Abstract
Gangliosides are structurally and functionally polymorphic sialic acid containing glycosphingolipids that are widely distributed in the human body. They play important roles in protecting us against immune attacks, yet they can become targets for autoimmunity and act as receptors for microbes, like the influenza viruses, and toxins, such as the cholera toxin. The expression patterns of gangliosides vary in different tissues, during different life periods, as well as in different animals. Antibodies against gangliosides (AGA) can target immune attack e.g., against neuronal cells and neutralize their complement inhibitory activity. AGAs are important especially in acquired demyelinating immune-mediated neuropathies, like Guillain–Barré syndrome (GBS) and its variant, the Miller–Fisher syndrome (MFS). They can emerge in response to different microbial agents and immunological insults. Thereby, they can be involved in a variety of diseases. In addition, antibodies against GM3 were found in the sera of patients vaccinated with Pandemrix®, who developed secondary narcolepsy, strongly supporting the autoimmune etiology of the disease.
Keywords: ganglioside, Guillain-Barre syndrome, complement, narcolepsy, sialic acid
Subject terms: Diagnostic markers, Autoimmunity, Prognostic markers
Introduction
The neuronal and immune systems represent the two most complicated systems in the vertebrate bodies. The brain has traditionally been considered as an immune privileged organ. However, both humoral and cell-mediated immunity can attack different cells and structures to cause autoimmune diseases. These include multiple sclerosis, narcolepsy, optic neuritis, limbic encephalitis, and Guillain–Barré syndrome. Immunoinflammatory mechanisms are believed to be involved in various forms of dementia and neurodegenerative diseases, as well.
Our understanding of many neurological and neurodegenerative pathological processes has increased a lot, despite the fact that the brain is not an easily accessible organ for studies, and that the actual disease processes have usually taken place before symptoms appear. The recent interest in the interactions between the nervous system and the immune system has been boosted by better opportunities to image intracerebral phenomena and by the identification of the network of dural lymphatic vessels.1,2
Molecular targets for neuronal autoimmunity are being better recognized, but in some diseases, like multiple sclerosis (MS) and narcolepsy, uncertainty about the real targets still exists. The targets are not always exclusively proteins, but could include lipids or glycolipids, as well. Our aim in the present review is to summarize and highlight the possibility that antiganglioside antibodies (AGAs) could have a pathophysiological role and help in the diagnostics or prognostic evaluation of the diseases. AGAs are autoantibodies produced against gangliosides, which are sialic acid containing glycosphingolipids (Box 1). Since their discovery, AGAs have been linked to a wide variety of pathologies, notably inflammatory neuropathies following an immunological insult.3,4 In recent years, gangliosides received renewed interest as targets for cancer immunotherapy. It is known that the GD3 ganglioside is strongly expressed by melanoma cells.5,6 The clearest pathophysiological role for AGAs has been demonstrated in Guillain-Barré Syndrome (GBS). GBS and AGA appearance have been associated with different infectious agents such as Campylobacter jejuni,7 cytomegalovirus,8 Epstein-Barr virus and Haemophilus influenzae.9
The present review will discuss the multiple roles of gangliosides and their key components, sialic acids, in shielding human and microbial cells from immune attack, determining species-specificity of certain infections and as targets for autoimmunity.
Box 1. Sphingolipids and sialic acids.
Sphingolipids are constituents of plasma membranes that fulfill various functions, like membrane stability, adhesion, signaling and cell protection. They also act as receptors for hormones or toxins.10 A particular derivative of sphingolipids, sphingosine-1-phosphate, has signaling functions to regulate the growth and survival of various cell types.11–13
Sialic acids are nine carbon acidic sugars that are enriched in the membranes of all vertebrate cells, and also in a few invertebrates. They can be attached to lipids or proteins.14 They owe their name to the term “saliva” (Greek σάλιο) because they were first identified in 1955 in the bovine salivary mucins.15 Given their negative charge, sialic acids can prevent unwanted interactions of erythrocytes in the blood circulation. They are necessary for maintaining the filtering function of the glomerular basement membranes of the kidney. They are also believed to affect neuronal plasticity.16 The functions of gangliosides remain related to the cells and the molecules which carry them.17 Sialic acids are also exploited by viruses like the influenza viruses, to be internalized into the host cells through interactions between the viral hemagglutinin and neuraminidase with sialic acids on the surfaces of e.g., the host respiratory epithelial cells.18
An overview of gangliosides
Structure, expression and function of gangliosides
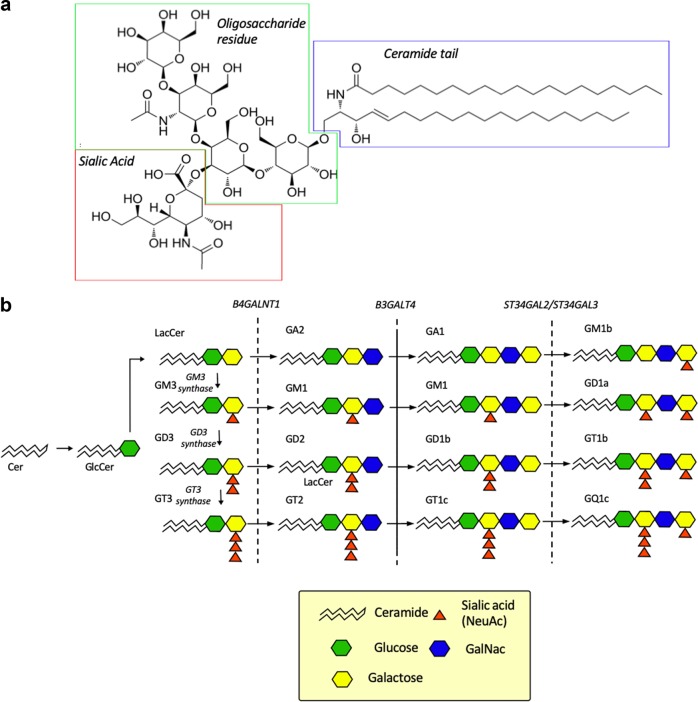
Glycosphingolipids contain a hydrophobic ceramide or sphingoid lipid tail, which is usually anchored to the outer leaflet of the plasma membrane (Fig. 1a). They also contain an oligosaccharide moiety and are classified according to this carbohydrate structure (ganglio, isoganglio, lacto etc.).5,19 Gangliosides are a peculiar and particularly important subclass of glycosphingolipids, because they contain negatively charged sialic acids (N-acetylneuraminic acid or N-glycolylneuraminic acid) linked to the lipooligosaccharide moiety. Gangliosides are named and classified according to the number of sialic acid residues attached (M for one, D for two, T for 3 and Q for 4) to the inner sugar moiety and according to their chromatographic mobility (Fig. 1b).20 The somewhat illogical numbering of the gangliosides (5−x) is based on the number (x) of the inner sugar moieties (glucose, galactose or GalNAc) according to the original experimental classification of Svennerholm.21 Thus, if x is 4, the gangliosides are: GM1, GD1, GT1, x = 3 for GM2, GD2, GT2, and x = 2 for GM3, GD3, and GT3 (Fig. 1b).
Fig. 1.
Structures and biosynthesis of gangliosides. a Structure of the GM1 ganglioside. The sialic acid (red) is linked to the oligosaccharide residue (green) ultimately connected to the ceramide tail (blue) that is embedded in the outer leaflet of the cell membrane. b A schematic representation of the biosynthetic pathway and structures of gangliosides. Gangliosides are sialylated glycosphingolipids. Their synthesis consists of the sequential addition of sugars and/or sialic acids (neuraminic acid; NeuAc) by two main groups of enzymes, i.e., sialyltransferases (GM3/GD3/GT3 synthases and ST3GAL2/ ST3GAL3) and glycosyltransferases (B4GALNT1 and B3GALT4).19,148 The letters M (1), D (2), T (3) and Q (4) indicate the number of sialic acid residues, respectively. The numbers, on the other hand, indicate indirectly the number of sugar residues subtracted from 5: e.g., GM1 contains 5–1 = 4 sugar residues, and GD3 contains 5–3 = 2 sugar residues. Abbreviations: beta-1,4-N-acetyl-galactosaminyl transferase 1 (B4GALNT1), beta-1,3-galactosyltransferase 4 (B3GALT4), ST3 beta-galactoside alpha-2,3-sialyltransferase 2 (ST3GAL2), ST3 beta-galactoside alpha-2,3-sialyltransferase 3 (ST3GAL3)
Gangliosides are ubiquitously expressed throughout the body tissues and fluids, but they are particularly abundant in the brain and in the nervous system. They participate in the maintenance and repair of neuronal cells22, memory formation23 and synaptic transmission24. They also take part in the development and regeneration of neurons.25,26 During brain development, ganglioside expression undergoes massive qualitative and quantitative changes.27 The ganglioside expression profile changes so that in embryonic rodent and human brain the predominant gangliosides are simple gangliosides like GM3 and GD3, while in adults more complex species such as GM1, GD1a, GD1b and GT1b predominate28 and comprise over the 90% of the adult brain gangliosides.20,29,30 This difference in ganglioside expression reflects changes in the expression of various glycosyltransferases, i.e., the enzymes needed for the synthesis of gangliosides.20,31 Vajn et al.29 have used immunochemical techniques to localize the presence of the above-mentioned gangliosides in the brains of C57bl/6 mice (Table 1).
