Abstract
Natural killer (NK) cells participate in early immune defenses against pathogens and tumors and play a major role as immune effector and regulatory cells. The NK cell-mediated elimination of an infected or cancerous cell is a highly regulated process that requires the formation of a cell contact, the establishment of an immunological synapse and the polarization and release of lytic granules. Additionally, the detachment of NK cells from target cells is important for NK cells to bind and kill other cells in a process called serial killing. However, very little is known about this detachment process. Here, we show that NK detachment is directly connected to the successful killing of a target cell. The inhibition of killing due to reduced NK cell cytotoxicity or increased target cell resistance results in defective detachment and prolonged contact times. This effect leads to sustained Ca2+ flux in NK cells and the hypersecretion of proinflammatory cytokines. Linking defective cytotoxicity with enhanced cytokine secretion via reduced detachment may explain inflammatory pathologies in several diseases.
Keywords: Cellular cytotoxicity, immunological synapse, adhesion
Subject terms: Cell death and immune response, Innate lymphoid cells
Introduction
Natural killer (NK) cells are innate lymphoid cells that play an important role in early and effective immune responses against pathogen-infected, transformed or stressed cells.1,2 NK cell effector functions include cellular cytotoxicity, but NK cells can also function as regulatory cells via the secretion of cytokines. Naïve NK cells can be activated by a variety of proinflammatory cytokines, such as IL-2, IL-15 or IL-21, which stimulate NK cell survival and proliferation and enhance NK cell cytotoxicity via the production of granzymes and perforin.3–5 One of the first events in NK cell activation is their contact with other cells and the establishment of an immunological synapse (IS).6 This process involves the engagement of adhesion molecules such as LFA-1,7,8 leading to spatial proximity between NK and target cells and allowing NK cell receptors to bind their ligands on the target cell. Depending on which receptors are engaged, the NK cell can be either activated or inhibited.9 Upon activation, a tight IS is formed, and the NK cell releases its cytotoxic granules containing perforin and granzymes into the sealed cleft between the cells.10 With the help of perforin, granzymes can enter the target cell and induce apoptosis by activating caspases and cleaving other substrates.11 One way by which NK cells prevent perforating their own cell membrane during this process is by translocating CD107a from the cytotoxic granules to the outer NK cell membrane, which inhibits the binding of perforin to the NK cell.12 Additionally, NK cells secrete proinflammatory cytokines such TNF-α and IFN-γ during this process, which are important for modulating the immune response.
To effectively clear an infection or cancerous cells, a fraction of particularly active NK cells can kill multiple target cells in a process called serial killing.13–16 These serial killer cells, which are characterized by the rapid formation of new cytotoxic granules, are able to effectively recycle secreted cytotoxic proteins.14,17,18 Interestingly, NK cells utilize granule-mediated cytotoxicity for their first killing events and then switch to the use of death receptor-mediated cytotoxicity for their final kill.16 As NK cells can form only one IS at a time, they first have to dissolve the IS and detach from the previous target cell to carry out serial killing. However, very little is known about this detachment process.
Previously, we used a flow cytometry-based assay to investigate the detachment of NK cells.19 Our data showed that activating signaling events are not only important for the initiation of the IS but also necessary for maintaining contact. Therefore, interfering with signaling molecules such as Src or the Syk kinases PLCγ or PI3K leads to the disruption of an established IS. Similarly, NK cells need ATP and functional actin dynamics to maintain the IS.19 While these data supported the idea that the maintenance of the IS is an active process, they did not address whether detachment is also regulated or a purely stochastic event. A prior publication showed that defects in CTL and NK cell cytotoxicity resulted in a prolonged synapse time and cytokine hypersecretion,20 suggesting that detachment is a regulated process.
Here, we investigate whether the cytotoxic activity of NK cells has an impact on their detachment. We found that cytokine-stimulated NK cells detached earlier than resting NK cells, which correlated with the cytotoxic activity of these cells. In contrast, NK cells detached later if their cytotoxicity was reduced or if the target cell was more resistant to NK cell killing, suggesting that detachment is regulated by the successful killing of the target cell. The detachment process is triggered by a mixture of apoptosis-induced loss of surface proteins on the target cell and a reduction in activating signaling in NK cells. Interestingly, delayed detachment due to reduced NK cell cytotoxicity or increased target cell resistance resulted in more sustained calcium signaling in the NK cells and the enhanced secretion of proinflammatory cytokines.
Results
Cytokine preactivation influences NK cell detachment from target cells
To test whether the cytotoxic activity of NK cells influences their detachment, we made use of the fact that NK cell effector functions can be boosted by preactivating NK cells with cytokines. To analyze the detachment of NK cells from their target cells, we used a flow cytometry-based detachment assay19 (Fig. S1). We isolated NK cells from healthy donors and tested them fresh, after 16 h of incubation with IL-2 or after 3 weeks of cultivation with NK cell growth medium containing IL-2 and IL-15 (see Materials and methods). We confirmed their cytotoxic activity against K562 cells in a standard chromium release assay (Fig. 1). As expected, freshly isolated NK cells showed significant cellular cytotoxicity. This activity was increased by a short incubation time with IL-2 and maximized by long-term cultivation in the presence of IL-2 and IL-15 (Fig. 1a). The half-life of NK cell detachment was not significantly reduced by the 16 h incubation with IL-2 (120.9 ± 47.27 min vs. 109.5 ± 31.31 min for IL-2-incubated cells vs. control cells, respectively). However, the cultivated NK cells showed significantly accelerated detachment and a half-life consistent with our previous findings19 (62.60 ± 17.08 min, Fig. 1b). These results suggest that the detachment process is influenced by the cytotoxic activity of NK cells.
Fig. 1.
