Liver transplantation (LT) provides a cure for various end-stage liver diseases. The liver graft becomes the recipient’s new metabolic center, and donor genetics plays a potent role in the development of complications in the recipient, such as metabolic disorders1 and even tumor recurrence.2 Notably, both the donor’s3 and recipient’s genetics4 contributes significantly to intrahepatic complications (e.g., liver fibrosis and de novo steatosis). Therefore, crosstalk between donor and recipient tissue remodels the architecture and homeostasis of the transplanted liver, which is still a great challenge to explore.
Macrophages are the cornerstones of liver architecture in homeostasis and disease.5 During the perioperative LT period, macrophages not only respond to ischemia/reperfusion injury but also become reorganized with the recipient’s peripheral blood monocytes. The interaction between the recomposed macrophages and other cells (e.g., hepatocytes and T cells) may affect immunological mechanisms6,7 and metabolic homeostasis.8 However, it is still unknown how the constitutional and functional shifting of macrophages under such circumstances takes place.
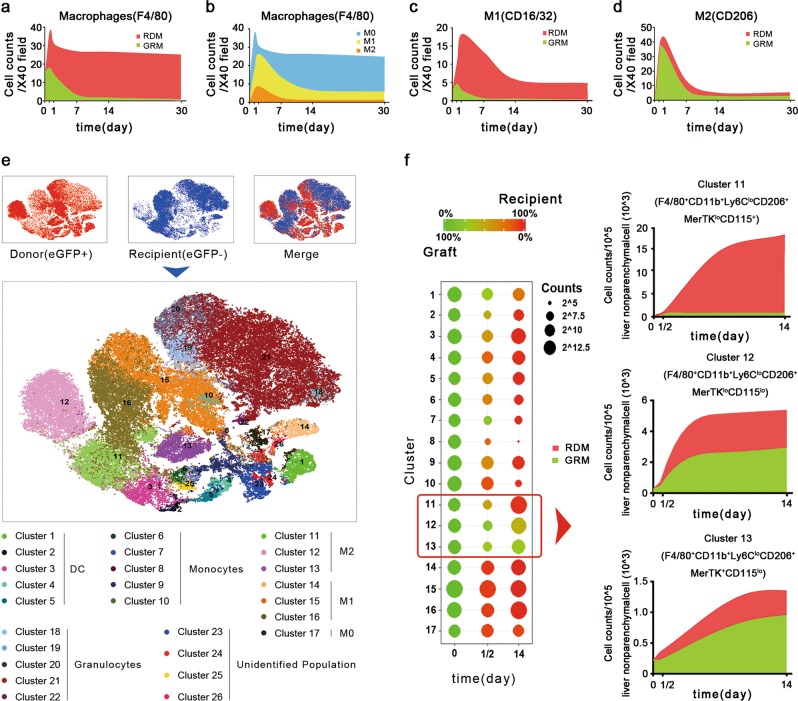
To better understand the impact of macrophages in the transplanted liver, in this study, we dynamically monitored the composition (graft- or recipient-derived) and polarization (e.g., M1 or M2) of macrophages during the perioperative period using an eGFP-labeled mouse model of orthotopic LT. Mice expressing eGFP were used as donors. Accordingly, liver macrophages were divided into graft-resident macrophages (GRMs) and recipient-derived macrophages (RDMs). The number of GRMs was adjusted according to the eGFP staining efficiency (~90% among all subtypes of macrophages). The mice were in good condition and were killed at day 0 (control) or at 12 h, 24 h, 7 days, 14 days, or 30 days after LT. During the first 12 h, hepatic macrophages sharply increased by ~2.4-fold due to the recruitment of RDMs. From 24 h to day 7, the macrophage population gradually decreased, and RDMs became predominant (>90%). From day 7 to day 30, the macrophage population remained steady, and RDMs took over the transplanted liver (~99%) (Fig. 1a).
Fig. 1.
Shift in the hepatic macrophage pool after liver transplantation. a The dynamic change in graft-resident macrophages (GRMs) and recipient-derived macrophages (RDMs), which are labeled green and red, respectively. b The dynamic change in M0, M1, and M2 macrophages, which are labeled blue, yellow, and brown, respectively. c The dynamic change in graft-resident and recipient-derived M1 macrophages, which are labeled green and red, respectively. d The dynamic change in graft-resident and recipient-derived M1 macrophages, which are labeled green and red, respectively. e Monocyte-derived cells were classified using CyTOF analysis. f Left: bubble chart displaying the source and level changes. Right: the dynamic change in graft-resident (green) and recipient-derived (red) M2 macrophages in clusters 11, 12, and 13
Upon a closer evaluation of the subtypes of macrophages, we observed dramatically elevated levels of both M1 and M2 macrophages, which reached maximum levels at 24 h. Thereafter, M1 and M2 macrophages decreased to the initial levels after days 7 and 14, respectively (Fig. 1b). The dynamic change in pro- and anti-inflammatory cytokines was consistent with the change in M1 and M2 macrophage populations (Fig. S1). Among M1 macrophages, RDMs were in charge of the dynamic change and became predominant after day 7 (>95%) (Fig. 1c). In contrast, GRMs were responsible for the dynamic change in M2 macrophages within the first week. Afterward, GRMs still accounted for almost half of the M2 macrophages, which remained at a steady level (Fig. 1d). More subgroups of monocyte-derived cells were detected using CyTOF in an independent group (day 0: control; 12 h: peak level; and day 14: steady level). We observed that RDMs accounted for the majority of cells, including monocytes, dendritic cells (DC), and M0 and M1 macrophages, at day 14 (15/17 clusters) (Fig. 1e). Among the three M2 macrophage subsets, clusters 11 and 13 were predominantly RDMs and GRMs, respectively, while cluster 12 was an equal mix of these two cell types (Fig. 1f).
