Research highlight
Ulcerative colitis and Crohn’s disease are the two major forms of inflammatory bowel disease (IBD) in humans.1 Chronic human IBD, such as Crohn’s disease, is characterized by features such as intestinal infiltration of immune cells, inflammatory cytokine milieu, intestinal fibrosis and weight loss among others, that lead to chronic and spontaneously recurring intestinal inflammation.2 Several mouse models of IBD have been used by researchers to understand the diverse aspects related to human IBD.1,2 For example, the mouse model of intra-rectal administration of the haptenating agent, 2,4,6-trinitrobenzene sulfonic acid (TNBS), which induces mucosal immunity to colonic immunogenic proteins in the host, recapitulates key immunological responses observed in Crohn’s disease, such as cytokine secretion patterns, intestinal fibrosis, and immune intolerance.2 The innate immune response is critical to initiate the cytokines production that prime T-cell differentiation in the TNBS model. The polarized T-cell responses are hallmarks of IBD and in the TNBS model TH1 and TH17-cell responses predominate as is observed with the signature cytokine secretion profile of IL12/IFNγ and IL23/IL17, respectively.2
Given the ability of dendritic cells (DCs) to modulate T-cell differentiation by secreting the key cytokines, exploring the recruitment, activation, roles and targeting of DCs in chronic IBD has been of considerable interest.3 Among the non-chemokine chemoattractants, DCs are well known to respond to the lipid mediator, Leukotriene B4 (LTB4).4 LTB4 is a potent chemoattractant produced in response to inflammation, largely by the cells of the myeloid lineage and to a lesser extent by the other immune and non-immune cells.5,6 The cognate G-protein coupled receptor (GPCR) for LTB4, BLT1, is expressed predominantly by the cells of the myeloid lineage and to a lesser extent by the other immune and non-immune cells.5,6 The binding of LTB4 to BLT1 activates the pathways leading to adhesion, polarization, and migration of target cells.6 Indeed, the LTB4-BLT1 signaling is required for the effective recruitment of neutrophils, macrophages and other myeloid-derived cells to the site of inflammation. In addition, the LTB4-BLT1 axis can also promote the recruitment of lymphocytes to the inflammation site in specific mouse models.5,6 Human monocyte-derived DCs that express BLT2 but not BLT1, are known to chemotax in response to LTB4.7 Importantly, the bone-marrow derived DCs from mouse respond to LTB4 by upregulating the chemokine GPCR CCR7, which binds and is activated by CCL19 and CCL21, and lack of BLT1 in such DCs impairs their ability to home to the draining lymph nodes.8 Additionally, LTB4-driven migration of DCs is important for the generation of TH2 type responses to airway hyperresponsiveness, which is dampened in the absence of BLT1 on DCs.9 Furthermore, lack of BLT1 in DCs dampens their ability to skew T-cell polarization to TH1 type in a mouse model of delayed-type hypersensitivity.10 Clearly, LTB4 acts on DCs to mediate two important and distinct cellular responses—(1) recruitment to the site of inflammation and (2) secretion of cytokines to skew T-cell polarization. However, the precise role of the LTB4-BLT1 axis in DC response in the context of IBD was not previously explored.
In a recent study by Zhou J et al., published in Cellular and Molecular Immunology, the authors provide insights into the role of the LTB4-BLT1 signaling in potentiating DCs to impact TH1 and TH17 responses in a model of TNBS-induced IBD in mice.11 Using BLT1−/− mice, authors establish that inability to sense LTB4 helps recover body weight in mice treated with TNBS. These results correlate with reduced frequencies of TH1 and TH17 cells in the colon as wells as reduced production of inflammatory cytokines associated with T-cell differentiation in the TNBS-treated BLT1−/− mice (summarized in Fig. 1). Further, the authors find that BLT1 on splenic DCs from TNBS-treated mice promotes the production inflammatory cytokines, such as IL12p70, IL6, and TNFα, which in-turn are known to promote TH1-cell differentiation. In fact, authors find that co-culture of LPS-treated DCs with naive T-cells promotes their differentiation to TH1 and TH17 cells, which in part depends on the LTB4-BLT1 signaling. Consistent with this, splenic DCs lacking BLT1 were found to be less effective in inducing differentiation of TH1 and TH17 cells in an in vivo mouse model where T-cells differentiate to respond to the ovalbumin challenge. Most strikingly, using a toxin-based depletion of DCs in vivo, the authors establish that replacing the endogenous DCs with the wild-type (WT) DCs was sufficient to induce colitis symptoms in response to the TNBS challenge, which was largely dampened when the DCs lacking BLT1 were introduced instead of WT DCs in the recipient mice. Finally, using small molecule inhibitor studies, authors propose that much of BLT1 activation leading to the production of pro-inflammatory cytokines appears to be dependent on the active Gαi and PLC signaling.