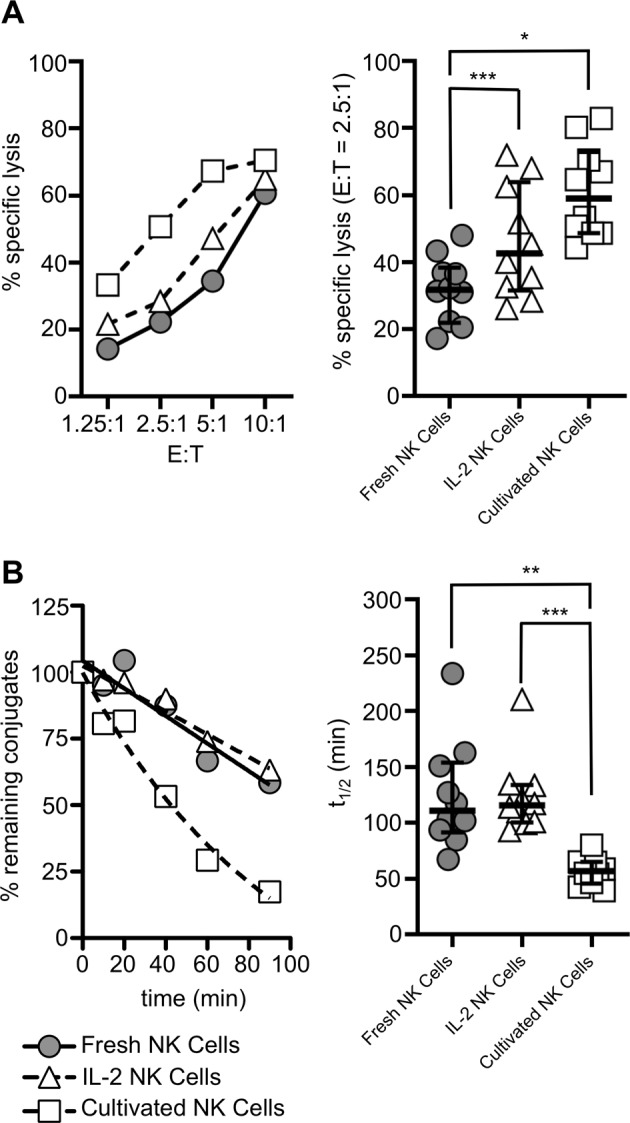
NK cell killing and detachment are influenced by NK cell preactivation. Freshly isolated NK cells were rested overnight (fresh NK cells), incubated for 16 h with 200 IU/ml IL-2 (IL-2 NK cells) or cultivated for 2–3 weeks in vericyte NK cell growth medium (cultivated NK cells). a Cytotoxicity against K562 target cells was tested in a standard chromium-51 release assay. b Detachment from K562 cells was tested in a detachment assay. The left panels show one representative experiment, and the right panels show the quantification of experimental results using NK cells from ten different donors. Values are shown as medians with interquartile ranges (*p < 0.05; **p < 0.01; ***p < 0.001)
Concanamycin A-treated NK cells have a prolonged conjugation time
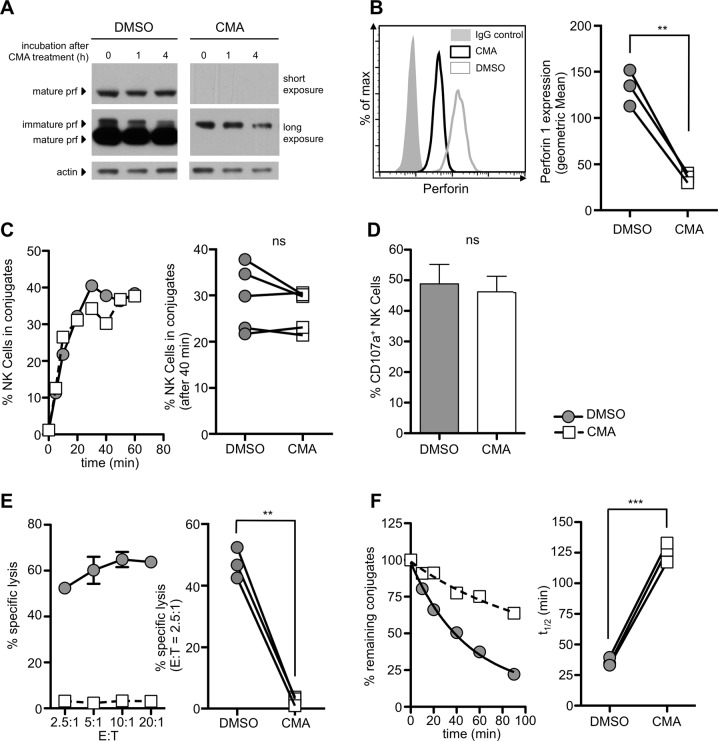
To more directly test the connection between cytotoxic activity and detachment, we wanted to block NK cell cytotoxicity. We therefore treated cultivated primary human NK cells with the vacuolar type H+-ATPase inhibitors concanamycin A (CMA) or bafilomycin A1. Treatment with these agents blocks the acidification of lytic granules and thereby inactivates cathepsin L, which is needed for the cleavage of immature perforin into its active and mature form.21–23 Indeed, after 3 h of treatment with 50 nM CMA or 50 nM bafilomycin, mature perforin could no longer be detected in the NK cells and did not reappear for at least 4 h, enabling us to pretreat the NK cells and perform functional assays without the inhibitors present (Fig. 2a, b, Fig. S2A). The absence of perforin had no effect on the formation of conjugates (Fig. 2c, Fig. S2B) or degranulation as a marker for the release of cytotoxic granules (Fig. 2d). However, without perforin, the lysis of K562 target cells in a 4 h 51Cr release assay was completely abrogated (Fig. 2e, Fig. S2C). Therefore, CMA- or bafilomycin-treated NK cells seemed fully functional in their ability to attach to target cells and degranulation, but due to the absence of perforin, the NK cells were unable to effectively kill the target cell. Interestingly, this effect inhibited the successful detachment of the NK cells and significantly increased the half-life of the conjugates (Fig. 2f, Fig. S2D). These data suggest that target cell death has a direct influence on the detachment of NK cells as a reduction in NK cell cytotoxicity resulted in decelerated detachment.
Fig. 2.