Table 1.
Areas of localization of the main gangliosides
| Ganglioside | Area of localization | Reference |
|---|---|---|
| GM1 |
Mainly in the white matter following the expression of MAG (a myelin marker). Present also in some nuclei, especially abundant in the hypothalamic nuclei but absent in amygdala and basal nuclei Abundant also in gut epithelial cells |
Vajn29 Aleadini149 |
| GD1a |
Predominantly expressed in the gray matter Strong presence in the olfactory bulb and substantia nigra Weak expression in thalamus (in the reticular nucleus) |
Vajn29 |
| GD1b |
Predominantly expressed in the gray matter (like GD1a) Strong expression in the epithalamus Widely expressed in both white matter and gray matter of the spinal cord |
Vajn29 |
| GT1b | Strong expression in the pallidus and raphe magnus | Vajn29 |
| GM3 |
A major endothelial cell ganglioside (angiogenesis suppressor) Especially present in “glycosignaling domains” Hypothalamus |
Hakomori150 |
| GQ1b |
Paranodal region Schwann cells |
Rodella151 Fehmi4 |
In cells, gangliosides are concentrated in the outermost leafleats of the plasma membranes (PMs) in transient structures referred to as lipid rafts.32,33 Gangliosides are attached to the membrane so that their hydrophobic ceramide tail is embedded in the lipid membrane, and the sugar moiety is protruding out from the membrane. Lipid rafts are specific and transient subdomains rich in glycosphingolipids (e.g., gangliosides), cholesterol and distinct proteins, e.g., caveolins, flotillins and glycophosphoinositol (GPI)-linked proteins. They also contain molecules involved in signaling, like low molecular weight and hetero-trimeric G proteins, EGF receptors, PDGF receptors, endothelin receptors, MAP kinase and protein kinase C.34 Gangliosides interact with phospholipids, cholesterol and transmembrane proteins of the lipid rafts both in cis and trans. They are involved in many cellular processes such as regulation of many cellular functions, neurotransmission, interaction with regulatory proteins of the nervous system and in cell-cell recognition, proliferation, as well as in the modulation of signal transduction pathways.10
Immunological roles of gangliosides
Gangliosides have multiple important structural and functional roles that are related with various immunological functions. Because of their location on the outer leaflet of the plasma membrane, gangliosides are critical components in the protection of host structures against the autologous immune system. By virtue of their robust negative charges, sialic acids shield self-surfaces and repulse the attachment of other cells or macromolecules to the cellular surfaces. On the other hand, certain viruses, bacteria and parasites, like blood merozoites of the deadliest malaria parasite Plasmodium falciparum, have learnt to exploit sialic acids/gangliosides as their receptors. Thus, gangliosides can function as attachment sites for pathogens. Some bacterial toxins (cholera toxin, Salmonella typhi toxin) also use sialic acids as their receptors. Since the binding of these toxins is specific for sialic acids in humans, the diseases they cause also occur primarily in humans.
Another special example is the influenza virus. Influenza viruses bind to sialic acids via their hemagglutinin (H) proteins, and after entry e.g., to respiratory epithelial cells, the neuraminidase (N) enzymes of the virus, will cut the sialic acids from the cell surface to prevent entry of further viruses to the cell. Of great importance, different types of influenza viruses carry H proteins with different sialic acid specificities. Thus, only certain influenza viruses (H1, H2, H3) are infecting humans, while H1 and H3 have more preference for pigs and all types from H1 to H16 can infect birds. The H5 and H7 types are particularly dangerous influenza virus types for birds and a potential risk for humans, as well.35 Variation in the H and N protein structures in pandemic influenza strains forces the adoption of new vaccines against the H and N proteins for seasonally occurring new types of influenza virus during epidemics.
Another major immunological function of gangliosides and sialic acids is to protect our cells from our own complement attack and from autoimmunity. In general, sialic acids are not very immunogenic, although in some cases and with specific sialic acids immunization may occur and cause immune damage (see below). Gangliosides function in the recognition and protection of host organs and tissues from complement attack by binding the complement regulatory protein factor H.36 The complement system has a potentially strong cytotoxic and inflammation-inducing activity. Sialic acids provide a robust protection against complement killing of autologous cells. This is mediated by the binding of the soluble complement inhibitor factor H to the surface sialic acids, when the surfaces are threatened by C3b deposition.37
Structural studies have shown that the specific targets for factor H include the α2–3 linked sialic acid glycans of the GD3 ganglioside.38 Thus, on surfaces, where the complement component C3b has become accidentally bound, the neighboring sialic acids provide the additional affinity to bind the complement inhibitory factor H instead of the activation promoting factor B, which does not bind to sialic acids.36
Gangliosides play an important role in maintaining the integrity of lipid rafts, particular cell membrane microdomains.22,33 Changes in ganglioside composition of lipid rafts may cause alterations in the modes of interactions with individual counterpart proteins, or phospholipid and cholesterol molecules.22 These may be involved in signaling, generation of focal synapse-like points of interactions and removal of membrane components by internalization or exocytosis.39 Such events may take place in T lymphocytes during their activation by various signals.40 Thus, defects in membrane lipid rafts could contribute to dysfunction of CD4+ T helper cells and consequent autoimmunity.
A set of mammalian proteins, more than 150, are anchored to the outer leaflets of cell membranes via a GPI-lipid anchor, which is a standard means of membrane protein anchoring in protozoan parasites. GPI-anchored proteins are enriched in lipid rafts and they can signal via cell membrane tyrosine kinases.41. In humans, GPI-anchored proteins include two complement-regulatory proteins: CD55 (decay accelerating factor; DAF) inhibiting the C3 convertases42 and CD59 (protectin) that inhibits the membrane attack complex formation by binding to the terminal C5b-8 and C5b-9 complexes.43 Alterations in ganglioside profiles may cause changes in the properties of these protective proteins. A deficiency in the anchoring mechanism causes a hematological disorder, paroxysmal nocturnal hemoglobinuria (PNH), where affected blood cells become the target for complement attack. A critical feature of the disease is also an increased tendency for thromboses. Most commonly, the disease is caused by an acquired mutation in the X-chromosomal PIG-A gene, whose product is involved in the GPI-anchor synthesis.44 When cells are attacked by the complement membrane attack complex, they release vesicles enriched in GPI-anchored proteins. Overall, the GPI-lipid anchored proteins together with the attached gangliosides are only relatively loosely bound to cell membranes and can easily become detached. For example, high density lipoproteins in human plasma can transfer GPI-anchored CD59 between cells.45 It is likely that the same applies for gangliosides, as well. Also, Helicobacter pylori, a long living companion in the human gastric ventricular mucosa, may acquire the human CD59 molecules to its surface to help the bacteria to survive attacks by the human complement system.46 Analogously, lipopolysaccharides (LPS) of Gram-negative bacteria may become transferred to the surfaces of human cells. Transfer of cell membrane glycolipids, including gangliosides and LPS, between human and microbial cells could—via an adjuvant type effect—be involved in the generation of antibody responses against these structures.
Pathologies involving antiganglioside antibodies (AGAS)
Neurological diseases
As stated above, gangliosides are particularly enriched on the outer leaflets of neuronal membranes. Lipid rafts and gangliosides therein have been suggested to serve important neuronal functions, such as modulation of ion channels and transporters, neuronal interactions and recognition, Ranvier node stability and synaptic transmission. In general, gangliosides seem to have important roles in the maintenance of the nervous system.
