Recent immunotherapy using chimeric antigen receptor-modified (CAR) T cells and immune checkpoint blockade therapies has shown remarkable outcomes in the field of cancer treatment.1,2 In particular, great success has been achieved in B cell acute lymphocytic leukemia (B-ALL) treated with anti-CD19 CAR T cells.2 However, the loss, mutation, and reduced expression of CD19 are crucial causes for the failure of CAR T cell treatment in leukemia patients.3,4 CD38, a 45-kDa type II glycoprotein, is detectable in B-ALL, and several studies have indicated the efficacy and safety of CD38 monoclonal antibodies in clinical applications, suggesting that CD38 represents a suitable therapeutic target for the treatment of B-ALL.5,6 In addition, we developed a CAR against CD38, and our preclinical study indicated that anti-CD38 CAR T (CAR T-38) cells could specifically lyse CD38-positive tumor cells.7 Herein, we present the anti-leukemia ability of CAR T-38 cells in an adult relapsed B-ALL patient after failure of bi-specific CD19/CD22 CAR T cell treatment, and this administration of CAR T-38 cells was accompanied by the occurrence of target-mediated toxicities and severe and uncontrollable cytokine release syndrome (CRS).
A 24-year-old female patient with relapsed and refractory B-ALL achieved minimal residual disease-negative complete remission 1 month after treatment with bi-specific CD19/CD22 CAR T cells and relapsed 5 months after therapy with CD19-negative and low but variable CD22 surface expression on leukemic blasts, and her disease relapsed with 63% CD38+ B-ALL cells in the bone marrow (BM) (Fig. S1). The detailed initial treatment is presented in Fig. S2. This patient was enrolled to receive CAR T-38 cell treatment on 1 December 2018, and additional detailed experimental protocols are given in the Supplementary Materials. After receiving a debulking treatment and a conditioning regimen (Fig. 1a), the patient had significantly decreased levels of CD38+ B-ALL cells in BM (14.52%). Then, the patient received 1 × 106/kg CAR T-38 cells (Fig. 1a), which were harvested after a total of 7 days of in vitro culture (Fig. S3a), containing 77.09% CAR-38-positive cells (Fig. S3b) and 97.18% CD3+ T cells with a CD4:CD8 percentage of 34.09:56.88 (Fig. S3c). There was up-regulated expression of CD107a on CAR T-38 cells after stimulation with CD38+ tumor cells (Fig. S3d–f). CD38+ B-ALL cells were decreased in both BM and peripheral blood (PB) following cell infusion (Fig. 1b–d).
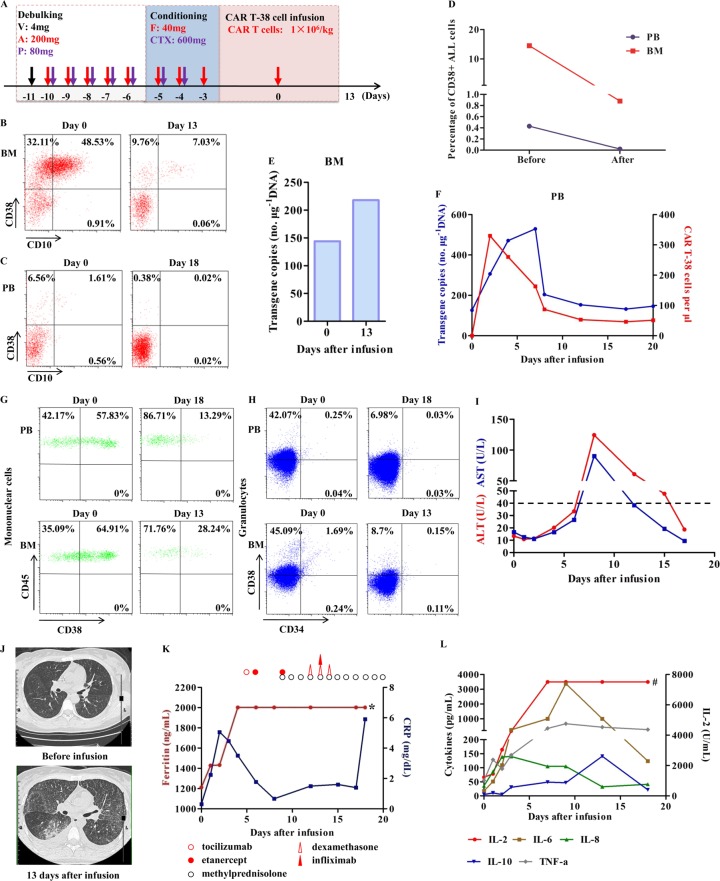
Fig. 1. Response and toxicities after the patient received the CAR T-38 cell treatment.
