DEAR EDITOR,
The Chinese tree shrew (Tupaia belangeri chinensis) is a small mammal closely related to primates. It has a small body size, low maintenance cost, and a relatively short reproductive cycle, all of which has made it the ideal model for the study of a variety of human diseases. In this study, we compared the anatomy of the skin of the Chinese tree shrew with that of the rhesus macaque, mouse and human, with the intention of providing the basic data required for the creation of skin disease models using this animal. Paraffin sections, hematoxylin-eosin (H&E) staining, masson staining and immunohistochemical techniques were used to examine the dorsal skin structure of the Chinese tree shrew. The epidermis was shown to be composed of 1–2 layers of cells. There were hair follicles, sebaceous glands and sweat glands in the dermis and the subcutaneous tissue, with apocrine glands being more common than eccrine glands. Both Keratin5 (KRT5) and Keratin10 (KRT10) were expressed in the skin of the Chinese tree shrew, with a localization in the cytoplasm. Overall, the skin morphology and histology of the Chinese tree shrew was basically the same as that of the human. We propose that the Chinese tree shrew has a strong potential to be used for creating animal models to help elucidate the molecular mechanisms underlying a variety of skin diseases.
Animal models have been used extensively with great benefit in the study of many human diseases (Yao et al., 2015). To date, a variety of animals, including fruit fly, zebrafish, frog, mouse, rat, rabbit, dog, pig, and rhesus macaque, have been used in studies looking at a variety of human diseases (Buchholz, 2015; Buffalo et al., 2019; Jiang et al., 2011; Xue et al., 2014). Each animal has its own merits and disadvantages when considering the wide range of human skin disease. For example, rodents are commonly used in biomedical researches of skin, such as psoriatic murine model induced by imiquimod (Chuang et al., 2018) and inflammatory mouse model of Behçet's disease induced by HSV-1 (Islam & Sohn, 2018), but the species disparity sometimes makes them difficult to extrapolate (van der Worp et al., 2010). Non-human primates (NHP), like rhesus macaques, are genetically closer to humans and have significant benefits in medical research (Buffalo et al., 2019; Zhang et al., 2014). NHP has been used to explore responses of Leishmania (Viannia) braziliensis cutaneous infection to N-methylglucamine antimoniate (Teva et al., 2005) and as SIVmac239-infected model for studying HIV infection (Zhang et al., 2019), to name a few. The NHP model has been proved to be the best model for biomedical researches. However, there are also some disadvantages of using NHP as the resources are not easy to obtain and the costs are relatively high. Therefore, there is an urgent need to find an appropriate animal which is not only genetically close to humans, but is also economic, easily obtainable and able to thrive in the laboratory.
The Chinese tree shrew is a small mammal that has a rat-sized body size (100−150 g), a low-cost of maintenance, a short reproductive cycle, and a moderate life span (6−8 years), as well as being closely related to primates (Fan et al., 2019; Xu et al., 2012; Yao, 2017; Zheng et al., 2014). During the past five decades, the Chinese tree shrew has been used as an animal model in the study of infectious diseases, myopia, cancer, metabolic diseases, and brain disorders (Guo et al., 2019; Xiao et al., 2017; Yao, 2017; Zheng et al., 2014). These Chinese tree shrew studies have increased our understanding of the basic biology of life and the molecular mechanisms underlying disease (Yao, 2017).
In this study, we examined the skin structure of the Chinese tree shrew and compared it to that of the rhesus macaque, mouse and human, to explore the potential for using the Chinese tree shrew in dermatological research. Chinese tree shrews were purchased from the Kunming Primate Research Center (KPRC) of the Chinese Academy of Sciences (CAS), Kunming Institute of Zoology (KIZ), CAS. A total of six animals of different ages (1 month, and 1, 3, 4, 4.5 and 6 years old) were used. We also obtained the skin tissues of two rhesus macaques (age <1 month), which had died from diarrhea, from KPRC, KIZ, CAS. Six-week old C57BL/6 mice ( n=3) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. Dorsal skin samples from these animals were fixed in 4% paraformaldehyde for later processing. All experimental procedures were performed according to the guidelines approved by the Institutional Review Board of KIZ, CAS (approved No. 2015-012).
