Abstract
Basic helix−loop−helix (bHLH) domain-containing transcription factors are known for their roles in regulating various plant growth and developmental processes. Previously, we showed that MdbHLH3 from apple (Malus domestica) has multiple functions, modulating both anthocyanin biosynthesis and cell acidification. Here, we show that MdbHLH3 also regulates ethylene biosynthesis and leaf senescence by promoting the expression of dehydratase-enolase-phosphatase complex 1 (MdDEP1). Therefore, we propose a model whereby MdbHLH3 acts as a crucial factor that modulates anthocyanin biosynthesis and cell acidification in addition to fruit ripening and leaf senescence by regulating distinct target genes.
Subject terms: Transcriptional regulatory elements, Plant signalling
Introduction
The basic helix−loop−helix (bHLH) superfamily contains transcription factors (TFs) that possess highly conserved alkaline helix-loop-helix domains1,2. Regarding the two conserved motifs of a bHLH TF, the basic region is responsible for DNA recognition and binding, whereas the hydrophobic residue-rich HLH region functions in dimerization3.
bHLH TFs have been widely studied in many animal and plant species. Previous studies have demonstrated that bHLH TFs play roles as key regulators of various plant growth and development processes. In apple (Malus domestica), MdbHLH3 has been identified as a multifunctional regulator. It was initially characterized by Xie et al.4 as an anthocyanin biosynthesis regulator. MdbHLH3 interacts with the MYB TF MdMYB1 with which it forms a complex to directly activate the downstream anthocyanin biosynthesis genes MdDFR and MdUFGT at low temperatures, thereby promoting anthocyanin accumulation and fruit coloration in apple. In addition, a previous study found that the glucose sensor MdHXK1 phosphorylates MdbHLH3, leading to enhanced transcription of downstream anthocyanin biosynthesis genes and increased anthocyanin accumulation5. MdbHLH3 was also characterized as a crucial component of the MYB-bHLH-WDR (MBW) complex, which controls vacuolar transport of anthocyanins6. Recently, we reported that the glucose-inhibited ubiquitin E3 ligase MdPUB29 ubiquitinates MdbHLH3 to influence ethylene biosynthesis and fruit ripening7. Overall, MdbHLH3 is considered a multifunctional regulator in apple that, in addition to regulating anthocyanin biosynthesis, is involved in processes such as environmental responses, cell acidification, ethylene biosynthesis, sugar homeostasis, and phytohormone signaling pathways.
Here, we report that apple dehydratase-enolase-phosphatase complex 1 (MdDEP1), initially characterized as active in the recycling of the ethylene precursor S-adenosylmethionine (SAM), is essential for regulating leaf senescence. Furthermore, MdbHLH3 was shown to mediate leaf senescence via direct activation of MdDEP1 expression. The exploitation of the multiple MdbHLH3-mediated functions in breeding programs is discussed.
Materials and methods
Plant materials and growth conditions
Tissue culture plantlets of the apple (Malus domestica) ‘Gala’ cultivar were maintained in vitro on MS medium supplemented with 0.2 mg L−1 IAA and 0.8 mg L−1 6-BA, with subculturing performed once a month. The plantlets were grown at room temperature under long-day conditions (16-h light/8-h dark). Calli induced from young embryos of ‘Orin’ apple (Malus domestica Borkh.) were proliferated at room temperature in darkness on MS medium supplemented with 1.5 mg L−1 6-BA and 0.5 mg L−1 IAA. The calli were subcultured at a minimum average of 5 day intervals in preparation for their use in genetic transformations and GUS assays.
Gene expression analysis
RNA extraction, cDNA synthesis, and quantitative real-time PCR (qRT-PCR) assays were carried out as previously described6. All study-associated primer sequences are listed in Supplementary Table 1.
Construction of plasmids and genetic transformation
The full-length MdDEP1 ORF was amplified by PCR and introduced into the pCXSN::Myc vector to overexpress MdDEP1 under the 35S promoter. The resulting construct was introduced into the maintained tissue culture of ‘Gala’ plantlets described above via Agrobacterium (LBA4404)-mediated transformation as described previously6.