Various diseases, especially those involving the neurological system and many neurodegenerative diseases may arise from disturbed ganglioside metabolism or alternatively from the action of antibodies directed against single or multiple targets. In 2004 mutations to GM3 synthase were identified as the cause of an autosomal recessive infantile-onset epilepsy syndrome.47 In addition, mutations in ST3GAL5, which codes for an enzyme necessary for ganglioside biosynthesis, result in an early-onset seizure disorder with motor and cognitive disturbances. Furthermore, mutations in B4GALNT1, a gene encoding for another enzyme of the ganglioside biosynthetic pathways, result in hereditary spastic paraplegia and intellectual deficits.10
In addition to gangliosidoses, ganglioside accumulation diseases, also diseases like infantile epilepsy, Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD) and multiple sclerosis (MS) may involve disturbed ganglioside metabolism.48 On the other hand, the peripheral neuropathy Guillain–Barré syndrome (GBS) is an example of a disease associated with production of autoreactive antiganglioside antibodies (AGAs).49 As studied by Ceccarelli et al. already in the 70’s, gangliosides can promote the regeneration of injured neurons.26 Exogenously administered gangliosides mimic endogenous gangliosides through their insertion into the plasma membranes after first adhering as micelles.25 During recent years, exogenous purified gangliosides have been used experimentally to treat certain neurological diseases.48
AGAs have been found in a variety of disorders classically considered to be of exclusive neurological interest. Anti-GM1 AGAs have been found in Alzheimer’s disease (AD) patients.50 AD has been also related to IgM anti-GD1b51,52 and anti-GQ1bα antibodies, although the latter association has received discordant views in the literature.53,54 It appears that GM1-amyloid-beta protein complexes have the capacity to increase Aβ assembly.55 To further complicate the picture, Svennerholm et al in 2002, reported a slowing of the degenerative process in Alzheimer’s disease patients treated with exogenous GM1.21 Similarly to AD, association to anti-GM1 antibodies has been found also in Parkinson disease56 and GM1 supplementation has been studied as a possible treatment strategy57. The presence of AGA in amyotrophic lateral sclerosis (ALS) has been investigated, but recent studies found no differences between ALS patients and healthy controls.58 AGA were also sporadically identified in cases of myasthenia gravis and Lambert–Eaton syndrome59,60 and in a single case of small fiber neuropathy61. Because of their ubiquitous nature in the neuronal system, it is not surprising that gangliosides have potentially multiple pathological roles in neurological diseases. Some of them play key roles in disease development, whereas in other cases ganglioside abnormalities are secondary to the actual disease process.
Immune-mediated diseases
The presence of AGAs has been investigated in many immune-mediated diseases, especially in those with neurological manifestations. In MS patients, AGAs have been detected in several studies, but their role in disease progression remains unclear.62 In fact, the presence of anti-GM1 antibodies appears to be unrelated to the level of brain atrophy.63,64 However, patients with primary MS have been shown to have higher plasma levels of anti-GM3 and anti-GQ1b antibodies compared to patients with relapsing-remitting MS, healthy controls and patients with other neurological diseases. This has led to the hypothesis that unconventional T cell reactions against GM3 and GQ1b could contribute to axonal damage.62,65
AGAs have been found in systemic lupus erythematosus (SLE) patients with neuropsychiatric manifestations of the disease. In particular, anti-GM1 antibodies were associated with neuropsychiatric manifestations, highlighting their predictive and, potentially, prognostic value.66,67 Also celiac disease (CD), which can have a variety of neurological manifestations, e.g cerebellar ataxia, peripheral neuropathy, epilepsy and depression68,69, has been associated with anti-GM1 antibodies.70 The trigger that induces the generation of AGAs is not known. However, it is possible that the formation of complexes between gliadin and GM1 leads to the generation of anti-GM1 antibodies as a “side product”.71,72 AGAs have also been found in patients with type 1 diabetes but their correlation with the development of diabetic neuropathy has not been confirmed.73,74
AGAs in healthy individuals
The presence of AGAs in the general population has not been studied thoroughly. However, estimates can be drawn from the data of healthy control groups from many AGA studies. The incidence of AGAs in healthy individuals in control groups appear to range from 1 to 9%.75,76 Sometimes, IgM class anti-GM1 antibodies can be found in the sera of healthy individuals after bacterial infections, while the presence of IgG antibodies, especially of IgG1 and IgG3 subclasses, are generally linked to pathological processes.77
Guillain–Barré syndrome
Gangliosides have many roles in the development of infectious diseases and neurological conditions triggered by infections. In the beginning of infection, gangliosides and other glycosphingolipids can serve as receptors for the entry of many viruses, and for binding of bacteria and fungi, or for toxins produced by bacteria. Pathogens may also carry structural elements on their surfaces, e.g., lipo-oligosaccharides (LOS) which resemble gangliosides. This structural similarity may protect pathogenic bacteria from both innate and adaptive immune defense reactions of the host. On the other hand, in the absence or upon loss of tolerance to these structures, the microbial infections may lead to production of autoantibodies against gangliosides. These would be due to immunological cross-reactions that lead to production of AGAs, which recognize also host gangliosides.
Since the first reports of the disease by Guillain, Barré and Strohl in 191678 our knowledge of Guillain-Barré syndrome (GBS) has exponentially increased over the last century.79 In particular, IgG AGAs are found in more than half of GBS patients and are strongly indicative of the disease, while the role of IgM is still not clear.80 GBS a is the most common cause of a flaccid pararalysis worldwide. It is considered to be a postinfectious, inflammatory, peripheral neuropathy.81 Clinically, it is characterized by weakness and areflexia or hyporeflexia in all four limbs.82,83 GBS has a plethora of variants but the most relevant ones are an acute inflammatory demyelinating polyneuropathy (AIDP), the axonal forms (AMAN and AMSAN), Miller–Fisher syndrome (MFS) and Bickerstaff brainstem encephalitis (BEE).
AIDP is the most common form of GBS. It is characterized by demyelination, probably mediated by antibodies against antigens in the paranodal region or directly on the Schwann cells. AIDP has been associated with antibodies against paranodal proteins like neurofascin 155 and contactin 1.4,84 Associations of AIDP with AGAs, such as GT1b85 are still controversial in the literature because a substantial number of AIDP cases could be reclassified as AMAN or AMSAN.86,87 As previously mentioned, axonal forms of the disease have been described, in which direct axonal damage is mediated by the attack of AGAs against gangliosides located in the nodes of Ranvier.4 Two main axonal forms of GBS are recognized: acute motor axonal neuropathy (AMAN) and acute motor and sensory axonal neuropathy (AMSAN). According to Hughes and Cornblath, antibodies to GM1, GM1b, GD1a, and GalNac-GD1a are in particular implicated in acute motor axonal neuropathy and also, with the exception of GalNac-GD1a, in acute motor and sensory axonal neuropathy.81,88,89
Miller–Fisher syndrome (MFS) was described for the first time in 1956 by Charles Fisher Miller.90 It is characterized by the clinical triad of ophthalmoplegia, ataxia and areflexia. Bickerstaff brainstem encephalitis (BEE), clinically very similar to MFS, is characterized by hypersomnolence, ophthalmoplegia and ataxia.82 Both syndromes are associated with anti-GQ1b antibodies, suggesting that they are two distinct manifestations of a single disease spectrum.91,92 Subsequent testing of the anti-GQ1b antibodies revealed that they react also with GD3 and GT1b gangliosides, making it difficult to identify a clear target for the immunological attack.93
As is apparent from the above, there is no doubt that anti-ganglioside antibodies may cause a variety of serious neurological diseases. However, antibodies alone rarely cause target cell damage. They recruit the complement system, leukocytes and other effector mechanisms into the area of antibody deposition. They mediate inflammation and cell injury, whose extent depends on the types of antibodies (class, subclass, target specificity and affinity), individual patterns or reactivity (including the MHC-type determining the extent of T cell help) and target cell sensitivity to damage. Importantly, sialic acids on gangliosides usually protect cells from complement attack, but this protection may fail, if AGAs neutralize the protective layer on cell surfaces. As pointed out above, the complement inhibitor factor H is a key player in protecting host cells from complement attack, because it binds directly to gangliosides in the context of prior antibody-mediated C3b deposition. Inhibition of factor H binding to neuronal cells predisposes them to complement-mediated cell damage and attack by inflammatory leukocytes.
