DEAR EDITOR,
Gap junctions regulate intercellular communication between Sertoli cells and germ cells in male testes and play vital functions in spermatogenesis. Many factors in animal breeding and husbandry can induce oxidative stress, which can impair the testis microenvironment and male animal fertility. However, the underlying mechanisms are largely unknown. Recently, we identified that androgen signals promote the expression of connexin-43 (Cx43), a key component of gap junctions, to regulate spermatogenesis. Thus, we asked whether hyperactive reactive oxygen species (ROS) can impair gap junctions by interfering with Cx43 in porcine testes. Using a porcine Sertoli cell in vitro system, we found that hyperactive ROS caused extensive apoptosis in Sertoli cells, remarkable decrease in Cx43 expression, and failed maintenance of co-cultured spermatogonial stem cells (SSCs), indicating that ROS impaired the function of Sertoli cells and promoted loss of SSCs. This observation provides a possible mechanism for the impact of ROS on fertility of male animals.
As germline stem cells residing in the testicular basal membrane, SSCs are responsible for producing functional sperm (Shinohara et al., 1999). The capacity of spermatogenesis determines the fertility of male animals. One spermatogonial stem cell in the seminiferous tubules is embraced by two Sertoli cells to form a unique structure called a niche. The testicular microenvironment is composed of several types of supporting cells. For example, specialized Sertoli cells are located at the base of testicular seminiferous tubules and exhibit multiple functions, such as protection of SSCs and provision of extrinsic signals for spermatogenesis (Naughton, 2006), Moreover, they promote germ-cell differentiation, meiosis, and transformation into spermatozoa (Phillips et al., 2010). Therefore, it is important to understand the physiological and metabolic characteristics of Sertoli cells in male reproduction. Many types of intercellular interactions between SSCs and Sertoli cells have been identified, including gap junctions (Xia et al., 2005). Gap junctions are a type of cellular interaction involved in diverse biological processes. They are closely related to spermatogenesis, with earlier studies revealing potential signaling pathways that influence the fate of spermatogonia and spermatocytes in differentiation and migration (Xia et al., 2005). Cx43 is an important gap junction protein (Laird et al., 1991). Cx43 is synthesized and trafficked through the endoplasmic reticulum like a typical integral membrane protein (Musil & Goodenough, 1993), and has been identified as a pivotal molecule regulating blood-testis barrier dynamics (Li et al., 2009). These observations indicate that Cx43 in Sertoli cells is closely associated with spermatogenesis.
Among the many harmful factors impacting livestock reproduction, oxidative stress has been well studied. ROS have been shown to decrease sperm and oocyte quality in rodents (Lane et al., 2014), porcines (Kang et al., 2013), bovines (Arias et al., 2017), and humans (Prasad et al., 2016). ROS exhibit diverse derivations, such as ultraviolet radiation, X-rays, gamma rays, and atmospheric pollutants (Nisar et al., 2013). These various sources of ROS imply an inevitable threat to male fertility in animal breeding and husbandry. In addition to the impact on spermatogonia, oxidative stress also affects the function of Sertoli cells (Liu et al., 2018). Sertoli cells reside in the basal membrane of seminiferous tubules and regulate germ cell fate via direct interaction or release of signaling molecules (Johnson et al., 2008). Many regulatory patterns have been identified, i.e., androgen controls the permeability of the blood-testis barrier to regulate SSC differentiation (Meng et al., 2005). Our previous studies revealed that androgen regulates ITGB1, a key molecule for SSC homing, via WT1 in Sertoli cells (Wang et al., 2019), and regulates Cx43 protein expression in Sertoli cells (Xia et al., 2020). These observations suggest that androgen signaling participates in the regulatory process of SSC fate via the intercellular molecules between Sertoli cells and SSCs. Thus, we asked whether oxidative stress affects these pivotal molecules in Sertoli cells, which directly influence spermatogonia fate.