For ‘Orin’ calli transformation, the PMdDEP1::udiA construct was used to transform the calli following an Agrobacterium-mediated method as described by Hu et al.7.
Chromatin immunoprecipitation (ChIP) qPCR and electrophoretic mobility shift assay (EMSA) analyses
The calli expressing 35S::Myc and 35S::MdbHLH3-Myc were subjected to ChIP-qPCR analysis. We used an anti-Myc antibody (Beyotime)6. The immunoprecipitated DNA samples were used as templates for qPCR assays.
EMSA was conducted as described by Hu et al.6. MdbHLH3-His recombinant protein was expressed in Escherichia coli strain BL21 and purified using glutathione sepharose beads (Thermo Scientific, San Jose, CA, USA). The EMSA probe biotin labeling kit (Beyotime) was used to label an oligonucleotide sequence corresponding to the MdDEP1 promoter, which was subsequently used for the binding assays with a LightShift chemiluminescent EMSA kit (Thermo).
GUS expression analysis
GUS reporter constructs were created containing the promoter sequence of MdDEP1, with cloning performed as previously described7.
Chlorophyll content determination
Determination of the chlorophyll content was performed as previously described7. The apple leaves used for chlorophyll content determination were obtained from five different developmental stages (T0, T1, T2, T4, and T6). These five stages were defined according to the characteristics of leaf age in reference to the second leaf from the bottom of the apple shoot (T0: 20 days; T1: 30 days; T2: 40 days; T4: 50 days; and T6: 60 days).
Statistical analysis
At least three biological replicates were included for all samples, and the data are presented as the means ± standard deviation unless otherwise indicated. Significant differences were determined with Student’s t-test (p ≤ 0.01, significantly different; p ≤ 0.001, very significantly different).
Results
MdbHLH3 influences leaf senescence in apple
Three apple lines harboring the 35S::MdbHLH3-GFP construct4 were further investigated. Phenotypic analysis of these lines showed that, compared to that of the wild-type (WT) control, MdbHLH3 overexpression was clearly associated with more severe leaf senescence symptoms starting at the T2 developmental stage (Fig. 1a), which became more severe in later stages. Moreover, faster chlorophyll degradation was observed in the MdbHLH3-overexpressing lines than in the WT control (Fig. 1b), and the transgenic lines produced a higher level of ethylene than did the control (Fig. 1c). Combined, these results point to a key role of MdbHLH3 in leaf senescence.