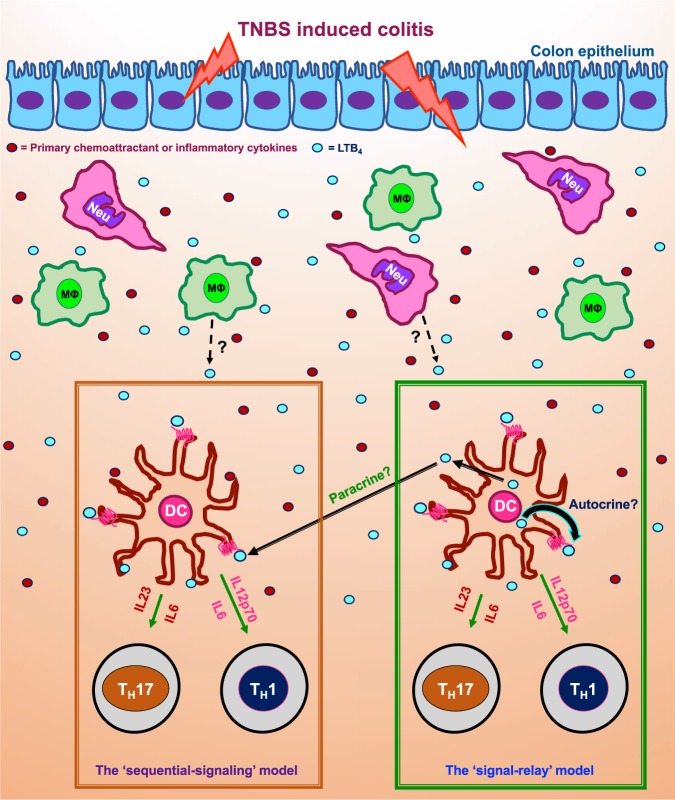
Fig. 1.
A depiction of the ‘sequential-signaling’ and the ‘signal-relay’ models derived from the highlighted study showing the production and action of LTB4 on BLT1 expressing DCs and its role in promoting TH1 and TH17-cells differentiation in a mouse model of TNBS-induced colitis. In the figure, ‘Neu’ stands for infiltrated neutrophil, ‘MØ’ stands for macrophage and ‘DC’ for dendritic cell
The production of LTB4 is known to promote neutrophil influx and the related colon injury parameters in response to IBD;12 however, the role for the LTB4-BLT1 axis in IBD beyond neutrophil influx wasn’t previously explored. Additionally, while the LTB4-BLT1 axis is known to promote DCs secretion of inflammatory cytokines to skew T cells to TH2 phenotype in a mouse model of allergen-induced airway hyperresponsiveness,9 in the case of IBD in mouse, the LTB4-BLT1 axis in DCs promotes TH1 as well as TH17 differentiation11 (summarized in Fig. 1; the ‘sequential-signaling’ model). The other fascinating and less explored aspect of the study by Zhou J et al., has to do with the potential for autocrine/paracrine LTB4-BLT1 signaling and its impact on the functional responses of DCs in a complex inflammatory condition such as the IBD. The autocrine/paracrine LTB4-BLT1 signaling in neutrophils is known to function as ‘signal-relay’ to promote directional migration to a primary chemoattractant source such as bacterial peptide and in vivo to a sterile injury.6,13,14 However, DCs are known to relay signals by sensing and in-turn producing chemokines to promote their self-recruitment to the site of inflammation;4 however, the role for non-chemokine chemoattractants such as LTB4 in relaying signals to drive DC migration and/or other functions is not known. In their study (see Fig. 1f and 2g in ref. 11) Zhou J et al., show that while recruitment of DCs is independent of BLT1 signaling in response to the TNBS-induced colitis, DCs from TNBS-treated mice detect self-generated LTB4 to drive the production of inflammatory cytokines that can drive TH1-cell differentiation.11 Therefore, it appears that the LTB4-BLT1 axis acts as a ‘relay’ not to recruit DCs, but to promote cytokine secretion in DCs to skew T-cell polarization in the TNBS-induced colitis model in mice (summarized in Fig. 1; the ‘signal-relay’ model), which is distinctly different from its known ‘relay’ mechanism to promote directional migration in neutrophils. However, more targeted studies are required to establish if the LTB4-BLT1 axis in DCs works as a ‘relay’ mechanism and how such a signaling axis contributes to different aspects of DC responses, i.e., impact on chemotactic recruitment over other responses such as cytokine secretion to drive T-cell polarization during inflammation. Overall, the LTB4-BLT1 axis might be used by innate immune cells for autocrine/paracrine signaling to promote not just directional migration, but potentially other key innate immune cell-type specific responses during inflammation.
