Basophils are rare granulocytes. Despite representing only ~0.5% of all leukocytes, basophils have several important physiological functions.1,2 Although basophils lack the classic features of professional antigen-presenting cells,3–7 through the secretion of cytokines, they orient the immune response by polarizing Th2 differentiation and supporting B-cell differentiation and class switching. Basophils are also critical for mediating protection against helminth infection.1,2,8,9
Basophils receive activation signals from diverse sources. It is well recognized that cytokines such as IL-3, granulocyte–macrophage colony-stimulating factor (GM-CSF), thymic stromal lymphopoietin (TSLP) and IL-33; various toll-like receptor ligands; allergen-bound IgE provide activation signals to basophils and induce the release of inflammatory mediators.10–15 In addition, several reports have also demonstrated the existence of anti-IgE autoantibodies that possess the capacity to induce basophil activation in patients with chronic spontaneous urticaria (CSU), atopic or non-atopic asthma or autoimmune disease.16–21 However, isolation and functional exploration of such anti-IgE IgG autoantibodies from either healthy donors or patients have not been attempted yet. By using a pooled normal IgG preparation from healthy donors, specifically intravenous immunoglobulin G (IVIG)22 that represents the complete IgG repertoire of a normal individual, we attempted to address this outstanding question in the field.
Recently, we reported that at a concentration (25 mg/0.5 million cells/mL) corresponding to the level of IgG reached in patients immediately following high-dose IVIG therapy, both IVIG and its F(ab’)2 fragments induce basophil activation.23 Mechanistically, IVIG induces basophil activation by signaling through basophil surface-bound IgE in an IL-3- and Syk-dependent mechanism.23 We first performed dose–response experiments with various concentrations of IVIG to determine whether a lower concentration of IVIG also induces basophil activation.
As a source of IVIG, Sandoglobulin® (CSL Behring, Switzerland) was used in our experiments. It was dialyzed several times against phosphate-buffered saline followed by final dialysis in RPMI-1640 medium at 4 °C to remove stabilizing agents. Peripheral blood basophils were isolated from the buffy bags of healthy donors obtained from the Centre Necker-Cabanel, EFS, Paris (INSERM-EFS ethical permission N°18/EFS/033). The cellular fractions that contained peripheral blood mononuclear cells and basophils were collected by Ficoll density gradient centrifugation. From these fractions, basophils were isolated by using a basophil isolation kit II (Miltenyi Biotec) and autoMACS® (Miltenyi Biotec). The purity of the isolated basophils was 96–97%.
Basophils (0.1 × 106 cells/well/200 μL) were seeded in 96-well U-bottomed plates. For dose–response experiments with IVIG, basophils were cultured either alone in serum-free X-VIVO 15 medium or with IL-3 (100 ng/mL, ImmunoTools) or IL-3 plus three different doses of IVIG (25, 15, or 10 mg/mL) for 24 h. IVIG was added to the basophils following 2 h of stimulation with IL-3. The basophils were analyzed for the expression of CD69 by flow cytometry (LSR II, BD Biosciences) using a CD69-APC/Cy7 MAb from BD Biosciences. Flow cytometry data were analyzed by BD FACS DIVA (BD Biosciences) and FlowJo (FlowJo LLC). Cell-free culture supernatants were used for cytokine analyses by ELISA (ELISA Ready-SET-Go, eBioscience Affymetrix).