Fig. 1. MdbHLH3 controls leaf senescence in apple.
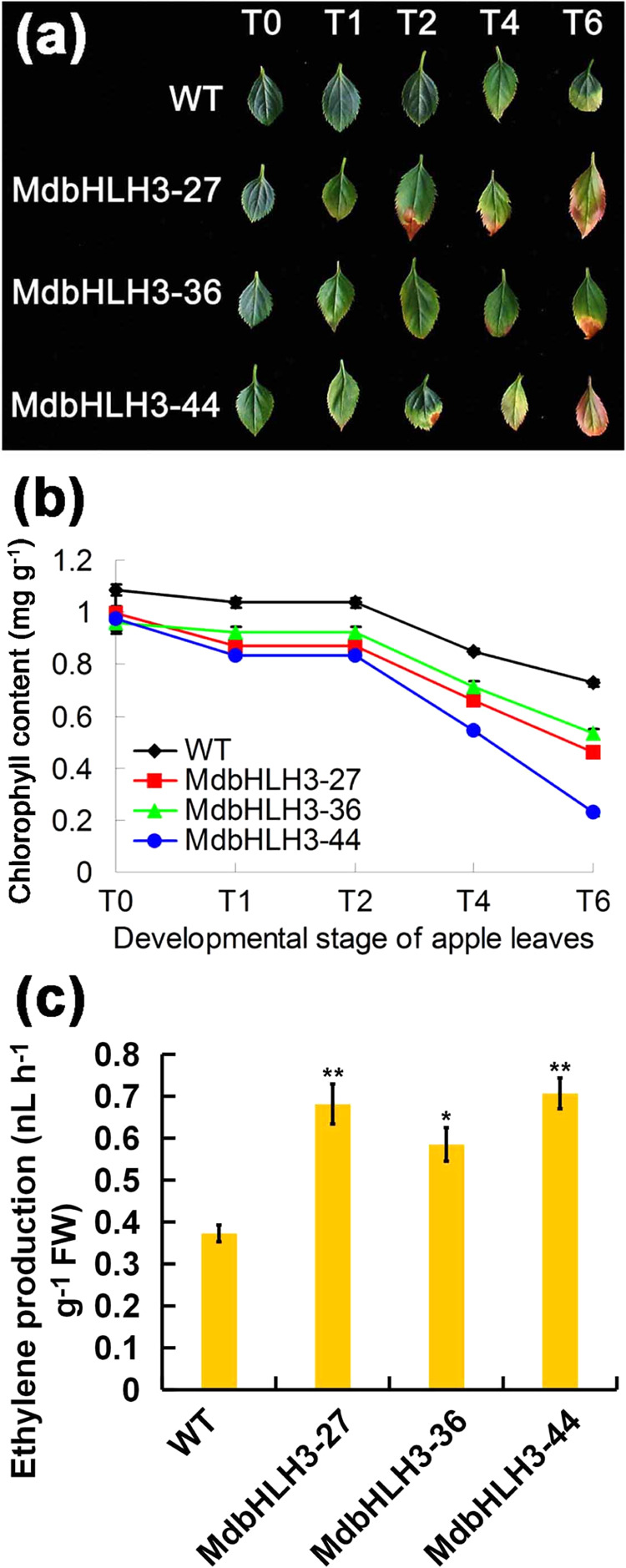
a Leaf senescence phenotype in the WT and three 35S::MdbHLH3 transgenic apple plantlets. Note: T0 to T6 represent the leaves in different developmental stages. b Chlorophyll content of the leaves shown in a. c Ethylene production in the WT and three 35S::MdbHLH3 transgenic apple plantlets. The data represent the means ± SE of three independent experiments. Significance was determined using Student’s t-test; *P < 0.01 and **P < 0.001
MdbHLH3 binds to the MdDEP1 promoter
To investigate the potential downstream target genes regulated by MdbHLH3, ChIP-seq combined with targeted sequence capture was performed. Among the identified candidates, MDP0000180420 encodes dehydratase/enolase/phosphatase MdDEP1, which functions in methionine salvaging (Yang cycle). The plant-specific DEP1 enzyme is well known for its trifunctional dehydratase, enolase, and phosphatase activities and converts 5-methylthioribulose-1-P (MTRu-1-P) directly to 1,2-dihydroxy-3-keto-5-methylthiopentene (DHKMP), the reciprocal third compound in the methionine salvage pathway8. MdDEP1 was identified through cDNA-AFLP, which is associated with low fruit acidity in apple9.
To confirm the role of MdbHLH3 in MdDEP1 gene expression regulation, we analyzed cis-elements within the MdDEP1 promoter region, which revealed two typical bHLH-binding E-boxes (5′-CATTTG-3′) (Fig. 2a). Chromatin immunoprecipitation PCR (ChIP-PCR) assays were carried out on apple leaf protoplasts individually transfected with the 35S::MdbHLH3-GFP construct or the 35S::GFP construct. The bHLH element-containing E-box2 region from the MdDEP1 promoter, but not the E-box1 region, was enriched in the ChIP protoplasts transfected with 35S::MdbHLH3-GFP compared with the protoplasts transfected with the 35S::GFP control (Fig. 2b).
Fig. 2. MdbHLH3 binds to the MdDEP1 promoter.