The dorsal skin samples, previously fixed in 4% paraformaldehyde, were paraffin embedded (vertical embedded) and 10-μm-thick sections were cut from the tissue blocks. The tissue sections were treated with either H&E staining or masson staining (Masson's Trichrome Stain Kit (Solarbio, G1340, China)). For the immunohistochemical staining, the prepared 10 μm sections were dewaxed, rehydrated, washed with PBS, infiltrated in citrate buffer and heated for 30 min for antigen retrieval. After blocking and further incubation with primary antibodies at 4 °C overnight, the reaction was developed using the Super Vision two-step kit (Boster, SV0001, SV0002, USA) which included the secondary antibodies followed by DAB (Vector Laboratories, PK-4002, USA) staining. Harris hematoxylin (Baso, BA4041, China) was used for nuclear staining. Primary antibodies were Keratin 5 (D4U8Q) mAb (Cell Signaling Technology, #25807, USA) and Cytokeratin 10 (4A27) mAb (Santa Cruze Biotechnology, sc-70907, USA), and were used for staining KRT5 and KRT10 in skin tissue sections, respectively.
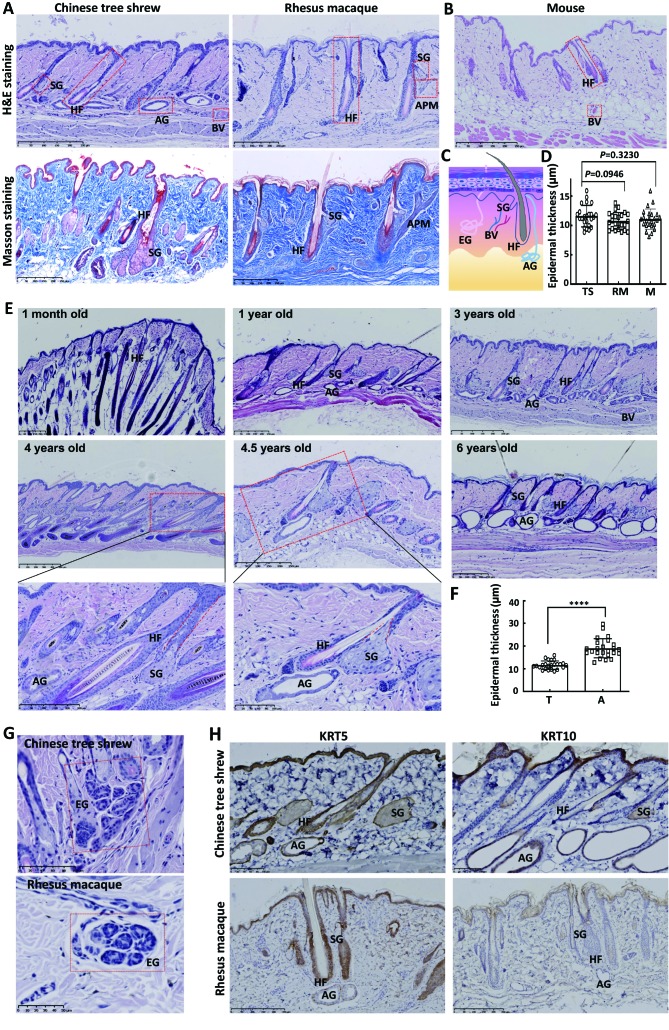
The skin of the Chinese tree shrew was composed of epidermis, dermis and the subcutaneous tissue (Figure 1A). As is known to all that human epidermis is composed of stratum corneum, stratum granulosum, stratum spinosum and stratum basal (Paus & Cotsarelis, 1999; Tobin, 2006). However, the epidermis of the Chinese tree shrew only consisted of 1–2 layers of keratinocyte cells, which was thinner than that of the human, but similar to those of the rhesus macaque and the mouse (Figure 1A–D). There were basal cells in the skin tissue composition of tree shrews because they are required for the replacement of dead cells in the epidermis. The dermis of the Chinese tree shrew showed multiple fibroblast fibers, together with blood vessels, epidermal appendages and inflammatory cells (Figure 1A). Collagen fibers, arranged in bundles, were the main fibers in the dermis, and showed blue by masson staining (Figure 1A). The subcutaneous tissue of the Chinese tree shrew was composed of loose connective and adipose tissues, which connected dermis and muscles. There were many sweat glands in the subcutaneous tissue, of which apocrine glands accounted for the highest proportion (Figure 1A). The composition of the epidermis, dermis and appendages of the rhesus macaque was similar to that of the Chinese tree shrew (Figure 1A).