Treatment of NK cells with concanamycin A (CMA) depletes mature perforin and inhibits NK cell detachment from target cells. a NK cells were treated with 50 nM CMA or solvent control for 3 h. Cells were then washed and incubated for up to 4 h without inhibitor. Cell lysates were analyzed for perforin expression by western blotting, and actin levels were determined as a loading control. The original blots are shown in Fig. S6. b Intracellular staining for perforin using CMA-treated and control NK cells and quantification of three independent experiments (right panel). c–f NK cells were pretreated with 50 nM CMA or DMSO for 3 h. The cells were washed before use, and the assays were performed without inhibitor. c The results of one representative conjugate formation assay and quantification of five independent experiments (right panel). d CD107a expression on NK cells after 4 h of coincubation with K562 cells (n = 3). e Standard 4 h chromium release assay against K562 target cells and quantification of three independent experiments (right panel). f Detachment assay against K562 cells. The left panel shows one representative experiment, and the right panel shows the quantification of the half-lives measured in three independent experiments (**p < 0.01, ***p < 0.001, ns = not significant)
Deletion of perforin inhibits NK cell detachment
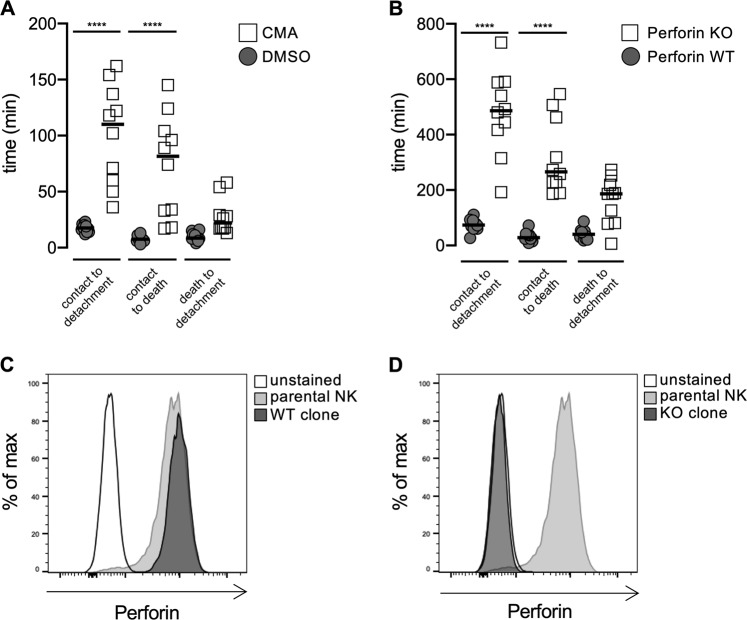
To confirm these FACS-based results, we incubated CMA-treated or control NK cells with HeLa target cells and analyzed the killing events by time-lapse video microscopy as previously described.16 For each killing event, we determined the time of contact between the NK and target cell, the time of target cell death and the time of detachment. Depletion of perforin by CMA treatment significantly increased the time from contact to detachment, confirming our FACS-based results (Fig. 3a). However, this effect was mainly due to an increase in the time from contact to target cell death. We had previously shown that perforin depletion by CMA results in slower death receptor-mediated killing of HeLa targets,16 explaining this increase in killing time. Interestingly, the time from target cell death to detachment was not significantly different between CMA-treated and control NK cells, confirming that detachment is regulated by target cell death.
Fig. 3.
Delayed killing and detachment in the absence of perforin. a Primary human NK cells were incubated with CMA or DMSO as a control for 3 h. Cells were washed, incubated with HeLa target cells and analyzed by video microscopy. The contact time, the time of target cell death and the time of detachment were analyzed for the first killing event for each NK cell. b–d Perforin was knocked out in primary human NK cells using CRISPR/Cas9. NK cell clones were generated and tested for perforin by intracellular FACS staining. Perforin WT (c) and perforin KO (d) clones were selected and tested as described in (a) (****p < 0.0001, ANNOVA)
We recently established CRISPR/Cas9-mediated deletion of perforin in human NK cell clones.16 We repeated our experiments using perforin KO or WT NK cell clones (Fig. 3b−d). The absence of perforin significantly increased the time from contact to detachment, which was mainly due to slower target cell killing.
NK cell detachment is decelerated in resistant target cells
To eliminate the possibility that other side effects of NK cell manipulation were responsible for the decelerated detachment instead of defective NK cytotoxicity, we repeated the experiments with K562 target cells that were more resistant to NK cell-mediated killing. To that end, we overexpressed CD107a on the surface of K562 cells to inhibit perforin12 or expressed the granzyme B inhibitor serpinB924 in K562 cells (Fig. S3). As expected, the transfected K562 target cells showed some reduction in NK cell-mediated lysis (Fig. 4a). Notably, overexpression of the surface protein CD107a did not significantly influence the formation of conjugates (Fig. 4b). Interestingly, detachment assays showed a clearly increased half-life when K562 cells overexpressed either CD107a (85.03 ± 22.43 min) or serpinB9 (115.8 ± 22.59 min) compared to the half-life of mock-transfected cells (60.07 ± 18.44 min) (Fig. 4c). As the NK cells in these experiments were not manipulated, these results exclude the possibility that side effects of CMA/bafilomycin treatment were responsible for the decelerated detachment previously observed. Therefore, NK cell detachment is decelerated if the NK cells are not able to efficiently kill the target cells because of either reduced NK cell cytotoxicity or increased target cell resistance.
Fig. 4.

Overexpression of serpinB9 (Sb9) and surface CD107a reduces NK cell-mediated killing and decelerates their detachment from target cells. K562 target cells were transfected with Sb9, surface CD107a, or empty vector. a A 4 h 51Cr release assay in NK cells with the indicated K562 target cells. b Conjugate formation between NK cells with the indicated K562 target cells. The left panels show one representative experiment, and the quantification of three independent experiments is shown in the right panels. c The detachment from target cells was analyzed in a detachment assay. The left panel shows the results of one representative experiment, and the right panel shows the quantification of the half-life (n = 4–11). Values are shown as medians with interquartile ranges (*p < 0.05, ***p < 0.001, ns = not significant)
Target cell death accelerates NK cell detachment
Our results suggest that target cell apoptosis is a necessary trigger to induce detachment. To directly test this idea, we induced target cell death via hydrogen peroxide (H2O2). K562 cells were treated with H2O2 for 15 min and washed thoroughly before their use in a detachment assay to avoid any effects of H2O2 on the NK cells. H2O2 treatment did not immediately induce cell death, whereas in the 2 h following treatment, approximately 35% of the K562 cells died (Fig. 5a). In the detachment assay, conjugates are first formed by a 30-min coincubation of NK cells and target cells (see Materials and methods). Importantly, during this initial time period, H2O2-treated K562 cells did not show defective conjugate formation (Fig. 5b). However, the increase in cell death of the H2O2-treated K562 cells during the next 90 min of the detachment assay clearly accelerated detachment and significantly decreased the half-life of the NK cell:target cell conjugates (Fig. 5c). This finding demonstrates that target cell death, even that induced by other means, induces NK cell detachment.
Fig. 5.
