine alone). Similarly, 500 nmol/L nifedipine did not increase the inhibition of basal aldosterone production from human adrenal slices produced by TTA-P2 (n = 5, p > 0.05 compared with TTA-P2 alone, Fig. 7B). To further test for drug additivity, we used lower concentrations of each antagonist, near the IC50 for T or L channel current inhibition (Fig.7C). As shown in Fig.7D, the inhibition of basal aldosterone secretion from human adrenal slices by 200 nmol/L TTA-P2 was additive to that of 100 nm/L nifedipine (n = 3). Drug additivity was also demonstrable against agonist-stimulated secretion. The inhibition of Ang II (10 nmol/L) or high K+(15mM)-stimulated aldosterone secretion from human adrenal slices induced by either TTA-P2(200 nmol/L) or nifedipine (100 nm/L) was less than that effected by these channel antagonists in combination (Fig.8).These data indicate that both T- and L-type calcium channels maybe necessary to drive aldosterone production.
Figure 7. Effect of combined exposure to nifedipine and TTA-P2 on the basal production of aldosterone from human adrenal slices.
Both nifedipine and TTA-P2 maximally inhibit aldosterone secretion. A, TTA-P2 does not alter nifedipine-induced inhibition of aldosterone production from human adrenal slices. B, Nifedipine does not alter TTA-P2 inhibition of aldosterone production from human adrenal slices (n = 5). C, Effects of different concentrations of nifedipine or TTA-P2 on CaV3.2/3 and CaV1.2/3 channel currents in H295R cells. D, 100 nm/L nifedipine and 200 nm/L TTA-P2 showed additive effect on basal aldosterone secretion. #, p < 0.05 compared with nifedipine or TTA-P2 alone.
Figure 8. Effect of combined exposure to nifedipine and TTA-P2 on Ang II or high K+-stimulated aldosterone secretion from human adrenal slices.
A, 100 nm/L nifedipine and 200 nm/L TTA-P2 showed additive inhibitory effect on Ang II–stimulated aldosterone secretion from human adrenal slices. #, p < 0.05 compared with nifedipine or TTA-P2 alone.
B, 100 nm/L nifedipine and 200 nm/L TTA-P2 showed additive inhibitory effect on High K+–stimulated aldosterone secretion. #, p < 0.05 compared with nifedipine or TTA-P2 alone.
With aging, the cellular capacity to synthesize aldosterone declines (Hegstad, et al. 1983; Nanba, et al. 2018). Less aldosterone synthase (CYP11B2) is expressed in the human ZG and more is expressed in aldosterone producing clusters (APCCs) found beneath the adrenal capsule (Nishimoto, et al. 2010; Nishimoto, et al. 2016). APCCs, formed by non-neoplastic cells, increase with age and harbor mutations in the CACNA1D gene that encodes Cav1.3 channels (Nanba, et al. 2017; Nishimoto, et al. 2015).
Therefore, we calculated the size effect (percent inhibition by nifedipine) as a function of patient age to determine if the nifedipine-induced inhibition of aldosterone production reported here increased with patient age. In the relatively small sample size of our study, we found no significant correlations with aging suggesting a role for normal human L-type calcium channels in the control of aldosterone synthesis (basal production:, R = −0.21; P = 0.56 (Fig. S4); Ang II stimulated: R = 0.23; P = 0.66 (data not shown); K-stimulated: R = 0.24; P = 0.64 (data not shown).