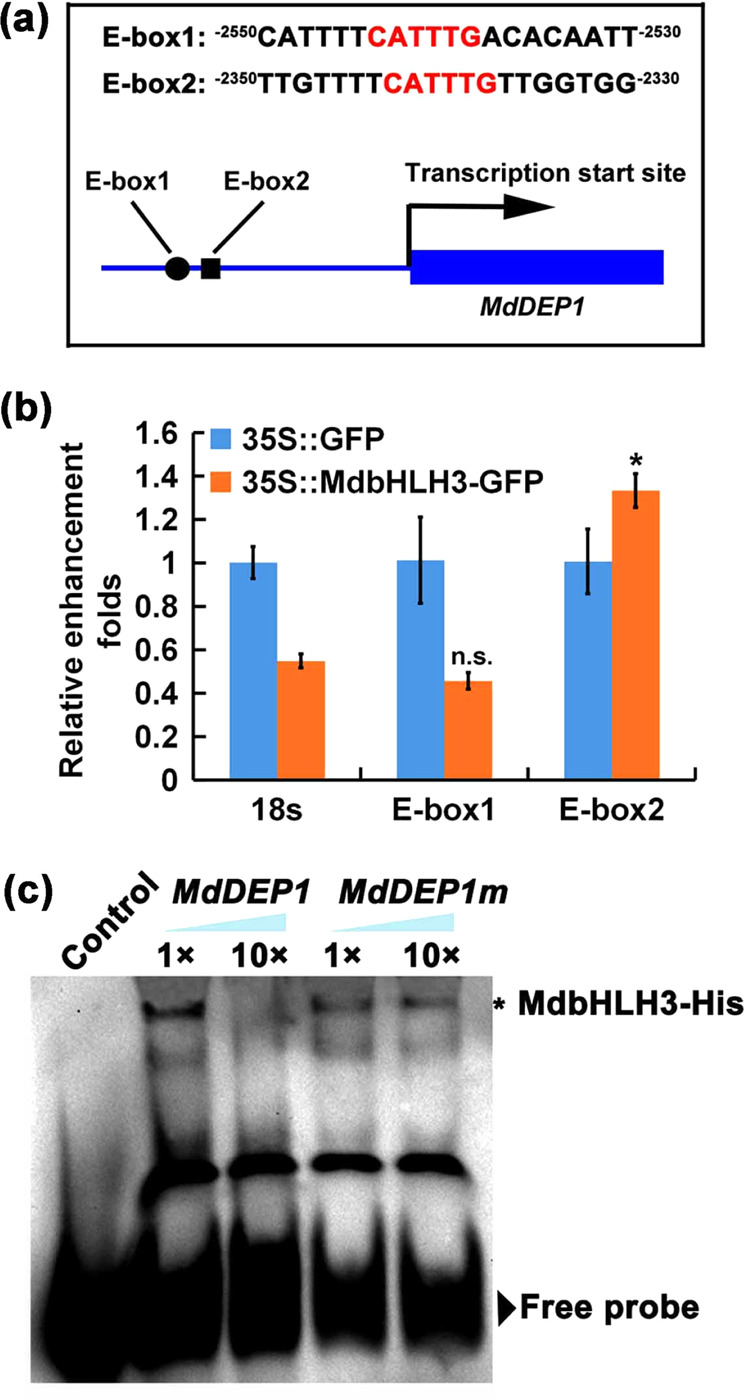
a The putative MdbHLH3 TF-binding element in the MdDEP1 promoter. The black box and cycle represent the two MdbHLH3-binding motifs. b The relative enrichment of the MdDEP1 gene promoter fragments. The MdbHLH3-DNA complex was coimmunoprecipitated from 35S::MdbHLH3-GFP-transfected apple leaf protoplasts using an anti-GFP antibody. 35S::GFP was used as a negative control. c Results from the EMSA assays of the interaction between MdbHLH3 and labeled E-box2 DNA probes in the MdDEP1 promoter
To confirm the direct binding of MdbHLH3 and the MdDEP1 promoter, the MdbHLH3-His fusion protein was subjected to an in vitro electrophoretic mobility shift assay (EMSA). We detected a specific DNA-MdbHLH3 protein complex using a probe representing the CATTTG-containing E-box2 sequence (Fig. 2c). Accumulation of this complex was reduced in proportion to the amount of unlabeled competitor probe with the same sequence included in the assay, whereas competition was absent in the assay with a probe comprising a different sequence (Fig. 2c). These results indicate that MdbHLH3 specifically binds to the bHLH recognition sequence in the MdDEP1 promoter.