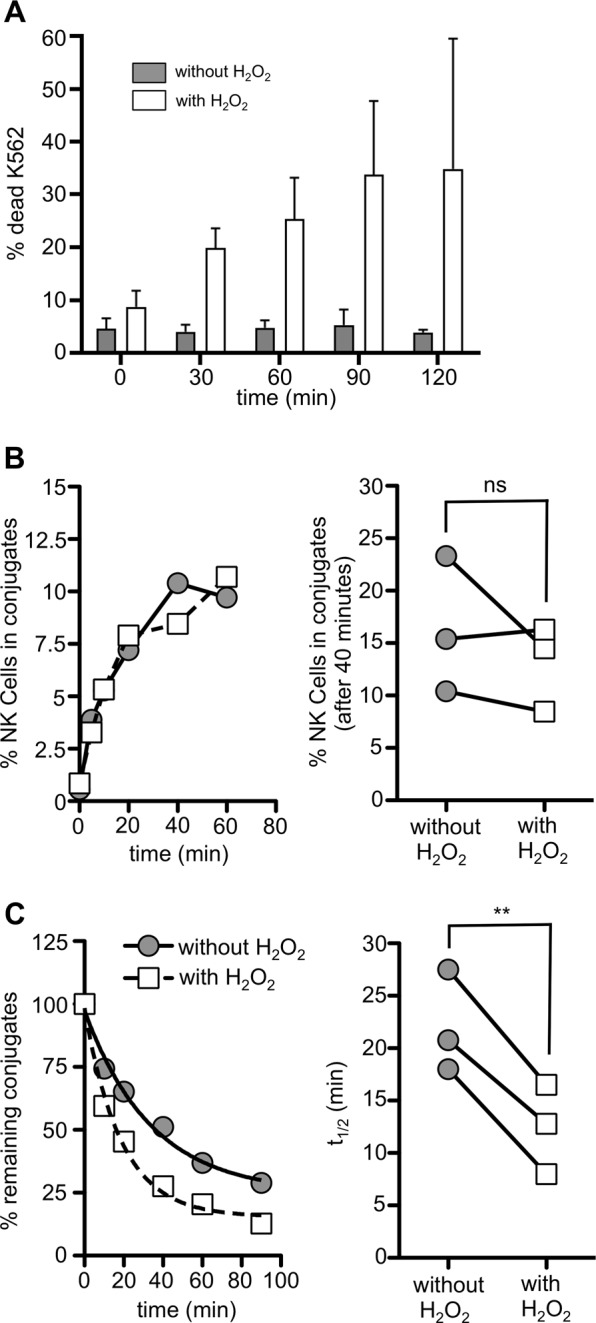
NK cell detachment is accelerated upon target cell death. a K562 cells were treated with 25 mM H2O2 for 15 min and then washed thoroughly. Samples were taken every 30 min and stained with the live/dead dye Zombie AquaTM. The percentages of dead H2O2-treated cells (white bars) and untreated cells (gray bars) (n = 2) are shown (b, c). The formation of conjugates was assessed in a conjugate assay, and the detachment of NK cells from K562 target cells was analyzed in a detachment assay. The left panels show the results of one representative experiment, and the right panels show the quantification of three independent experiments with NK cells from three different donors (**p < 0.01, ns = not significant)
NK cell-mediated loss of target cell adhesion molecules and ligands that activate NK cell receptors
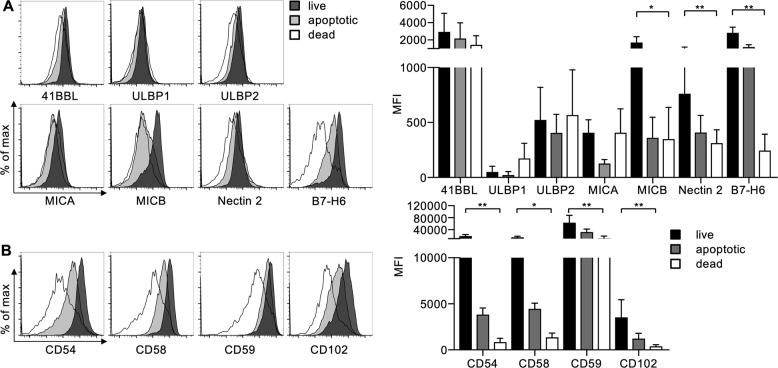
As the IS is actively maintained by the NK cell19 and target cell death can induce detachment, we wanted to investigate how target cell death is sensed by the NK cell. Therefore, we investigated alterations on the target cell membrane that occur during the process of NK cell-mediated killing. We coincubated cultivated NK cells with K562 cells and compared the expression of several adhesion proteins and ligands that activate NK cell receptors on killed, early apoptotic and surviving target cells (Fig. S4). The expression of several of the ligands that activate NK cell receptors remained unchanged on dying target cells. Interestingly, the expression of the NKp30 ligand B7H6, the DNAM-1 ligand Nectin 2, and the NKG2D ligand MICB were significantly decreased on dying K562 cells (Fig. 6a). Additionally, we found that the expression of the adhesion molecules CD54, CD58, CD59, and CD102 was strongly reduced in dead cells (Fig. 6b). We had previously shown that activating receptors on NK cells are downregulated upon their engagement25 and thereby reduced on detached NK cells.19 This finding suggests that a reduction in NK cell activation and the loss of adhesion are responsible for NK cell detachment.
Fig. 6.
K562 cells lose activating NK cell ligands and adhesion molecules after NK cell-mediated killing. NK cells and K562 target cells were coincubated for 2 h and stained with the live/dead dye Zombie Aqua and Annexin V to distinguish live, early apoptotic and dead target cells (see Fig. S4). Cells were stained with antibodies against the indicated a ligands for activating NK cell receptors and b adhesion proteins. Expression levels of the indicated proteins were analyzed by subtracting the median fluorescence of the respective control stained sample from that of the specifically stained sample. Left panels show histograms from one representative experiment. Right panels show the quantification of independent experiments with NK cells from donors (n = 4, except CD58 and CD59 n = 3 and 41BBL n = 2). Values are shown as the mean ± SD (*p < 0.05, **p < 0.01 Friedman test with Dunn’s multiple comparison)
NK cells produce more cytokines if detachment is decelerated
NK cells not only are cytotoxic cells but also fulfill important regulatory functions via the release of cytokines. Therefore, we were interested in investigating the effect of delayed detachment on cytokine production. NK cells were treated with CMA or coincubated with CD107a-serpinB9-overexpressing K562 cells (Fig. S3) to interfere with target cell killing and induce delayed detachment. Under these conditions, we observed significantly increased levels of TNF-α and IFN-γ compared to those in DMSO control-treated NK cells that were coincubated with wild-type K562 cells (Fig. 7). These data show that NK cells that fail to efficiently kill target cells can still significantly influence immune responses by enhancing the secretion of proinflammatory cytokines.
Fig. 7.