Discussion
Here, we report the novel findings that in human adrenal glomerulosa cells both basal and stimulated rates of aldosterone production depend on voltage-gated calcium currents through T-type as well as L-type calcium channels. These findings are surprising because patch-clamp studies consistently report the basal membrane potential of ZG cells to be very hyperpolarized (around −80 mV), a potential at which high threshold L-type calcium channels are not expected to be active. In contrast, low threshold T-type calcium channels are activated at more hyperpolarized potentials and have a permissive window of steady-state activity at more hyperpolarized potentials(Perez-Reyes 2003). When stimulated by low physiological concentrations of Ang II or small increases in extracellular potassium, the membrane potential of ZG cells can rapidly reach the permissive voltage window for persistent T-type, but not L-type, calcium channel activity, that allows steady-state calcium influx(Rossier 2016). Therefore, in primary cultured ZG cells(Lotshaw 2001; Python, et al. 1993; Rossier et al. 1996b; Rossier, et al. 1998; Rossier, et al. 1993) or H295R cells(Akizuki et al. 2008), it is not surprising that Ang II- or K+-stimulated aldosterone secretion consistently has been reported to be efficiently inhibited by various T-type calcium channel blockers, and not inhibited by highly selective L-type calcium channel inhibitors such as nifedipine. Nevertheless, previously we reported that mouse ZG cells when retained within an adrenal slice spontaneously generate Vm oscillations (Hu et al. 2012), of high amplitude, that can reach depolarized membrane voltages close to but always below 0 mV, voltages at which L-type calcium channels are active. Therefore, the contribution of L-type calcium channels to the regulation of aldosterone production previously may have been underestimated in studies of dispersed ZG cells that have lost the intrinsic ability to oscillate persistently.
Indeed, here we find that within human adrenal slices the L-type specific blocker nifedipine significantly inhibited secretagogue-stimulated aldosterone secretion from ZG cells. Although some dihydropyridines that are considered to be specific L-type channel inhibitors, such as nicardipine and niguldipine also inhibit T-type calcium channels, nifedipine discriminates between T- and L-type calcium channel families(Stengel et al. 1998). Nifedipine selectively inhibits L-type calcium channels in human adrenocortical H295R cells(Akizuki et al. 2008; Imagawa et al. 2006; Rossier et al. 1996b; Yang et al. 2016) and in primary cultured human adrenal glomerulosa cells(Payet et al. 1994), and at 500nM in our study had no effect on recombinant CaV3.2 and CaV3.3 channel currents expressed in human adrenal cells. Therefore, inhibition of L-type calcium channel activity should account for the nifedipine-induced reduction of aldosterone production reported here. Similar to previous studies(Dreyfus, et al. 2010; Enyeart and Enyeart 2015), 2μm TTAP2 fully inhibits T-type calcium channels, and has no effect on L-type calcium channels.
That nifedipine also inhibited aldosterone secretion from unstimulated human adrenal slices implies that L-type calcium channels are active even under basal condition. Again, we hypothesize that if human ZG cells within adrenal slices spontaneously generate Vm oscillations, as mouse ZG cells, this would enable L-type calcium channels to participate in regulating basal production. The direct proof of this prediction requires voltage recordings of human ZG cells by patch clamp. However, unfortunately we found that obtaining a gigaseal on adult human ZG cells (data not shown) was not possible. Rather, we used the very limited human adrenal samples for aldosterone secretion experiments. It is surprising that the degree of inhibition of aldosterone secretion evoked by nifedipine was equivalent to that of TTA-P2. At concentrations that produced maximal inhibition of L or T channel currents, no additivity of drug effects or synergy was observed, whereas at near half-maximal concentrations, nifedipine and TTA-P2 showed additive inhibitory effects on both basal and agonist-stimulated aldosterone secretion. Together, these observations suggest that in the human ZG layer, L- and T-type calcium channel flux may not be redundant but rather may each subserve a unique function that is required for the control of aldosterone production. Therefore, we propose a model for the regulation of aldosterone secretion from human ZG cells by T- and L-type channels that can explain our findings. We predict that like mouse ZG cells, human ZG cells may also spontaneously oscillate, allowing the unique biophysical properties of each channel class to have distinct roles in controlling the oscillatory cycle. We hypothesize that by opening at more hyperpolarized potentials T-type channels may be principally responsible for recruiting L-type channels that, in turn, may provide the major calcium source of calcium required for the production of aldosterone; AngII or high K+ may increase oscillation frequency and thus control the magnitude of calcium entry and the production of aldosterone.
