The generation and function of plasma cells have been well recognized as the central events in humoral immunity. In particular, long-lived plasma cells, characterized by a long lifespan and the lack of cell division, persistently secrete high-affinity antibodies to maintain serum antibody titers in the immune response and autoimmune inflammation. Although the significant expansion of plasma cells is observed during the aging process, autoimmune diseases and chronic infections, the expanding functional diversity and underlying mechanisms of plasma cells remain incompletely understood. Here, we describe recent advances in revealing new functional subsets and phenotypic features of plasma cells in aging and autoimmunity.
Hematopoietic stem cells (HSCs) include lymphoid-biased HSCs (Ly-HSCs) for lymphopoiesis and myeloid-biased counterparts (My-HSCs) for myelopoiesis. Aging results in decreased lymphopoiesis and increased myelopoiesis in the bone marrow with a prominent expansion of My-HSCs, common myeloid progenitors (CMPs), granulocyte-macrophage progenitors (GMPs) and mature myeloid cell subsets.1 Recently, Pioli et al. described a novel function of plasma cells in the age-related increase in myelopoiesis in the bone marrow.2 Significant accumulation of surface IgM+IgA−, IgM−IgA+, and IgM+IgA+ plasma cells was observed in the bone marrow of middle-aged and old mice. The depletion of plasma cells from old mice with anti-mouse CD138 antibodies reduced myelopoiesis to its levels observed in young animals. Anti-mouse CD138 antibody treatment effectively removed B220−CD138+ plasma cells without affecting B220+CD138int IgD− plasmablast counterparts, unlike the effects of isotype-matched antibody-treated old mice. Old mice subjected to plasma cell depletion exhibited dramatically decreased numbers of My-HSCs, CMPs, monocytes, and granulocytes, while no significant change in the numbers of Ly-HSCs was observed. Importantly, the depletion of plasma cells did not alter either lymphopoiesis or myelopoiesis in young bone marrow, indicating the key effects of plasma cell accumulation in age-related myeloid skewing in the bone marrow. Consistently, in vitro experiments confirmed the reduced proportions of CD19+ B lymphocytes but increased CD11b+ myeloid lineage output from Ly-HSCs cocultured with old plasma cells. Furthermore, the addition of old plasma cells significantly enhanced CD11b+ cell production from My-HSCs and suppressed CD19+ B cell generation in common lymphoid progenitor (CLP) cultures. However, supplementation with young plasma cells had no effects on the differentiation of Ly-HSCs and My-HSCs in vitro. Further elucidation of the transcriptional signatures of plasma cells isolated from young and old mice indicated age-associated transcriptional profiles in plasma cells. Notably, increased levels of the pathogen sensors Tlr4, Naip2, and Nod2 and the effector molecules Erk1, Elk1, and Tbk1 were detected in old plasma cells. Accordingly, old plasma cells expressed persistently higher levels of TLR4 than young plasma cells with or without LPS stimulation. In addition, the natural TLR4 ligand LPS has been shown to induce expression of the proinflammatory cytokines Il1b, Il6, Tnf and the chemokines Ccl3 and Cxcl10 in old plasma cells. Interestingly, the depletion of plasma cells with anti-CD138 antibody also triggered alterations in the bone marrow stromal microenvironment. After plasma cell depletion, decreased levels of the inflammatory molecules Il23, Tnfsf14, Tirap, Ly96, Tlr4, Tlr5, Itgb2, and Fos were observed in stromal cells from the bone marrow of old mice. Moreover, blockade of IL-1β and TNF-α markedly decreased myelopoiesis in the bone marrow of old mice, indicating that the proinflammatory cytokines IL-1β and TNF-α contribute significantly to the aging-related inflammatory milieu and myelopoiesis in mouse bone marrow. Together, these seminal findings have identified the novel function of aging-associated plasma cells in modulating both the hematopoietic microenvironment and HSC fate decisions.