Acknowledgements
Dr. Christina Stuelten is thanked for her constructive inputs on this write-up. This work was supported by the Intramural Research Program of the Center for Cancer Research, NCI, National Institutes of Health.
Competing interests
The author declares no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mizoguchi A. Animal models of inflammatory bowel disease. Prog. Mol. Biol. Transl. Sci. 2012;105:263–320. doi: 10.1016/B978-0-12-394596-9.00009-3. [DOI] [PubMed] [Google Scholar]
- 2.Kiesler P, Fuss IJ, Strober W. Experimental models of inflammatory bowel diseases. Cell Mol. Gastroenterol. Hepatol. 2015;1:154–170. doi: 10.1016/j.jcmgh.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaistha A, Levine J. Inflammatory bowel disease: the classic gastrointestinal autoimmune disease. Curr. Probl. Pediatr. Adolesc. Health Care. 2014;44:328–334. doi: 10.1016/j.cppeds.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Tiberio, L., et al. Chemokine and chemotactic signals in dendritic cell migration. Cell Mol Immunol.15, 346–352 (2018). [DOI] [PMC free article] [PubMed]
- 5.Yokomizo T. Two distinct leukotriene B4 receptors, BLT1 and BLT2. J. Biochem. 2015;157:65–71. doi: 10.1093/jb/mvu078. [DOI] [PubMed] [Google Scholar]
- 6.Subramanian BC, Majumdar R, Parent CA. The role of the LTB4-BLT1 axis in chemotactic gradient sensing and directed leukocyte migration. Semin. Immunol. 2017;33:16–29. doi: 10.1016/j.smim.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin EH, Lee HY, Bae YS. Leukotriene B4 stimulates human monocyte-derived dendritic cell chemotaxis. Biochem. Biophys. Res. Commun. 2006;348:606–611. doi: 10.1016/j.bbrc.2006.07.084. [DOI] [PubMed] [Google Scholar]
- 8.Del Prete A, et al. Regulation of dendritic cell migration and adaptive immune response by leukotriene B4 receptors: a role for LTB4 in up-regulation of CCR7 expression and function. Blood. 2007;109:626–631. doi: 10.1182/blood-2006-02-003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyahara N, et al. Leukotriene B4 receptor 1 expression on dendritic cells is required for the development of Th2 responses and allergen-induced airway hyperresponsiveness. J. Immunol. 2008;181:1170–1178. doi: 10.4049/jimmunol.181.2.1170. [DOI] [PubMed] [Google Scholar]
- 10.Toda A, et al. Attenuated Th1 induction by dendritic cells from mice deficient in the leukotriene B4 receptor 1. Biochimie. 2010;92:682–691. doi: 10.1016/j.biochi.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Zhou, J., et al. BLT1 in dendritic cells promotes Th1/Th17 differentiation and its deficiency ameliorates TNBS-induced colitis. Cell Mol Immunol. 2018 Apr 18 10.1038/s41423-018-0030-2. PMID: 29670278. [DOI] [PMC free article] [PubMed]
- 12.Cuzzocrea S, et al. 5-Lipoxygenase modulates colitis through the regulation of adhesion molecule expression and neutrophil migration. Lab. Invest. 2005;85:808–822. doi: 10.1038/labinvest.3700276. [DOI] [PubMed] [Google Scholar]
- 13.Afonso PV, et al. LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev. Cell. 2012;22:1079–1091. doi: 10.1016/j.devcel.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lammermann T, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]