The samples used in this study were obtained from patients spanning a broad age range, and would be expected to contain an age-dependent increase in APCCs. Indeed, with tissue remaining from some patient samples, we detected a very large CYP11B2 expressing APCCs in one of three samples (Fig. S5). Approximately one-third of APCCs (Nishimoto et al. 2015) harbor mutations in Cav1.3 channels that lower the voltage-dependence for channel activation, or reduce channel inactivation (Azizan, et al. 2013; Scholl, et al. 2013), biophysical alterations that would make mutant channels more likely to participate in regulating calcium at hyperpolarized potentials. If mutant channels were the sole target of nifedipine action reported here, we would have expected an age-dependent increase in the efficacy of nifedipine, which we did not observe (Fig.S4). Thus, we conclude that normal CaV1.3 channels as well as mutant CaV1.3 channels participated in the response to nifedipine. The relative contribution of each channel type to the inhibition of aldosterone synthesis induced by nifedipine in normal adrenal tissue is worthy of a future expanded study with a larger sample size.
In conclusion, our data demonstrate that L-type calcium channels play an important role in the regulation of aldosterone production from human ZG cells within a tissue context, and that both T- and L-type calcium channels critically contribute to aldosterone production from human adrenals.
Supplementary Material
Figure S1. Positive controls for CaV1.2 and CaV1.3 antibodies. (A) The HEK293 cells transfected with CaV1.2 channels showed strong immunofluorescence signals, while the un-transfected cells and cells transfected with CaV1.3 show no signals. (B) The HEK293 cells transfected with CaV1.3 showed strong immunofluorescence signals, while the un-transfected cells and cells transfected with CaV1.2 showed no signals.
Figure S2. Positive controls for CaV3.1 , CaV3.2 and CaV3.3 antibodies. The HEK293 cells transfected with CaV3.1 channels showed strong immunofluorescence signals, while the un-transfected cells and cells transfected with CaV3.2/3.3 channels show no signals. (B) The HEK293 cells transfected with CaV3.2 channels showed strong immunofluorescence signals, while the un-transfected cells and cells transfected with CaV3.1/3.3 show no signals. (C) The HEK293 cells transfected with CaV3. 3 channels showed strong immunofluorescence signals, while the un-transfected cells and cells transfected with CaV3.1/3.2 showed no signals.
Figure S3. Effects of nifedipine (500 nmol/L) or TTA-P2(2 μmol/L) on CaV3.2/3 and CaV1.2 /3 channel currents in H295R cells. A, Effects of L-type Ca2+ channel inhibitor nifedipine and T-type Ca2+ channel inhibitor TTA-P2 on CaV3.2 channel currents (n = 4). B, Effects of 500 nmol/L nifedipine and 2 μmol/L TTA-P2 on CaV3.3 channel currents(n = 5). C, Effects of 500 nmol/L nifedipine and 2 μmol/L TTA-P2 on CaV1.2 channel currents(n = 4). D, Effects of 500 nmol/L nifedipine and 2 μmol/L TTA-P2 on CaV1.3 channel currents(n = 6). Calcium currents were elicited by 50-ms steps (in 5-mV increments, −70 to +30 mV for CaV3.2/3.3; −50 to +50 mV for CaV1.2/1.3) from a holding potential of -90 mV at 10 s intervals.
Figure S4. Effect of nifedipine on basal secretion from adrenal slices at different ages. No correlation was observed between the inhibitory effect of 500nM/L nifedipine and age (R = -0.211; P = 0.56).
Figure S5. Aldosterone-producing cell clusters (APCCs) in human adrenal slices. Representative immunofluorescence images of S10 and S15 adrenals showing continuous CYP11B2 expression in the zona glomerulosa (S10: 47 year-old, male; S15: 54 year-old, female). Representative immunofluorescence images of S27 adrenal showing discontinuous CYP11B2 expression in the zona glomerulosa with APCCs (S27: 56 year-old, male )
Funding
This work was supported by the National Natural Science Foundation of China (NSFC 31771282 and 31271240) and the National Key R&D Program of China (2016YFA0100802) awarded to C. Hu, and by grant from the National Institutes of Health (HL138241) awarded to P. Q. Barrett.