1. Comparison of the skin morphology and histology, and KRT5 and KRT10 expression pattern of the Chinese tree shrew and rhesus macaque.
A: Hematoxylin and eosin (H&E) staining (upper) and masson staining (below) for the dorsal skin of the Chinese tree shrew and rhesus macaque. B: H&E staining for the dorsal skin of mouse. C: Cartoon of normal human skin. D: Comparison of the epidermal thickness among the Chinese tree shrew, rhesus macaque and mouse. TheP-values were based on Student’s t-test. E: H&E staining for the dorsal skin tissues of the Chinese tree shrews with different ages. The pilosebaceous unit and the apocrine glands were further demonstrated in the boxed skin tissues of the Chinese tree shrews of 4 years old and 4.5 years old, respectively. The hair follicle cycle in the skin tissues of 1-month old and 4 years old tree shrews indicated the late-anagen stage. F: Comparison of the epidermal thickness between telogen and anagen stages. ****:P<0.000 1, Student’st-test. G: H&E staining for the eccrine gland of the Chinese tree shrew (upper) and rhesus macaque (below). H: The expression of KRT5 and KRT10 in the skin tissues of the Chinese tree shrew and rhesus macaque. The labeled abbreviations are hair follicle (HF), sebaceous gland (SG), apocrine gland (AG), eccrine gland (EG), arrector pili muscle (APM), and blood vessel (BV), tree shrew (TS), rhesus macaque (RM), mouse (M), telogen (T), anagen (A).
The appendages of the Chinese tree shrew included hair follicles, sebaceous glands and sweat glands. Sweat glands and sebaceous glands increased in size and became more abundant with age (Figure 1E). The sweat glands were significantly distributed around the top of the hair bulbs (Figure 1E), just as eccrine sweat glands associated with the hair follicle within a defined compartment of dermal white adipose tissue in the human (Poblet et al., 2018), to form an “adnexal skin unit” along with the pilosebaceous unit. It was possible that there was some relationship between each component of this superstructure to form a common homeostatic tissue environment (Poblet et al., 2018). The mature hair follicles in the skin tissue of the Chinese tree shrew contained melanocytes. The sebaceous gland of the Chinese tree shrew was composed of acini and the short duct opening into the hair follicle as well as the epidermis (Figure 1E). However, in the human, the duct of the sebaceous gland normally opens into the hair follicle and goes directly into the epidermis under pathological conditions (Zouboulis et al., 2016). Surprisingly, the apocrine glands in the dorsal skin of the Chinese tree shrew were more prominent, while the eccrine glands were few (Figure 1E). This pattern was different to that seen in the rhesus macaque and human where eccrine glands are dominant (Best et al., 2019). By contrast, in the mouse eccrine glands are only present in the paw pad (Chee et al., 2017). The concentration of apocrine glands in the dorsal skin of the Chinese tree shrew may make it a suitable model animal for the study of apocrine gland-related diseases. In mammals, the hair follicle is a cyclical organ (Baker & Murray, 2012; Paus & Cotsarelis, 1999), and this is the same in the Chinese tree shrew (Figure 1E). As the thickness of the epidermis changed in the hair follicle cycle, it was significantly thicker in the anagen than the telogen (Figure 1E, F). The arrector pili muscle in the skin of the rhesus macaque was more obvious than that of the Chinese tree shrew, while sweat glands were uncommon in the rhesus macaque (Figure 1A). The eccrine glands were the dominant sweat glands in the rhesus macaque (Figure 1G). In the dorsal skin sections taken from the mouse, there were almost no sweat glands, and the sebaceous glands were also not obvious (Figure 1B).