Infections associated with GBS development
Campylobacter jejuni
Numerous infectious agents have been associated with the onset of GBS and consequently with the production of AGAs. The most important pathogen associated with GBS is Campylobacter jejuni.9,94 Almost three-quarters of the GBS patients developed the disease after a gastrointestinal or respiratory infection.94
The onset of GBS can be explained by the molecular mimicry that occurs between gangliosides and the campylobacter’s lipo-oligosaccharides (LOS).7 Although GM1 in particular is indicated as the target for AGAs in GBS, there are many other homologs between the campylobacter LOS and human gangliosides.95 As stated by Shahrizaila and Yuki the association between GBS and C. jejuni is the is the first human autoimmune disorder that fulfills the criteria to conclude that GBS is caused by molecular mimicry.96 The criteria include (1) the establishment of an epidemiological association between infectious agent and the immune-mediated disease. In general, epidemiological studies have shown 30–35% positivity for C. jejuni in GBS patients as compared to 4.4% in controls.97 The second (2) criterium is to identify antibodies or T cells directed against the target antigen. As previously stated, AGAs were identified in patients with GBS and recognized as the key pathological element in the pathogenesis. Depending on the ganglioside targeted a different manifestation of GBS may occur. Interestingly, also activated T cells and macrophages have been identified around the nerves of GBS patients.88 Apparently, patients develop T cell responses against the exogenous LOS and endogenous glycolipids (gangliosides). Further studies should address the nature of the responding T cells. Possible candidates include innate lymphoid cells, gamma-delta T cells or NK cells. As the third criterium (3) microbial mimics to the target antigens have been identified. They include the low molecular weight LOS of C. jejuni. It has been observed that only certain specific C. jejuni serotypes are associated with GBS, in particular O:19 and O:41.94,98 These serotypes correspond to the development of AGAs especially against GM1 but also to other gangliosides, like GD1a and GD3. As the final criterium (4) it has been shown that GBS can be induced in a rabbit model by an injection with either the GM1 ganglioside or the homologous LOS of C. jejuni.99 The antibodies were found to disrupt lipid rafts, nodal structures and ion channel clusters in the peripheral motor nerves.99 Interestingly, in the animal model the neuronal damage could be inhibited with the synthetic serine protease inhibitor nafamostat mesilate that also acts as a complement inhibitor.100
Pathogens other than C. jejuni
GBS and AGA development can be a post-infectious condition associated with many pathogens other than C. jejuni. These include M. pneumoniae, H. influenzae, cytomegalovirus and Epstein–Barr virus.9,101–103 M. pneumoniae has been linked to the development of GBS. These patients developed antibodies not only against gangliosides but also against anti-galactocerebrosides.104–106 H. influenzae has GM1-like structures on its surface that can elicit a mimicry-type antibody response similar to the one seen in C. jejuni infections.107–109 Analyses of the the antecedent infections in GBS and MFS have indicated that C. jejuni is the most common causative agent for infections preceding the occurrence of the neurological disease, being usually observed in approximately 21–32% of the cases.101,110
Interestingly, it has become clear that also viral infections can predispose to GBS, although the mechanisms may be different than in the case of bacterial infections. Cytomegalovirus has been mainly associated with the development of IgM class anti-GM2 antibodies.111,112 The Varicella-Zoster virus, also known as HHV-3, has been associated with GBS in rare cases.113,114 Recently, GBS has been associated with arboviruses infections, like Zika virus and chikungunya, but the mechanisms are still debated.49
Zika virus (ZIKV) has caused recent outbreaks in Latin America, Africa and French Polynesia. It has been associated with neurological sequelae such as microcephaly in newborns and GBS in adults.115,116 Microcephaly seems to be related to a direct neurotropic effect of the virus on the neural cells of the newborn, given its ability to cross the placenta, while the pathogenesis of GBS is far from being understood.115,117 An autoimmune etiology, through a mechanism of molecular mimicry similar to the one observed in C. jejuni, has been hypothesized, but the mechanism may differ.118 In a few studies, AGAs have been found in GBS patients having antibodies against ZIKV. Nico et al. reported anti-GD3 antibodies in acute ZIKV infections.119 Baskar and colleagues showed anti-GM1 antibodies in 50% of ZIKV-positive patients.120 However, Cao-Lormeau and his team showed that only a fraction of patients with GBS after ZIKV had AGAs, and their sera (anti-GA1 and GD1a) displayed no antibody competition between ZIKV proteins and GA1, a.k.a. asialo GM1.116 In addition to ZIKV, also other arbovirus infections have been associated with GBS, like dengue, West Nile and chikungunya with sporadic reports in the literature. Case reports have been published on GBS in patients with hepatitis E121 or herpes simplex virus infection.122 Parvovirus B19 infections are associated with a broad spectrum of neurological complications. Anti-GD1b AGAs were described in a case report by Sequeira et al. in a 23-year-old immunocompetent woman with a PB19 infection associated with hemolytic anemia and cranial polyneuropathy.123
While in some cases the associations between viral infections and GBS may be coincidental, it is possible that viral infections could lead to autoimmunity e.g., by exposing endogenous structures. One possibility is that a viral infection of cells may force them to release cell membrane particles containing gangliosides, e.g., from the membrane rafts, which then, in association with viral structures, could lead to a bystander-type autoimmune response.
Possible functional roles of AGAs
It has been suggested that in Guillain-Barré syndrome AGAs play a role in the destruction of the neuromuscular junction (NMJ) of nerve cells. NMJ is rich in gangliosides and resides outside the blood-nerve barrier thus making it more vulnerable to attack by antibodies. In the Miller-Fisher variant of GBS, characterized by ataxia, areflexia and ophtalmoplegia, high titer AGAs against GQ1b are present in the majority of patients in the acute phase. These AGAs cross-react often with GT1a, and may react with GD3 and GD1b gangliosides, as well. They activate the human complement system and mediate complement-dependent injury in the motor nerve terminal.124,125 Complement activation will also attract leukocytes to neurons and contribute to activation of neutrophils and macrophages. Often, also T lymphocytes have been detected in the area of damage indicating a cell-mediated immune response. The activated immune cells can release cytokines and oxygen radicals to further damage the target cells. As a result, the protective myelin sheath and even the neuronal axons may become destroyed.
Experimental data by Kanda has revealed that AGAs in vitro can disrupt the integrity of the blood nerve barrier (BNB), possibly foreshadowing a similar effect on the blood brain barrier (BBB).126 Breakdown of BBB or changes in its permeability can be critical events in the pathogenesis of many neurological diseases and AGAs may be key players of the neurological damage.127,128
Secondary narcolepsy following Pandemrix® vaccination
Type I narcolepsy (NT1) is a chronic sleep disorder characterized by excessive daytime sleepiness, cataplexy and, commonly, parasomnias.129,130 The prevalence of narcolepsy is circa 30 per 100,000. It occurs in a bimodal distribution with two peaks at the ages of 15 and 35 years.130,131 Cataplexy is defined as a sudden loss of bilateral muscular tone, usually brought on by a strong emotional response. It is essentially unique to narcolepsy type I.132 A clue to the etiology of narcolepsy was obtained, when narcoleptic dogs were found to be genetically deficient in the orexin receptor type 2.133 Human NT1 is caused by a reduced orexinergic signaling, probably due to a loss of orexinergic neurons in the hypothalamus.131,132 The neuropeptide product of the neurons, orexin, is also known as hypocretin, encoded by the HCRT gene. It is involved in maintaining the sleep-wake cycle and regulating muscle tone and appetite.
There are different genetic traits related to the human narcoleptic phenotype, i.e., mutations in the T-cell-receptor α chain and purinergic receptor subtype 2Y11.131 It associates strongly with the HLA- DQB1*06:02 allele, present in practically all narcoleptic patients.134 However, the etiology of NT1 is still under investigation, but in the majority of cases it is an autoimmune disease. No direct evidence is available for the pathogenetic mechanisms in humans but evidence from studies in mice indicate that CD8+T cells can mediated killing of orexinergic neurons to give a narcolepsy-like phenotype.135
Surprisingly, narcolepsy has been associated with the pandemic H1N1 flu vaccine. In Finland and Sweden, between October 2009 and August 2010, and later in other countries, after the vaccination campaign against the H1N1-type influenza A with the Pandemrix vaccine (GSK), a clear increase in narcolepsy cases was reported. The cases were observed in particular in children and adolescents, ranging from 4 to 19 years of age. The vaccination increased the risk of developing narcolepsy 6.5–14.4-fold in vaccinated subjects compared to unvaccinated ones.136–138 An increase in narcolepsy was not restricted to the flu vaccine. Interestingly, in China, in the absence of vaccination, a 6-fold increase in the incidence of narcolepsy was observed after the 2009 influenza pandemics.139
The mechanisms and potential autoantigen underlying the development of H1N1 vaccine- or virus infection-induced narcolepsy have remained elusive. The receptor for the H1N1-virus hemagglutinin H1 is sialic acid, α2–3 or α2–6 linked to galactose, structures commonly present on gangliosides, like GD3. Furthermore, the other main protein on the virus particle surface is neuraminidase N1, capable of cleaving sialic acids. A molecular mimicry between orexin and the viral proteins leading to T cell cross-reactivity has been suggested.140 Pandemrix is an AS03-adjuvanted vaccine that contains α-tocopherol, which in vitro can increase the expression of orexin, upregulating the Nrf2 pathway.141 In this way it can potentially favor cross-reactive antigen presentation by MHC class II.142 Another influenza A (H1N1) vaccine, Arepanrix (also by GSK) with a similar composition, same adjuvant and antigenicity as Pandemrix,143 did not show any increase in narcolepsy incidence.144 This could have been due to a different protein antigen composition in the vaccine.145
In our own study137 we found AGAs, in particular anti-GM3 and anti-GM4, in the sera of 18.1% of patients with Pandemrix-induced narcolepsy (compared to 7.3% in unvaccinated controls). The origin of these antibodies could be an immune response against the vaccine adjuvant (squalene) preparation that is from shark liver, a source rich in the GM3 and GM4 gangliosides. The role of anti-ganglioside antibodies in the pathological process is not clear. While the disease most likely is a specific autoimmune response to distinct self antigen(s) expressed by the orexin producing neurons the anti-ganglioside antibodies could have an additional role. A model for the potential disease development is depicted in Fig. 2. Anti-GM3 or anti-GM4 antibodies could (1) directly damage the hypothalamic neurons by binding to their gangliosides and activating the complement system or inflammatory cells. This is unlikely to be a major mechanism given the presence of these autoantibodies only in a proportion of the patients. On the other hand, they are frequently observed in the context of neurological disorders and could have an effect on the aforesaid nervous damage. (2) Since GM3 is a major endothelial ganglioside, anti-GM3 AGAs could damage the cerebral endothelium and BBB increasing its permeability to immune cells and mediators. (3) Furthermore, anti-GM3 autoantibodies could bind to lipid raft gangliosides and activate a signaling pathway that leads to the recruitment of molecules like integrins that can potentially facilitate the chemotaxis of the leukocytes.146 A similar effect has been observed in melanoma cell lines, in which GD3 activation leads to the recruitment of β1 integrins.147 These potential mechanisms could play a role in any disease, where antiganglioside antibodies are formed (Table 2).