MdbHLH3 transcriptionally activates and positively regulates MdDEP1 gene expression
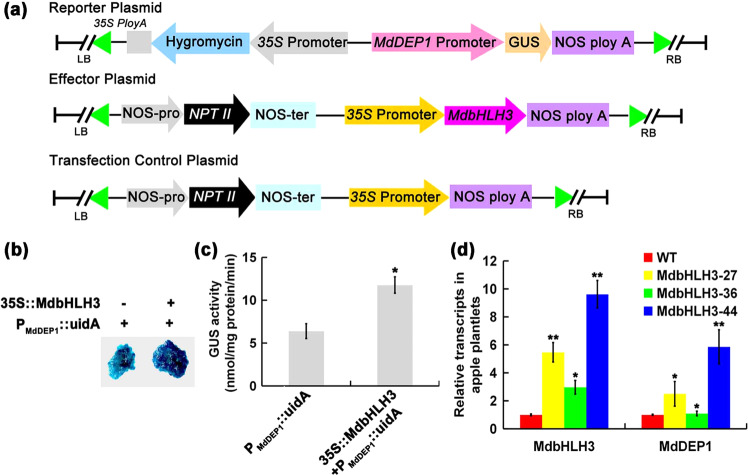
GUS assays were performed to verify the transcriptional activation of MdDEP1 by MdbHLH3. Calli were transformed with the construct PMdDEP1::udiA alone or in combination with the 35S::MdbHLH3 construct. Transgenic calli expressing PMdDEP1::uidA and 35S::MdbHLH3 exhibited much higher GUS enzyme activity than those expressing PMdDEP1::uidA alone (Fig. 3a–c), thus indicating that MdbHLH3 enhanced GUS expression by interacting with the MdDEP1 promoter. The results from the qRT-PCR analysis showed that the transgenic MdbHLH3-overexpressing plantlets possessed a higher level of MdDEP1 transcript than was expressed in the WT plantlets (Fig. 3d), confirming that MdDEP1 expression was positively regulated by MdbHLH3.
Fig. 3. MdbHLH3 transcriptionally activates and positively regulates MdDEP1 gene expression.
a The effector and reporter constructs in the binary vectors were introduced into apple calli for the GUS activity assays. b PMdDEP1::uidA transgenic apple calli with or without the 35S::MdbHLH3 effector were grown at 25 °C in the dark and stained to visualize the GUS activity. c GUS activity in the transgenic apple calli as labeled. d Relative transcripts of MdbHLH3 and MdDEP1 in transgenic apple lines overexpressing MdbHLH3 (MdbHLH3-27, MdbHLH3-36, MdbHLH3-44) and the WT control. The data represent the means ± SE of three independent experiments. Statistical significance was determined using Student’s t-test; *P < 0.01 and **P < 0.001
MdDEP1 regulates leaf senescence in apple
Since MdbHLH3 modulates leaf senescence and positively regulates MdDEP1 gene expression, it is reasonable to assume that MdDEP1 influences leaf senescence. To further validate the role of MdDEP1 in leaf senescence, a Myc-tagged MdDEP1 fusion protein with expression driven by the 35S promoter (35S::MdDEP1-Myc) was expressed in apple plants. Several independent transgenic lines were identified by qRT-PCR analysis (Supplementary Fig. 1), of which three (MdDEP1-OVX1, MdDEP1-OVX2, and MdDEP1-OVX4) exhibited higher MdDEP1 expression levels. In addition to enhanced ethylene production, these MdDEP1-overexpressing lines showed more severe leaf senescence symptoms compared to those shown in the WT control, commencing at the T2 developmental stage (Fig. 4a–c). At later developmental stages, leaf yellowing and the degradation of chlorophyll were further enhanced in the MdDEP1-overexpressing lines (Fig. 4a, b). These results support the conclusion that MdDEP1 also plays a key role in leaf senescence in apple.
