Abstract
Innate lymphoid cells (ILCs), as an important component of the innate immune system, arise from a common lymphoid progenitor and are located in mucosal barriers and various tissues, including the intestine, skin, lung, and adipose tissue. ILCs are heterogeneous subsets of lymphocytes that have emerging roles in orchestrating immune response and contribute to maintain metabolic homeostasis and regulate tissue inflammation. Currently, more details about the pathways for the development and differentiation of ILCs have largely been elucidated, and cytokine secretion and downstream immune cell responses in disease pathogenesis have been reported. Recent research has identified that several distinct subsets of ILCs at skin barriers are involved in the complex regulatory network in local immunity, potentiating adaptive immunity and the inflammatory response. Of note, additional studies that assess the effects of ILCs are required to better define how ILCs regulate their development and functions and how they interact with other immune cells in autoimmune-related and inflammatory skin disorders. In this review, we will distill recent research progress in ILC biology, abnormal functions and potential pathogenic mechanisms in autoimmune-related skin diseases, including systemic lupus erythematosus (SLE), scleroderma and inflammatory diseases, as well as psoriasis and atopic dermatitis (AD), thereby giving a comprehensive review of the diversity and plasticity of ILCs and their unique functions in disease conditions with the aim to provide new insights into molecular diagnosis and suggest potential value in immunotherapy.
Keywords: ILC, SLE, scleroderma, psoriasis, atopic dermatitis, autoimmunity
Subject terms: Autoimmunity, Innate immunity, Immunological disorders
Introduction
It has long been recognized that the human immune system is composed of two parts: the innate immune system and the adaptive immune system. The innate immunity is the first-line defender against pathogenic microbial infection before the adaptive immune system is activated, which quickly responds to pathogens without specificity. Innate lymphoid cells (ILCs), as newly described lymphoid cells, have greatly enhanced our knowledge about the immune system in the past 10 years. ILCs, as an important component of the innate immune system, promote host defense against pathogens and microorganisms,1 maintain tissue and organ homeostasis,2 and promote tissue remodeling, healing and repair,3 as well as tumor development.4 On the other hand, transcription abnormalities and functional dysregulation of ILCs play an important role in the pathogenesis of autoimmune diseases, which are related to immune tolerance and autoimmunity.
ILCs have been found in the mucosal system and visceral organs in humans and mice and are strategically enriched at mucosal sites. They are particularly abundant in the skin, lung, and intestinal mucosa and rich in adipose tissue and lymph nodes. ILCs are innate cells devoid of recombination activating gene (RAG)-dependent rearrangements; therefore, ILCs cannot express diversified antigen-specific recognition receptors, thus differing from T cells and B cells.5 Recently, three heterogeneous subsets of ILCs, termed ILC1s, ILC2s and ILC3s, have been identified on the basis of transcription factors and the expression of effector cytokines. ILC1s are similar to T helper (Th) 1 cells, which secrete type 1 cytokines such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α. ILC1s require the Th1 cell-associated transcription factor T-bet6 for their development. They contribute an essential role in early host protection and viral immunosurveillance at sites of initial infection.7 ILC2s are defined by the capacity to secrete interleukin-5 (IL-5),8 IL-9,9 IL-13,8 and amphiregulin10 and are important in host resistance against nematodes11 and mediating type 2 immunity.12 They are composed of natural helper cells,13 nuocytes12 and innate helper 2 cells,14 utilizing GATA-binding protein 3 (GATA3)15 and retinoic acid-related orphan receptor α (RORα)16 for their differentiation. ILC3s can be divided into natural cytotoxicity receptor (NCR)+ ILC3s and NCR− ILC3s17 and produce IL-17 and/or IL-2218 and require retinoic acid-related orphan receptor γt (RORγt).19 These different ILC populations have distinct patterns of cytokine production that are similar to the cytokine secretion profiles of Th cell subsets. In summary, ILCs are a heterogeneous population of non-B and non-T lymphocytes, but they are innate immune cells that have adaptive immune functions and are activated before T cells.20
The epithelial immune system has been shown to involved in immune surveillance of pathogens and other external factors, maintaining a dynamic balance of commensal bacteria and tissue homeostasis.21 It is easily exposed to antigens and leads to active responses of ILCs at barrier surfaces. Studies indicate that ILCs have a high frequency in some autoimmune-related and inflammatory skin diseases and exert regulatory functions on other dermal immune cell populations.22,23 Nevertheless, deeper mechanism remains incompletely understood, and further work remains to direct ILC development in human diseases.
Based on many speculative models and studies on human diseases, we suggest that different subsets and transcriptional regulation networks of ILCs could be critical to autoimmune-related skin diseases and inflammatory diseases; hence, we aim to give a comprehensive review of recent research progress on the pathogenic role of ILCs in these diseases. In this review, we will discuss the current knowledge about the contribution of ILCs to the impaired immune response and epithelial dysfunction in autoimmune-related skin diseases and inflammatory diseases. In each section, we will summarize the available mechanistic evidence for ILC dysregulation and how cytokines and cellular molecules work in the cutaneous immune network. Finally, we will discuss the potential of ILCs as therapeutic targets in the context of immune-mediated diseases.
ILC development
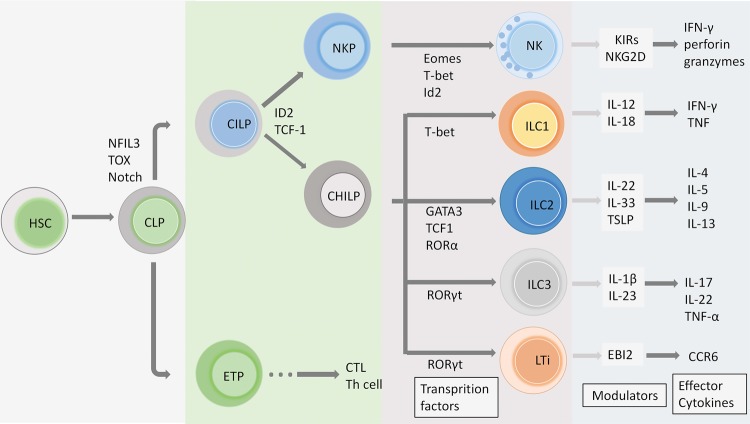
Critically, the nomenclature and classification of three major groups of ILCs were proposed according to their phenotypical and functional characteristics in 2013.24 Later, single-cell RNA-sequencing confirmed 15 transcriptionally homogeneous clusters of ILCs on the basis of three classical subsets, with distinct marker gene expression and indexed protein markers.25 Collectively, the updated categorization of ILC groups includes five main subsets, natural killer (NK) cells, ILC1s, ILC2s, ILC3s, and lymphoid tissue inducer (LTi) cells, and this categorization was recently approved by the International Union of Immunological Societies.20 NK cells and LTi cells were identified before the conception of ILCs. NK cells are now known as the prototypical ILC subset, were found in the spleen of adult mice in 197526 and are involved in immune responses against cancer cells and viral infection. LTi cells are instrumental in the initiation of lymph nodes, accumulating during late fetal and early neonatal life, and were identified as a novel subset of the hematopoietic lineage in 1997.27 Although several lymphocytes that lack RAG and differ from B cells and T cells have already been discovered, the ILCs were only discovered in the laboratory setting in the last 10 years and have been extensively studied since. The following detail the development (Fig. 1) and major content of each ILC subset and the extended biological role of ILCs in homeostatic or pathological conditions.
Fig. 1.
The development of ILCs. ILCs differentiate from hematopoietic stem cells (HSCs) and then generate CLPs in the bone marrow at the early stage. CLPs are a cellular source of early T cell progenitors (ETPs) and CILPs, and ILCs and NK cells share some notable parallels with T cell lineage commitment. The transcriptional factor Id2 and T cell factor 1 (TCF-1) are involved in CILP regulation and differentiation into NKPs and CHILPs. These transcription factors, such as T-bet, GATA3, and RORγt, described above, drive the generation of different ILC subsets. Downstream cytokines of the ILC group participate in orchestrating the immune network, and several modulators have been proven to regulate ILC functions in disease conditions
ILCs originate from the common lymphoid progenitor (CLPs), which reside in fetal liver and adult bone marrow and can give rise to common innate lymphoid progenitors (CILPs), as well as B and T cells lineages.28,29 Through lineage tracing and transfer studies in mice, CILPs have been found that located in the downstream of CLP and have restricted potential to generate ILCs and NK cells.29 CILPs differentiate into different progenitors, including NK cell precursors (NKPs) cells and common helper innate lymphoid progenitors (CHILPs).30 CHILPs give rise to LTi progenitors and ILC precursors, developing to LTi cell and ILC1, ILC2, and ILC3, respectively.31 Inhibitor of DNA binding 2 (Id2) has a high expression in all ILC lineages but not T or B cells, and contribute to immune cell fate decisions.32 Id2 expression is pivotal in transcriptional network establishing ILC fate.33 ILC lineages have been defined by distinct transcription factors. T-bet is necessary for development of ILC1 and NK cells, while Eomes is uniquely required for NK differentiation.34 GATA3, potently regulated by thymic stromal lymphopoietin (TSLP), are essential for ILC2 development35 and present similar fate decision of Th2 cells.15 On the other hand, ILC3s are dependent on RORγt for their development and function.36
ILC subsets
NK cells and ILC1s
NK cells originate from bone marrow and secondary lymphoid tissues, are present in many peripheral tissues, with a high frequency in the lung, uterus and liver, and include a large diversity of cell types.37–39 There are few major subsets of NK cells: CD56bright, CD56dim NK cells and other diverse subsets that are driven by killer cell immunoglobulin-like receptors,40 NK group 2, member A41 and epigenetic reprogramming.42 In recent studies, NK cells have been regarded as an important part of orchestrating early viral immunity and human cancer, and currently, more mechanisms of NK cell-mediated immunosurveillance and immunotherapy have been revealed.43,44 Moreover, their functions are broader than originally appreciated, being involved in transplantation and degeneration of injured axons. NK cells help clear damaged axons to reduce postinjury hypersensitivity, suggesting their therapeutic potential to resolve painful neuropathy.45 Viral immune-evasion restrains NK cell effector function via NK cell receptors, suggesting their instrumental role in innate immunity and the host-pathogen interplay.46 NK cells contribute to the recruitment of conventional dendritic cells into the tumor microenvironment via the NK cell-derived chemokines CC-type chemokine ligand 5 and lymphotactin and may be useful in therapeutic strategies for cancer immunology.47 Recent studies have found that trifunctional NK cell engagers, connecting NK cells with cancer cells by targeting NKp46, CD16 and tumor antigen, promote antibody-dependent cell-mediated cytotoxicity and enhance the antitumor efficacy of NK cells. Remarkably, trifunctional NK cell engagers have been proven to have efficacy superior to that of therapeutic antibodies in vitro and in vivo.48 Moreover, the cytotoxic activity of NK cells regulate their detachment from target cells, thus leading to sustained calcium signaling and hypersecretion of pro-inflammatory cytokines, such as TNF-α and IFN-γ, suggesting a role in inflammatory pathologies in several diseases.49
ILC1s were first named by Bernink in 2013 to delineate a subset of lineage negative (Lin−) CD127+c-Kit−NKp44− ILCs in tonsils and inflamed intestinal mucosa that expressed T-bet and produced the pro-inflammatory cytokine IFN-γ,50 which was similar but distinct from NK cells that T-bet and Eomes drive NK cell differentiation;51 in fact, the substantial functional and phenotypic overlap between ILC1s and NK cells delayed studies on ILC1s. Recent research has shown that promyelocytic leukaemia zinc finger (PLZF) contribute to map the lineage divergence between ILC1s and classical NK cells, which is located, as expected, at the initial branching of the two subsets.52 ILC1s are important components of the first line of immune defense against viral infection along with NK cells and even initiate the immune response prior to NK cells. Orr-El Weizman found that ILC1s were the first and main source of IFN-γ in the early stages of viral infection driven by conventional dendritic cells in a signal transducer and activator of transcription (STAT4)-dependent manner.7 In addition to host protection, ILC1s also play a nonredundant role in hapten-specific memory responses. Interestingly, haptens can induce the migration of IL-7Rα+ ILC1s to skin-draining lymph nodes in a CXC chemokine receptor (CXCR) type 3-dependent fashion. The microenvironment of the liver, which is rich in CXCR6 and IL-7, supports ILC1 residency and longevity.53 Although we usually define ILC1s as members of the innate immune system, evidence, as mentioned above, has emerged that ILC1s possess adaptive immune features.