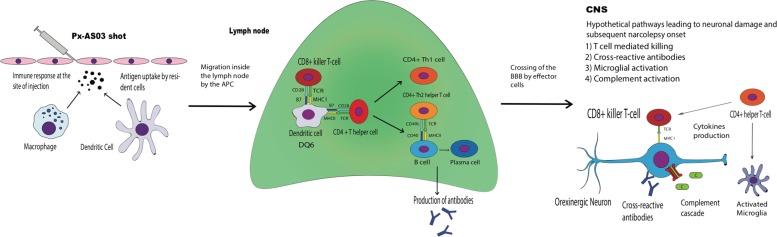
Fig. 2.
A model for the pathogenesis of secondary narcolepsy following Pandemrix® vaccination. In susceptible recipients, i.e., in those, who harbor the HLA- DQB1*06:02 allele, antigen presenting cells (APCs, dendritic cells and macrophages) gather at the injection site after the vaccine shot. Following the uptake of and response to vaccine components (e.g., H1 and N1 proteins, squalene, α-tocopherol, possible nucleoprotein or ganglioside impurities) the APCs migrate to the lymph node presenting the antigens to the T-cells through the MHC class II, inducing their activation. Eventually, through a process of molecular mimicry this mechanism could lead to a targeted attack against hypothalamic orexinergic neurons and to the subsequent narcolepsy onset. Antiganglioside antibodies could emerge upon complex formation between H1N1 proteins and the gangliosides, which would be picked up by the ganglioside responding B cells. T cells specific for the H1N1 proteins would provide the necessary help for antibody formation by B cells, and subsequently by plasma cells. Antiganglioside antibodies have an ability to both activate complement and to neutralize the complement inhibitory gangliosides on neuronal cell surfaces
Table 2.
Disease associations of antiganglioside antibodies (AGA)
| Disease(s) associated | AGAs | References |
|---|---|---|
| Alzheimer’s disease | GM1, GD1b (IgM strongly correlates with AD), GQ1bα (association still debated) |
Chapman50, Hatziflippu52, Koutsouraki51 |
| Multiple sclerosis | GM3, GQ1b | Pender65 |
| Systemic lupus erythematosus | GM1 |
Costellat152, Galeazzi67 |
| Celiac disease | GM1 (or GM1-gliadin complexes) | Przybylska70 |
| HIV (asymptomatic patients) | GM2, GD1a, GQ1b | Nicolae153 |
| Parvovirus B19 | GD1b | Sequeira123 |
| Type I diabetes | GM1, GM2, GM3, GD1b, GD1a | Lucchetta73 |
| Secondary narcolepsy | GM3, GM4 | Saariaho137 |
| Guillain–Barré syndrome |
AIDP: GT1b AMAN: GM1, GM1b, GD1a, GalNac-GD1a AMSAN: GM1, GM1b, GD1a MF: GQ1b BE: GQ1b |
Hughes and Cornblath88, Naik85 |
| Breast cancer | GM3, GD3, 9-O-Ac GD3, 9-O-Ac GT3 | Groux-Degroote154 |
Conclusions
Gangliosides and especially their key variable components, sialic acids, are at the border of immune tolerance. In general, sialic acids protect and shield human cell and tissue structures against immune attacks. In consequence, certain microbes have learnt to mimic or exploit these structures to escape immune attack or to use them as receptors for entry into human tissues. On some occasions, however, the tolerance is broken, and the immune system succeeds in responding to the microbial mimics of sialic acids with consequent autoimmunity as a byproduct. Examples include neuronal autoimmune diseases like Guillain–Barré and Miller–Fisher syndromes.
The role of AGAs in the pathogenesis of many different diseases is not fully understood. AGAs could (i) participate in the direct damage to the structure that they bind to, (ii) be a sequela of different types of infectious diseases, (iii) facilitate many immune-mediated pathological mechanisms, including complement activation and the enhancement of its functional effects because of neutralization of a basic complement control mechanism of the cells.
Relative to proteins, immune responses to carbohydrates and glycolipids have often received less attention in studies of autoimmunity despite the fact that they could have a direct or an important adjunct role. Future studies should address the abilities of antibodies to neutralize the functions of gangliosides, including their roles as receptors or protective molecules against immune attack. Therapeutic inhibition of complement activation could be considered in cases, where the protective ability of gangliosides has become compromised, and complement causes serious injury. Because of varying expression during different life periods, gangliosides could explain relative sensitivities to diseases in different age groups, especially in the elderly people. Their roles as receptors for infectious agents and initiators of immunological cross-reactions still needs further exploration. Also, studies on the roles of gangliosides in T cell-mediated immune responses and in regulating activities of nonconventional T cells, NK cells and innate lymphoid cells could prove useful and interesting in the future.
Acknowledgements
This work was supported by the Sigrid Jusélius Foundation, the Academy of Finland (292393) and Helsinki University Central Hospital (TYH2018313, TYH2019311) grants.
Competing interests
The authors declare no competing interests.
References
- 1.Aspelund A, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louveau A, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, Shao C, Yang C, Kang X, Zhang G. Association of anti-gangliosides antibodies and anti-CMV antibodies in Guillain-Barré syndrome. Brain Behav. 2017;7:1–6. doi: 10.1002/brb3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fehmi, J., Scherer, S. S., Willison, H. J. & Rinaldi, S. Nodes, paranodes and neuropathies. J. Neurol. Neurosurg. Psychiatry 1–11 10.1136/jnnp-2016-315480 (2017). [DOI] [PubMed]
- 5.Groux-Degroote S, Guérardel Y, Delannoy P. Gangliosides: structures, biosynthesis, analysis, and roles in cancer. ChemBioChem. 2017;18:1146–1154. doi: 10.1002/cbic.201600705. [DOI] [PubMed] [Google Scholar]
- 6.Ohmi Y, et al. Differential roles of gangliosides in malignant properties of melanomas. PLoS ONE. 2018;13:e0206881. doi: 10.1371/journal.pone.0206881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moran AP, Annuk H, Prendergast MM. Antibodies induced by ganglioside-mimicking Campylobacter jejuni lipooligosaccharides recognise epitopes at the nodes of Ranvier. J. Neuroimmunol. 2005;165:179–185. doi: 10.1016/j.jneuroim.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Sivadon V, et al. Prévalence et caractéristiques des syndromes de Guillain-Barré associés à Campylobacter jejuni et au cytomégalovirus en région parisienne. Pathol. Biol. 2005;53:536–538. doi: 10.1016/j.patbio.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Tam CC, et al. Guillain-Barrè syndrome and preceding infection with Campylobacter, influenza and Epstein-Barr virus in the general practice research database. PLoS ONE. 2007;2:1–6. doi: 10.1371/journal.pone.0000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnaar RL. Gangliosides of the vertebrate nervous system. J. Mol. Biol. 2016;428:3325–3336. doi: 10.1016/j.jmb.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 12.Spiegel, S. & Milstien, S. An enigmatic signalling lipid. 10.1038/nrm1103 (1884). [DOI] [PubMed]
- 13.Kolesnick R. Critical review: the therapeutic potential of modulating the ceramide/sphingomyelin pathway. J. Clin. Invest. 2002;110:3–8. doi: 10.1172/JCI0216127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varki, A. & Schauer, R. Sialic Acids. Essentials of Glycobiology (Cold Spring Harbor Laboratory Press, 2009). [PubMed]
- 15.Blix G, Lindberg E, Odin L, Werner I. Sialic acids. Nature. 1955;175:340–341. doi: 10.1038/175340a0. [DOI] [PubMed] [Google Scholar]
- 16.Varki A. Sialic acids in human health and disease. Trends Mol. Med. 2009;14:351–360. doi: 10.1016/j.molmed.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnaar RL, Gerardy-Schahn R, Hildebrandt H. Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 2014;94:461–518. doi: 10.1152/physrev.00033.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krammer F, et al. Influenza. Nat. Rev. Dis. Prim. 2018;4:3. doi: 10.1038/s41572-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsen ASB, Færgeman NJ. Sphingolipids: membrane microdomains in brain development, function and neurological diseases. Open Biol. 2017;7:170069. doi: 10.1098/rsob.170069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu RK, Tsai Y-T, Ariga T, Yanagisawa M. Structures, biosynthesis, and functions of gangliosides–an overview. J. Oleo Sci. 2011;60:537–544. doi: 10.5650/jos.60.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Svennerholm L. Ganglioside designation. Adv. Exp. Med. Biol. 1980;125:11. doi: 10.1007/978-1-4684-7844-0_2. [DOI] [PubMed] [Google Scholar]