a Schematic of the clinical protocol. A, cytosine arabinoside; CTX, cyclophosphamide; Dex, dexamethasone; F, fludarabine; P, methylprednisolone; V, vindesine. b, c Response in BM on day 13 and in PB on day 18. Numbers represent the percentage of positive cells. The gating strategy is shown in Fig. S5a. d The change in the percentage of CD38+ B-ALL cells in PB and BM before (on day 0) and after CAR T-38 cell infusion (PB on day 18 and BM on day 13). e The CAR gene copy number in the genomic DNA in BM analyzed using Q-PCR. f Persistence of infused CAR T-38 cells in PB. The blue line indicates the CAR gene copy number in the genomic DNA detected by Q-PCR, and the red line indicates the number of CAR T-38 cells per microliter (μL) PB by flow cytometry. g, h CD38 expression on mononuclear cells and granulocytes in PB on days 0 and 18 and in BM on days 0 and 13. The gating strategies are shown in Fig. S5b, c. i The patient suffered from transient liver damage that was accompanied by an elevation in aspartate aminotransferase (AST, blue line) and alanine aminotransferase (ALT, red line) 9 days after the CAR T-38 cell infusion. The dotted black line indicates the normal maximum levels of ALT and AST. j A CT scan showed that the patient had diffuse damage in both lungs, mainly including interstitial exudation, 13 days after the CAR T-38 cell infusion. k Variation in the serum levels of ferritin (red line) and C-reactive protein (CRP, blue line) in PB after the infusion of CAR T-38 cells. Multiple cycles of tocilizumab, etanercept, infliximab, and steroids, including methylprednisolone and dexamethasone, were subsequently administered, and their detailed doses are presented in Fig. S4. * indicates that the serum ferritin level was outside the maximum range (>2000 ng/mL) 4 days after the CAR T-38 cell infusion. l Changes in the serum IL-2, IL-6, IL-8, IL-10 and TNF-α levels in PB after CAR T-38 cell infusion. # indicates that the serum IL-2 level was outside the maximum range (>7500 ng/mL) 7 days after the cell infusion.
After cell culture in vitro, we found negligible numbers of CAR T-38 cells expressing CD38 (Fig. S3g). Similar to other studies on CAR T-38 cells,7,8 the reduced CD38+ CAR T cells should be caused by CAR T-38-mediated “self-lysis.” In addition, a high background level of CD107a expression by CAR T-38 cells was observed (Fig. S3f) compared with that on bi-specific CD19/20 CAR T cells generated from the patient (Fig. S3h–j), and this might have been due to ongoing CAR T-38 cell activation against CD38+ T cells in cell culture.
After the infusion, CAR T-38 cells could migrate to BM (Fig. 1e), and a rapid increase in CAR T cells in PB was observed at both the genomic and cellular levels (Fig. 1f). Then, both the CAR gene copy numbers and CAR T cell numbers in PB declined to close to baseline within ~10 days. As reported, CD38 is detected on activated T cells.9 Accordingly, the CD38 expression by the infused CAR T-38 cells could be enhanced upon their recognition and activation by other CD38+ cells, indicating that “self-lysis” potentially restricted the expansion of CAR T-38 cells.
A reduction in the percentage of CD38+ mononuclear cells and granulocytes in PB and BM (Fig. 1g, h), transient liver damage (Fig. 1i), and lung diffuse damage (Fig. 1j) were observed after the patient received the CAR T-38 cell infusion. These observations might have relevance for the occurrence of target-mediated toxicity, which results when these CAR T cells damage healthy cells, because CD38 is not only expressed on hematological malignancies but is also expressed on normal cells, such as mononuclear cells and granulocytes in PB and BM, liver and lung smooth muscle cells, and pancreatic β cells.10 CARs with high affinity have been reported to poorly distinguish cells expressing the target at any level, including levels undetectable by flow cytometry in normal cells, whereas low-affinity CARs could reduce the toxicity aimed at normal cells; however, both types of CARs presented similar robust anti-tumor efficacy,11 indicating that screening for the optimal affinity of CAR T-38 cells needs to be performed to reduce target-mediated toxicity in future studies.