Although the pathogenic role of plasma cells in autoimmune development has been extensively studied, functional subsets of plasma cells remain partially characterized. Recently, we revealed the pathogenic role of the long-lived TLR4+CXCR4+ plasma cell subset in both patients with systemic lupus erythematosus (SLE) and mice with experimental lupus.3 TLR4+CXCR4+ plasma cells, which predominantly secreted anti-dsDNA IgG autoantibodies and triggered nephritis development in immunodeficient RAG-2 mice after their adoptive transfer, were significantly expanded in the peripheral blood and renal tissues of active SLE patients and lupus mice. In addition, a long-term BrdU incorporation assay identified TLR4+CXCR4+ plasma cells as long-lived plasma cells in lupus mice. Remarkably, the blockade of TLR4 with CLI-095 abrogated autoantibody secretion in TLR4+CXCR4+ plasma cells and ameliorated renal damage in lupus mice, indicating the critical role of TLR4 signaling in modulating autoantibody secretion in long-lived plasma cells. Moreover, CXCR4 expression may contribute to the recruitment of long-lived plasma cells towards inflamed kidneys during lupus pathogenesis. Thus, these findings highlight the pathogenic role of long-lived autoreactive plasma cells in autoimmune development.
Novel subsets of plasma cells with regulatory functions in infections and autoimmune diseases via the secretion of inhibitory cytokines, including IL-10 and IL-35, were recently characterized.4–6 Notably, hypoxia-inducible factor-1α (HIF-1α) has been identified as a critical transcription factor in IL-10-producing B cells in autoimmune disease.7 Mice with impaired plasma cell differentiation (Prdm1f/fMb1Cre/+ ) showed exacerbated experimental autoimmune encephalomyelitis (EAE) development.4 Previous studies revealed that CD138hi plasmablasts in the draining lymph nodes, but not those in the spleen, were the dominant IL-10-producing B cell subset and negatively regulated autoimmune inflammation in EAE.4 However, splenic CD138hi plasma cells with increased secretion of IL-35 were shown to possess a regulatory function during EAE development and Salmonella infection.5 Recently, Lino et al. defined a natural regulatory plasma cell subset characterized by expression of the inhibitory receptor lymphocyte-activation gene 3 (LAG-3) that developed at steady state and quickly responded to Salmonella infection by producing IL-10.8 These LAG-3+ B cell counterparts exhibited key attributes of plasma cells with a phenotype of B220−CD138hiBlimp-1hiKi-67− and were the major IL-10-producing B cell subset in the spleen and bone marrow. Further transcriptome analysis revealed the functional features of LAG-3+CD138hi plasma cells with enriched gene expression of Il10, Lag3, Cd200, Cd273, Klf4, and Bhlhe40 compared with that of LAG-3− plasma cell counterparts. Although LAG-3+CD138hi plasma cells showed the increased transcription of Klf4, which is expressed in long-lived plasma cells in the bone marrow, it is currently unclear whether these nondividing LAG-3+CD138hi plasma cells represent a subset of long-lived plasma cell compartments in vivo. Interestingly, single-cell BCR sequencing revealed a distinct BCR repertoire with increased VH7, VH10, and VH11 segments in LAG-3+CD138hi plasma cells that corresponded to the typical BCR features in B-1a cells.9,10 The enrichment of VH11+Vk14+ BCRs, which were otherwise expressed by B-1a cells to recognize phosphatidylcholine (PtC), was confirmed by flow cytometric analysis of IL-10-producing LAG-3+CD138hi plasma cells. Mechanistically, deficiency of Cd19 and Btk significantly reduced the frequency and number of LAG-3+CD138hi plasma cells in vivo. In contrast, Il10, TCRαβ or Cd72 deficiency markedly expanded LAG-3+CD138hi plasma cells. This elegant study has demonstrated the regulatory role of LAG-3+CD138hi plasma cells in preventing excessive immunopathology.
A recent study by Lam et al. reported that the survival of long-lived plasma cells was modulated by pyruvate generation.11 Long-lived plasma cells were found to harbor a potent mitochondrial respiratory capacity when compared with short-lived plasma cells. The blockade of pyruvate import with UK5099 skewed the metabolic profiles of long-lived plasma cells, which adopted the metabolic features of short-lived plasma cells. Moreover, depletion of the mitochondrial pyruvate carrier Mpc2 substantially diminished the frequency of long-lived plasma cells in mice. In addition, long-lived plasma cells showed elevated expression levels of the glucose transporter GLUT1 and enhanced uptake of the metabolizable fluorescent glucose analog 2NBDG. Further studies on metabolic fates with 13C glucose showed substantial 13C enrichment in mannose for antibody glycosylation in long-lived plasma cells. These findings reveal the unique metabolic pathways in long-lived plasma cells. However, the distinct metabolic features of age-associated plasma cells, long-lived autoreactive plasma cells and regulatory plasma cells remain to be investigated.