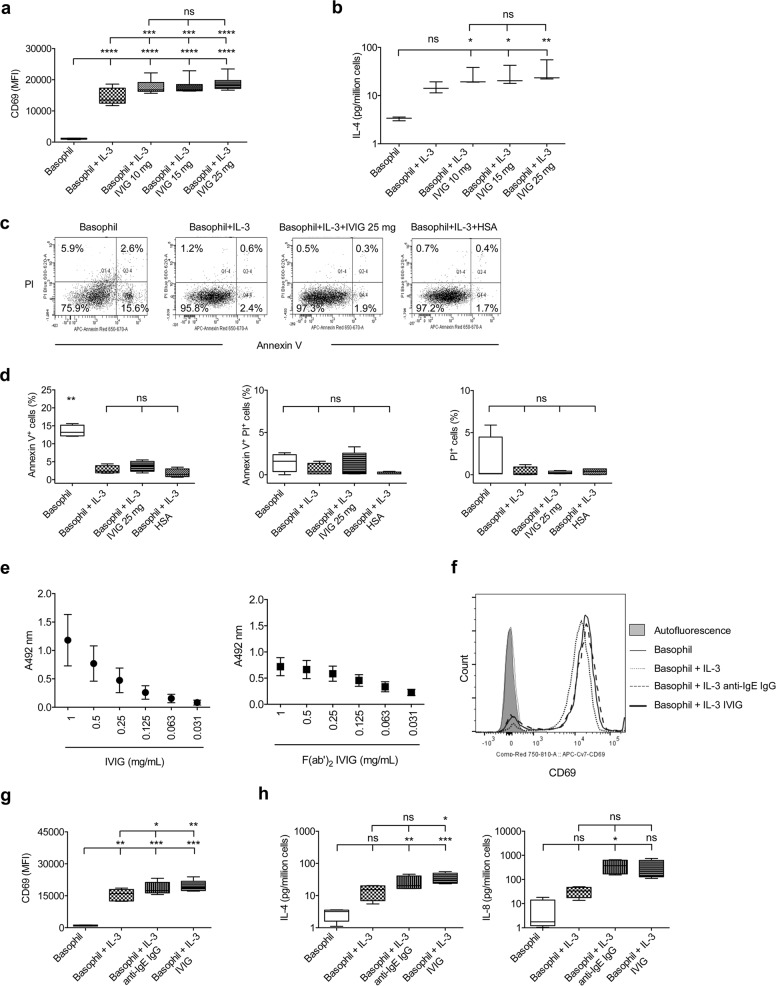
As IVIG did not modify the activation status of either resting basophils or basophils primed with the cytokines IL-33, TSLP, IL-25, or GM-CSF,23 we used IL-3 for priming throughout the experiments. The dose–response experiments revealed that even at a concentration of 10 mg/mL, which corresponds to circulating IgG levels in healthy donors, IVIG could induce basophil activation, as shown by the enhanced expression of CD69 and secretion of IL-4 (Fig. 1a, b). These results suggest that the occurrence of anti-IgE IgG autoantibodies is a common feature in the healthy population.
Fig. 1. Anti-IgE IgG autoantibodies isolated from IVIG induce basophil activation.
a, b Basophils isolated from healthy donors were cultured either alone (0.1 × 106 cells/well/200 μL) or with IL-3 (100 ng/mL). Three different concentrations of IVIG (25, 15, or 10 mg/ml) were added to the cells following 2 h of stimulation with IL-3. After 24 h, the expression of CD69 (MFI, median fluorescence intensity; n = 7 donors) and the concentration of IL-4 (n = 3 donors) in the culture supernatants were analyzed. c, d The viability of basophils was analyzed by Annexin V and PI staining. Representative dot plots (c) and data from four experiments (d) using different donors are presented. human serum albumin (HSA) used at 10 mg/mL. e The recognition of IgE by IVIG (left panel) or (Fab’)2 fragments (right panel) was evaluated by ELISA. Data (mean ± SEM) are from two experiments. f, g Basophils were cultured either alone or with IL-3 (100 ng/mL). IVIG (25 mg/mL) or anti-IgE IgG (1.5 mg/mL) was added to the cells following 2 h of stimulation with IL-3. After 24 h, the expression (MFI, median fluorescence intensity) of CD69 was analyzed. Representative histogram overlays (f) and data from five donors (g) are presented. h The amounts (pg) of IL-4 and IL-8 in culture supernatants from the above experiments were measured by ELISA. Data from four donors are presented. All data (except in panel e) are plotted using a box and whisker plot, wherein the whiskers denote the minimum and maximum values, and the dividing line in the box symbolizes the median. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant; one-way ANOVA with Tukey’s multiple comparison test.