Cytokeratin is a characteristic marker of epithelial cells and has a great significance in the epidermis and adnexal diseases (Moll et al., 1982). We studied the expression of KRT5 and KRT10 in the skin of the Chinese tree shrew. KRT5 dimerizes with keratin 14 forming the intermediate filaments to make up the cytoskeleton of basal epithelial cells, and has an active role in epidermolysis bullosa simplex, Dowling-Degos disease and some other dermatologic diseases (Lane et al., 1992; Tryon et al., 2019). KRT10 was observed in a group of tough, fibrous proteins that form the structural framework of keratinocytes and was produced in keratinocytes in the outer layer of the skin (Müller et al., 2006). KRT10 is associated with epidermolytic hyperkeratosis (Morais et al., 2009; March et al., 2019). We found that KRT5 and KRT10 were both expressed in the skin of the Chinese tree shrew. Specifically, KRT5 was expressed in the whole epidermis except for the stratum corneum (Figure 1H), while KRT10 was expressed mainly in the outer layer of the epidermis (Figure 1H). In terms of the epidermal appendages, KRT5 was expressed in the outer root sheath of hair follicles, sebaceous glands (especially in the basal cells in the outer layer) and sweat glands (the outer wall of the gland expressing more) (Figure 1H). In contrast, KRT10 was expressed mainly in the follicular epithelium above the follicular infundibulum, sebaceous and sweat glands, especially in the internal wall of these glands (Figure 1H). The expression pattern showed that both KRT5 and KRT10 were expressed in the cytoplasm (Figure 1H). The expression patterns of KRT5 and KRT10 in the skin of the Chinese tree shrew were similar to those observed in the rhesus macaque (Figure 1H) and human (Zhong et al., 2000).
In conclusion, we showed that the skin histology of the Chinese tree shrew was generally like that of human skin. There was a consistent composition of epidermal appendages and a similar expression pattern of some keratins between the Chinese tree shrew and human. The similarity of the skin structure of the Chinese tree shrew relative to non-human primates and human may provide a solid basis for using this animal in further studies of human skin diseases, following the recent study into creating a Chinese tree shrew model of basal cell carcinoma (Jiang et al., 2017) and successful genetic modifications of this animal (Li et al., 2017). All those features of the Chinese tree shrew have laid the foundation for the use of this animal as a model for the study of human skin diseases.
COMPETING INTERESTS
The authors have no conflicts of interest to declare.
AUTHORS' CONTRIBUTIONS
X.Y.M. and Y.G.Y. designed this study. J. Z., R.C.L. and X.Y.M. performed the experiments and drafted the initial manuscript. L.B.L. provided key samples. Y.G.Y. and M.Z. revised the manuscript. All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We thank Zhao-Yuan Wang, Bing-Xi Yan and Yuan Zhou from Department of Dermatology, Second Affiliated Hospital, Zhejiang University School of Medicine for critical comments and Dr. Ian Logan for language editing this manuscript.
Funding Statement
This study was supported by the Chinese Academy of Sciences (CAS zsys-02) and CAS "Light of West China" Program (xbzg-zdsys-201909)
Contributor Information
Yong-Gang Yao, Email: yaoyg@mail.kiz.ac.cn.
Min Zheng, Email: minz@zju.edu.cn.