Fig. 4. MdDEP1 regulates leaf senescence in apple.
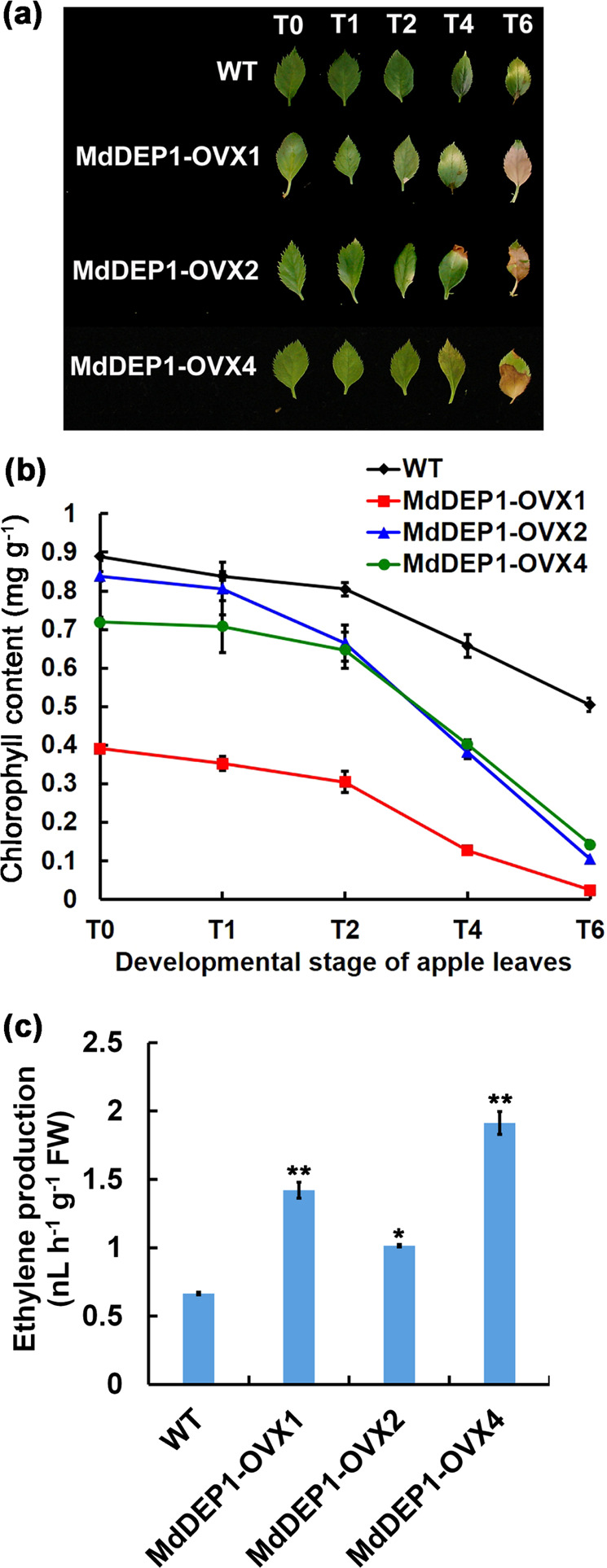
a Leaf senescence phenotype in the WT and three 35S::MdbHLH3 transgenic apple plantlets. Note: T0 to T6 represent the leaves in different developmental stages. b Chlorophyll content of the leaves shown in a. c Ethylene production in the WT and three 35S::MdDEP-Myc transgenic apple plantlets. The data represent the means ± SE of three independent experiments. Statistical significance was determined using Student’s t-test; *P < 0.01 and **P < 0.001
Discussion
The Yang cycle indicates that 5’-methylthioadenosine (MTA) is recycled during the production of methionine from ethylene, nicotianamide, or polyamines8. This cycle plays important roles in ethylene-mediated leaf senescence8,10. Among those enzymes active in the Yang cycle, the plant-specific enzyme DEP1 possesses phosphatase, enolase, and dehydratase activities and converts 5-methylthioribulose-1-P (MTRu-1-P) directly to 1,2-dihydroxy-3-keto-5-methylthiopentene (DHKMP), the reciprocal third compound in this pathway8. The present study provides evidence that MdbHLH3 controls leaf senescence by directly activating MdDEP1 expression in apple.