- 22.Ohmi Y, et al. Gangliosides are essential in the protection of inflammation and neurodegeneration via maintenance of lipid rafts: elucidation by a series of ganglioside-deficient mutant mice. J. Neurochem. 2011;116:926–935. doi: 10.1111/j.1471-4159.2010.07067.x. [DOI] [PubMed] [Google Scholar]
- 23.Rahmann H. Brain gangliosides and memory formation. Behav. Brain Res. 1995;66:105–116. doi: 10.1016/0166-4328(94)00131-X. [DOI] [PubMed] [Google Scholar]
- 24.Palmano K, Rowan A, Guillermo R, Guan J, McJarrow P. The role of gangliosides in neurodevelopment. Nutrients. 2015;7:3891–3913. doi: 10.3390/nu7053891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mocchetti I. Exogenous gangliosides, neuronal plasticity and repair, and the neurotrophins. Cell. Mol. Life Sci. 2005;62:2283–2294. doi: 10.1007/s00018-005-5188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ceccarelli, B., Aporti, F. & Finesso, M. Effects of brain gangliosides on functional recovery in experimental regeneration and reinnervation. Ganglioside Funct. 275–293 10.1007/978-1-4614-4614-9_17 (1976). [DOI] [PubMed]
- 27.Ngamukote, S., Yanagisawa, M., Ariga, T., Ando, S. & Yu, R. K. Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. J. NeuroChem. 2327–2341 10.1111/j.1471-4159.2007.04910.x (2007). [DOI] [PubMed]
- 28.Yuki N. Molecular mimicry between gangliosides and lipopolysaccharides of Campylobacter jejuni isolated from patients with Guillain-Barré syndrome and Miller Fisher syndrome. J. Infect. Dis. 1997;176(Suppl 2):S150–S153. doi: 10.1086/513800. [DOI] [PubMed] [Google Scholar]
- 29.Vajn K, Viljetić B, Degmečić IV, Schnaar RL, Heffer M. Differential distribution of major brain gangliosides in the adult mouse central nervous system. PLoS ONE. 2013;8:1–11. doi: 10.1371/journal.pone.0075720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanda T, Yoshino H, Ariga T, Yamawaki M, Yu RK. Glycosphingolipid antigens in cultured microvascular bovine brain endothelial cells: Sulfoglucuronosyl paragloboside as a target of monoclonal IgM in demyelinative neuropathy. J. Cell Biol. 1994;126:235–246. doi: 10.1083/jcb.126.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furukawa K, Ohmi Y, Ohkawa Y, Tajima O, Furukawa K. Glycosphingolipids in the regulation of the nervous system. Adv. Neurobiol. 2014;9:307–320. doi: 10.1007/978-1-4939-1154-7_14. [DOI] [PubMed] [Google Scholar]
- 32.Sonnino S, Mauri L, Chigorno V, Prinetti A. Gangliosides as components of lipid membrane domains. Glycobiology. 2007;17:1030. doi: 10.1093/glycob/cwl052. [DOI] [PubMed] [Google Scholar]
- 33.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 34.Pike LJ. Lipid rafts. J. Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Hampson AW, Mackenzie JS. The influenza viruses. Med. J. Aust. 2006;185:S39–S43. doi: 10.5694/j.1326-5377.2006.tb00705.x. [DOI] [PubMed] [Google Scholar]
- 36.Meri, S. Self-nonself discrimination by the complement system. FEBS Lett. 2418–2434 10.1002/1873-3468.12284 (2016). [DOI] [PubMed]
- 37.Meri S, Pangburn MK. Discrimination between activators and nonactivators of the alternative pathway of complement: Regulation via a sialic acid/polyanion binding site on factor H. Proc. Natl Acad. Sci. USA. 1990;87:3982–3986. doi: 10.1073/pnas.87.10.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blaum BS, et al. Structural basis for sialic acid-mediated self-recognition by complement factor H. Nat. Chem. Biol. 2015;11:77–82. doi: 10.1038/nchembio.1696. [DOI] [PubMed] [Google Scholar]
- 39.Jury EC, Kabouridis PS, Flores-Borja F, Mageed RA, Isenberg DA. Altered lipid raft-associated signaling and ganglioside expression in T lymphocytes from patients with systemic lupus erythematosus. J. Clin. Invest. 2004;113:1176–1187. doi: 10.1172/JCI200420345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janes PW, Ley SC, Magee AI, Kabouridis PS. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin. Immunol. 2000;12:23–34. doi: 10.1006/smim.2000.0204. [DOI] [PubMed] [Google Scholar]
- 41.Štefanová I, Hořejší V, Ansotegui IJ, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991;254:1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- 42.Medof EM, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of Decay-Accelerating Factor (DAF) into their membranes. J. Exp. Med. 1984;160:1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meri S, et al. Human protectin (CD59), an 18-20 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990;70:1–9. [PMC free article] [PubMed] [Google Scholar]
- 44.Takeda J, et al. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell. 1993;73:703–711. doi: 10.1016/0092-8674(93)90250-T. [DOI] [PubMed] [Google Scholar]
- 45.Väkevä A, Jauhiainen M, Ehnholm C, Lehto T, Meri S. High-density lipoproteins can act as carriers of glycophosphoinositol lipid-anchored CD59 in human plasma. Immunology. 1994;82:28–33. [PMC free article] [PubMed] [Google Scholar]
- 46.Rautemaa R, Rautelin H, Kokkola A, Kärkkäinen P, Meri S. Survival of Helicobacter pylori from complement lysis by binding of GPI-anchored protectin (CD59) Gastroenterology. 2001;120:470–479. doi: 10.1053/gast.2001.21197. [DOI] [PubMed] [Google Scholar]
- 47.Simpson MA, et al. Infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of GM3 synthase. Nat. Genet. 2004;36:1225–1229. doi: 10.1038/ng1460. [DOI] [PubMed] [Google Scholar]
- 48.Ariga T. Pathogenic role of ganglioside metabolism in neurodegenerative diseases. J. Neurosci. Res. 2014;92:1227–1242. doi: 10.1002/jnr.23411. [DOI] [PubMed] [Google Scholar]
- 49.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;388:717–727. doi: 10.1016/S0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- 50.Chapman J, Sela BA, Wertman E, Michaelson DM. Antibodies to ganglioside GM1 in patients with Alzheimer’s disease. Neurosci. Lett. 1988;86:235–240. doi: 10.1016/0304-3940(88)90577-0. [DOI] [PubMed] [Google Scholar]
- 51.Koutsouraki E, et al. The probable auto-antigenic role of lipids (anti-ganglioside antibodies) in the pathogenesis of Alzheimer’s disease. J. Alzheimers Dis. 2014;42(Suppl 3):S163–S166. doi: 10.3233/JAD-132633. [DOI] [PubMed] [Google Scholar]
- 52.Hatzifilippou E, Koutsouraki E, Costa VG, Baloyannis SJ. Antibodies against gangliosides in patients with dementia. Am. J. Alzheimers Dis. Other Demen. 2014;29:660–666. doi: 10.1177/1533317514534953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ariga T, et al. Anti-Chol-1 antigen, GQ1bα, antibodies are associated with Alzheimer’s disease. PLoS ONE. 2013;8:e63326. doi: 10.1371/journal.pone.0063326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miura Y, et al. Autoantibodies to GM1 and GQ1bα are not biological markers of Alzheimer’s disease. J. Alzheimers Dis. 2014;42:1165–1169. doi: 10.3233/JAD-140474. [DOI] [PubMed] [Google Scholar]
- 55.Yanagisawa K. GM1 ganglioside and Alzheimer’s disease. Glycoconj. J. 2015;32:87–91. doi: 10.1007/s10719-015-9579-5. [DOI] [PubMed] [Google Scholar]
- 56.Zappia M, et al. Anti-GM1 ganglioside antibodies in Parkinson’s disease. Acta Neurol. Scand. 2002;106:54–57. doi: 10.1034/j.1600-0404.2002.01240.x. [DOI] [PubMed] [Google Scholar]
- 57.Schneider, J. S. GM1 ganglioside in the treatment of Parkinson’s disease. Ann. N.Y. Acad. Sci.845, 363–373 (1998). [DOI] [PubMed]
- 58.Kollewe K, et al. Anti-ganglioside antibodies in amyotrophic lateral sclerosis revisited. PLoS ONE. 2015;10:1–11. doi: 10.1371/journal.pone.0125339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scaioli V, Andreetta F, Mantegazza R. Unusual neurophysiological and immunological findings in myasthenia gravis: a case report. J. Peripher. Nerv. Syst. 2004;9:92–97. doi: 10.1111/j.1085-9489.2004.09207.x. [DOI] [PubMed] [Google Scholar]