The patient immediately experienced high-grade fever accompanied by chills after the infusion, and no obvious improvement was observed from day 1 to day 4 after symptomatic treatment (Fig. S4). The serum concentrations of C-reactive protein, ferritin, and cytokines, including interleukin-2 (IL-2), IL-6, IL-8, IL-10, and tumor necrosis factor-α, significantly increased after CAR T-38 cell infusion (Fig. 1k, l), indicating that CRS occurred in the patient.12 Although multiple cycles of tocilizumab, etanercept, infliximab, and steroids were administered subsequently, the patient’s fever and CRS were not ameliorated (Fig. 1k, l and Fig. S4). Unfortunately, the patient abandoned any treatment 20 days after the cell infusion. In summary, although the potent and specific anti-tumor activity of CAR T-38 cells was established in a relapsed B-ALL patient, further experiments, including experiments optimizing the affinity of CAR T-38 cells to reduce target-mediated toxicity and knocking out the CD38 gene in CAR T-38 cells by using gene editing technology, such as CRISPR/Cas9, to avoid the “self-lysis” of CAR T-38 cells, are required to improve the safety and persistence of these cells in future clinical studies.
Supplementary information
Acknowledgements
We are grateful to the patient and patient’s next of kin for allowing us to share her story. This work was supported by the grants from the National Natural Science Foundation of China for the Grants 81830002 (W.H.) and 81903151 (Y.G.) and the National Key Research and Development Program of China for the Grant 2017YFC0909803 (Y.W.).
Author contributions
Z.W. and W.H. conceived and designed the experiment and analyzed the data; C.T., Y.W. and D.T. performed the experiments; K.F., H.J., Y.L. and Q.Y. provided management of patient (patient enrollment, treatment, data collection); and Y.G. analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Yelei Guo, Kaichao Feng, Chuan Tong
Contributor Information
Zhiqiang Wu, Email: wuzhiqiang1006@163.com.
Weidong Han, Email: hanwdrsw69@yahoo.com.
Supplementary information
The online version of this article (10.1038/s41423-019-0355-5) contains supplementary material.
References
- 1.Li X, et al. Emerging predictors of the response to the blockade of immune checkpoints in cancer therapy. Cell. Mol. Immunol. 2019;16:28–39. doi: 10.1038/s41423-018-0086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai H, et al. Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. Oncoimmunology. 2015;4:e1027469. doi: 10.1080/2162402X.2015.1027469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sotillo E, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5:1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orlando EJ, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat. Med. 2018;24:1504–1506. doi: 10.1038/s41591-018-0146-z. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Z, et al. Heterogeneity of CD34 and CD38 expression in acute B lymphoblastic leukemia cells is reversible and not hierarchically organized. J. Hematol. Oncol. 2016;9:94. doi: 10.1186/s13045-016-0310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lokhorst HM, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N. Engl. J. Med. 2015;373:1207–1219. doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- 7.Gao, Z. et al. Blocking CD38-driven fratricide among T cells enables effective antitumor activity by CD38-specific chimeric antigen receptor T cells. J. Genet. Genomics. 10.1016/j.jgg.2019.06.007 (2019). [DOI] [PubMed]
- 8.Mihara K, et al. Activated T-cell-mediated immunotherapy with a chimeric receptor against CD38 in B-cell non-Hodgkin lymphoma. J. Immunother. 2009;32:737–743. doi: 10.1097/CJI.0b013e3181adaff1. [DOI] [PubMed] [Google Scholar]
- 9.Morandi F, et al. CD38: a target for immunotherapeutic approaches in multiple myeloma. Front. Immunol. 2018;9:2722. doi: 10.3389/fimmu.2018.02722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malavasi F, et al. Evolution and function of the ADP ribosylcyclase/CD38 gene family in physiology and pathology. Physiol. Rev. 2008;88:841–886. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, et al. Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 2015;75:3596–3607. doi: 10.1158/0008-5472.CAN-15-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DW, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.