DEP1 is a plant-specific trifunctional enzyme that converts MTRu-1-P directly to KMTB in Arabidopsis8. Consistent with its role in the Yang cycle, apple plantlets overexpressing MdDEP1 accumulated a significantly higher amount of methionine (Supplementary Fig. 2). This accumulation may explain the higher ethylene production observed in the MdDEP1 transgenic plantlets compared to the level produced in the WT control (Fig. 4c). On the other hand, our previous study showed that MdbHLH3 controls ethylene levels by directly activating the expression of the ethylene biosynthetic genes MdACO1, MdACS1, and MdACS5A7. These results suggest that MdbHLH3 either directly or indirectly controls ethylene production underlying leaf senescence through multiple biological pathways (Fig. 5).
Fig. 5. Model demonstrating the multifaceted roles of MdbHLH3 in apple.
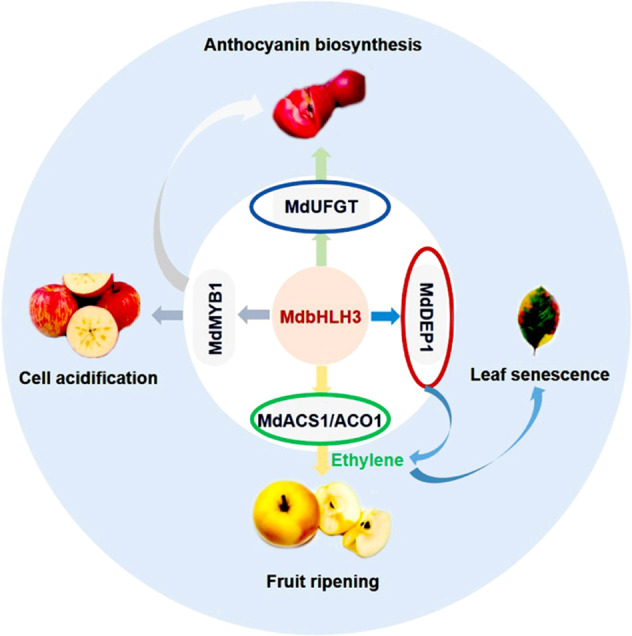
MdbHLH3 interacts with the MYB TF MdMYB1, forming a complex that directly activates the downstream anthocyanin biosynthetic MdUFGT gene, thereby enhancing anthocyanin content and fruit coloration; MdbHLH3 controls the expression levels of genes encoding vacuolar transporters, including MdVHAs, MdVHP1, and MdtDT, resulting in vacuolar acidification and anthocyanin accumulation; MdbHLH3 controls the ethylene level by directly activating the expression of the ethylene biosynthetic genes MdACO1, MdACS1, and MdACS5A7; and MdbHLH3 modulates leaf senescence by directly activating MdDEP1 expression
Additionally, MdbHLH3 interacts with the MYB TF MdMYB1, forming a complex that directly activates MdDFR and MdUFGT, downstream anthocyanin biosynthetic genes, in apple4 at low temperatures, thereby enhancing anthocyanin content and fruit coloration. Moreover, the glucose sensor MdHXK1 phosphorylates MdbHLH3, resulting in enhanced transcription of downstream anthocyanin biosynthesis genes and increased anthocyanin accumulation5. MdbHLH3 was also shown to be a crucial component of the MYB-bHLH-WDR (MBW) complex that controls expression levels of genes encoding vacuolar transporters, including MdVHAs, MdVHP1, and MdtDT, resulting in vacuolar acidification and anthocyanin accumulation6.