Here, we investigated the impact of oxidative stress on Sertoli cells using an in vitro system and determined the expression of Sertoli cell markers and apoptosis ratio. The results demonstrated that the hyperactive ROS disturbed the expression of Cx43 in Sertoli cells and affected the co-cultured SSCs in this system, suggesting that the hyperactive ROS impaired the function of Cx43. The detailed materials and methods are available in the Supplementary Online file.
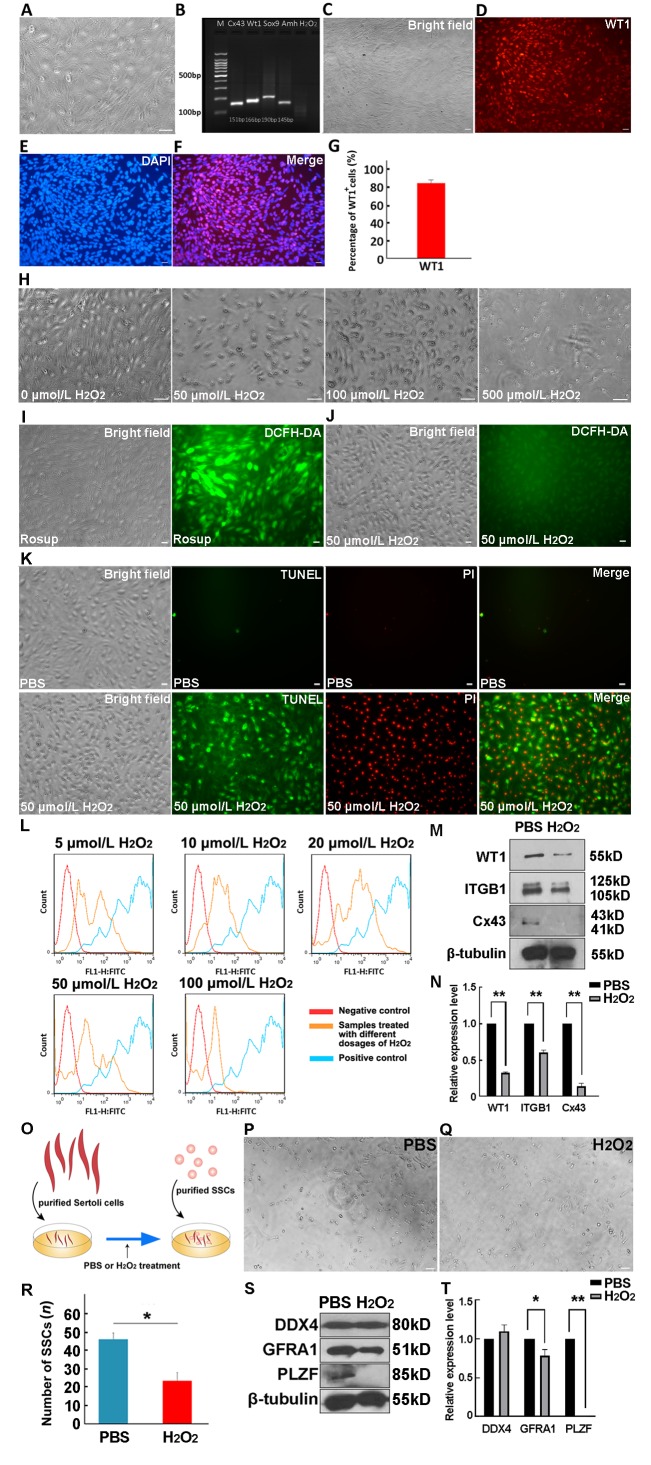
After purification differential adhesion and hypotonic treatment to remove germ cells, followed by 24 h culture, Sertoli cells displayed polygonal morphology and the nucleus was clearly visible (Figure 1A). Reverse Transcription-polymerase chain reaction (RT-PCR) verified the expression of Sertoli cell markers Cx43, WT1, AMH, and SOX9 (Figure 1B). The expression of WT1 was also detected using immunofluorescence to identify the purity of the isolated cells (Figures 1C–F). Statistical analysis revealed that the purity of the Sertoli cells was over 80% (Figure 1G). Collectively, purified Sertoli cells from 7-d-old porcines were obtained.