Footnotes
Declaration of interest
The authors declare no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported
References
- Akizuki O, Inayoshi A, Kitayama T, Yao K, Shirakura S, Sasaki K, Kusaka H & Matsubara M 2008. Blockade of T-type voltage-dependent Ca2+ channels by benidipine, a dihydropyridine calcium channel blocker, inhibits aldosterone production in human adrenocortical cell line NCI-H295R. Eur J Pharmacol 584 424–434. [DOI] [PubMed] [Google Scholar]
- Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, Maniero C, Garg S, Bochukova EG, Zhao W, et al. 2013. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet 45 1055–1060. [DOI] [PubMed] [Google Scholar]
- Barrett PQ, Ertel EA, Smith MM, Nee JJ & Cohen CJ 1995. Voltage-gated calcium currents have two opposing effects on the secretion of aldosterone. Am J Physiol 268 C985–992. [DOI] [PubMed] [Google Scholar]
- Barrett PQ, Guagliardo NA, Klein PM, Hu C, Breault DT & Beenhakker MP 2016. Role of voltage-gated calcium channels in the regulation of aldosterone production from zona glomerulosa cells of the adrenal cortex. J Physiol 594 5851–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB & Weissmann P 2002. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension 40 892–896. [DOI] [PubMed] [Google Scholar]
- Cherradi N, Rossier MF, Vallotton MB & Capponi AM 1996. Calcium stimulates intramitochondrial cholesterol transfer in bovine adrenal glomerulosa cells. J Biol Chem 271 25971–25975. [DOI] [PubMed] [Google Scholar]
- Cohen CJ, McCarthy RT, Barrett PQ & Rasmussen H 1988. Ca channels in adrenal glomerulosa cells: K+ and angiotensin II increase T-type Ca channel current. Proc Natl Acad Sci U S A 85 2412–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Liu X, Yang T, Mei YA & Hu C 2014. Exposure to extremely low-frequency electromagnetic fields inhibits T-type calcium channels via AA/LTE4 signaling pathway. Cell Calcium 55 48–58. [DOI] [PubMed] [Google Scholar]
- Douma S, Petidis K, Doumas M, Papaefthimiou P, Triantafyllou A, Kartali N, Papadopoulos N, Vogiatzis K & Zamboulis C 2008. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet 371 1921–1926. [DOI] [PubMed] [Google Scholar]
- Dreyfus FM, Tscherter A, Errington AC, Renger JJ, Shin HS, Uebele VN, Crunelli V, Lambert RC & Leresche N 2010. Selective T-type calcium channel block in thalamic neurons reveals channel redundancy and physiological impact of I(T)window. J Neurosci 30 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyeart JJ & Enyeart JA 2015. Adrenal fasciculata cells express T-type and rapidly and slowly activating L-type Ca2+ channels that regulate cortisol secretion. Am J Physiol Cell Physiol 308 C899–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young WF Jr., VM Montori & Endocrine S 2008. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 93 3266–3281. [DOI] [PubMed] [Google Scholar]
- Hannemann A & Wallaschofski H 2012. Prevalence of primary aldosteronism in patient’s cohorts and in population-based studies--a review of the current literature. Horm Metab Res 44 157–162. [DOI] [PubMed] [Google Scholar]
- Hegstad R, Brown RD, Jiang NS, Kao P, Weinshilboum RM, Strong C & Wisgerhof M 1983. Aging and aldosterone. Am J Med 74 442–448. [DOI] [PubMed] [Google Scholar]
- Helton TD, Xu W & Lipscombe D 2005. Neuronal L-type calcium channels open quickly and are inhibited slowly. J Neurosci 25 10247–10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Rusin CG, Tan Z, Guagliardo NA & Barrett PQ 2012. Zona glomerulosa cells of the mouse adrenal cortex are intrinsic electrical oscillators. J Clin Invest 122 2046–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa K, Okayama S, Takaoka M, Kawata H, Naya N, Nakajima T, Horii M, Uemura S & Saito Y 2006. Inhibitory effect of efonidipine on aldosterone synthesis and secretion in human adrenocarcinoma (H295R) cells. J Cardiovasc Pharmacol 47 133–138. [DOI] [PubMed] [Google Scholar]