References
- 1.Baker RE, Murray PJ Understanding hair follicle cycling: a systems approach. Current Opinion in Genetics & Development. 2012;22(6):607–612. doi: 10.1016/j.gde.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Best A, Lieberman DE, Kamilar JM Diversity and evolution of human eccrine sweat gland density. Journal of Thermal Biology. 2019;84:331–338. doi: 10.1016/j.jtherbio.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Buchholz DR More similar than you think: frog metamorphosis as a model of human perinatal endocrinology. Developmental Biology. 2015;408(2):188–195. doi: 10.1016/j.ydbio.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Buffalo EA, Movshon JA, Wurtz RH From basic brain research to treating human brain disorders. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(52):21617–26172. doi: 10.1073/pnas.1919895116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chee MK, Jo SK, Sohn KC, Kim CD, Lee JH, Lee YH Effects of Brn2 overexpression on eccrine sweat gland development in the mouse paw. Biochemical and Biophysical Research Communications. 2017;490(3):901–905. doi: 10.1016/j.bbrc.2017.06.138. [DOI] [PubMed] [Google Scholar]
- 6.Chuang SY, Lin CH, Sung CT, Fang JY Murine models of psoriasis and their usefulness for drug discovery. Expert Opinion on Drug Discovery. 2018;13(6):551–562. doi: 10.1080/17460441.2018.1463214. [DOI] [PubMed] [Google Scholar]
- 7.Fan Y, Ye MS, Zhang JY, Xu L, Yu DD, Gu TL, Yao YL, Chen JQ, Lv LB, Zheng P, Wu DD, Zhang GJ,Yao YG Chromosomal level assembly and population sequencing of the Chinese tree shrew genome. Zoological Research. 2019;40(6):506–521. doi: 10.24272/j.issn.2095-8137.2019.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo L, Frost MR, Siegwart JT Jr, Norton TT Gene expression signatures in tree shrew sclera during recovery from minus-lens wear and during plus-lens wear. Molecular Vision. 2019;25:311–328. [PMC free article] [PubMed] [Google Scholar]
- 9.Islam SMS, Sohn S HSV-induced systemic inflammation as an animal model for Behçet's disease and therapeutic applications. Viruses. 2018;10(9):511. doi: 10.3390/v10090511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang HJ, Feng F, Dong ED Model animals and animal models of human diseases. Chinese Bulletin of Life Sciences. 2011;23(3):234–238. [Google Scholar]
- 11.Jiang LP, Shen QS, Yang CP, Chen YB Establishment of basal cell carcinoma animal model in Chinese tree shrew (Tupaia belangeri chinensis) . Zoological Research. 2017;38(4):180–190. doi: 10.24272/j.issn.2095-8137.2017.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lane EB, Rugg EL, Navsaria H, Leigh IM, Heagerty AH, Ishida-Yamamoto A, Eady RA A mutation in the conserved helix termination peptide of keratin 5 in hereditary skin blistering. Nature. 1992;356(6366):244–246. doi: 10.1038/356244a0. [DOI] [PubMed] [Google Scholar]
- 13.Li CH, Yan LZ, Ban WZ, Tu Q, Wu Y, Wang L, Bi R, Ji S, Ma YH, Nie WH, Lv LB, Yao YG, Zhao XD, Zheng P Long-term propagation of tree shrew spermatogonial stem cells in culture and successful generation of transgenic offspring. Cell Research. 2017;27(2):241–252. doi: 10.1038/cr.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller FB, Huber M, Kinaciyan T, Hausser I, Schaffrath C, Krieg T, Hohl D, Korge BP, Arin MJ A human keratin 10 knockout causes recessive epidermolytic hyperkeratosis. Human Molecular Genetics. 2006;15(7):1133–1141. doi: 10.1093/hmg/ddl028. [DOI] [PubMed] [Google Scholar]
- 15.March OP, Lettner T, Klausegger A, Ablinger M, Kocher T, Hainzl S, Peking P, Lackner N, Rajan N, Hofbauer JP, Guttmann-Gruber C, Bygum A, Koller U, Reichelt J Gene editing- mediated disruption of epidermolytic ichthyosis-associated KRT10 alleles restores filament stability in keratinocytes . Journal of Investigative Dermatology. 2019;139(8):1699–1710. doi: 10.1016/j.jid.2019.03.1146. [DOI] [PubMed] [Google Scholar]
- 16.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R The catalog of human cytokeratins: Patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 17.Morais P, Mota A, Baudrier T, Lopes JM, Cerqueira R, Tavares P, Azevedo F Epidermolytic hyperkeratosis with palmoplantar keratoderma in a patient with KRT10 mutation. European Journal of Dermatology. 2009;19(4):333–336. doi: 10.1684/ejd.2009.0684. [DOI] [PubMed] [Google Scholar]