Finally, our laboratory recently demonstrated a role for MdbHLH3 in fruit ripening and ethylene biosynthesis upon ubiquitination of the ubiquitin E3 ligase MdPUB29, which can be inhibited by glucose7. Combined, these findings support the notion that MdbHLH3 is a multifunctional regulator in apple and is involved in many other processes aside from anthocyanin biosynthesis, such as environmental responses, cell acidification, ethylene biosynthesis, sugar homeostasis, and phytohormone signaling pathways. Therefore, it is reasonable to conclude that MdbHLH3 acts as a multifaceted regulator that functions in anthocyanin biosynthesis, cell acidification, fruit ripening, and leaf senescence by regulating different target genes (Fig. 5).
Leaf senescence is a complicated and crucial physiological process in plants8,10. Elucidating this mechanism is a key step to understanding a series of biological phenotypes11. Additionally, leaf senescence is a major target of breeding programs for many crop plants. Our findings add to the current understanding of how the MdbHLH3-MdDEP1 regulatory module affects leaf senescence. Taken together, these results describe a novel molecular mechanism of these important processes in plants. The results of this study may also contribute to the development of biotechnological approaches and tools for future phytoremediation applications.
Supplementary information
Acknowledgements
We would like to thank Dr. Mengxia Zhang at Cornell University for providing linguistic assistance during the preparation of this manuscript. This work was financially supported by grants from the National Key Research and Development Program of China (2018YFD1000200), the National Natural Science Foundation of China (31972375), the Ministry of Agriculture of China (CARS-28), Shandong Province (SDAIT-06-03), and Nanjing Agricultural University (ZW201805).
Author contributions
Y.J.H. and D.G.H. conceived and designed the study. D.G.H., C.H.S., Q.Y.Z., and K.D.G. performed the experiments. D.G.H. and Y.J.H. wrote the paper. All authors discussed the results and commented on the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41438-020-0273-9).
References
- 1.Duek PD, Fankhauser C. bHLH class transcription factors take centre stage in phytochrome signalling. Trends Plant Sci. 2005;10:51–54. doi: 10.1016/j.tplants.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Feller A, Machemer K, Braun EL, Grotewold E. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J. 2011;66:94–116. doi: 10.1111/j.1365-313X.2010.04459.x. [DOI] [PubMed] [Google Scholar]
- 3.Murre C, et al. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989;58:537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- 4.Xie XB, et al. The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell Environ. 2012;35:1884–1897. doi: 10.1111/j.1365-3040.2012.02523.x. [DOI] [PubMed] [Google Scholar]
- 5.Hu DG, et al. Glucose sensor MdHXK1 phosphorylates and stabilizes MdbHLH3 to promote anthocyanin biosynthesis in apple. PLoS Genet. 2016;12:e1006273. doi: 10.1371/journal.pgen.1006273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu DG, et al. MdMYB1 regulates anthocyanin and malate accumulation by directly facilitating their transport into vacuoles in apples. Plant Physiol. 2016;170:1315–1330. doi: 10.1104/pp.15.01333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu DG, et al. The regulatory module MdPUB29-MdbHLH3 connects ethylene biosynthesis with fruit quality in apple. New Phytol. 2019;221:1966–1982. doi: 10.1111/nph.15511. [DOI] [PubMed] [Google Scholar]
- 8.Pommerrenig B, et al. Phloem-specific expression of Yang cycle genes and identification of novel Yang cycle enzymes in Plantago and Arabidopsis. Plant Cell. 2011;23:1904–1919. doi: 10.1105/tpc.110.079657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao YX, Li M, Liu Z, Hao YJ, Zhai H. A novel gene, screened by cDNA-AFLP approach, contributes to lowering the acidity of fruit in apple. Plant Physiol. Biochem. 2007;45:139–145. doi: 10.1016/j.plaphy.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Grbić V, Bleecker AB. Ethylene regulates the timing of leaf senescence in Arabidopsis. Plant J. 1995;8:595–602. doi: 10.1046/j.1365-313X.1995.8040595.x. [DOI] [Google Scholar]
- 11.Giovannoni J, et al. The epigenome and transcriptional dynamics of fruit ripening. Annu. Rev. Plant Biol. 2017;68:61–84. doi: 10.1146/annurev-arplant-042916-040906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.