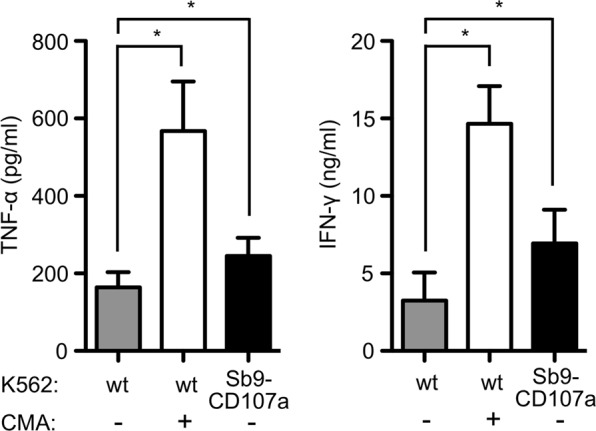
Defective killing results in enhanced cytokine secretion. NK cells (pretreated with CMA or solvent control) were coincubated with the indicated K562 target cells for 16 h, and the production of TNF-α (left panel) or IFN-γ (right panel) was assessed by ELISA. Values are shown as the mean ± SD (*p < 0.05)
Intracellular calcium level remains high if NK cell detachment is decelerated
If NK cell detachment is decelerated because of reduced cytotoxicity or target cell resistance, NK cells continue to receive signals via activating receptors. Calcium is an important second messenger during NK cell activation that activates transcription factors such as NFAT, CREB and NFκB, thereby enhancing the expression of cytokines.26 Therefore, we measured cytosolic calcium levels and compared those in free NK cells and NK cells in conjugates during a detachment assay (see Materials and methods and Fig. S5). The calcium levels in NK cells in conjugates were clearly higher than those in free NK cells (Fig. S5A), confirming ongoing signaling in NK cells during target cell contact. During the detachment assay, the calcium level in conjugated NK cells did not remain constant but decreased over time. Interestingly, calcium levels in NK cells treated with CMA remained higher than those in DMSO-treated NK cells, and the rate of this decrease was significantly reduced (Fig. 8). Notably, CMA did not affect calcium signaling when NK cells were stimulated by activating receptor crosslinking or ionomycin (Fig. S5B, C). These results suggest that delayed detachment due to reduced NK cell cytotoxicity increased sustained calcium signaling in the NK cells, which may be the cause of enhanced cytokine secretion.
Fig. 8.
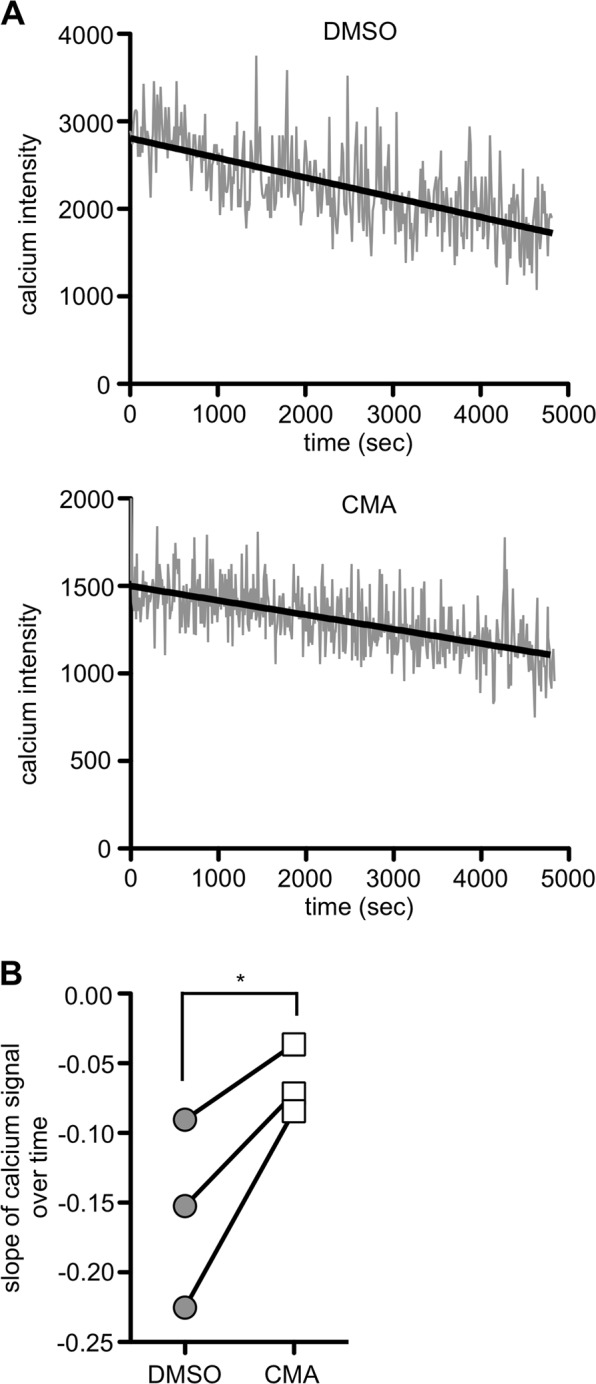
Defective killing is associated with sustained Ca2+ flux. NK cells were pretreated for 2 h with 50 nM CMA or DMSO as a control and loaded with the calcium binding dye Fluo-8. Cells were allowed to form conjugates with K562 target cells for 30 min. Samples were subsequently diluted and agitated to prevent the formation of new conjugates. a The Ca2+ flux in NK cells in conjugates was analyzed over 90 min by flow cytometry. The Ca2+ level was plotted against time, and a regression line was calculated. b Slope of the regression line for the calcium signal over time in DMSO- and CMA-treated NK cells for three independent experiments using NK cells from different donors (*p < 0.05)
Discussion
The efficiency of NK cell cytotoxicity depends on several factors. Preactivation with cytokines and chemokines modulates NK cell reactivity, target cell contact stimulates the multistep process of IS formation, and intracellular signaling events stimulate the degranulation of cytotoxic granules and the production of cytokines. However, the last step in NK cell killing, termination of the IS and detachment from the target cell, is poorly understood. We had previously shown that maintenance of the IS is an active and regulated process.19 Another publication showed that the synapse time in CTLs and NK cells was prolonged upon defective cytotoxicity.20 Here, we confirm these findings and show that NK cell detachment is highly regulated and dependent on successful cytotoxicity.
Inhibition of the V-H+-ATPase with CMA or bafilomycin A1 effectively interfered with NK cell cytotoxicity and thereby inhibited detachment. Preventing the acidification of lytic granules inhibits the last step of perforin maturation,22,23 and we detected only uncleaved, immature perforin after inhibition of the V-H+-ATPase for 3 h. However, perforin turnover is slow, and preventing the de novo production of mature perforin would not result in the complete disappearance of mature perforin in such a short time. The low pH in the lytic granules is necessary not only for the activity of cathepsin L, which cleaves perforin and generates the mature form, but also to keep mature perforin inactive and prevent damage to the cytotoxic cell itself.27,28 Therefore, it is likely that safeguard measures exist to actively degrade mature perforin to prevent damage to the cell if the acidic environment of the lytic granules is lost.