In summary, the expanding pool of plasma cell subsets may harbor phenotypic, metabolic and transcriptional diversity related to their anti- or proinflammatory functions in aging and autoimmunity, as revealed by recent findings that TLR-4-expressing long-lived plasma cells persistently secrete autoantibodies and proinflammatory cytokines, while LAG-3-expressing regulatory plasma cells produce IL-10 and suppress autoimmune inflammation (Fig. 1). Thus, further characterization of plasma cell subsets is needed to address the key metabolic, transcriptomic and epigenetic events that modulate their differentiation and functional diversity in immune homeostasis and disease.12
Fig. 1.
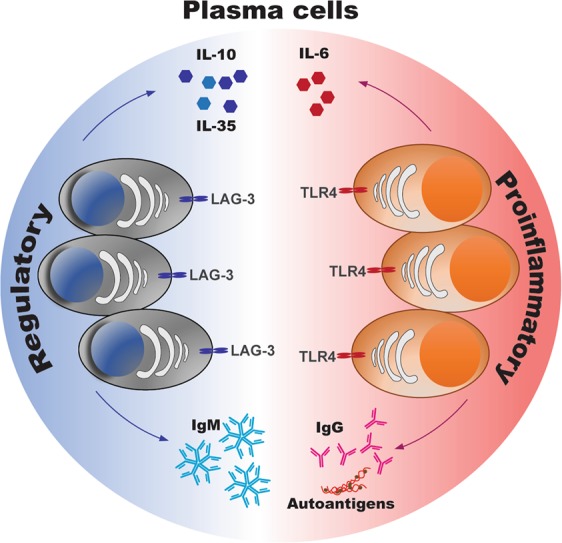
The diverse functions of plasma cells in immunity and inflammation.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 91842304, 81771761, and 81901635), the Hong Kong SAR Government (No. 17114515), HKU Seed Funding for Strategic Interdisciplinary Research Scheme and the Hong Kong Croucher Foundation (260960116).
Competing interests
The authors declare no competing interests.
References
- 1.Morrison SJ, et al. The aging of hematopoietic stem cells. Nat. Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 2.Pioli PD, et al. Plasma cells are obligate effectors of enhanced myelopoiesis in aging bone marrow. Immunity. 2019;51:351–366.e6. doi: 10.1016/j.immuni.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma K, et al. TLR4+CXCR4+ plasma cells drive nephritis development in systemic lupus erythematosus. Ann. Rheum. Dis. 2018;77:1498–1506. doi: 10.1136/annrheumdis-2017-212652. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto M, et al. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity. 2014;41:1040–1051. doi: 10.1016/j.immuni.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Shen P, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507:366. doi: 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin, X. et al. IL-10-producing regulatory B cells restrain the T follicular helper cell response in primary Sjögren’s syndrome. Cell Mol. Immunol. 10.1038/s41423-019-0227-z. (2019). [DOI] [PMC free article] [PubMed]
- 7.Meng X, et al. Hypoxia-inducible factor-1α is a critical transcription factor for IL-10-producing B cells in autoimmune disease. Nat. Commun. 2018;9:251. doi: 10.1038/s41467-017-02683-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lino AC, et al. LAG-3 inhibitory receptor expression identifies immunosuppressive natural regulatory plasma cells. Immunity. 2018;49:120–133.e9. doi: 10.1016/j.immuni.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graf R, et al. BCR-dependent lineage plasticity in mature B cells. Science. 2019;363:748–753. doi: 10.1126/science.aau8475. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, et al. New insights into the significance of the BCR repertoire in B-1 cell development and function. Cell Mol. Immunol. 2019;16:772–773. doi: 10.1038/s41423-019-0249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam WY, et al. Mitochondrial pyruvate import promotes long-term survival of antibody-secreting plasma cells. Immunity. 2016;45:60–73. doi: 10.1016/j.immuni.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H, et al. Epigenetic regulation in B-cell maturation and its dysregulation in autoimmunity. Cell Mol. Immunol. 2018;15:676–684. doi: 10.1038/cmi.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]