- Lalevee N, Resin V, Arnaudeau S, Demaurex N & Rossier MF 2003. Intracellular transport of calcium from plasma membrane to mitochondria in adrenal H295R cells: implication for steroidogenesis. Endocrinology 144 4575–4585. [DOI] [PubMed] [Google Scholar]
- Lifton RP, Dluhy RG, Powers M, Rich GM, Cook S, Ulick S & Lalouel JM 1992. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature 355 262–265. [DOI] [PubMed] [Google Scholar]
- Lotshaw DP 2001. Role of membrane depolarization and T-type Ca2+ channels in angiotensin II and K+ stimulated aldosterone secretion. Mol Cell Endocrinol 175 157–171. [DOI] [PubMed] [Google Scholar]
- Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, Gabetti L, Mengozzi G, Williams TA, Rabbia F, et al. 2017. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J Am Coll Cardiol 69 1811–1820. [DOI] [PubMed] [Google Scholar]
- Nanba K, Vaidya A & Rainey WE 2018. Aging and Adrenal Aldosterone Production. Hypertension 71 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanba K, Vaidya A, Williams GH, Zheng I, Else T & Rainey WE 2017. Age-Related Autonomous Aldosteronism. Circulation 136 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, et al. 2010. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab 95 2296–2305. [DOI] [PubMed] [Google Scholar]
- Nishimoto K, Seki T, Hayashi Y, Mikami S, Al-Eyd G, Nakagawa K, Morita S, Kosaka T, Oya M, Mitani F, et al. 2016. Human Adrenocortical Remodeling Leading to Aldosterone-Producing Cell Cluster Generation. Int J Endocrinol 2016 7834356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, Liu CJ, Sanjanwala AR, Edwards MA, Gomez-Sanchez CE, et al. 2015. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci U S A 112 E4591–4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payet MD, Durroux T, Bilodeau L, Guillon G & Gallo-Payet N 1994. Characterization of K+ and Ca2+ ionic currents in glomerulosa cells from human adrenal glands. Endocrinology 134 2589–2598. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E 2003. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev 83 117–161. [DOI] [PubMed] [Google Scholar]
- Python CP, Rossier MF, Vallotton MB & Capponi AM 1993. Peripheral-type benzodiazepines inhibit calcium channels and aldosterone production in adrenal glomerulosa cells. Endocrinology 132 1489–1496. [DOI] [PubMed] [Google Scholar]
- Rossi GP 2011. A comprehensive review of the clinical aspects of primary aldosteronism. Nat Rev Endocrinol 7 485–495. [DOI] [PubMed] [Google Scholar]
- Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, et al. 2006. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol 48 2293–2300. [DOI] [PubMed] [Google Scholar]
- Rossier MF 2016. T-Type Calcium Channel: A Privileged Gate for Calcium Entry and Control of Adrenal Steroidogenesis. Front Endocrinol (Lausanne) 7 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier MF, Burnay MM, Brandenburger Y, Cherradi N, Vallotton MB & Capponi AM 1996a. Sources and sites of action of calcium in the regulation of aldosterone biosynthesis. Endocr Res 22 579–588. [DOI] [PubMed] [Google Scholar]
- Rossier MF, Burnay MM, Vallotton MB & Capponi AM 1996b. Distinct functions of T- and L-type calcium channels during activation of bovine adrenal glomerulosa cells. Endocrinology 137 4817–4826. [DOI] [PubMed] [Google Scholar]
- Rossier MF, Ertel EA, Vallotton MB & Capponi AM 1998. Inhibitory action of mibefradil on calcium signaling and aldosterone synthesis in bovine adrenal glomerulosa cells. J Pharmacol Exp Ther 287 824–831. [PubMed] [Google Scholar]
- Rossier MF, Python CP, Capponi AM, Schlegel W, Kwan CY & Vallotton MB 1993. Blocking T-type calcium channels with tetrandrine inhibits steroidogenesis in bovine adrenal glomerulosa cells. Endocrinology 132 1035–1043. [DOI] [PubMed] [Google Scholar]
- Scholl UI, Goh G, Stolting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, et al. 2013. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet 45 1050–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier AD, Wang H, Talley EM, Perez-Reyes E & Barrett PQ 2001. alpha1H T-type Ca2+ channel is the predominant subtype expressed in bovine and rat zona glomerulosa. Am J Physiol Cell Physiol 280 C265–272. [DOI] [PubMed] [Google Scholar]