We then investigated whether the induction of basophil activation by IVIG is due to nonspecific cell activation caused by cell death. Therefore, we measured the viability of cells by Annexin V staining. Basophils were cultured in medium alone or with IL-3, IL-3 plus IVIG (25 mg/mL), or IL-3 plus human serum albumin (HSA, 10 mg/mL, Laboratoire Français du Fractionnement et des Biotechnologies, France) for 24 h. HSA was used as an irrelevant protein control for IVIG. The cells were stained with Annexin V (APC-Annexin V, BD Biosciences) and propidium iodide (PI; InvitrogenTM). We indeed confirmed that basophil activation induced by IVIG was not because of nonspecific cell activation caused by cell death. In fact, cell viability as analyzed by Annexin V–PI staining was similar between the IL-3-treated, IVIG-treated, and HSA-treated cells (Fig. 1c, d).
These data prompted us to investigate the anti-IgE IgG autoantibodies in IVIG. An ELISA performed with recombinant human IgE-kappa (AbD Serotec) revealed that both IVIG and its F(ab’)2 fragments recognize IgE in a dose-dependent manner (Fig. 1e). Therefore, we next aimed to isolate anti-IgE IgG autoantibodies from IVIG by affinity chromatography. The IgE myeloma protein was coupled to cyanogen bromide-activated sepharose 4B (Sigma-Aldrich). IVIG at a concentration of 60 mg/mL was added to the column and incubated at ambient temperature for 4 h on a rotator. After draining the unbound IgG, the column was washed several times with phosphate-buffered saline. The anti-IgE IgG autoantibodies were eluted by using 3 M potassium thiocyanate, dialyzed against phosphate-buffered saline and concentrated. The IgG concentration was measured by using a spectrophotometer (NanoDrop Technologies).
Based on the amount of eluted IgG, we estimated that anti-IgE IgG represent nearly 0.3–0.5% of total IgG (IVIG). We then investigated whether this isolated anti-IgE IgG is functional. IL-3-primed basophils from healthy individuals were stimulated with either IVIG (25 mg/0.5 × 106 cells/mL) or isolated anti-IgE IgG (1.5 mg/0.5 × 106/mL) for 24 h. This concentration of anti-IgE IgG was selected according to the level of IgG and the number of basophils in the circulation of healthy individuals. We found that isolated anti-IgE IgG was able to induce basophil activation similar to that of IVIG even at a 16-fold lower concentration: both induced similar levels of CD69 expression (Fig. 1f, g) and IL-4 and IL-8 secretion (Fig. 1h).
The site of recognition of anti-IgE IgG autoantibodies from IVIG is not known. We used the IgE myeloma protein for the isolation of anti-IgE IgG, and yet these isolated IgG autoantibodies induced the activation of basophils from various donors carrying distinct IgE molecules, implying that anti-IgE IgG does not signal basophils by interacting with the Fab region of basophil-bound IgE. It is likely that these antibodies may recognize either the Cε2 or Cε4 domain of IgE but not the Cε3 domain (which is the binding site of FcεRI and hence inaccessible for recognition by anti-IgE IgG), as indicated by a previous report;24 however, we cannot rule out the possibility of recognition of the Cε1 domain.