1. Hyperactive reactive oxygen species (ROS) disturbed expression of Cx43 in Sertoli cells and affected co-cultured SSCs.
A: Purified Sertoli cells were plated on dishes for culture. B: Expression levels of Sertoli cell markers, Cx43, WT1, AMH, and Sox9, were detected in purified Sertoli cells by RT-PCR, n=3. C−F: Purity of Sertoli cells was determined using IF staining against WT1 (C: Bright field; D: WT1; E: DAPI; F: Merge). G: Percentage of WT1+ cells is presented as mean percentage±SEM, n=5. H: Morphology of Sertoli cells treated with 0, 50 μmol/L H2O2, 100 μ mol/L H2O2, or 500 μmol/L H2O2 is shown. I, J: Images of DCFH-DA fluorescence were taken for positive control group (Rosup provided by the Reactive Oxygen Species Assay Kit, Beyotime) (I) and 50 μmol/L H2O2-treated group, n=5 (J). K: Apoptosis signal detection using Annexin V-FITC/PI kit. PBS- and 50 μmol/L H2O2-treated groups, n=5. L: ROS levels in Sertoli cells treated with different concentrations of H2O2 (5, 10, 20, 50, or 100 μmol/L, n=3) were analyzed by flow cytometry. M, N: Sertoli cells treated with PBS or 50 μmol/L H2O2 for 48 h were harvested to detect protein levels of WT1, ITGB1, and Cx43 using Western blotting, with data presented as means±SEM, n=3, **: P<0.01 (N). O: Schematic of Sertoli cell and SSC co-culture system. P, Q: Morphology of co-culture of SSCs maintained with PBS- (P) or H2O2- (Q) treated Sertoli cells for 48 h is displayed. R: Number of SSCs in PBS- or H2O2-treated Sertoli cells was statistically analyzed, n=3. S, T: Protein levels of DDX4, GFRA1, and PLZF in PBS- and H2O2-treated SSCs were analyzed using Western blotting; Data are presented as mean percentage±SEM, n=3, *: P<0.05, **:P<0.01 (t-test) (T). Scale bars: 20 μm.
To investigate the impact of ROS on Sertoli cell growth status, 1×105 Sertoli cells cultured in 24-well plates were supplemented with various doses of H2O2. No obvious morphological changes were observed at low H2O2 doses (10 μmol/L and 20 μmol/L, data not shown). However, as the dosage increased, cell number decreased and cell morphology changed and began to shrink. Obvious impact on cell morphology was observed at a H2O2 concentration of 50 μmol/L. The cell structure also changed, with a large number of cells showing atrophy when the H2O2 concentration increased to 100 μmol/L, and extensive Sertoli cell death observed when the H2O2 concentration increased to 500 μmol/L (Figure 1H). We then performed dichloro-dihydro-fluorescein diacetate (DCFH-DA) fluorescent dye staining to detect ROS levels; however, only a weak ROS signal was detected at the dose of 50 μmol/L H2O2 (Figure 1I, J). Subsequently, the Sertoli cells (1×104) were stained with Annexin V-FITC/PI for apoptosis ratio analysis under ROS stress. We found that when cells were treated with 50 μmol/L H2O2, the number of apoptotic cells increased significantly (Figure 1K). In addition, many necrotic cells were observed in the group treated with 250 μmol/L H2O2 (data not shown), indicating that a high dose of H2O2 was lethal to Sertoli cells. We further clarified the dosage effect of H2O2 on Sertoli cells using flow cytometry. Two major populations were observed based on ROS levels, with the proportion of the higher-level ROS population increasing in the 20 μmol/L H2O2 group, but decreasing in the 50 μmol/L H2O2 and 100 μmol/L H2O2 groups (Figure 1L). This was likely due to increasing ROS levels causing extensive death of Sertoli cells. It is also worth noting that the induced ROS signal clearly appeared under 5 μmol/L H2O2 (Figure 1L), indicating that Sertoli cells were sensitive to H2O2 stimulation.