- 18.Paus R, Cotsarelis G The biology of hair follicles. New England Journal of Medicine. 1999;341(7):491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 19.Poblet E, Jimenez F, Escario-Travesedo E, Hardman JA, Hernández-Hernández I, Agudo-Mena JL, Cabrera-Galvan JJ, Nicu C, Paus R Eccrine sweat glands associate with the human hair follicle within a defined compartment of dermal white adipose tissue. British Journal of Dermatology. 2018;178(5):1163–1172. doi: 10.1111/bjd.16436. [DOI] [PubMed] [Google Scholar]
- 20.Teva A, Porrozzi R, Oliveira-Neto MP, Grimaldi Jr GJ Responses of Leishmania (Viannia) braziliensis cutaneous infection to N-methylglucamine antimoniate in the rhesus monkey (Macaca mulatta) model . Journal of Parasitology. 2005;91(4):976–978. doi: 10.1645/GE-3486RN.1. [DOI] [PubMed] [Google Scholar]
- 21.Tobin DJ Biochemistry of human skin-our brain on the outside. Chemical Society Reviews. 2006;35(1):52–67. doi: 10.1039/B505793K. [DOI] [PubMed] [Google Scholar]
- 22.Tryon RK, Tolar J, Preusser SM, Riddle MJ, Keene DR, Bower M, Thyagarajan B, Ebens CL A homozygous frameshift variant in the KRT5 gene is compatible with life and results in severe recessive epidermolysis bullosa simplex . JAAD Case Report. 2019;5(7):576–579. doi: 10.1016/j.jdcr.2019.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O'Collins V, Macleod MR Can animal models of disease reliably inform human studies? PLoS Medicine. 2010;7(3):e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao J, Liu R, Chen CS Tree shrew (Tupaia belangeri) as a novel laboratory disease animal model . Zoological Research. 2017;38(3):127–137. doi: 10.24272/j.issn.2095-8137.2017.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu L, Chen SY, Nie WH, Jiang XL, Yao YG Evaluating the phylogenetic position of Chinese tree shrew (Tupaia belangeri chinensis) based on complete mitochondrial genome: implication for using tree shrew as an alternative experimental animal to primates in biomedical research . Journal of Genetics and Genomics. 2012;39(3):131–137. doi: 10.1016/j.jgg.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Xue LX, Zhang FZ, Sun RJ, Dong ED Research status and perspective of disease animal models in China. Scientia Sinica Vitae. 2014;44(9):851–860. doi: 10.1360/N052014-00186. [DOI] [Google Scholar]
- 27.Yao YG, Chen YB, Liang B The 3rd symposium on animal models of primates - the application of non-human primates to basic research and translational medicine. Journal of Genetics and Genomics. 2015;42(6):339–341. doi: 10.1016/j.jgg.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Yao YG Creating animal models, why not use the Chinese tree shrew (Tupaia belangeri chinensis)? . Zoological Research. 2017;38(3):118–126. doi: 10.24272/j.issn.2095-8137.2017.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang MX, Song TZ, Zheng HY, Wang XH, Lu Y, Zhang HD, Li T, Pang W, Zheng YT Superior intestinal integrity and limited microbial translocation are associated with lower immune activation in SIVmac239-infected northern pig-tailed macaques (Macaca leonina) . Zoological Research. 2019;40(6):522–531. doi: 10.24272/j.issn.2095-8137.2019.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang XL, Pang W, Hu XT, Li JL, Yao YG, Zheng YT Experimental primates and non-human primate (NHP) models of human diseases in China: current status and progress. Zoological Research. 2014;35(6):447–464. doi: 10.13918/j.issn.2095-8137.2014.6.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng YT, Yao YG, Xu L. 2014. Basic Biology and Disease Models of Tree Shrews. Kunming: Yunnan Science and Technology Press, 1–475. (in Chinese)
- 32.Zhong BY, Cheng B, Mai Y, Diao QC, Yin R Study on the distribution of hair keratin in normal hair follicles by immunohistochemical staining. Acta Academiae Medicinae Militaris Tertiae. 2000;22(11):1087–1089. [Google Scholar]
- 33.Zouboulis CC, Picardo M, Ju Q, Kurokawa I, Törőcsik D, Bíró T, Schneider MR Beyond acne: current aspects of sebaceous gland biology and function. Reviews in Endocrine and Metabolic Disorders. 2016;17(3):319–334. doi: 10.1007/s11154-016-9389-5. [DOI] [PubMed] [Google Scholar]