ILC2s
ILC2s were first reported in splenocytes from immune-deficient (RAG KO) mice. These cells were IL-25-responsive and produced IL-13. They were detected as non-T/non-B cells54 and could initiate worm expulsion and protect against helminth parasite infection.55 Later, in 2010, natural helper cells,13 nuocytes12 and innate helper 2 cells14 were combined into a group: ILC2s or ‘group 2 innate lymphoid cells’.24 ILC2s are regarded as a unique subset and produce the Th2 cell-associated cytokines with large amounts of IL-5, IL-9, and IL-13 but very little IL-4.35,56,57 In some cases, IL-33, usually work as an ILC activator, did not induce high level of IL-4 production.58 We suppose the potential mechanisms were certain trigger alone may be not sufficient to induce IL-4 production and it requires additional signals for exerting a strong synergistic effect. Quantification of human ILC2s are significant elevated by the co-stimulation of IL-2 plus IL-1β or IL-33. Moreover, cytokines produced by ILC2s including IL-4, IL-5, and IL-13 are remarkable increased.59 Evidence also demonstrates that both freshly isolated ILC2s and presented ILC2s are triggered by TSLP and are responded with the increasing of IL-4, IL-5 and IL-13.55 In addition, ILC2-derived IL-4 has the capacity to antagonize oral tolerance to food allergy and impair the suppressive function of allergen-specific Treg cells.60 It also has been proved that IL-4 plays an important role in activating ILC2s and promoting type 2 cytokine production.61 Thus, IL-4 may work as a vital regulator in an autocrine manner.
ILC2s are usually located throughout mucosal and barrier surfaces in the intestines and airways and also reside in the lymph nodes and liver. Evidence shows that liver-resident ILC2s are significantly upregulated by IL-33 and play a potentially synergistic role in the pathogenesis of liver fibrosis.62 Small intestinal tuft cell and ILC2s were activated by natural intestinal parasites and promoted small intestinal remodeling.63,64 In addition, ILC2s also contribute to pathological inflammation in allergic asthma, and the mechanism is intriguing; recently, the neuropeptide neuromedin U was found to increase ILC2-induced inflammation as a modulator after allergic sensitization.65 The β2-adrenergic receptor is regarded as a negative regulator in molecular pathways.66 Interestingly, both the neuropeptide neuromedin U and β2-adrenergic receptor provide evidence of a neuronal-derived regulatory circuit in type 2 inflammatory regulation.65–67 Dysregulated ILC2 responses were found to be critical to obesity, and IL-33 plays a role in limiting adiposity in mice by eliciting beiging of white adipose tissue, indicating that ILC2s can regulate adipose function and metabolic homeostasis.68
ILC3s
RORγt is a ligand-dependent nuclear hormone receptor69 and is regarded as a central molecule for distinguishing ILC3s from other subpopulations.70 Group 3 ILCs were initially found to express NK cell receptors, such as NKp44, in human tonsils and to produce the Th17-associated cytokines IL-17 and/or IL-22.70,71 The subset of ILC3s can be classified by the cell surface expression of NKp4672 (in mice) and NKp4473 (in humans) and can be further divided into NCR+ ILC3s or NCR− ILC3s or termed IL-17- or IL-22-producing cells on the basis of cytokine secretion.73 They are predominantly located in the intestinal lamina propria. In addition to RORγt, another transcription factor that is essential for LTi-like cells and NCR+ ILC3s is the ligand-activated arylhydrocarbon receptor (AHR), which activated by environmental clues, endogenous factors as well as microbial metabolites.74,75 Genetic or pharmacological activation of AHR have been reported to regulate ILC2-ILC3 balance and host immunity.76
Recent studies indicate that ILC3s are relevant to human cancer and involved in intestinal homeostasis. Carrega P found that NCR+ ILC3s were recruited in human non-small-cell lung cancer tissue and that their presence was linked with intratumoral lymphoid structures.77 Additionally, more detailed mechanisms of ILC3 regulation in intestinal inflammation and homeostasis have been revealed recently. A study discovered that ILC3s regulate epithelial cell glycosylation in the intestinal microenvironment.78 NKp46− and NKp46+ ILC3 cells constitute most NKR-P1B+ lymphocytes in human intestine. While, the deletion of NKR-P1B results in a higher frequency and number of ILC3 and γδ T cells in the gut lamina propria and may contribute to innate immunity.79 Neuroimmune regulation plays an important role in intestinal homeostasis through the glial-ILC3 regulatory arm in a myeloid differentiation primary response gene 88-dependent manner.80,81 Interestingly, dissection of the vagus nerve is correlated with a decreased number of ILC3s, and acetylcholine upregulates the protecting conjugate in tissue regeneration biosynthetic pathway in mouse and human ILC3s. Moreover, the protecting conjugate in tissue regeneration can control macrophage responses and infections.82 Mechanistically, complex class II+ ILC3s regulate host-microbe symbiosis via repressing pathologic CD4+ T cell responses in inflammatory conditions, indicating a novel role in maintenance intestinal homeostasis and potential target for chronic intestinal inflammation treatment.83
ILCs in autoimmune-related and inflammatory skin diseases
As discussed above, abnormal populations and dysregulation of ILCs are instrumental factors in the development of many diseases, such as autoimmune diseases, inflammatory diseases and asthma. ILCs are mainly tissue-resident lymphocytes located in the mucosal barriers of the intestine, skin and lung and play a role in immunity, inflammation and tissue homeostasis. The skin, as the primary interface between the host and the environment, provides an early and prompt defensive immune response to protect epithelial integrity by serving as a physical and immunological barrier containing cells from both the innate and the adaptive immune systems. The local interplay between keratinocytes and immune cells, such as naive T cell by cell contact and costimulatory signaling, contribute to the disease pathogenesis under pro-inflammatory conditions.84 A much more heterogeneous population of immunological cells resides in the dermis, including dermal subsets of dendritic cells, mast cells, CD4+ and CD8+ T cells, B cells, macrophages, NK cells and the newly identified ILCs.85,86
Skin-resident ILCs locate at surface barrier and perform an important role in regulating microbial commensalism and cutaneous inflammation. As a large epithelial surface for interaction with microbes, mammalian skin protects body health against pathogenic microorganisms, but also facilitate host-microbe symbiosis.87 Furthermore, follicular and the interfollicular epithelial surface support a vast interface for microbes habitat, as well as host immunity.88 Kobayashi et al. have found that a population of skin-resident RORγt+ ILCs located around hair follicles in close proximity to sebaceous glands. More detailed researches have been reported that sebaceous function, especially free fatty acid production, were regulated by TNF-producing ILC via Notch signaling and were altered to significantly antimicrobial activity against skin-associated Gram-positive cocci. ILCs deficiency results in alteration of the microbial landscape by regulating sebocyte growth and antimicrobial lipids production, highlighting the significant role of ILC in skin commensals and barrier immunity.89 In addition, it is recently reported that atopic dermatitis (AD)-like murine model with filaggrin mutation do not develop skin inflammation under germ-free compared to SPF conditions. This model develops spontaneous AD-like skin inflammation with a significant elevation in dermal ILC2 numbers, indicting a crucial role for the microbiome in promoting type 2 innate immune responses.90
Furthermore, there may exit genetic association between ILC and autoimmune diseases and inflammatory diseases. ILC super-enhancers, demarcating cohorts of cell identity genes, are enriched for autoimmune-associated single nucleotide polymorphisms (SNPs). ILC3-specific super-enhancers had the most autoimmune SNPs, including IL23R and STAT3, perhaps revealing critical regulatory elements for differential gene expression in autoimmune diseases.91 Several susceptibility loci have been uniquely associated with psoriasis are involved in innate immune responses, with roles in IFN-mediated antiviral responses (DDX58), macrophage activation (ZC3H12C), and NF-κB signaling (CARD14 and CARM1).92 In addition, RUXN3, identified as psoriasis susceptibility loci that regulate T cell function, is also essential for ILC development and orchestrate RORγt expression in ILC3 cells.93 Consistent with dysregulation of ILC3s in psoriasis patients, RUXN3 may be a potential genetic regulator of ILC3s in psoriasis, still, more direct evidences are needed. ADAM17 deficiency leads dampened Notch signaling and results in increasing production of TSLP in atopic barrier immunity.94 Most importantly, Notch deficiency induces keratinocyte-mediated release of TSLP.95 As a fact of TSLP receptor expressed on ILC2s, TSLP is involved in inflammatory skin of AD associated with enhanced production of IL-5 and IL-13 from ILC2s.96 Notch have been recognized as a common regulator in ILC lineage differentiation.97 In addition, evidence links Notch signaling to ILC2 plasticity that Notch could change the cytokine responsiveness of ILC2.98 In line with these researches, ILCs are now a focus of skin homeostasis research as a critical factor in the innate immunity that protects against invading pathogens and in the regulatory functions in cutaneous inflammation and the pathogenesis of autoimmune diseases.