Our data show a clear correlation between successful cytotoxicity and detachment. Failure to kill, by manipulating either NK cells or target cells, interfered with detachment, while preincubation with H2O2 to promote target cell apoptosis accelerated detachment. Therefore, detachment is dependent on the successful killing of the target cell, as was already suggested by Jenkins et al.20 How does the NK cell “know” that its degranulation was effective and that the target cell is dead? After they are triggered by the appropriate ligand, several NK cell receptors are internalized,25 and we previously showed that freshly detached NK cells express a significantly lower level of the activating receptors 2B4, DNAM-1 and NKG2D.19 This apoptosis-independent reduction in surface receptors will decrease activating signaling over time, which may be why we detected a decline in calcium flux during ongoing attachment. In addition, we observed a reduction in the activating ligands MICB, Nectin 2, and B7-H6 on target cells after apoptosis, which may indicate an apoptosis-dependent mechanism to downregulate activating signaling after successful killing. MICB can be shed by several enzymes,29–32 and B7-H6 is shed by the metalloproteases ADAM-17 and ADAM-10,33 which are activated during the process of apoptosis. Additionally, a recent study found that the Fc receptor CD16 is shed during NK cell activation,34 which is necessary for successful NK cell detachment and serial killing during antibody-dependent cellular cytotoxicity. In addition to a reduction in activating receptors or ligands, we demonstrated the loss of adhesion molecules on dying target cells. CD54 and CD102 are ligands for LFA-1, which is essential for the formation of the IS. The activity of LFA-1 is regulated by inside-out signaling,35 in which signals from activating NK cell receptors stimulate a conformational change in LFA-18 that results in its binding to CD54 on the target cell with high affinity. Therefore, the loss of an activating signaling due to internalization or the shedding of receptors or ligands will reduce inside-out signaling. In addition, the binding partners for LFA-1 and other adhesion receptors are reduced on dying target cells. This will greatly reduce the adhesiveness of the NK cell, ultimately resulting in its detachment. This process would explain why detachment is dependent on the successful killing of the target cell. Such a mechanism would ensure that the NK cell stays connected to the target cell until the killing mechanism is completed. Notably, this system only works in lytic cell−cell contacts. How nonlytic synapses are dissolved requires further investigation.
Failure to kill a target cell results in a prolonged contact time,20 which enhances Ca2+ flux in NK cells. Calcium, an important second messenger, is released from intracellular stores and activates transcription factors such as NFAT, CREB and NFκB to produce cytokines.26 Our data show that the hypersecretion of TNF-α and IFN-γ is the result of ongoing signaling during prolonged conjugation with the target cell. Calcium is also crucial for the maintenance of the IS in T cells,36 and manipulation of intracellular calcium levels with the calcium releasing drugs Ionomycin or thapsigargin resulted in the instant disruption of the IS in NK cells (data not shown). As the actin cytoskeleton is important for IS stability19 and sensitive to cytosolic calcium,37 we speculate that high calcium levels caused by ongoing signaling stabilize the IS, prevent NK cell detachment and activate cytokine transcription.
Our results are consistent with those of a previous study showing that cytotoxic T cells and NK cells from patients suffering from FHL-2 (type 2 familial hemophagocytic lymphohistiocytosis), who lack perforin due to a genetic mutation, had a clearly longer synapse time and secreted high amounts of proinflammatory cytokines.20 Therefore, failure to kill resulting in reduced detachment and cytokine hypersecretion could cause the observed hyperinflammatory state of these FHL-2 patients.20,38 Additionally, other instances of failure to kill, for example, by the antiapoptotic mechanism employed by virally infected cells or resistance mechanisms in tumor cells, would likely produce a similar outcome of delayed detachment and enhanced cytokine secretion. This effect may contribute to the proinflammatory environment during viral infection and cancer. A recent study demonstrated that NK cells show altered metabolism during obesity that results in defective NK cell cytotoxicity due to a failure in granule polarization, while conjugate formation is intact.39 Our data suggest that this effect would result in enhanced cytokine secretion. Indeed, NK cells have been linked to obesity-related inflammation and insulin resistance.40 Therefore, defective cytotoxicity and enhanced cytokine secretion due to reduced detachment may contribute to inflammatory pathologies in several diseases.
Materials and methods
Cells and cell culture
The K562 human cell line was maintained in Iscove’s modified Dulbecco’s medium (IMDM) (Thermo Fisher, USA). All media were supplemented with 10% heat-inactivated FCS (Thermo Fisher, USA) + 100 U/ml penicillin and 100 μg/ml streptomycin (P/S, Thermo Fisher, USA). K562 cells were stably transfected with serpinB9 or surface CD107a12 using the retroviral PhoenixAMPHO system and selected with 0.5 µg/ml puromycin (Merck Millipore, Germany). Transduced cells were sorted for a purity of ~97% on a BD FACSJazzTM. Primary NK cells were isolated from the blood of healthy donors as previously described.41 Briefly, peripheral blood mononuclear cells (PBMCs) were obtained via density centrifugation, and NK cells were isolated with a Dynabeads® Untouched™ Human NK Cell Kit (Thermo Fisher, Germany). Primary NK cells were cultivated in IMDM + 10% FCS + 1% P/S and supplemented with irradiated K562mbIL15-41BBL feeder cells and recombinant IL-2 (200 IU/ml, NIH Cytokine Repository, USA). At days 1 and 7, 100 ng/ml recombinant human IL-21 (Miltenyi, Germany) was added. If indicated, NK cells were incubated in vericyte NK cell growth medium (Medicyte, Germany). All cells were incubated in a humidified incubator at 37 °C and 5% CO2. CRISPR/Cas9-mediated deletion of perforin and the generation of NK cell clones were performed as previously described.16
Antibodies and reagents
The following antibodies were used in this study: anti-serpinB9 (7D8, Abcam USA); anti-B7H6 (875001); anti-ULBP1 (170818), anti-ULBP2 (165903), anti-MICA (159227), and anti-MICB (236511) from R&D Systems (USA); anti-CD107a (H4A3), anti-NTB-A (NT-7), anti-perforin (dG9), anti-Nectin-2, anti-CD102 (CBR-IC2/2), anti-41BBL (5F4), and IgG-control (MOPC21) from BioLegend (USA); anti-CD58 (MEM-63) and anti-CD59 (MEM-43) from Immunotools (Germany); anti-CD54 (H458, BD, USA); anti-perforin (Pf-344, Mabtech, Germany, Western blot); and anti-actin (Sigma Aldrich, USA, Western blot).
Other reagents used in this study were concanamycin A and ionomycin (Sigma Aldrich, USA), brefeldin A1 (BioViotica, Germany), Zombie Aqua dye and Annexin V FITC (BioLegend, USA), CellTracker Deep Red (Thermo Fisher Scientific), H2O2 (AppliChem, Germany), and DMSO (Avantor, USA).