- 60.Mitsui Y, et al. Sensorimotor polyneuropathy associated with chronic lymphocytic leukemia, IgM antigangliosides antibody and human T-cell leukemia virus I infection. Muscle Nerve. 1999;22:1461–1465. doi: 10.1002/(SICI)1097-4598(199910)22:10<1461::AID-MUS19>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 61.Favoni V, Liguori R, Incensi A, Fileccia E, Donadio V. The incidental finding of elevated anti GQ1B antibodies in a patient with selective small fiber neuropathy. J. Neurol. Sci. 2018;388:192–194. doi: 10.1016/j.jns.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 62.Sadatipour BT, Greer JM, Pender MP. Increased circulating antiganglioside antibodies in primary and secondary progressive multiple sclerosis. Ann. Neurol. 1998;44:980–983. doi: 10.1002/ana.410440621. [DOI] [PubMed] [Google Scholar]
- 63.Giovannoni G, Morris PR, Keir G. Circulating antiganglioside antibodies are not associated with the development of progressive disease or cerebral atrophy in patients with multiple sclerosis. Ann. Neurol. 2000;47:684–685. doi: 10.1002/1531-8249(200005)47:5<684::AID-ANA27>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 64.Valentino P, et al. Anti-GM1 antibodies are not associated with cerebral atrophy in patients with multiple sclerosis. Mult. Scler. J. 2009;15:114–115. doi: 10.1177/1352458508096685. [DOI] [PubMed] [Google Scholar]
- 65.Pender MP, et al. Increased circulating T cell reactivity to GM3 and GQ1b gangliosides in primary progressive multiple sclerosis. J. Clin. Neurosci. 2003;10:63–66. doi: 10.1016/S0967-5868(02)00270-9. [DOI] [PubMed] [Google Scholar]
- 66.Costallat LT, de Oliveira RM, Santiago MB, Cossermelli W, Samara AM. Neuropsychiatric manifestations of systemic lupus erythematosus: the value of anticardiolipin, antigangliosides and antigalactocerebrosides antibodies. Clin. Rheumatol. 1990;9:489–497. doi: 10.1007/BF02030510. [DOI] [PubMed] [Google Scholar]
- 67.Galeazzi M, et al. Anti-ganglioside antibodies in a large cohort of European patients with systemic lupus erythematosus: clinical, serological, and HLA class II gene associations. European Concerted Action on the Immunogenetics of SLE. J. Rheumatol. 2000;27:135–141. [PubMed] [Google Scholar]
- 68.Siqueira Neto JI, Leite Vieira Costa AC, Magalhães FG, Sampaio Silva G. Neurological manifestations of celiac disease. Arq. Neuropsiquiatr. 2004;62:969–972. doi: 10.1590/S0004-282X2004000600007. [DOI] [PubMed] [Google Scholar]
- 69.Bushara KO. Neurologic presentation of celiac disease. Gastroenterology. 2005;128:S92–S97. doi: 10.1053/j.gastro.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 70.Przybylska-Feluś M, et al. Anti-GM1 ganglioside antibodies, neuron specific enolase and interleukin 10 concentrations as potential markers of autonomic nervous system impairment in celiac disease - preliminary findings. Pol. Arch. Intern. Med. 2016;126:763–771. doi: 10.20452/pamw.3512. [DOI] [PubMed] [Google Scholar]
- 71.Sabayan B, Foroughinia F, Imanieh M-H. Can Campylobacter jejuni play a role in development of celiac disease? A hypothesis. World J. Gastroenterol. 2007;13:4784–4785. doi: 10.3748/wjg.v13.i35.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alaedini A, Latov N. Transglutaminase-independent binding of gliadin to intestinal brush border membrane and GM1 ganglioside. J. Neuroimmunol. 2006;177:167–172. doi: 10.1016/j.jneuroim.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 73.Lucchetta M, et al. Anti-ganglioside autoantibodies in type 1 diabetes. Muscle Nerve. 2010;41:50–53. doi: 10.1002/mus.21326. [DOI] [PubMed] [Google Scholar]
- 74.Ge S, et al. Associations of serum anti-ganglioside autoantibodies and inflammatory markers in diabetetic peripheral neuropathy. Diabetes Res Clin. Pr. 2016;115:68–76. doi: 10.1016/j.diabres.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 75.Conrad K, et al. A new line immunoassay for the multiparametric detection of antiganglioside autoantibodies in patients with autoimmune peripheral neuropathies. Ann. N.Y. Acad. Sci. 2007;1109:256–264. doi: 10.1196/annals.1398.031. [DOI] [PubMed] [Google Scholar]
- 76.Johannis W, Renno JH, Klatt AR, Wielckens K. Anti-glycolipid antibodies in patients with neuropathy: a diagnostic assessment. J. Clin. Neurosci. 2014;21:488–492. doi: 10.1016/j.jocn.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 77.Lardone RD, Alaniz ME, Irazoqui FJ, Nores GA. Unusual presence of anti-GM1 IgG-antibodies in a healthy individual, and their possible involvement in the origin of disease-associated anti-GM1 antibodies. J. Neuroimmunol. 2006;173:174–179. doi: 10.1016/j.jneuroim.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 78.Guillain, G., Barré, J. A. & Strohl, A. [Sur un syndrome de radiculo-névrite avec hyperalbuminose du liquide céphalo-rachidien sans réaction cellulaire. Remarques sur les caractères cliniques et graphiques des réflexes tendineux]. Ann. Med. Interne40, 1462–1470 (1916). [PubMed]
- 79.Goodfellow JA, Willison HJ. Guillain-Barré syndrome: a century of progress. Nat. Rev. Neurol. 2016;12:723–731. doi: 10.1038/nrneurol.2016.172. [DOI] [PubMed] [Google Scholar]
- 80.Koga M, Takahashi M, Yokoyama K, Kanda T. Ambiguous value of anti-ganglioside IgM autoantibodies in Guillain-Barré syndrome and its variants. J. Neurol. 2015;262:1954–1960. doi: 10.1007/s00415-015-7806-4. [DOI] [PubMed] [Google Scholar]
- 81.Fujimura H. The Guillain-Barré syndrome. Handb. Clin. Neurol. 2013;115:383–402. doi: 10.1016/B978-0-444-52902-2.00021-7. [DOI] [PubMed] [Google Scholar]
- 82.Wakerley BR, et al. Guillain–Barré and Miller Fisher syndromes—new diagnostic classification. Nat. Rev. Neurol. 2014;10:537–544. doi: 10.1038/nrneurol.2014.138. [DOI] [PubMed] [Google Scholar]
- 83.Vucic S, Kiernan MC, Cornblath DR. Guillain-Barré syndrome: an update. J. Clin. Neurosci. 2009;16:733–741. doi: 10.1016/j.jocn.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 84.Doppler K, et al. Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathy. Brain. 2016;139:2617–2630. doi: 10.1093/brain/aww189. [DOI] [PubMed] [Google Scholar]
- 85.Naik Gs, et al. Anti-ganglioside antibodies profile in guillain-barré syndrome: correlation with clinical features, electrophysiological pattern, and outcome. Neurol. India. 2017;65:1001. doi: 10.4103/neuroindia.NI_1226_15. [DOI] [PubMed] [Google Scholar]
- 86.Kuwabara S, Yuki N. Axonal Guillain-Barré syndrome: concepts and controversies. Lancet Neurol. 2013;12:1180–1188. doi: 10.1016/S1474-4422(13)70215-1. [DOI] [PubMed] [Google Scholar]
- 87.Uncini A, Kuwabara S. Nodopathies of the peripheral nerve: an emerging concept. J. Neurol. Neurosurg. Psychiatry. 2015;86:1186–1195. doi: 10.1136/jnnp-2014-310097. [DOI] [PubMed] [Google Scholar]
- 88.Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet. 2005;366:1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 89.Yuki N. Guillain-Barré syndrome and anti-ganglioside antibodies: a clinician-scientist’s journey. Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci. 2012;88:299–326. doi: 10.2183/pjab.88.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fisher M. An unusual variant of acute idiopathic polyneuritis (syndrome of sphthalmoplegia, Ataxia and areflexia) N. Engl. J. Med. 1956;255:57–65. doi: 10.1056/NEJM195607122550201. [DOI] [PubMed] [Google Scholar]
- 91.Shahrizaila N, Yuki N. Bickerstaff brainstem encephalitis and Fisher syndrome: anti-GQ1b antibody syndrome. J. Neurol. Neurosurg. Psychiatry. 2013;84:576–583. doi: 10.1136/jnnp-2012-302824. [DOI] [PubMed] [Google Scholar]
- 92.Kuwabara S. Fisher syndrome and Bickerstaff brainstem encephalitis. Brain Nerve. 2015;67:1371–1376. doi: 10.11477/mf.1416200308. [DOI] [PubMed] [Google Scholar]
- 93.Willison HJ. Fine specificity of anti-GQ1b antibodies and clinical features. J. Neurol. Sci. 2001;185:1–2. doi: 10.1016/S0022-510X(01)00465-8. [DOI] [PubMed] [Google Scholar]