ILCs in systemic lupus erythematosus (SLE)
SLE is a chronic, multifactorial autoimmune disease and complicated immune disorder that has not been characterized extensively. Autoreactive T and B cell clones, increased autoantibodies and cytokines, and abnormal signal transduction constitute essential elements of the pathogenesis of lupus.99,100 High levels of IFN-α in peripheral blood have been noted in SLE for many years.101 Recently, study has found that IFN-α is able to promote transitional B cells survival and enhance pro-inflammatory activities and could contribute to the breach of B cell tolerance in this condition.102,103 As a result, research interests in recent decades have focused mainly on studying autoantibody-mediated tissue damage and identifying the role of adaptive immunity in SLE, often neglecting the involvement of cells central to innate immunity.
Currently, ILCs, especially ILC1s, are regarded as emerging modulators of immunity in SLE, and evidence suggests that ILC2s are potential protective factors. Hou recently found that ILC1s and ILC3s were significantly increased in the peripheral blood, whereas circulating ILC2s were decreased.22 Recently, evidence has shown that kidney-residing ILC2s are decreased in the MRL/MpJ-Faslpr lupus mouse model and are inhibited by the T cell- and myeloid cell-derived pro-inflammatory cytokines IFN-γ and IL-27. Furthermore, these ILC2s could be effectively expanded by IL-33 treatment and later had moderate beneficial effects on renal outcome and improve survival.104 Interestingly, another study found that ILC1s were markedly altered in the peripheral blood of SLE patients and then became the dominant ILC subset but were reduced after steroid and cyclophosphamide treatment. Moreover, after treatment with SLE plasma or IL-2/IL-12/IL-18, increased IFN-γ was secreted by ILC1s, which has been implicated in the pathogenesis of SLE.105 In addition, SLE patients with moderate and severe disease activities had a decreased frequency of ILC2s and ILC3s in one study.105 Blokland et al. also found that ILC1 frequencies were increased in blood circulation of SLE patients. Conversely, ILC2s or ILC3s did not differ between patients with SLE and healthy controls in peripheral blood; however the IFN signature was associated with elevated Fas expression on ILC2 and ILC3 subsets in circulation.106 In the present study, the divergent results in different ILC populations may be associated with the heterogeneity of and different treatments used for SLE patients, as SLE is not only the prototypical systemic autoimmune disease but also one of the most heterogeneous illnesses.
In addition, NK cells stand at the crossroads of innate and adaptive immunity as the third main lymphocyte lineage after T and B cells and work as an important element in the pathogenesis of SLE. A previous study found that NK activity could be inhibited by serum from SLE patients and was correlated with the presence of clinically active disease.106 Schepis D et al. showed that the CD56bright NK cell count was decreased in patients with active SLE, but the specific mechanism behind this finding remains unclear.107 Alterations in several cytokines, such as a reduction in IL-2, increased TNF-α and IFN-γ and may modulate NK cell phenotype and function in SLE by enhancing local autoimmune injury.108 A recent study reported that two clusters of NK cells were found in the kidneys of patients with lupus nephritis, including tissue-resident CD56brightCD16− NK cells and CD56dimCD16+ NK cells. These dividing cytotoxic T lymphocytes and NK cells were increased in patient kidneys, indicating a role of cytotoxic activity in lupus nephritis.109 Although circulating cytokines and antibodies could affect local skin immunity, ILC frequency and functions are poorly understood in lesional and nonlesional skin in SLE patients. Nevertheless, more powerful evidence is required to explore the mechanism of ILCs in SLE.
ILCs in psoriasis
Psoriasis is an immune-mediated, inflammatory and predominantly skin-tropic disease with multifactorial symptoms and is mediated by cells and molecules of both the innate and adaptive immune systems.110 Both genetic and environmental factors have been linked with the onset of psoriasis. HLA-Cw6, as a major disease allele of PSORS1, confers the strongest genetic linkage to susceptibility to psoriasis.111 Infections,112 trauma,113 some drugs such as β-adrenergic-blocking agents114 and a wealth of external elements contribute to psoriasis lesions and disease aggravation. Currently, it is widely held that divergent factors trigger plasmacytoid dendritic cells and then activate autoaggressive Th cells through IL-23, IL-12, IL-6, and TNF-α, leading to T lymphocyte subset dysfunction and immune responses in psoriatic plaques.114 ILCs are also an important source of the Th17 representative cytokines TNF-α, IL-17, and IL-22, suggesting a synergistic role between ILCs and Th17 cells in psoriasis via the innate immune response.13,57 The latest research has begun to discuss this possible relationship (Fig. 2).
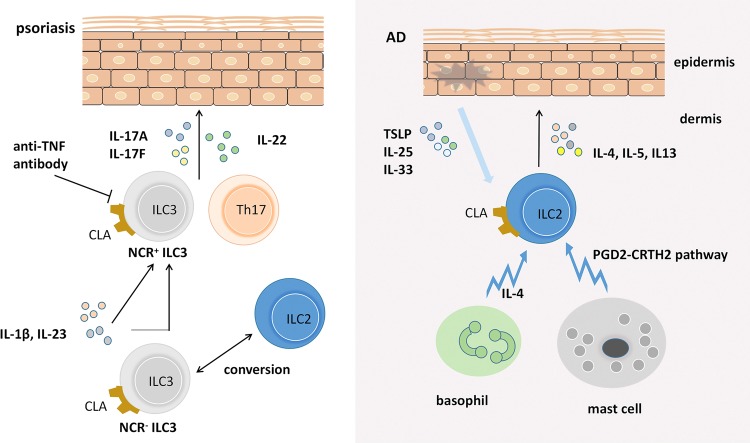
Fig. 2.
The pathogenic role of ILCs in psoriasis and AD. ILC3s are elevated in the skin of psoriasis patients. RORγt+ ILC3s secret IL-17A, IL-17F, and IL-22 and become a critical factor in promoting psoriasiform lesions. However, anti-TNF treatment can decrease the elevated level of circulating NCR+ ILC3s. Notably, ILCs can change phenotype. NCR− ILC3s in healthy skin can be converted to NCR+ ILC3s when cultured with IL-1β plus IL23. In addition, ILC2s play a central role in the skin in AD in both mouse models and humans through the secretion of IL-4, IL-5, and IL-13. Emerging evidence shows that IL-25, IL-33, and TSLP are all involved in the modulation of ILC2s in inflamed skin. In addition, ILC2s interact with mast cells and basophils and participate in driving pathology in atopic dermatitis through cell interactions
ILCs are proposed to contribute to the pathogenesis of psoriasis plaque formation and are a source of psoriasis-related cytokine in a murine model. The use of ILCs in inducing murine psoriasis was first uncovered in a murine model induced by topical application of the Toll-like receptor 7 agonist imiquimod (IMQ). There is mounting evidence that the Th17 signature cytokines IL-17A, IL-17F, and IL-22 play a predominant role in psoriasis, and Th17 cells act as a primary source of these pathogenic cytokines.110,115 However, skin-invading γδ T cells and RORγt+ ILCs secrete IL-17A, IL-17F, and IL-22 and then become an essential predisposing factor in the formation of acute psoriasiform lesions in mice, indicating that dysregulated innate immunity participates in psoriatic plaque formation.116 This research demonstrates that mice topically administered IMQ develop psoriasiform skin inflammation to the same extent as Rag1−/− mice lacking T and NK T cells. However, Rag2−/−Il2rg−/− mice, which additionally lack NK cells and ILCs, do not develop IMQ-driven plaques, indicating a unique function of ILCs in this pathogenic condition. Previously, evidence confirmed that RORγt, now also regarded as a transcription factor of group 3 ILCs,19 is a key regulator in Th17 differentiation and IL-17 secretion,117 and more in-depth research and relevant evidence are required in human diseases.
To interrogate whether ILC3s are present in human skin and altered in psoriasis, Villanova et al. performed flow cytometric analysis and found that NKp44+ ILC3s were increased in the skin and blood of psoriasis patients, with a significant enrichment of the ILC population in the skin compared with the blood.118 Almost at the same time, similar results showed that a significantly elevated proportion of NKp44+ ILC3s was located both in human skin lesions and in peripheral blood from psoriasis patients compared with healthy individuals, and skin NKp44+ ILC3s were correlated with psoriasis severity.17 Moreover, recent research has confirmed the role of ILC3s in the pathogenesis of psoriasis. Intradermal injection of human CD3−RORγt+NKp44+ ILC3s leads to the development of lesions with psoriatic histological features in humanized severe combined immunodeficient mice using healthy human skin grafts.17 These results highlight that ILC3s not only are increased in psoriasis but also drive the development of the human psoriatic phenotype independent of Th17 cells.