Detachment assay
The detachment assay was performed as described19 (Fig. S1). Briefly, 1 × 106 NK cells and target cells were stained for 30 min with 0.25 µM PKH26 or PKH67 membrane dyes, respectively (Fig. 2 and Fig. S2), or 10 µM CellTracker Orange, 25 µM CellTracker Violet or 5 µM CFSE (all other experiments). Stained NK and K562 cells were coincubated for 30 min in 100 μl NK cell medium to form conjugates (Fig. S1A, D). The cells were diluted 25-fold in NK cell medium and rotated at 37 °C to allow NK cells to detach but prevent the formation of new conjugates (Fig. S1E). Samples were collected for 0–90 min, and the reaction was stopped by vortexing and the addition of 2% paraformaldehyde. Cells were directly analyzed by FACS, and conjugates were determined as double-positive events. The half-life was determined by GraphPad Prism using the formula for a one-phase exponential decay.
Conjugate assay
Differently stained NK and K562 cells were washed thoroughly and resuspended in NK cell medium (IMDM, 10% FCS, 1% P/S). A total of 5 × 104 NK cells were mixed with 1 × 105 K562 cells in 100 µl of medium, centrifuged (20 × g, 1 min), and coincubated for 0−90 min. The reaction was stopped by brief vortexing and the addition of 100 µl of 4% paraformaldehyde (PFA, Sigma Aldrich, USA). Cells were analyzed immediately using a BD FACSCalibur or BD LSRFortessa, and conjugates were determined as double-positive events. Data were analyzed using FlowJo (Tree Star) software, and statistics were calculated using GraphPad Prism software.
Chromium release assay
Cytotoxicity was determined using a standard 4 h chromium release assay as described before.41 Briefly, 5 × 105 target cells were labeled in 100 µl of medium with 100 µCi 51Cr (Hartmann Analytic) for 1 h at 37 °C and 5% CO2. A total of 5 × 104 target cells were added to each well of a 96-well U-bottom plate and mixed with NK cells at ratios from 20:1 to 1.25:1 (depending on the experiment). Supernatants were harvested after 4 h and analyzed in a Wizard2 (Perkin Elmer) gamma counter. The percent of specific lysis was calculated as follows: (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100.
SDS-PAGE and western blotting
Identical numbers of cells were lysed, and proteins were separated using 4–12% Bis-Tris NuPAGE gels (Life Technologies, USA). Proteins were transferred to a PVDF membrane (Millipore, Germany) and blocked with 5% nonfat dry milk for 1 h. The membranes were incubated with primary antibody overnight at 4 °C, washed and incubated with the appropriate secondary antibody for at least 1 h at room temperature.
CD107a assay
NK cells and K562 cells were stained as described above and coincubated in the presence of CD107a antibody at a ratio of 1:2. If necessary, NK cells were pretreated with the indicated inhibitors and washed thoroughly before coincubation. After 2 h, the cells were fixed with 2% paraformaldehyde and directly analyzed using an LSRFortessa or FACSCalibur.
Calcium detachment assay
A total of 1 × 106 NK cells were stained with 4.8 µM calcium binding dye Fluo-8 (Thermo Fisher, USA) in calcium staining medium (IMDM without phenol red, 2.5 mM probenecid (Sigma Aldrich, USA), 5 mM HCl, 10% FCS, 1% P/S) for 30 min at 37 °C and then washed twice. K562 cells were stained with intracellular fluorescence dyes as described above and coincubated with NK cells in calcium assay medium (IMDM without phenol red, 2.5 mM probenecid, 5 mM HCl, 2% FCS, 10 mM Hepes (Thermo Fisher, USA)) for 30 min. Then, the cells were diluted 1:25, aliquoted in 22 equal samples and rotated at 37 °C. All samples were measured successively, and every 4 min, the current sample was exchanged with a new sample.
ELISA
NK cells were pretreated with 50 nM concanamycin A or DMSO for 3 h and coincubated with wild-type K562 cells or CD107a-serpinB9-overexpressing K562 cells for 16 h. Supernatants were collected and analyzed using an IFN-γ or TNF ELISA Max Kit (BioLegend, USA) according to the manufacturer’s instructions and measured using a Tecan M200 Pro.
Statistical analysis
Statistical analyses were performed using GraphPad Prism v.5.0f and v.7.0e (GraphPad Software, Inc., San Diego, CA, USA). Differences between two groups were analyzed using the paired, two-tailed Student’s t test. As shown in Fig. 5, differences between three groups were compared using Friedman with Dunn’s multiple comparisons test. p values < 0.05 indicated significant differences and marked as follows: *<0.05; **<0.01; ***<0.001; ns = not significant.
Supplementary information
Acknowledgements
We thank Clarissa Liesche for the serpinB9 cDNA and all members of our lab for their help and discussions.
Author contributions
M.A., P.N., D.U., I.P. and S.S. performed the experiments; M.A., P.N. and C.W. planned the experiments and analyzed the data; and M.A. and C.W. wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Moritz Anft, Petra Netter
Supplementary information
The online version of this article (10.1038/s41423-019-0277-2) contains supplementary material.