As a key gap junction protein, the expression level of Cx43 is closely related to Sertoli cell function (Xia et al., 2005). Thus, we treated Sertoli cells with phosphate-buffered saline (PBS) or 50 μmol/L H2O2 for 48 h to test the possible effects of ROS on Cx43 expression. Western blotting revealed that Cx43 expression was significantly down-regulated in the 50 μmol/L H2O2-treated group compared with the PBS-treated group (Figure 1M). In addition, the expression levels of WT1, a Sertoli cell marker, and ITGB1, a key surface molecule for Sertoli cell-SSC interaction, were significantly decreased (Figure 1M, N). Collectively, these observations indicate that the structure and function of Sertoli cells were impaired under ROS stress.
As an important component of the testicular microenvironment, the damage induced by H2O2 should affect SSC maintenance. To test this hypothesis, Sertoli cells were treated with PBS or 50 μmol/L H2O2 for 24 h and then co-cultured with purified SSCs for 48 h (Figure 1O). Compared with the PBS-treated group, the number of SSCs decreased significantly in the H2O2-treated group (Figure 1P, R). Further analysis demonstrated that the expression levels of SSC markers GFRA1 and PLZF were down-regulated after H2O2 treatment (Figure 1S, T), thus indicating that the number of undifferentiated spermatogonia was reduced. However, there was no significant difference in the level of DDX4 in the PBS and H2O2 treatment groups, which may be due to an increase in the number of differentiated spermatogonia in the H2O2treated group. These results suggest that the impaired Sertoli cells affected the maintenance of SSCs and probably led to differentiation.
Although in vitro culture of porcine SSCs is still a challenging project, recent studies have taken a step forward in the long-term maintenance and establishment of porcine SSC lines (Sun et al., 2019). In this study, a co-culture system was used to explore the impact of a representative harmful factor, ROS, on Sertoli cell function. Although high levels of ROS in germlines are known to be risky, knowledge regarding the impact of ROS on Sertoli cells is limited. In particular, the influence on Sertoli cell-germ cell interactions remains poorly identified. This directly determines the capacity of spermatogenesis, as demonstrated by the successful establishment of tree shrew spermatogonial stem cells with Sertoli feeder cells in culture systems (Li et al., 2017).
ROS are mainly produced by mitochondria during cell metabolism in various cell types (Scherz-Shouval et al., 2007). Studies have demonstrated that ROS play regulatory roles in various stem cells. For example, ROS are instantaneously generated during embryoid development and regulate cardiotypic development in embryonic stem cell-derived embryoid bodies (Sauer et al., 2000). ROS signaling regulates the cellular pathways involved in neuronal differentiation and neuronal stem cell proliferation (Vieira et al., 2011). Increased ROS levels drive hematopoietic stem cell differentiation (Ludin et al., 2014). However, ROS may play very different roles in different types of stem cells. In some types of stem cells, ROS induce apoptosis, while in others, ROS may promote self-renewal. In male germlines, the effects of ROS are interesting. Although ROS are generally considered harmful to spermatogenesis, previous study has reported that the self-renewal of SSCs requires a certain level of ROS, with no significant effects observed at 30 μmol/L H2O2, but with proliferation inhibited by the addition of >100 μmol/L H 2O2 (Morimoto et al., 2015). However, our data revealed that even low doses of H2O2 disturbed Sertoli cell maintenance and inhibited expression of key surface functional proteins, indicating that SSCs and Sertoli cells may have different tolerances to ROS stress. In the co-culture system, we observed a reduced number of SSCs maintained with H2O2-treated Sertoli cells. Expression levels of undifferentiated markers GFRA1 and PLZF decreased markedly, but total germ cell marker DDX4 was not altered. Based on our previous studies (Wang et al., 2019; Xia et al., 2020), loss of ITGB1 or Cx43 facilitates SSC differentiation. Thus, impaired Sertoli cell function by ROS can lead to loss of SSCs and promotion of SSC differentiation, and differentiated germ cells possibly compensate the expression of DDX4. Notably, some studies have reported that antioxidants, such as lycopene (Krishnamoorthy et al., 2013) and genistein (Zhang et al., 2017), eliminate ROS in Sertoli cells and rescue spermatogenesis, indicating some potential ways to protect fertility of boars.