In addition, mature ILC subtypes have the capacity to modify their phenotype and function in response to local environmental cues, sharing a similar feature of plasticity with Th cells. A study revealed that ILC2s and NCR− ILC3s constitute the major ILC groups in healthy skin. Interestingly, NCR− ILC3s in healthy skin can be converted to NCR+ ILC3s through culture with IL-1β plus IL-23, which have already been found to be elevated in psoriatic lesions and implicated in the pathogenesis of psoriasis.17 A similar phenotype switching of ILCs has also been described in the intestinal lamina propria, and such a switch is reversible in specific microenvironments.119 A type of ILC2, inflammatory ILC2s, also expresses RORγt and is able to secrete IL-17 and respond to IL-25, and they have been referred to as transient ILC progenitors that could develop into IL-33-responsive natural ILC2s and ILC3-like cells.120 Studies have discovered that NKp44− ILC3s may be derived from ILC2s and are elevated in psoriasis lesions, corresponding with a decrease in the frequency of CRTH2+ ILC2s. Moreover, data show that these IL-17-producing ILC3s can switch back to ILC2-like cells when cultured with IL-1β and IL-4, suggesting a potential target for therapeutic intervention.73
Several studies have demonstrated the possible mechanism for the role of ILCs in the development of inflammation in psoriasis patients. Bruggen et al. performed ILC in situ mapping in human skin and revealed that psoriasis lesions contained a prominent population of TBET+ ILC1s and RORC+ ILC3s with nearly absent GATA3+ ILC2s, identified on the basis of different transcription factors between different ILC subpopulations. Using immunofluorescence in situ staining, these ILCs were found to reside beneath the dermoepidermal junction and in close proximity to T lymphocytes. The intimate contact between ILCs and T cells suggests a potential functional relationship between these cells and provides new insight into the shared mechanisms between psoriasis mechanism and innate immunity.121 A numerical increase in ILC3s was later found in both nonlesional and lesional psoriatic skin, whereas altered cytokine levels were not observed before cutaneous changes. Additionally, the cytokine IL-23 and the NK group 2 member D ligand MICA, which are both remarkably upregulated in lesions, were shown to have the potential to activate group 3 ILCs in lesional psoriatic skin.122 Thus, we hypothesize that the ILC imbalance acts before the IL-23/IL-17 axis and IL-22 production changes and that ILCs have emerging roles in the early stage of psoriasis. Previous studies both noticed that peripheral blood ILC subsets express skin-homing markers cutaneous lymphocyte antigen (CLA) at a high frequency,17,118 which indicates a potential source of skin ILCs. However, the deeper mechanism remains unclear. A Lin−CD123+CD127low population with characteristics of ILCs transmigrated through endothelial cells in response to CXCR4-stromal cell-derived factor-1 under inflammatory conditions in psoriasis.73,121 Taken together, these results suggest that ILCs, especially ILC3s, play a predominate role in the early part of psoriasis pathogenesis. Deeper insights into how ILCs make a substantial contribution may reveal potential immunopathological hallmarks of psoriasis and therapeutic approaches in the future.
ILCs in AD
AD, also known as atopic eczema, is a chronic, relapsing inflammatory skin disease that was originally regarded as a childhood disorder and includes a familial propensity to become IgE-sensitized against environmental allergens.73,121 It is now recognized as a lifelong disposition with persistent pruritus and recurrent eczematous lesions, and both epidermal barrier dysfunction and immune system dysregulation, especially Th2 cell adaptive immune responses, contribute to AD pathogenesis based on strong heritability.123 The gene encoding filaggrin (FLG), an epidermal structural protein, is strongly associated with AD as an important player in deficient barrier function.124,125 A genome-wide association analysis revealed new candidate genes related to innate immune signals and the T cell response.123 Notably, ILCs, especially ILC2s, were found to act as a critical predisposing factor in the pathogenesis of AD skin lesions and work as members of the signaling pathways involved (Fig. 2).
Numerous studies have demonstrated that ILC2s are involved in skin inflammation and are significantly increased in lesional AD skin compared to healthy human skin, as well as being increased in an AD-like dermatitis mouse model. Elevated ILC2s have been reported in the skin of AD patients and are identified by the specific markers CD25 (IL-2Ra) and IL-33R (ST2) expressed by lineage negative cells.126 Similar results are observed in the AD murine model: vitamin D analog calcipotriol (MC903)-treated naïve C57BL/6 wild-type mice develop AD-like dermatitis associated with Th2 cell-associated cytokine production and elevated serum IgE. Histopathologic changes exist similar to those observed in human atopic lesions, including epidermal hyperplasia and mononuclear leukocyte and granulocyte infiltration. Cutaneous CD25+IL-33R+ group 2 ILCs were increased on flow cytometric analysis, as was the expression of the effector cytokines IL-5 and IL-13, which are typical type 2 cytokines produced by Th2 cells and are now also regarded as innate sources. The data resemble those in the skin-draining lymph nodes. More strikingly, after intradermal injection of purified ILC2s sorted from MC903-treated C57BL/6 wild-type mice into naïve C57BL/6 wild-type mice, the recipients develop AD-like inflammatory response and histological features in the skin.126 On the other hand, the number of CRTH2+IL-7Rα+ ILC2s are elevated in skin suction blisters from nonlesional skin of patients with FLG mutations compared with the number in those of patients without FLG mutations.127 Furthermore, a filaggrin-mutant mouse model, also called “flaky tail” mice, shows spontaneous AD-like inflammation and pro-inflammatory cytokine production, eliciting ILC2 alterations and Th2 cell responses.127 Therefore, these results demonstrate ILC2 dysregulation in AD patients and murine models; thus, ILC2s have become a promising means to study the pathogenesis of AD, and the deeper mechanism remains to be discussed.
Notably, ILC2s have been found to play a central role in AD independent of adaptive immunity. Increasing numbers of ILC2s and cytokine secretion have been observed in lymphocyte-deficient Rag1−/− mice. The effective depletion of CD25+IL-33R+ ILCs by anti-CD25 or anti-CD90.2 monoclonal antibodies reduced skin lesions in a Rag1−/− mouse model of AD-like skin inflammation, indicating that the ILC2-mediated immune response plays a major role in AD independent of adaptive immunity.126 Moreover, RORα-deficient bone marrow chimaera mice that lack ILC2s have ameliorated cellular infiltration in the skin and markedly reduced ear swelling. In addition, the increased susceptibility of Rag1−/−Flgft/ft mice to contact hypersensitivity inflammation reveals that innate immunity mediates spontaneous inflammatory lesions. Furthermore, in this model, an adaptive immune response is required for the progression from dermatitis to pulmonary inflammation.127 In a recently described Zn2+-finger DHHC-domain-containing protein 13 (ZDHHC)-deficient mouse model, which features mutations of DHHC-domain-containing palmitoyl acyltransferases, animals develop an impaired skin barrier and abnormal filaggrin due to the deficiency of ZDHHC. Moreover, the Rag1−/−;Zdhhc13k/k double-mutant mice generated to further explore adaptive immune functions in AD still develop inflamed skin with substantial mast cell accumulation and abundant ILC2s, highlighting ILC2s as a main pro-inflammatory cell, and the initiation of dermatitis may activate the innate immune response independent of adaptive immunity.128 Therefore, ILC2s are linked with the pathogenesis of AD, and moreover, it is interesting that ILC2s can be self-governed without adaptive immunity in murine models.
To investigate the modulators that promote ILC2 activation in the context of AD-like inflammation, studies have focused on whether and to what extent ILC2s respond to IL-25, IL-33, and TSLP, given their expression of the membrane-bound IL-33 receptor ST2, IL-25R, and TSLP receptor. Studies have demonstrated that epithelium derived IL-25 and IL-33 can induce the accumulation of ILC2s in the lung tissue and local lymph nodes.129,130 Moreover, Kim et al. found that ILC2s and the cytokines IL-5 and IL-13 were increased in AD lesion and draining lymph nodes.96 This finding was critically dependent on TSLP, rather than IL-25 and IL-33, which have been proven to be associated with ILC2 activation in intestinal lymphoid tissue and the lung parenchyma.2 Skin-specific expression of IL-33 was also proved to be a critical activator of ILC2s to induce AD-like dermatitis with eosinophil infiltrates with the establishment of a transgenic mouse model that overexpresses IL-33 in keratinocytes.126 Another relevant study confirmed the role of Lin−CD45hiIL-7Rα+CRTH2+ ILC2s in allergic skin inflammation and further indicated that the initiating cytokines IL-25 and IL-33 played a predominate role over TSLP in a BALB/c mouse model of dermatitis.128 Discrepancies seem to exist in mice with different genetic backgrounds, and the role of IL-33 remains a matter of debate. However, what does make sense is the extent that ILC2s are dependent on IL-33 or TSLP in humans. Collectively, IL-25, IL-33 and TSLP are all involved in the modulation of ILC2s in inflammatory skin from patients with AD. Additionally, ILC2s express the skin-homing receptors CLA, complete cytogenetic response 10 and complete cytogenetic response 4 and infiltrate AD skin.131 Further studies need to focus on more detailed mechanisms and their therapeutic implications.
ILC2s also interact with multiple cell populations to drive pathology in AD, especially mast cells and basophils. A unique population of skin-resident CD103+ ILC2s, called dermal ILC2s, promote eosinophil influx and cutaneous inflammation. In assessing the skin by multiphoton microscopy, a novel interaction was uncovered between these dermal ILC2s and mast cells. Human mast cell-derived prostaglandin D2 triggers ILC2 migration and cross-regulation through a prostaglandin D2-CRTH2-dependent pathway in the skin.132 Interactions may also exist between ILC2s and basophils,96 while the upstream innate cellular mechanism remains unclear. Later, a close proximity of ILC2s and basophils was reported in the dermis of human AD lesions. Kim et al. found that basophil-derived IL-4 was necessary for skin inflammation and ILC2 proliferation in murine AD-like skin lesions.133 In addition, basophils were positively correlated with the frequency of skin ILC2s but not ILC3s in the lesions of AD patients.134 Basophils have been report to be accumulated in the skin biopsies of patients with AD and are responsive for AD-related chemokine expression.135,136 Skin basophils significantly expressed IL-4 following ex vivo stimulation, indicating a potential therapeutic target in AD.134 On the other hand, dermal ILC2s could act as immune-regulatory factors in steady-state conditions, producing IL-13, which is capable of promoting homeostatic function.23 There is indeed a need to focus on the regulation and functional interactions of ILC2s in cutaneous inflammation, aiming to inhibit the switch to a pro-inflammatory phenotype for potential therapies in the future.