References
- 1.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 3.London L, Perussia B, Trinchieri G. Induction of proliferation in vitro of resting human natural killer cells: IL 2 induces into cell cycle most peripheral blood NK cells, but only a minor subset of low density T cells. J. Immunol. 1986;137:3845–3854. [PubMed] [Google Scholar]
- 4.Parrish-Novak J, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis DM, et al. The human natural killer cell immune synapse. Proc. Natl. Acad. Sci. USA. 1999;96:15062–15067. doi: 10.1073/pnas.96.26.15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann SC, Cohnen A, Ludwig T, Watzl C. 2B4 engagement mediates rapid LFA-1 and actin-dependent NK cell adhesion to tumor cells as measured by single cell force spectroscopy. J. Immunol. 2011;186:2757–2764. doi: 10.4049/jimmunol.1002867. [DOI] [PubMed] [Google Scholar]
- 8.Urlaub D, Hofer K, Muller ML, Watzl C. LFA-1 activation in NK cells and their subsets: influence of receptors, maturation, and cytokine stimulation. J. Immunol. 2017;198:1944–1951. doi: 10.4049/jimmunol.1601004. [DOI] [PubMed] [Google Scholar]
- 9.Watzl C. How to trigger a killer: modulation of natural killer cell reactivity on many levels. Adv. Immunol. 2014;124:137–170. doi: 10.1016/B978-0-12-800147-9.00005-4. [DOI] [PubMed] [Google Scholar]
- 10.Mace EM, et al. Cell biological steps and checkpoints in accessing NK cell cytotoxicity. Immunol. Cell Biol. 2014;92:245–255. doi: 10.1038/icb.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prager, I. & Watzl, C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol.105, 1319–1329 (2019). [DOI] [PubMed]
- 12.Cohnen A, et al. Surface CD107a/LAMP-1 protects natural killer cells from degranulation-associated damage. Blood. 2013;122:1411–1418. doi: 10.1182/blood-2012-07-441832. [DOI] [PubMed] [Google Scholar]
- 13.Choi PJ, Mitchison TJ. Imaging burst kinetics and spatial coordination during serial killing by single natural killer cells. Proc. Natl. Acad. Sci. USA. 2013;110:6488–6493. doi: 10.1073/pnas.1221312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanherberghen B, et al. Classification of human natural killer cells based on migration behavior and cytotoxic response. Blood. 2013;121:1326–1334. doi: 10.1182/blood-2012-06-439851. [DOI] [PubMed] [Google Scholar]
- 15.Bhat R, Watzl C. Serial killing of tumor cells by human natural killer cells-enhancement by therapeutic antibodies. PLoS ONE. 2007;2:e326. doi: 10.1371/journal.pone.0000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prager, I. et al. NK cells switch from granzyme B to death receptor-mediated cytotoxicity during serial killing. J. Exp. Med.216, (2019). 10.1084/jem.20181454. [DOI] [PMC free article] [PubMed]
- 17.Li P, Katirai F, Zheng F, Gong F. Recycling and reutilization of cytotoxic molecules, a new type of energy conservation of NK cells? Med. Hypotheses. 2011;76:293–295. doi: 10.1016/j.mehy.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Liu D, et al. Rapid biogenesis and sensitization of secretory lysosomes in NK cells mediated by target-cell recognition. Proc. Natl. Acad. Sci. USA. 2005;102:123–127. doi: 10.1073/pnas.0405737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Netter P, Anft M, Watzl C. Termination of the activating NK cell immunological synapse is an active and regulated process. J. Immunol. 2017;199:2528–2535. doi: 10.4049/jimmunol.1700394. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins MR, et al. Failed CTL/NK cell killing and cytokine hypersecretion are directly linked through prolonged synapse time. J. Exp. Med. 2015;212:307–317. doi: 10.1084/jem.20140964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konjar S, et al. Human and mouse perforin are processed in part through cleavage by the lysosomal cysteine proteinase cathepsin L. Immunology. 2010;131:257–267. doi: 10.1111/j.1365-2567.2010.03299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kataoka T, et al. Acidification is essential for maintaining the structure and function of lytic granules of CTL. Effect of concanamycin A, an inhibitor of vacuolar type H(+)-ATPase, on CTL-mediated cytotoxicity. J. Immunol. 1994;153:3938–3947. [PubMed] [Google Scholar]
- 23.Kataoka T, et al. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J. Immunol. 1996;156:3678–3686. [PubMed] [Google Scholar]
- 24.Bird CH, et al. Selective regulation of apoptosis: the cytotoxic lymphocyte serpin proteinase inhibitor 9 protects against granzyme B-mediated apoptosis without perturbing the Fas cell death pathway. Mol. Cell Biol. 1998;18:6387–6398. doi: 10.1128/MCB.18.11.6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandusky MM, Messmer B, Watzl C. Regulation of 2B4 (CD244)-mediated NK cell activation by ligand-induced receptor modulation. Eur. J. Immunol. 2006;36:3268–3276. doi: 10.1002/eji.200636146. [DOI] [PubMed] [Google Scholar]
- 26.Feske S. Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 27.Voskoboinik I, et al. Calcium-dependent plasma membrane binding and cell lysis by perforin are mediated through its C2 domain: a critical role for aspartate residues 429, 435, 483, and 485 but not 491. J. Biol. Chem. 2005;280:8426–8434. doi: 10.1074/jbc.M413303200. [DOI] [PubMed] [Google Scholar]
- 28.Praper T, et al. Human perforin permeabilizing activity, but not binding to lipid membranes, is affected by pH. Mol. Immunol. 2010;47:2492–2504. doi: 10.1016/j.molimm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Kaiser BK, et al. Disulphide-isomerase-enabled shedding of tumour-associated NKG2D ligands. Nature. 2007;447:482–486. doi: 10.1038/nature05768. [DOI] [PubMed] [Google Scholar]
- 30.Boutet P, et al. Cutting edge: the metalloproteinase ADAM17/TNF-alpha-converting enzyme regulates proteolytic shedding of the MHC class I-related chain B protein. J. Immunol. 2009;182:49–53. doi: 10.4049/jimmunol.182.1.49. [DOI] [PubMed] [Google Scholar]
- 31.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 32.Waldhauer I, et al. Tumor-associated MICA is shed by ADAM proteases. Cancer Res. 2008;68:6368–6376. doi: 10.1158/0008-5472.CAN-07-6768. [DOI] [PubMed] [Google Scholar]
- 33.Schlecker E, et al. Metalloprotease-mediated tumor cell shedding of B7-H6, the ligand of the natural killer cell-activating receptor NKp30. Cancer Res. 2014;74:3429–3440. doi: 10.1158/0008-5472.CAN-13-3017. [DOI] [PubMed] [Google Scholar]
- 34.Srpan K, et al. Shedding of CD16 disassembles the NK cell immune synapse and boosts serial engagement of target cells. J. Cell Biol. 2018;217:3267–3283. doi: 10.1083/jcb.201712085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abram CL, Lowell CA. The ins and outs of leukocyte integrin signaling. Annu. Rev. Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bunnell SC, Kapoor V, Trible RP, Zhang W, Samelson LE. Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity. 2001;14:315–329. doi: 10.1016/S1074-7613(01)00112-1. [DOI] [PubMed] [Google Scholar]
- 37.Furukawa R, et al. Calcium regulation of actin crosslinking is important for function of the actin cytoskeleton in Dictyostelium. J. Cell Sci. 2003;116(Pt 1):187–196. doi: 10.1242/jcs.00220. [DOI] [PubMed] [Google Scholar]
- 38.Henter JI, et al. Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood. 1991;78:2918–2922. doi: 10.1182/blood.V78.11.2918.2918. [DOI] [PubMed] [Google Scholar]
- 39.Michelet X, et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 2018;19:1330–1340. doi: 10.1038/s41590-018-0251-7. [DOI] [PubMed] [Google Scholar]
- 40.Wensveen FM, et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat. Immunol. 2015;16:376–385. doi: 10.1038/ni.3120. [DOI] [PubMed] [Google Scholar]
- 41.Messmer B, Eissmann P, Stark S, Watzl C. CD48 stimulation by 2B4 (CD244)-expressing targets activates human NK cells. J. Immunol. 2006;176:4646–4650. doi: 10.4049/jimmunol.176.8.4646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.