The precise function of anti-IgE IgG autoantibodies in healthy individuals is not clear at this stage. The isolation and characterization of such anti-IgE IgG monoclonal antibodies by using techniques, such as phage-display technology, should provide further insight into these antibodies.25 Since anti-IgE autoantibodies induce IL-4 secretion in basophils, this function might support Th2 and B-cell differentiation. In the context of IVIG immunotherapy, however, we propose that IL-4 induction in basophils may help in the suppression of effector Th1 and Th17 cells22 and in the upregulation of inhibitory FcγRIIB expression on phagocytic cells to reduce the inflammatory response to IgG–autoantigen immune complexes.26 Thus, distinct natural autoantibodies diversify the effects of IVIG on granulocytes.27
Acknowledgements
This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM), Sorbonne Université, Université Paris Descartes, ANR-19-CE17-0021(BASIN), and CSL Behring, Switzerland. C.G. and A.K. were the recipients of fellowships from La Fondation pour la Recherche Médicale (FDM20150633674 and FDT201805005552, respectively), France. We thank the staff of the Centre d’Histologie, d’Imagerie et de Cytométrie, and Centre de Recherche des Cordeliers for their help, and F. Carrère at Royan Hospital for providing the IgE myeloma serum.
Author contributions
J.B. designed the study. C.G. and A.K. performed the experiments. C.G., A.K., J.D., S.V.K., and J.B. analyzed the data. A.C. provided essential tools. J.B. drafted the paper, and all the authors edited and approved the final version of the paper.
Competing interests
This work is supported in part by a research grant from CSL Behring, Switzerland.
Footnotes
These authors contributed equally: Caroline Galeotti, Anupama Karnam
References
- 1.Karasuyama H, Miyake K, Yoshikawa S, Yamanishi Y. Multifaceted roles of basophils in health and disease. J. Allergy Clin. Immunol. 2018;142:370–380. doi: 10.1016/j.jaci.2017.10.042. [DOI] [PubMed] [Google Scholar]
- 2.Voehringer D. Protective and pathological roles of mast cells and basophils. Nat. Rev. Immunol. 2013;13:362–375. doi: 10.1038/nri3427. [DOI] [PubMed] [Google Scholar]
- 3.Eckl-Dorna J, et al. Basophils are not the key antigen-presenting cells in allergic patients. Allergy. 2012;67:601–608. doi: 10.1111/j.1398-9995.2012.02792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitzmuller C, et al. Human blood basophils do not act as antigen-presenting cells for the major birch pollen allergen Bet v 1. Allergy. 2012;67:593–600. doi: 10.1111/j.1398-9995.2011.02764.x. [DOI] [PubMed] [Google Scholar]
- 5.Sharma M, et al. Circulating human basophils lack the features of professional antigen presenting cells. Sci. Rep. 2013;3:1188. doi: 10.1038/srep01188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyake K, et al. Trogocytosis of peptide-MHC class II complexes from dendritic cells confers antigen-presenting ability on basophils. Proc. Natl Acad. Sci. USA. 2017;114:1111–1116. doi: 10.1073/pnas.1615973114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephen-Victor E, et al. Demystification of enigma on antigen-presenting cell features of human basophils: data from secondary lymphoid organs. Haematologica. 2017;102:e233–e237. doi: 10.3324/haematol.2016.163451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurram RK, Zhu J. Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses. Cell. Mol. Immunol. 2019;16:225–235. doi: 10.1038/s41423-019-0210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma M, Bayry J. Autoimmunity: basophils in autoimmune and inflammatory diseases. Nat. Rev. Rheumatol. 2015;11:129–131. doi: 10.1038/nrrheum.2014.199. [DOI] [PubMed] [Google Scholar]