Collectively, the impact of ROS on two pivotal surface proteins in Sertoli cells was revealed, and an in vitro model confirmed that this damage affected the maintenance of spermatogonia, implying potential damage to the testicular niche. However, further studies using in vivo models are required, including studies on the link between ROS dosage in Sertoli cells and SSC fate.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS' CONTRIBUTIONS
K.Z. proposed the ideas. D.C.Z. drafted the manuscript. R.C., Y.H.C., J.J.W. and C.Y. revised the manuscript. All authors read and approved the final version of the manuscript.
Funding Statement
This work was supported by a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and National Innovation and Entrepreneurship Training Program for Undergraduates (201910307021Y)
References
- 1.Arias ME, Andara K, Briones E, Felmer R Bovine sperm separation by Swim-up and density gradients (Percoll and BoviPure): Effect on sperm quality, function and gene expression. Reproductive Biology. 2017;17(2):126–132. doi: 10.1016/j.repbio.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Johnson L, Thompson Jr D, Varner DD Role of Sertoli cell number and function on regulation of spermatogenesis. Animal Reproduction Science. 2008;105(1-2):23–51. doi: 10.1016/j.anireprosci.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 3.Kang JT, Kwon DK, Park SJ, Kim SJ, Moon JH, Koo OJ, Jang G, Lee BC Quercetin improves the in vitro development of porcine oocytes by decreasing reactive oxygen species levels . Journal of Veterinary Science. 2013;14(1):15–20. doi: 10.4142/jvs.2013.14.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishnamoorthy G, Selvakumar K, Venkataraman P, Elumalai P, Arunakaran J Lycopene supplementation prevents reactive oxygen species mediated apoptosis in Sertoli cells of adult albino rats exposed to polychlorinated biphenyls. Interdisciplinary Toxicology. 2013;6(2):83–92. doi: 10.2478/intox-2013-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laird DW, Puranam KL, Revel JP Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochemical Journal. 1991;273(1):67–72. doi: 10.1042/bj2730067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lane M, McPherson NO, Fullston T, Spillane M, Sandeman L, Kang WX, Zander-Fox L Oxidative stress in mouse sperm impairs embryo development, fetal growth and alters adiposity and glucose regulation in female offspring. PLoS One. 2014;9(7):e100832. doi: 10.1371/journal.pone.0100832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li CH, Yan LZ, Ban WZ, Tu Q, Wu Y, Wang L, Bi R, Ji S, Ma YH, Nie WH, Lv LB, Yao YG, Zhao XD, Zheng P Long-term propagation of tree shrew spermatogonial stem cells in culture and successful generation of transgenic offspring. Cell Research. 2017;27(2):241–252. doi: 10.1038/cr.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li MWM, Mruk DD, Lee WM, Cheng CY Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(25):10213–10218. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu LQ, Chang XL, Zhang YB, Wu CH, Li R, Tang LM, Zhou ZJ Fluorochloridone induces primary cultured Sertoli cells apoptosis: Involvement of ROS and intracellular calcium ions-mediated ERK1/2 activation. Toxicology in Vitro. 2018;47:228–237. doi: 10.1016/j.tiv.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Ludin A, Gur-Cohen S, Golan K, Kaufmann KB, Itkin T, Medaglia C, Lu XJ, Ledergor G, Kollet O, Lapidot T Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bone marrow microenvironment. Antioxidants & Redox Signaling. 2014;21(11):1605–1619. doi: 10.1089/ars.2014.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE Androgens regulate the permeability of the blood-testis barrier. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(46):16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morimoto H, Kanatsu-Shinohara M, Shinohara T ROS-generating oxidase Nox3 regulates the self-renewal of mouse spermatogonial stem cells . Biology of Reproduction. 2015;92(6):147. doi: 10.1095/biolreprod.114.127647. [DOI] [PubMed] [Google Scholar]