ILCs in other autoimmune-related and inflammatory diseases
Systemic sclerosis (SSc), also known as scleroderma, is an immune-mediated disease featuring fibroblast activation, progressive tissue fibrosis of the skin and internal organs and vasculopathy.137 Skin tightness and itching are early features, and later, vasculature and musculoskeletal damage and fibrosis are involved.138 Genetic clues as well as chemical exposure and several chemotherapy drugs might be important drivers of molecular and clinical diversity within SSc.139,140 Notably, ANAs and anti-RNA polymerase III antibodies141 are specific hallmarks of SSc; moreover, reactive CD4 T cells are observed,142 and autoimmunity might be central to the initiation or progression of the disease. Increased proportions of ILC1s and NKp44+ ILC3s have been reported in the peripheral blood of individuals with SSc compared with healthy controls, while NKp44− ILC3 frequencies are decreased. Interestingly, the elevated ILC1s were predominantly attributable to changes in CD4+ ILC1s, which have lower expression of T-bet than CD4− ILC1s and alteration of IL-6Rα.143 Moreover, deficiency of the transcription factor T-bet has been proven to increase sensitivity to bleomycin-induced dermal sclerosis in a mouse model, and this also occurs in mouse models lacking T and B cells.144 Expansion of ILC2 numbers has also been revealed in the skin and blood of SSc patients; in addition, skin ILC2s have fibrotic manifestations, and circulating ILC2s correlate with the extent of skin fibrosis and interstitial lung disease.145 The expression of the skin-homing marker CLA on resident ILCs provides potential insights into the source of cutaneous ILC2s, which are activated locally in the skin.17,131
Anti-neutrophil cytoplasmic autoantibody-associated vasculitis (AAV) is a group of autoimmune diseases characterized by necrotizing small-vessel vasculitis that largely affects the kidneys, respiratory tract and skin and circulating autoantibodies to myeloperoxidase or proteinase 3, which are helpful for the classification of AAV.146,147 The major subgroups of AAV are microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA) and eosinophilic GPA.148 A study has demonstrated that the total ILCs in peripheral blood are markedly decreased in patients with GPA and MPA during the acute phase but are restored in the remission phase. Notably, ILC1s have a higher frequency in acute-phase GPA patients than in GPA patients in the remission phase. Decreased frequencies of ILC2s and NKp44− ILC3s can be seen during the acute phase in both GPA and MPA compared with the respective frequencies in healthy controls and matched patients the remission phase.149 However, more details need to be understood, and the function of ILCs in the pathogenesis of AAV, especially MPA and GPA, needs to be assessed.
Allergic contact dermatitis is a series of inflammatory reactions in the skin with contact with low-molecular-weight organic chemicals or metal ions, ultimately resulting in the development of intensely pruritic erythema, edema and even vesicles.150 The hapten-mediated activation of the innate immune system and cutaneous antigen-presenting cells play a critical role in skin inflammation.151 Increasing numbers of ILCs and their respective marker cytokines have been reported, with the number of NK cells and the expression of IFN-γ and TNF being highest 24 h after allergen challenge and paralleling the strongest skin inflammatory response. Moreover, depletion of ILCs, especially ILC2s, leads to increased contact hypersensitivity responses, providing compelling evidence for the role of ILCs in inflammation associated with contact dermatitis.152 A recent study revealed that ILC2 deficiency elicited an obvious decrease in cutaneous infiltrating Th2 cell numbers following allergen rechallenge in sensitized animals, demonstrating that ILC2s are important for orchestrating efficient localized memory Th2 cell responses to allergens.152
Applications of ILCs in mouse models and emerging therapeutic targets
Previous studies have shown that ILCs play critical, nonredundant roles in the activation of the immune response and pro-inflammatory cytokine secretion. Research and early stage trials have been performed on murine models based on their similar physiological and pathological states to those seen in human diseases. With more in-depth investigations, ILCs have been observed to induce an inflammatory response in vivo, suggesting their role in disease pathogenesis and their increased potential for establishing animal disease models. On the other hand, ongoing studies continue to reveal emerging therapies for immune regulation and the inflammatory response in the skin targeting the ILC group. Several questions and specific mechanism of ILC in the pathogenesis of SLE, scleroderma and allergic contact dermatitis are still remain addressed, limiting the therapeutic applications. However, abundant evidences suggest that ILC contribute to skin inflammation in psoriasis and AD patients. These findings establish fundamental therapeutic ideas as target interventions, and actually evidence has already shown ILC could serve as promising therapeutic targets in disease models.
It is intriguing to find ILC prompt the devolopment of psoriasis and targeting the corresponding cytokines has potential treatment effect. Intradermal injection of specific ILC subsets has been used to precisely define the function of ILCs in skin immunity, and evidence has demonstrated that human ILC3s have the capacity to induce psoriatic lesions on healthy human skin grafts in recipient mice.153 As suggested by studies of ILC activity in human psoriasis, ILC3 alterations and production of downstream cytokines production, such as IL-22, TNF-α and IL-17, are important in disease conditions. Using an IMQ-induced psoriasis mouse model, an IL-22 neutralizing antibody was shown to have the capacity to improve keratinocyte dysregulation and neutrophil infiltration in skin inflammation.154 The elevated level of circulating NCR+ ILC3s decreased following treatment with an anti-TNF antibody, suggesting a potential role of these cells as a therapeutic target or biomarker in psoriasis.118 Recently, a novel RORγt inhibitor significantly blocked the development of psoriatic skin inflammation in mice by suppressing all subsets of IL-17-producing cells, including Th17 cells, dermal γδ T cells and ILC3s, and followed with dose-dependently decreasing of IL-17 production,155 suggesting its promise in therapeutic interventions for psoriasis. Actually, clinical trials of several anti-IL-17 antibodies in psoriasis patients are ongoing. Furthermore, drugs like Secukinumab,156 Ixekizumab,157 and Brodalumab158 that target IL-17A or IL-17F provide new biologic therapies with high effectiveness for psoriasis patients.
Nevertheless, some efforts have focused on ILC-relevant therapy in AD. After injecting ILC2s into the dermis, AD-like inflammatory responses and histological changes similar to dermatitis in AD patients can be seen in mice.126 Moreover, models with ILC-related gene mutations, such as RORα-deficient bone marrow chimaera mice,126 which have a deficiency of one type of ILC, also broaden the scope of new potential animal models that can be used to explore the complex immune network. A synthetic RORα/γ inverse agonist (SR1001) treatment suppressed MC903-induced TSLP expression and reversed impaired keratinocyte differentiation and epidermal barrier disruption, thus alleviating skin inflammatory responses in the MC903-induced AD mouse model and acting as a potential therapeutic compound in AD patients.159 Moreover, IL-5 and IL-13 are type 2 cytokines that are markedly elevated in lesions, regional lymph nodes and peripheral blood, corresponding with an increase in ILC2s. Anti-IL-5 antibody, as a neutralizing monoclonal antibody used to treat a spontaneous itchy dermatitis mouse model, reduces epidermis thickening and reduces eosinophil infiltrates in inflamed skin.160 However, a clinical trial in patients with AD did not show a promising improvement after the use of an anti-human IL-5 antibody (mepolizumab).161 Perhaps the low dose of mepolizumab administered was responsible for the limited decreases in eosinophil levels. However, evidence has shown that mepolizumab is an available strategy and a well-tolerated targeted therapy that can reduce asthma exacerbations and blood eosinophil counts and benefit quality of life; the lowest dose of mepolizumab (75 mg) has been identified to be close to the plateau of the dose-response curve.162,163 However, previous studies have revived interest in the biological targeting of IL-5 and other potential molecules.
Concluding remarks
In summary, emerging evidence suggests that ILCs play an important, nonredundant role in the abnormal immune response and epithelial dysfunction seen in conditions of autoimmunity and chronic inflammatory disorders (Table 1). The study of ILCs has been rapidly increasing recently, but is still in its infancy, and most available evidence has been obtained from murine models or from small numbers of human patients. Abundant studies have revealed the key role of ILCs in lung and intestine inflammation, while the locations and functional potential of ILCs in the skin remain poorly understood. The identification of different ILC subsets that reside at the skin barrier indicates that productive immune responses against invading microbes and regulatory functions are initiated in the dermis. Currently, ILC populations are known to be altered in many autoimmune-related and inflammatory skin diseases, and dysregulation of ILCs can result in complex immune responses via cytokine secretion and cell interactions.
Table 1.