- 10.Voehringer D. Basophil modulation by cytokine instruction. Eur. J. Immunol. 2012;42:2544–2550. doi: 10.1002/eji.201142318. [DOI] [PubMed] [Google Scholar]
- 11.Siracusa MC, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leyva-Castillo JM, et al. Skin thymic stromal lymphopoietin initiates Th2 responses through an orchestrated immune cascade. Nat. Commun. 2013;4:2847. doi: 10.1038/ncomms3847. [DOI] [PubMed] [Google Scholar]
- 13.Sharma M, et al. Regulatory T cells induce activation rather than suppression of human basophils. Sci. Immunol. 2018;3:aan0829. doi: 10.1126/sciimmunol.aan0829. [DOI] [PubMed] [Google Scholar]
- 14.Jiao D, et al. NOD2 and TLR2 ligands trigger the activation of basophils and eosinophils by interacting with dermal fibroblasts in atopic dermatitis-like skin inflammation. Cell. Mol. Immunol. 2016;13:535–550. doi: 10.1038/cmi.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suurmond J, et al. Activation of human basophils by combined toll-like receptor- and FcepsilonRI-triggering can promote Th2 skewing of naive T helper cells. Eur. J. Immunol. 2014;44:386–396. doi: 10.1002/eji.201343617. [DOI] [PubMed] [Google Scholar]
- 16.MacGlashan D. Autoantibodies to IgE and FcepsilonRI and the natural variability of spleen tyrosine kinase expression in basophils. J. Allergy Clin. Immunol. 2019;143:1100–1107 e1111. doi: 10.1016/j.jaci.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritter C, Battig M, Kraemer R, Stadler BM. IgE hidden in immune complexes with anti-IgE autoantibodies in children with asthma. J. Allergy Clin. Immunol. 1991;88:793–801. doi: 10.1016/0091-6749(91)90187-S. [DOI] [PubMed] [Google Scholar]
- 18.Chan YC, et al. “Auto-anti-IgE”: naturally occurring IgG anti-IgE antibodies may inhibit allergen-induced basophil activation. J. Allergy Clin. Immunol. 2014;134:1394–1401 e1394. doi: 10.1016/j.jaci.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shakib F, Smith SJ. In vitro basophil histamine-releasing activity of circulating IgG1 and IgG4 autoanti-IgE antibodies from asthma patients and the demonstration that anti-IgE modulates allergen-induced basophil activation. Clin. Exp. Allergy. 1994;24:270–275. doi: 10.1111/j.1365-2222.1994.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 20.Iwamoto I, et al. Relationship between anti-IgE autoantibody and severity of bronchial asthma. Int. Arch. Allergy Appl. Immunol. 1989;90:414–416. doi: 10.1159/000235064. [DOI] [PubMed] [Google Scholar]
- 21.Gruber BL, Kaufman LD, Marchese MJ, Roth W, Kaplan AP. Anti-IgE autoantibodies in systemic lupus erythematosus. Prevalence and biologic activity. Arthritis Rheum. 1988;31:1000–1006. doi: 10.1002/art.1780310810. [DOI] [PubMed] [Google Scholar]
- 22.Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int. Immunol. 2017;29:491–498. doi: 10.1093/intimm/dxx039. [DOI] [PubMed] [Google Scholar]
- 23.Galeotti C, et al. Intravenous immunoglobulin induces IL-4 in human basophils by signaling through surface-bound IgE. J. Allergy Clin. Immunol. 2019;144:524–535 e528. doi: 10.1016/j.jaci.2018.10.064. [DOI] [PubMed] [Google Scholar]
- 24.Shakib F, Powell-Richards A. Elucidation of the epitope locations of human autoanti-IgE: recognition of two epitopes located within the C epsilon 2 and the C epsilon 4 domains. Int. Arch. Allergy Appl. Immunol. 1991;95:102–108. doi: 10.1159/000235413. [DOI] [PubMed] [Google Scholar]
- 25.Vogel M, Horn MP. Isolation of natural anti-fcepsilonrialpha autoantibodies from healthy donors. Methods Mol. Biol. 2017;1643:5–22. doi: 10.1007/978-1-4939-7180-0_2. [DOI] [PubMed] [Google Scholar]
- 26.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Gunten S, Simon HU. Granulocyte death regulation by naturally occurring autoantibodies. Adv. Exp. Med. Biol. 2012;750:157–172. doi: 10.1007/978-1-4614-3461-0_12. [DOI] [PubMed] [Google Scholar]