- 13.Musil LS, Goodenough DA Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993;74(6):1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- 14.Naughton KC, Jain S, Strickland AM, Gupta A, Milbrandt J Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biology of Reproduction. 2006;74(2):314–321. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- 15.Nisar NA, Sultana M, Waiz HA, Para PA, Dar SA Oxidative stress - threat to animal health and production. International Journal of Livestock Research. 2013;3(2):76–83. [Google Scholar]
- 16.Phillips BT, Gassei K, Orwig KE Spermatogonial stem cell regulation and spermatogenesis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365(1546):1663–1678. doi: 10.1098/rstb.2010.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prasad S, Tiwari M, Pandey AN, Shrivastav TG, Chaube SK Impact of stress on oocyte quality and reproductive outcome. Journal of Biomedical Science. 2016;23(1):36. doi: 10.1186/s12929-016-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sauer H, Rahimi G, Hescheler J, Wartenberg M Role of reactive oxygen species and phosphatidylinositol 3-kinase in cardiomyocyte differentiation of embryonic stem cells. FEBS Letters. 2000;476(3):218–223. doi: 10.1016/S0014-5793(00)01747-6. [DOI] [PubMed] [Google Scholar]
- 19.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. The EMBO Journal. 2007;26(7):1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinohara T, Avarbock MR, Brinster RL β1-and α6-integrin are surface markers on mouse spermatogonial stem cells . Proceedings of the National Academy of Sciences of the United States of America. 1999;96(10):5504–5509. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun YZ, Liu ST, Li XM, Zou K Progress in in vitro culture and gene editing of porcine spermatogonial stem cells . Zoological Research. 2019;40(5):343–348. doi: 10.24272/j.issn.2095-8137.2019.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vieira HLA, Alves PM, Vercelli A Modulation of neuronal stem cell differentiation by hypoxia and reactive oxygen species. Progress in Neurobiology. 2011;93(3):444–455. doi: 10.1016/j.pneurobio.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Wang JJ, Li JM, Xu W, Xia Q, Gu YZ, Song WX, Zhang XY, Yang Y, Wang W, Li H, Zou K Androgen promotes differentiation of PLZF+ spermatogonia pool via indirect regulatory pattern. Cell Communication and Signaling. 2019;17(1):57. doi: 10.1186/s12964-019-0369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia Q, Zhang DC, Wang JJ, Zhang XY, Song WX, Chen R, Li H, Xie WH, Zou K Androgen indirectly regulates gap junction component connexin 43 through Wilms tumor-1 in Sertoli cells . Stem Cells and Development. 2020;29(3):169–176. doi: 10.1089/scd.2019.0166. [DOI] [PubMed] [Google Scholar]
- 25.Xia WL, Mruk DD, Lee WM, Cheng CY Cytokines and junction restructuring during spermatogenesis-a lesson to learn from the testis. Cytokine & Growth Factor Reviews. 2005;16(4−5):469–493. doi: 10.1016/j.cytogfr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Zhang LD, Gao M, Zhang TD, Chong T, Wang ZM, Zhai XQ, Wu ZZ, Li HC Protective effects of Genistein against Mono-(2-ethylhexyl) phthalate-induced oxidative damage in prepubertal sertoli cells. BioMed Research International. 2017;2017:2032697. doi: 10.1155/2017/2032697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.