ILC subtypes in different autoimmune-related and inflammatory diseases
| Disease | ILC subset | Cell surface markers | Tissue samples | Function | Model | Reference |
|---|---|---|---|---|---|---|
| Psoriasis | ILC1s(-) |
Lin− CD127+ CRTH2 CD117-CD161+ ILCs |
Human lesional skin | - (no significant difference) | Psoriasis patients | 17 |
| ILC2s(-) | Lin− CD127+ CRTH2+ ILCs | Human lesional skin | - (no significant difference) | Psoriasis patients | 17 | |
| ILC3s(↑) | RORγt+ ILCs | Mouse lesional skin | Psoriatic plaque formation | IMQ-induced Rag1-/- and Rag2−/−Il2rg−/− mice | 19 | |
| NKp44+ ILC3s | Human lesional skin and blood | Correlate with psoriasis severity | Psoriasis patients | 17 | ||
| CD3− RORγt+ NKp44+ ILC3s | Human blood | Induce psoriatic lesions | Intradermal injection into healthy human skin grafts on severe combined immunodeficient mice | 153 | ||
| c-kit+ CRTH2- ILCs | Human lesional skin | Resident c-kit+ CRTH2- ILCs are derived from ILC2s and could switch back to ILC2-like cells | Cutaneous ILCs cultured in vitro | 72 | ||
| NCR+ ILC3s | Human skin | NCR+ ILC3s elevated both in lesional and nonlesional skin | Psoriasis patient | 122 | ||
| AD | ILC1s(-) | Lin− IL-7Rα+ T-bet+ ILCs | Mouse skin | - (no significant difference) | Flgft/ft mice | 127 |
| ILC2s(↑) | Lin− CD25+ IL-33R+ ILC2s | Human lesional skin | Higher frequency of ILC2s in the skin of patients | AD patients | 126 | |
| Lin− CD25+ IL-33R+ ILC2s | Mouse lesional skin | Promoting AD-like disease | MC903-treated naïve C57BL/6 mice | |||
| CRTH2+ IL-7Rα+ ILC2s | Human nonlesional skin | FLG mutations in AD patients are associated with ILC2s frequency in nonlesional skin | AD patients | 126 | ||
| Lin− CD127+ CD25+ CD90+ ILC2s | Mouse lesional skin | Increasing ILC2s serve as pro-inflammatory factors | Filaggrin-mutant mouse model | 127 | ||
| Lin− CD25+ IL-33R+ ILCs | Mouse lesional skin | ILC2s develop AD-like dermatitis independent of adaptive immunity | Rag1−/−mouse model, Rag1−/−Flgft/ft mice, Rag1−/−; Zdhhc13k/k double-mutant mice | 127,128,131 | ||
| ILC3s(-) | Lin− IL-7Rα+ ST2- RORγT+ ILCs | Mouse skin | - (no significant difference) | Flgft/ft mice | 127 | |
| SLE | ILC1s(↑) | Lin− CD127+ CRTH2- CD117- ILCs | Human blood | Increasing in patients and decreasing after treatment | SLE patients | 104 |
| ILC2s(↓) | Lin− CD127+ CD25hi GATA3+ ILCs | Human blood | Benefit renal outcome and improve survival | SLE patients | 22 | |
| ILC3s(?) | Lin− CD127+ CD117+ CRTH2- ILCs | Human blood | Remains unclear | SLE patients | 22,106 | |
| NK cells(↓) | CD56brightNK cell | Human blood | Local autoimmune injury | SLE patients | 106 | |
| NK cells(↑) | CD56bright CD16- NK cells and CD56dim CD16+ NK cells | Human kidney | Cytotoxic activity | Lupus nephritis patients | 107 |
Alterations in the ILC population and different subset percentages have been reported in both peripheral blood and skin in SLE patients.22,104,106 However, studies have been limited to identifying whether ILCs play a predominate role in disease pathogenesis or the specific factors that lead ILCs to become imbalanced. NK cells, ILC1s and ILC2s may be potential regulatory factors in lupus, but more powerful evidence is required. Notably, ILC function seems more relevant in inflamed skin. In psoriasis patients, NCR+ ILC3s are elevated and associated with disease severity.17 Evidence shows that ILC3s may be regulated by CXCR4-stromal cell-derived factor-1164 under inflammatory conditions and act before IL-23 and IL-17A changes, indicating emerging roles for ILC3s in the initial stage of psoriasis.122 Although ILC3s are the predominant ILC type in psoriasis, ILC2s play a predominant role in the pathogenesis of AD. IL-25,131 IL-33,131,160 and TSLP126 are all involved in the modulation of ILC2s in an adaptive immune response-independent manner.126,128 Moreover, abundant evidence has revealed that in other pathological conditions in human skin, ILCs also play an provital role in orchestrating the immune response. Taken together, the findings so far suggest that ILCs can serve as promising therapeutic targets in autoimmunity and inflammatory skin diseases by modulating transcription factors and effector cytokines (Table 2). Deciphering ILC biology and function in epithelial immune barriers will be important in the future, and additional studies are required to explore specific ILC-directed treatment strategies.
Table 2.
Emerging targets of ILCs in disease therapy
| ILC subsets | Transcription factor | Effector cytokines | Therapeutic targets | Treatment efficacy | Reference |
|---|---|---|---|---|---|
| ILC1s | T-bet | IFN-γ | – | – | – |
| ILC2s | RORα, GATA3 | IL-4,IL-5, IL-13 | RORα/γ inverse agonist (SR1001) | Reversing impaired keratinocyte differentiation and epidermal barrier disruption | 107 |
| Anti-IL-5 antibody | Reducing thickened epidermis changes and eosinophils infiltrates | 159 | |||
| ILC3s | RORγt, AHR, T-bet | IL-17, IL-22 | RORγt inhibitor | Suppressing the IL-17 production | 155 |
| IL-22 neutralizing antibody and neutrophil infiltration | Improving keratinocyte dysregulation | 154 | |||
| Anti-TNF antibody | Elevated circulating NCR+ILC3s are decreased | 118 |
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81972943, No.81830097) and Hunan Talent Young Investigator (No. 2019RS2012).
Author contributions
S.Q.Z. wrote the manuscript, Q.W.L. did the editing, H.J.W. and Q.J.L. revised the manuscript.
Competing interests
The authors declare no competing interests.
Contributor Information
Haijing Wu, Email: Chriswu1010@126.com.
Qianjin Lu, Email: qianlu5860@csu.edu.cn.
References
- 1.Minton K. Innate lymphoid cells: ILC diversity maintained by microbiota. Nat. Rev. Immunol. 2016;16:593. doi: 10.1038/nri.2016.101. [DOI] [PubMed] [Google Scholar]
- 2.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 2016;17:765–774. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 3.Tait WE, Artis D. Innate lymphoid cells: Balancing immunity, inflammation, and tissue repair in the intestine. Cell Host Microbe. 2012;12:445–457. doi: 10.1016/j.chom.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tugues S, et al. Innate lymphoid cells as regulators of the tumor microenvironment. Semin. Immunol. 2019;41:101270. doi: 10.1016/j.smim.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Spits H, Cupedo T. Innate lymphoid cells: Emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 6.Powell N, et al. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells. Immunity. 2012;37:674–684. doi: 10.1016/j.immuni.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weizman OE, et al. ILC1 confer early host protection at initial sites of viral infection. Cell. 2017;171:795–808. doi: 10.1016/j.cell.2017.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brestoff JR, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilhelm C, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat. Immunol. 2011;12:1071–1077. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monticelli LA, et al. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc. Natl Acad. Sci. USA. 2015;112:10762–10767. doi: 10.1073/pnas.1509070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasuda K, et al. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc. Natl Acad. Sci. USA. 2012;109:3451–3456. doi: 10.1073/pnas.1201042109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moro K, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 14.Price AE, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl Acad. Sci. USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoyler T, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong SH, et al. Transcription factor RORalpha is critical for nuocyte development. Nat. Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teunissen M, et al. Composition of innate lymphoid cell subsets in the human skin: Enrichment of NCR(+) ILC3 in lesional skin and blood of psoriasis patients. J. Invest. Dermatol. 2014;134:2351–2360. doi: 10.1038/jid.2014.146. [DOI] [PubMed] [Google Scholar]
- 18.Ciccia F, et al. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann. Rheum. Dis. 2015;74:1739–1747. doi: 10.1136/annrheumdis-2014-206323. [DOI] [PubMed] [Google Scholar]
- 19.Montaldo E, et al. Human RORgammat(+)CD34(+) cells are lineage-specified progenitors of group 3 RORgammat(+) innate lymphoid cells. Immunity. 2014;41:988–1000. doi: 10.1016/j.immuni.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Vivier E, et al. Innate lymphoid cells: 10 years on. Cell. 2018;174:1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Tlaskalova-Hogenova H, et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol. Lett. 2004;93:97–108. doi: 10.1016/j.imlet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Hou M, Liu S. Innate lymphoid cells are increased in systemic lupus erythematosus. Clin. Exp. Rheumatol. 2019;37:676–679. [PubMed] [Google Scholar]
- 23.Roediger B, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat. Immunol. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spits H, et al. Innate lymphoid cells-a proposal for uniform nomenclature. Nat. Rev. Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 25.Gury-BenAri M, et al. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell. 2016;166:1231–1246. doi: 10.1016/j.cell.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 26.Kiessling R, et al. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 1975;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 27.Mebius RE, et al. Developing lymph nodes collect CD4+ CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/S1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 28.Ding L, Morrison S. J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klose C, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 30.Carotta S, et al. Identification of the earliest NK-cell precursor in the mouse BM. J. Exp. Med. 2011;203:1105–1116. doi: 10.1182/blood-2010-11-318956. [DOI] [PubMed] [Google Scholar]
- 31.Constantinides M. G., et al. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rawlins E. L., et al. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009;136:3741–3745. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boos M. D., et al. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J. Exp. Med. 2007;204:1119–1130. doi: 10.1084/jem.20061959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daussy C, et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J. Exp. Med. 2014;211:563–577. doi: 10.1084/jem.20131560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mjosberg J, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 36.Luci C, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat. Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 37.Hudspeth K, et al. Human liver-resident CD56(bright)/CD16(neg) NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J. Autoimmun. 2016;66:40–50. doi: 10.1016/j.jaut.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Male V, et al. The effect of pregnancy on the uterine NK cell KIR repertoire. Eur. J. Immunol. 2011;41:3017–3027. doi: 10.1002/eji.201141445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marquardt N, et al. Human lung natural killer cells are predominantly comprised of highly differentiated hypofunctional CD69(-)CD56(dim) cells. J. Allergy Clin. Immunol. 2017;139:1321–1330. doi: 10.1016/j.jaci.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 40.Beziat V, et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013;121:2678–2688. doi: 10.1182/blood-2012-10-459545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li F, et al. Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenterology. 2013;144:392–401. doi: 10.1053/j.gastro.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 42.Schlums H, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42:443–456. doi: 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krneta T, et al. The breast tumor microenvironment alters the phenotype and function of natural killer cells. Cell. Mol. Immunol. 2016;13:628–639. doi: 10.1038/cmi.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng X, et al. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat. Immunol. 2019;20:1656–1667. doi: 10.1038/s41590-019-0511-1. [DOI] [PubMed] [Google Scholar]