- Stengel W, Jainz M & Andreas K 1998. Different potencies of dihydropyridine derivatives in blocking T-type but not L-type Ca2+ channels in neuroblastoma-glioma hybrid cells. Eur J Pharmacol 342 339–345. [DOI] [PubMed] [Google Scholar]
- Stowasser M 2001. Primary aldosteronism: rare bird or common cause of secondary hypertension? Curr Hypertens Rep 3 230–239. [DOI] [PubMed] [Google Scholar]
- Wiederkehr A, Szanda G, Akhmedov D, Mataki C, Heizmann CW, Schoonjans K, Pozzan T, Spat A & Wollheim CB 2011. Mitochondrial matrix calcium is an activating signal for hormone secretion. Cell Metab 13 601–611. [DOI] [PubMed] [Google Scholar]
- Williams TA, Monticone S, Schack VR, Stindl J, Burrello J, Buffolo F, Annaratone L, Castellano I, Beuschlein F, Reincke M, et al. 2014. Somatic ATP1A1, ATP2B3, and KCNJ5 mutations in aldosterone-producing adenomas. Hypertension 63 188–195. [DOI] [PubMed] [Google Scholar]
- Xu W & Lipscombe D 2001. Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci 21 5944–5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, He M & Hu C 2018. Regulation of aldosterone production by ion channels: From basal secretion to primary aldosteronism. Biochim Biophys Acta 1864 871–881. [DOI] [PubMed] [Google Scholar]
- Yang T, Zhang HL, Liang Q, Shi Y, Mei YA, Barrett PQ & Hu C 2016. Small-Conductance Ca2+-Activated Potassium Channels Negatively Regulate Aldosterone Secretion in Human Adrenocortical Cells. Hypertension 68 785–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Positive controls for CaV1.2 and CaV1.3 antibodies. (A) The HEK293 cells transfected with CaV1.2 channels showed strong immunofluorescence signals, while the un-transfected cells and cells transfected with CaV1.3 show no signals. (B) The HEK293 cells transfected with CaV1.3 showed strong immunofluorescence signals, while the un-transfected cells and cells transfected with CaV1.2 showed no signals.
Figure S2. Positive controls for CaV3.1 , CaV3.2 and CaV3.3 antibodies. The HEK293 cells transfected with CaV3.1 channels showed strong immunofluorescence signals, while the un-transfected cells and cells transfected with CaV3.2/3.3 channels show no signals. (B) The HEK293 cells transfected with CaV3.2 channels showed strong immunofluorescence signals, while the un-transfected cells and cells transfected with CaV3.1/3.3 show no signals. (C) The HEK293 cells transfected with CaV3. 3 channels showed strong immunofluorescence signals, while the un-transfected cells and cells transfected with CaV3.1/3.2 showed no signals.
Figure S3. Effects of nifedipine (500 nmol/L) or TTA-P2(2 μmol/L) on CaV3.2/3 and CaV1.2 /3 channel currents in H295R cells. A, Effects of L-type Ca2+ channel inhibitor nifedipine and T-type Ca2+ channel inhibitor TTA-P2 on CaV3.2 channel currents (n = 4). B, Effects of 500 nmol/L nifedipine and 2 μmol/L TTA-P2 on CaV3.3 channel currents(n = 5). C, Effects of 500 nmol/L nifedipine and 2 μmol/L TTA-P2 on CaV1.2 channel currents(n = 4). D, Effects of 500 nmol/L nifedipine and 2 μmol/L TTA-P2 on CaV1.3 channel currents(n = 6). Calcium currents were elicited by 50-ms steps (in 5-mV increments, −70 to +30 mV for CaV3.2/3.3; −50 to +50 mV for CaV1.2/1.3) from a holding potential of -90 mV at 10 s intervals.
Figure S4. Effect of nifedipine on basal secretion from adrenal slices at different ages. No correlation was observed between the inhibitory effect of 500nM/L nifedipine and age (R = -0.211; P = 0.56).
Figure S5. Aldosterone-producing cell clusters (APCCs) in human adrenal slices. Representative immunofluorescence images of S10 and S15 adrenals showing continuous CYP11B2 expression in the zona glomerulosa (S10: 47 year-old, male; S15: 54 year-old, female). Representative immunofluorescence images of S27 adrenal showing discontinuous CYP11B2 expression in the zona glomerulosa with APCCs (S27: 56 year-old, male )