It was astonishing to find that the donor Kupffer cell population disappeared in such a short time. Kupffer cells account for the majority (~80%) of all macrophages in the body and may locally proliferate and self-renew.5 Although blood monocytes are recruited into the liver, they never contribute to the Kupffer cell pool. Under the circumstance of LT, however, recipient blood-recruited monocytes replaced graft-resident Kupffer cells and occupied the transplanted liver within approximately 1–2 weeks. The crosstalk between graft-resident cells (e.g., hepatocytes and natural killer cells) and recipient-derived cells (e.g., RDM and DC) initiates tissue remodeling and probably leads to the achievement of a new homeostatic balance, providing a favorable or unfavorable environment for the development or progression of some diseases. After the remodeling period, more attention should be paid to RDMs than to GRMs after LT in the study of various macrophage-related diseases, including inflammation, hepatic steatosis, fibrosis, and tumor recurrence.5,8,9 How a genetic match between donor and recipient plays a role in this complex system of remodeling and the long-term outcome remains an open question.
It was also notable that the sources of the macrophage subtypes were different. M1 macrophages were predominantly derived from recipient blood-recruited monocytes, possibly as a response to ischemia/reperfusion injury and surgical stress. In contrast, M2 macrophages were mostly polarized from GRMs in the early posttransplantation period. After 2 weeks, RDMs became the sole source of M1 macrophages, whereas M2 macrophages seemed to originate equally from GRMs and RDMs. Therefore, the strategic targeting of M1 RDMs and M2 GRMs may help attenuate ischemia/reperfusion injury and improve prognosis. Investigation of diseases associated with the M2 subtype after LT (e.g., liver fibrosis and tumor recurrence) should consider both the donor and recipient genetic backgrounds.5,10
In conclusion, this study is the first to explore the time-dependent development of the hepatic macrophage pool following LT. It was striking that RDMs took over the transplanted liver (>99%) so quickly, but GRMs still played a role in the polarization of M2 macrophages. Because of the heterogeneity of macrophages, differing genetics between the donor and recipient may have a considerable impact on the polarization and function of macrophages in response to specific environmental signals. The interplay between RDMs and graft-resident cells requires further investigation.
Supplementary information
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81771713), the Zhejiang Provincial Natural Science Foundation of China (LR18H030001), and the Fundamental Research Funds for the Central Universities (2019QNA7030).
Author contributions
Q.L. designed the experiments; H.H., X.Z., C.Z., and H.C. performed the experiments; H.H. wrote the draft; and Q.L. and S.Z. reviewed and revised the manuscript.
Competing interests
The authors declare no competing interests.
Contributor Information
Qi Ling, Email: lingqi@zju.edu.cn.
Shusen Zheng, Email: zyzss@zju.edu.cn.
Supplementary information
The online version of this article (10.1038/s41423-019-0253-x) contains supplementary material.
References
- 1.Ling Q, et al. Donor graft microRNAs: a newly identified player in the development of new-onset diabetes after liver transplantation. Am. J. Transpl. 2017;17:255–264. doi: 10.1111/ajt.13984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu X, et al. Donor miR-196a-2 polymorphism is associated with hepatocellular carcinoma recurrence after liver transplantation in a Han Chinese population. Int. J. Cancer. 2016;138:620–629. doi: 10.1002/ijc.29821. [DOI] [PubMed] [Google Scholar]
- 3.Dunn W, et al. Donor PNPLA3 rs738409 genotype affects fibrosis progression in liver transplantation for hepatitis C. Hepatology. 2014;59:453–460. doi: 10.1002/hep.26758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.John BV, et al. Recipient but not donor adiponectin polymorphisms are associated with early posttransplant hepatic steatosis in patients transplanted for non-nonalcoholic fatty liver disease indications. Exp. Clin. Transpl. 2018;16:439–445. doi: 10.6002/ect.2018.0070. [DOI] [PubMed] [Google Scholar]
- 5.Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 2017;17:306–321. doi: 10.1038/nri.2017.11. [DOI] [PubMed] [Google Scholar]
- 6.Chu Z., et al. Primed macrophages directly and specifically reject allografts. Cell Mol Immunol 2019. in press. [DOI] [PMC free article] [PubMed]
- 7.Iske J, et al. Composite tissue allotransplantation: opportunities and challenges. Cell Mol. Immunol. 2019;16:343–349. doi: 10.1038/s41423-019-0215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazankov K, et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019;16:145–159. doi: 10.1038/s41575-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura K, et al. Macrophage heme oxygenase-1-SIRT1-p53 axis regulates sterile inflammation in liver ischemia-reperfusion injury. J. Hepatol. 2017;67:1232–1242. doi: 10.1016/j.jhep.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeung OW, et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J. Hepatol. 2015;62:607–616. doi: 10.1016/j.jhep.2014.10.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.