- 45.Davies AJ, et al. Natural killer cells degenerate intact sensory afferents following nerve injury. Cell. 2019;176:716–728. doi: 10.1016/j.cell.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aguilar OA, et al. A viral immunoevasin controls innate immunity by targeting the prototypical natural killer cell receptor family. Cell. 2017;169:58–71. doi: 10.1016/j.cell.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Bottcher JP, et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172:1022–1037. doi: 10.1016/j.cell.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gauthier L, et al. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell. 2019;177:1701–1713. doi: 10.1016/j.cell.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 49.Anft, M. et al. NK cell detachment from target cells is regulated by successful cytotoxicity and influences cytokine production. Cell. Mol. Immunol.10.1038/s41423-019-0277-2 (2019). [DOI] [PMC free article] [PubMed]
- 50.Bernink JH, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol. 2013;14:221–229. doi: 10.1038/ni.2534. [DOI] [PubMed] [Google Scholar]
- 51.Jiang X, et al. Single line or parallel lines: NK cell differentiation driven by T-bet and Eomes. Cell. Mol. Immunol. 2012;9:193–194. doi: 10.1038/cmi.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Constantinides MG, et al. PLZF expression maps the early stages of ILC1 lineage development. Proc. Natl Acad. Sci. USA. 2015;112:5123–5128. doi: 10.1073/pnas.1423244112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, et al. Memory formation and long-term maintenance of IL-7Ralpha(+) ILC1s via a lymph node-liver axis. Nat. Commun. 2018;9:4854. doi: 10.1038/s41467-018-07405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fort MM, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/S1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 55.Fallon PG, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Price, A. E. et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity (2010). [DOI] [PMC free article] [PubMed]
- 57.Guo L, et al. Cytokine-induced cytokine production by conventional and innate lymphoid cells. Trends Immunol. 2012;33:598–606. doi: 10.1016/j.it.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doherty TA, et al. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J. Allergy Clin. Immunol. 2013;132:205–213. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohne Y, et al. IL-1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat. Immunol. 2016;17:646–655. doi: 10.1038/ni.3447. [DOI] [PubMed] [Google Scholar]
- 60.Noval RM, et al. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J. Allergy Clin. Immunol. 2016;138:801–811. doi: 10.1016/j.jaci.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bal SM, et al. IL-1beta, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat. Immunol. 2016;17:636–645. doi: 10.1038/ni.3444. [DOI] [PubMed] [Google Scholar]
- 62.Tan Z, et al. Interleukin-33 drives hepatic fibrosis through activation of hepatic stellate cells. Cell. Mol. Immunol. 2018;15:388–398. doi: 10.1038/cmi.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.von Moltke J, et al. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529:221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schneider C, et al. A Metabolite-Triggered tuft Cell-ILC2 circuit drives small intestinal remodeling. Cell. 2018;174:271–284. doi: 10.1016/j.cell.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wallrapp A, et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature. 2017;549:351–356. doi: 10.1038/nature24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moriyama S, et al. Beta2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science. 2018;359:1056–1061. doi: 10.1126/science.aan4829. [DOI] [PubMed] [Google Scholar]
- 67.Cardoso V, et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature. 2017;549:277–281. doi: 10.1038/nature23469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brestoff JR, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kurebayashi S, et al. Retinoid-related orphan receptor gamma (RORgamma) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc. Natl Acad. Sci. USA. 2000;97:10132–10137. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cupedo T, et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC + CD127+ natural killer-like cells. Nat. Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 71.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang, Q. et al. Circadian rhythm-dependent and circadian rhythm-independent impacts of the molecular clock on type 3 innate lymphoid cells. Sci. Immunol. 4, eaay7501, 10.1126/sciimmunol.aay7501 (2019). [DOI] [PMC free article] [PubMed]
- 73.Bernink JH, et al. C-Kit-positive ILC2s exhibit an ILC3-like signature that may contribute to IL-17-mediated pathologies. Nat. Immunol. 2019;20:992–1003. doi: 10.1038/s41590-019-0423-0. [DOI] [PubMed] [Google Scholar]
- 74.Stockinger B, et al. External influences on the immune system via activation of the aryl hydrocarbon receptor. Semin. Immunol. 2011;23:99–105. doi: 10.1016/j.smim.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 75.Qiu J, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li S, et al. Aryl hydrocarbon receptor signaling cell intrinsically inhibits intestinal group 2 innate lymphoid cell function. Immunity. 2018;49:915–928. doi: 10.1016/j.immuni.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carrega P, et al. NCR(+)ILC3 concentrate in human lung cancer and associate with intratumoral lymphoid structures. Nat. Commun. 2015;6:8280. doi: 10.1038/ncomms9280. [DOI] [PubMed] [Google Scholar]
- 78.Goto Y, et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science. 2014;345:1254009. doi: 10.1126/science.1254009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abou-Samra E, et al. NKR-P1B expression in gut-associated innate lymphoid cells is required for the control of gastrointestinal tract infections. Cell. Mol. Immunol. 2019;16:868–877. doi: 10.1038/s41423-018-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ibiza S, et al. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature. 2016;535:440–443. doi: 10.1038/nature18644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Godinho-Silva C, et al. Light-entrained and brain-tuned circadian circuits regulate ILC3s and gut homeostasis. Nature. 2019;574:254–258. doi: 10.1038/s41586-019-1579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dalli J, et al. Vagal regulation of group 3 innate lymphoid cells and the immunoresolvent PCTR1 controls infection resolution. Immunity. 2017;46:92–105. doi: 10.1016/j.immuni.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hepworth MR, et al. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4(+) T cells. Science. 2015;348:1031–1035. doi: 10.1126/science.aaa4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Orlik, C. et al. Keratinocytes costimulate naive human T cells via CD2: A potential target to prevent the development of proinflammatory Th1 cells in the skin. Cell. Mol. Immunol.10.1038/s41423-019-0261-x (2019). [DOI] [PMC free article] [PubMed]
- 85.Nestle FO, et al. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kabashima K, et al. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019;19:19–30. doi: 10.1038/s41577-018-0084-5. [DOI] [PubMed] [Google Scholar]
- 87.Grice EA, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gallo RL. Human skin is the largest epithelial surface for interaction with microbes. J. Invest. Dermatol. 2017;137:1213–1214. doi: 10.1016/j.jid.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kobayashi T, et al. Homeostatic control of sebaceous glands by innate lymphoid cells regulates commensal bacteria equilibrium. Cell. 2019;176:982–997. doi: 10.1016/j.cell.2018.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schwartz C, et al. Spontaneous atopic dermatitis in mice with a defective skin barrier is independent of ILC2 and mediated by IL-1beta. Allergy. 2019;74:1920–1933. doi: 10.1111/all.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koues OI, et al. Distinct gene regulatory pathways for human innate versus adaptive lymphoid cells. Cell. 2016;165:1134–1146. doi: 10.1016/j.cell.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsoi LC, et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat. Genet. 2012;44:1341–1348. doi: 10.1038/ng.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ebihara T, et al. Runx3?specifies lineage commitment of innate lymphoid cells. Nat. Immunol. 2015;16:1124–1133. doi: 10.1038/ni.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murthy A, et al. Notch activation by the metalloproteinase ADAM17 regulates myeloproliferation and atopic barrier immunity by suppressing epithelial cytokine synthesis. Immunity. 2012;36:105–119. doi: 10.1016/j.immuni.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 95.Demehri S, et al. Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol. 2008;6:e123. doi: 10.1371/journal.pbio.0060123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Siracusa MC, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cherrier M, et al. Notch, Id2, and RORgammat sequentially orchestrate the fetal development of lymphoid tissue inducer cells. J. Exp. Med. 2012;209:729–740. doi: 10.1084/jem.20111594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang K, et al. Cutting edge: Notch signaling promotes the plasticity of group-2 innate lymphoid cells. J. Immunol. 2017;198:1798–1803. doi: 10.4049/jimmunol.1601421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.D’Cruz DP, et al. Systemic lupus erythematosus. Lancet. 2007;369:587–596. doi: 10.1016/S0140-6736(07)60279-7. [DOI] [PubMed] [Google Scholar]
- 100.Dorner T, Furie R. Novel paradigms in systemic lupus erythematosus. Lancet. 2019;393:2344–2358. doi: 10.1016/S0140-6736(19)30546-X. [DOI] [PubMed] [Google Scholar]
- 101.Chiche L, et al. Modular transcriptional repertoire analyses of adults with systemic lupus erythematosus reveal distinct type I and type II interferon signatures. Arthritis Rheumatol. 2014;66:1583–1595. doi: 10.1002/art.38628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu M, et al. Type I interferons promote the survival and proinflammatory properties of transitional B cells in systemic lupus erythematosus patients. Cell. Mol. Immunol. 2019;16:367–379. doi: 10.1038/s41423-018-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chang NH, et al. Interferon-alpha induces altered transitional B cell signaling and function in Systemic Lupus Erythematosus. J. Autoimmun. 2015;58:100–110. doi: 10.1016/j.jaut.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 104.Duster M, et al. T cell-derived IFN-gamma downregulates protective group 2 innate lymphoid cells in murine lupus erythematosus. Eur. J. Immunol. 2018;48:1364–1375. doi: 10.1002/eji.201747303. [DOI] [PubMed] [Google Scholar]
- 105.Guo C, et al. Innate lymphoid cell disturbance with increase in ILC1 in systemic lupus erythematosus. Clin. Immunol. 2019;202:49–58. doi: 10.1016/j.clim.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blokland S, et al. Increased expression of Fas on group 2 and 3 innate lymphoid cells is associated with an interferon signature in systemic lupus erythematosus and Sjogren’s syndrome. Rheumatol. (Oxf.) 2019;58:1740–1745. doi: 10.1093/rheumatology/kez116. [DOI] [PubMed] [Google Scholar]
- 107.Schepis D, et al. Increased proportion of CD56bright natural killer cells in active and inactive systemic lupus erythematosus. Immunology. 2009;126:140–146. doi: 10.1111/j.1365-2567.2008.02887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Waldmann TA. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 109.Arazi A, et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 2019;20:902–914. doi: 10.1038/s41590-019-0398-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lowes MA, et al. Immunology of psoriasis. Annu. Rev. Immunol. 2014;32:227–255. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nair RP, et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am. J. Hum. Genet. 2006;78:827–851. doi: 10.1086/503821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tervaert WC, Esseveld H. A study of the incidence of haemolytic streptococci in the throat in patients with psoriasis vulgaris, with reference to their role in the pathogenesis of this disease. Dermatologica. 1970;140:282–290. doi: 10.1159/000252565. [DOI] [PubMed] [Google Scholar]
- 113.Thorarensen SM, et al. Physical trauma recorded in primary care is associated with the onset of psoriatic arthritis among patients with psoriasis. Ann. Rheum. Dis. 2017;76:521–525. doi: 10.1136/annrheumdis-2016-209334. [DOI] [PubMed] [Google Scholar]
- 114.Brauchli YB, et al. Association between beta-blockers, other antihypertensive drugs and psoriasis: Population-based case-control study. Br. J. Dermatol. 2008;158:1299–1307. doi: 10.1111/j.1365-2133.2008.08563.x. [DOI] [PubMed] [Google Scholar]
- 115.Becher B, Pantelyushin S. Hiding under the skin: interleukin-17-producing gammadelta T cells go under the skin? Nat. Med. 2012;18:1748–1750. doi: 10.1038/nm.3016. [DOI] [PubMed] [Google Scholar]
- 116.Pantelyushin S, et al. Rorgammat+ innate lymphocytes and gammadelta T cells initiate psoriasiform plaque formation in mice. J. Clin. Invest. 2012;122:2252–2256. doi: 10.1172/JCI61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 118.Villanova F, et al. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J. Invest. Dermatol. 2014;134:984–991. doi: 10.1038/jid.2013.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bernink JH, et al. Interleukin-12 and -23 control plasticity of CD127(+) group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity. 2015;43:146–160. doi: 10.1016/j.immuni.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 120.Huang Y, et al. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat. Immunol. 2015;16:161–169. doi: 10.1038/ni.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bruggen MC, et al. In situ mapping of innate lymphoid cells in human skin: Evidence for remarkable differences between normal and inflamed skin. J. Invest. Dermatol. 2016;136:2396–2405. doi: 10.1016/j.jid.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 122.Dyring-Andersen B, et al. Increased number and frequency of group 3 innate lymphoid cells in nonlesional psoriatic skin. Br. J. Dermatol. 2014;170:609–616. doi: 10.1111/bjd.12658. [DOI] [PubMed] [Google Scholar]
- 123.Paternoster L, et al. Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat. Genet. 2015;47:1449–1456. doi: 10.1038/ng.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Palmer CN, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 125.Rodriguez E, et al. Meta-analysis of filaggrin polymorphisms in eczema and asthma: Robust risk factors in atopic disease. J. Allergy Clin. Immunol. 2009;123:1361–1370. doi: 10.1016/j.jaci.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 126.Kim BS, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 2013;5:116r–170r. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saunders SP, et al. Spontaneous atopic dermatitis is mediated by innate immunity, with the secondary lung inflammation of the atopic march requiring adaptive immunity. J. Allergy Clin. Immunol. 2016;137:482–491. doi: 10.1016/j.jaci.2015.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen LY, et al. Protein palmitoylation by ZDHHC13 protects skin against microbial-driven dermatitis. J. Invest. Dermatol. 2017;137:894–904. doi: 10.1016/j.jid.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 129.Li Y, et al. Kinetics of the accumulation of group 2 innate lymphoid cells in IL-33-induced and IL-25-induced murine models of asthma: a potential role for the chemokine CXCL16. Cell. Mol. Immunol. 2019;16:75–86. doi: 10.1038/s41423-018-0182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barlow JL, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J. Allergy Clin. Immunol. 2013;132:933–941. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 131.Salimi M, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xue L, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J. Allergy Clin. Immunol. 2014;133:1184–1194. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim BS, et al. Basophils promote innate lymphoid cell responses in inflamed skin. J. Immunol. 2014;193:3717–3725. doi: 10.4049/jimmunol.1401307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mashiko S, et al. Increased frequencies of basophils, type 2 innate lymphoid cells and Th2 cells in skin of patients with atopic dermatitis but not psoriasis. J. Dermatol. Sci. 2017;88:167–174. doi: 10.1016/j.jdermsci.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 135.Jiao D, et al. NOD2 and TLR2 ligands trigger the activation of basophils and eosinophils by interacting with dermal fibroblasts in atopic dermatitis-like skin inflammation. Cell. Mol. Immunol. 2016;13:535–550. doi: 10.1038/cmi.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Irani AM, et al. Immunohistochemical detection of human basophils in late-phase skin reactions. J. Allergy Clin. Immunol. 1998;101:354–362. doi: 10.1016/S0091-6749(98)70248-9. [DOI] [PubMed] [Google Scholar]
- 137.Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390:1685–1699. doi: 10.1016/S0140-6736(17)30933-9. [DOI] [PubMed] [Google Scholar]
- 138.Foocharoen C, et al. Clinical characteristics of scleroderma overlap syndromes: Comparisons with pure scleroderma. Int. J. Rheum. Dis. 2016;19:913–923. doi: 10.1111/1756-185X.12884. [DOI] [PubMed] [Google Scholar]
- 139.Rezaei R, et al. Genetic implications in the pathogenesis of systemic sclerosis. Int. J. Rheum. Dis. 2018;21:1478–1486. doi: 10.1111/1756-185X.13344. [DOI] [PubMed] [Google Scholar]
- 140.De Martinis M, et al. An overview of environmental risk factors in systemic sclerosis. Expert Rev. Clin. Immunol. 2016;12:465–478. doi: 10.1586/1744666X.2016.1125782. [DOI] [PubMed] [Google Scholar]
- 141.Airo’ P, et al. Malignancies in Italian patients with systemic sclerosis positive for anti-RNA polymerase III antibodies. J. Rheumatol. 2011;38:1329–1334. doi: 10.3899/jrheum.101144. [DOI] [PubMed] [Google Scholar]
- 142.Joseph CG, et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science. 2014;343:152–157. doi: 10.1126/science.1246886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Roan F, et al. CD4+ group 1 innate lymphoid cells (ILC) form a functionally distinct ILC subset that is increased in systemic sclerosis. J. Immunol. 2016;196:2051–2062. doi: 10.4049/jimmunol.1501491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Aliprantis AO, et al. Transcription factor T-bet regulates skin sclerosis through its function in innate immunity and via IL-13. Proc. Natl Acad. Sci. USA. 2007;104:2827–2830. doi: 10.1073/pnas.0700021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wohlfahrt T, et al. Type 2 innate lymphoid cell counts are increased in patients with systemic sclerosis and correlate with the extent of fibrosis. Ann. Rheum. Dis. 2016;75:623–626. doi: 10.1136/annrheumdis-2015-207388. [DOI] [PubMed] [Google Scholar]
- 146.Cohen TJ, Damoiseaux J. Antineutrophil cytoplasmic autoantibodies: How are they detected and what is their use for diagnosis, classification and follow-up? Clin. Rev. Allergy Immunol. 2012;43:211–219. doi: 10.1007/s12016-012-8320-4. [DOI] [PubMed] [Google Scholar]
- 147.Lyons PA, et al. Genetically distinct subsets within ANCA-associated vasculitis. N. Engl. J. Med. 2012;367:214–223. doi: 10.1056/NEJMoa1108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sunderkotter CH, et al. Nomenclature of cutaneous vasculitis: dermatologic addendum to the 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheumatol. 2018;70:171–184. doi: 10.1002/art.40375. [DOI] [PubMed] [Google Scholar]
- 149.Braudeau C, et al. Persistent deficiency of circulating mucosal-associated invariant T (MAIT) cells in ANCA-associated vasculitis. J. Autoimmun. 2016;70:73–79. doi: 10.1016/j.jaut.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 150.Mowad CM. Patch testing: pitfalls and performance. Curr. Opin. Allergy Clin. Immunol. 2006;6:340–344. doi: 10.1097/01.all.0000244794.03239.8e. [DOI] [PubMed] [Google Scholar]
- 151.Kaplan DH, et al. Early immune events in the induction of allergic contact dermatitis. Nat. Rev. Immunol. 2012;12:114–124. doi: 10.1038/nri3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rafei-Shamsabadi DA, et al. Lack of type 2 innate lymphoid cells promotes a type I-Driven enhanced immune response in contact hypersensitivity. J. Invest. Dermatol. 2018;138:1962–1972. doi: 10.1016/j.jid.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Keren A, et al. Innate lymphoid cells 3 induce psoriasis in xenotransplanted healthy human skin. J. Allergy Clin. Immunol. 2018;142:305–308. doi: 10.1016/j.jaci.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 154.Van Belle AB, et al. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J. Immunol. 2012;188:462–469. doi: 10.4049/jimmunol.1102224. [DOI] [PubMed] [Google Scholar]
- 155.Imura C, et al. A novel RORgammat inhibitor is a potential therapeutic agent for the topical treatment of psoriasis with low risk of thymic aberrations. J. Dermatol. Sci. 2019;93:176–185. doi: 10.1016/j.jdermsci.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 156.Rompoti, N. et al. Real world data from a single Greek center on the use of secukinumab in plaque psoriasis: effectiveness, safety, drug survival, and identification of patients that sustain optimal response. J. Eur. Acad. Dermatol. Venereol. (2020). [DOI] [PubMed]
- 157.Merola, J. F. et al. Ixekizumab improves secondary lesional signs, pain and sexual health in patients with moderate-to-severe genital psoriasis. J. Eur. Acad. Dermatol. Venereol. 10.1111/jdv.16181 (2020). [DOI] [PMC free article] [PubMed]
- 158.Bilal J, et al. A systematic review and meta-analysis of the efficacy and safety of the interleukin (IL)-12/23 and IL-17 inhibitors ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab and tildrakizumab for the treatment of moderate to severe plaque psoriasis. J. Dermatolog. Treat. 2018;29:569–578. doi: 10.1080/09546634.2017.1422591. [DOI] [PubMed] [Google Scholar]
- 159.Dai J, et al. Topical ROR inverse agonists suppress inflammation in mouse models of atopic dermatitis and acute irritant dermatitis. J. Invest. Dermatol. 2017;137:2523–2531. doi: 10.1016/j.jid.2017.07.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Imai Y, et al. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc. Natl Acad. Sci. USA. 2013;110:13921–13926. doi: 10.1073/pnas.1307321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Oldhoff JM, et al. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy. 2005;60:693–696. doi: 10.1111/j.1398-9995.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- 162.Pavord ID, et al. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 163.Ortega HG, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 164.Mora-Velandia LM, et al. A human lin(-) CD123(+) CD127(low) population endowed with ILC features and migratory capabilities contributes to immunopathological hallmarks of psoriasis. Front Immunol. 2017;8:176. doi: 10.3389/fimmu.2017.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]