- 94.Prendergast MM, Lastovica AJ, Moran AP. Lipopolysaccharides from Campylobacter jejuni O: 41 Strains associated with Guillain-Barre syndrome exhibit mimicry of GM1 ganglioside. Infect. Immun. 1998;66:3649–3655. doi: 10.1128/IAI.66.8.3649-3655.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moran AP, Prendergast MM. Molecular mimicry in Campylobacter jejuni lipopolysaccharides and the development of Guillain-Barré syndrome. J. Infect. Dis. 1998;178:1549–1551. doi: 10.1086/314462. [DOI] [PubMed] [Google Scholar]
- 96.Yuki N, Shahrizaila N. Guillain-Barré syndrome animal model: the first proof of molecular mimicry in human autoimmune disorder. J. Biomed. Biotechnol. 2011;2011:10–14. doi: 10.1155/2011/829129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Poropatich KO, Walker CLF, Black RE. Quantifying the association between Campylobacter infection and Guillain-Barré syndrome: a systematic review. J. Health Popul. Nutr. 2010;28:545–552. doi: 10.3329/jhpn.v28i6.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Allos BM, Lippy FT, Carlsen A, Washburn RG, Blaser MJ. Campylobacter jejuni strains from patients with Guillain-Barre syndrome. Emerg. Infect. Dis. 1998;4:263–268. doi: 10.3201/eid0402.980213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yuki N, et al. Animal model of axonal Guillain-Barré syndrome induced by sensitization with GM1 ganglioside. Ann. Neurol. 2001;49:712–720. doi: 10.1002/ana.1012. [DOI] [PubMed] [Google Scholar]
- 100.Phongsisay V, et al. Complement inhibitor prevents disruption of sodium channel clusters in a rabbit model of Guillain-Barré syndrome. J. Neuroimmunol. 2008;205:101–104. doi: 10.1016/j.jneuroim.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 101.Jacobs BC, et al. The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurology. 1998;51:1110–1115. doi: 10.1212/WNL.51.4.1110. [DOI] [PubMed] [Google Scholar]
- 102.Rho YIl. Overlapping Guillain-Barré syndrome and Bickerstaff’s brainstem encephalitis associated with Epstein Barr virus. Korean J. Pediatr. 2014;57:457. doi: 10.3345/kjp.2014.57.10.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rodríguez Y, et al. Guillain–Barré syndrome, transverse myelitis and infectious diseases. Cell. Mol. Immunol. 2018;15:547–562. doi: 10.1038/cmi.2017.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Groot RCA, Meyer Sauteur PM, Unger WWJ, van Rossum AMC. Things that could be Mycoplasma pneumoniae. J. Infect. 2017;74:S95–S100. doi: 10.1016/S0163-4453(17)30198-6. [DOI] [PubMed] [Google Scholar]
- 105.Meyer Sauteur PM, et al. Severe childhood Guillain-Barré syndrome associated with Mycoplasma pneumoniae infection: a case series. J. Peripher. Nerv. Syst. 2015;20:72–78. doi: 10.1111/jns.12121. [DOI] [PubMed] [Google Scholar]
- 106.Meyer Sauteur PM, et al. Mycoplasma pneumoniae triggering the Guillain-Barré syndrome: a case-control study. Ann. Neurol. 2016;80:566–580. doi: 10.1002/ana.24755. [DOI] [PubMed] [Google Scholar]
- 107.Nafissi S, Vahabi Z, Sadeghi Ghahar M, Amirzargar AA, Naderi S. The role of cytomegalovirus, Haemophilus influenzae and Epstein Barr virus in Guillain Barre syndrome. Acta Med. Iran. 2013;51:372–376. [PubMed] [Google Scholar]
- 108.Mori M, et al. Haemophilus influenzae infection and Guillain-Barré syndrome. Brain. 2000;123:2171–2178. doi: 10.1093/brain/123.10.2171. [DOI] [PubMed] [Google Scholar]
- 109.Mori M, et al. Haemophilus influenzae has a GM1 ganglioside-like structure and elicits Guillain-Barré syndrome. Neurology. 1999;52:1282–1284. doi: 10.1212/WNL.52.6.1282. [DOI] [PubMed] [Google Scholar]
- 110.Koga M, et al. Antecedent infections in Fisher syndrome: A common pathogenesis of molecular mimicry. Neurology. 2005;64:1605–1611. doi: 10.1212/01.WNL.0000160399.08456.7C. [DOI] [PubMed] [Google Scholar]
- 111.Jacobs BC, van Doorn PA, Groeneveld JH, Tio-Gillen AP, van der Meché FG. Cytomegalovirus infections and anti-GM2 antibodies in Guillain-Barré syndrome. J. Neurol. Neurosurg. Psychiatry. 1997;62:641–643. doi: 10.1136/jnnp.62.6.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Irie S, et al. Association of anti-GM2 antibodies in Guillain-Barré syndrome with acute cytomegalovirus infection. J. Neuroimmunol. 1996;68:19–26. doi: 10.1016/0165-5728(96)00059-8. [DOI] [PubMed] [Google Scholar]
- 113.Science M, et al. Central nervous system complications of Varicella-Zoster virus. J. Pediatr. 2014;165:779–785. doi: 10.1016/j.jpeds.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 114.Tatarelli P, et al. Guillain-Barré syndrome following chickenpox: A case series. Int. J. Neurosci. 2016;126:478–479. doi: 10.3109/00207454.2015.1033621. [DOI] [PubMed] [Google Scholar]
- 115.Satterfield-Nash A, et al. Health and development at age 19–24 months of 19 children who were born with microcephaly and laboratory evidence of congenital Zika virus infection during the 2015 Zika virus outbreak—Brazil, 2017. Mmwr. Morb. Mortal. Wkly. Rep. 2017;66:1347–1351. doi: 10.15585/mmwr.mm6649a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cao-Lormeau VM, et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Anaya JM, et al. Zika virus and neurologic autoimmunity: the putative role of gangliosides. BMC Med. 2016;14:1–3. doi: 10.1186/s12916-016-0601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lucchese G, Kanduc D. Zika virus and autoimmunity: from microcephaly to Guillain-Barré syndrome, and beyond. Autoimmun. Rev. 2016;15:801–808. doi: 10.1016/j.autrev.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 119.Nico D, et al. Prevalence of IgG autoantibodies against GD3 Ganglioside in acute Zika virus infection. Front. Med. 2018;5:25. doi: 10.3389/fmed.2018.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baskar, D., Amalnath, D., Mandal, J., Dhodapkar, R. & Vanathi, K. Antibodies to Zika virus, Campylobacter jejuni and gangliosides in Guillain-Barre syndrome: a prospective single-center study from southern India. Neurol. India66, 1324–1331 [DOI] [PubMed]
- 121.Stevens O, Claeys KG, Poesen K, Saegeman V, Van Damme P. Diagnostic challenges and clinical characteristics of hepatitis e virus-associated guillain-Barré syndrome. JAMA Neurol. 2017;74:26–33. doi: 10.1001/jamaneurol.2016.3541. [DOI] [PubMed] [Google Scholar]
- 122.Miyaji K, et al. Altered gene expression of glycosyltransferases and sialyltransferases and total amount of glycosphingolipids following herpes simplex virus infection. Carbohydr. Res. 2016;434:37–43. doi: 10.1016/j.carres.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 123.Sequeira, J., Calado, A., Dias, M. & Manita, M. Parvovirus B19 infection associated with hemolytic anemia and cranial polyneuropathy. J. Neurovirol. 10.1007/s13365-017-0562-8 (2017). [DOI] [PubMed]
- 124.Plomp JJ, Willison HJ. Pathophysiological actions of neuropathy-related anti-ganglioside antibodies at the neuromuscular junction. J. Physiol. 2009;587:3979–3999. doi: 10.1113/jphysiol.2009.171702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.O’Hanlon GM, et al. Anti-GQ1b ganglioside antibodies mediate complement-dependent destruction of the motor nerve terminal. Brain. 2001;124:893–906. doi: 10.1093/brain/124.5.893. [DOI] [PubMed] [Google Scholar]
- 126.Kanda T, Iwasaki T, Yamawaki M, Tai T, Mizusawa H. Anti-GM1 antibody facilitates leakage in an in vitro blood-nerve barrier model. Neurology. 2000;55:585–587. doi: 10.1212/WNL.55.4.585. [DOI] [PubMed] [Google Scholar]
- 127.Desai BS, Monahan AJ, Carvey PM, Hendey B. Blood-brain barrier pathology in Alzheimer’s and Parkinson’s disease: implications for drug therapy. Cell Transplant. 2007;16:285–299. doi: 10.3727/000000007783464731. [DOI] [PubMed] [Google Scholar]
- 128.Platt MP, Agalliu D, Cutforth T. Hello from the other side: how autoantibodies circumvent the blood-brain barrier in autoimmune encephalitis. Front. Immunol. 2017;8:1–15. doi: 10.3389/fimmu.2017.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee, J., Na, G., Joo, E. Y., Lee, M. & Lee, J. Clinical and polysomnographic characteristics of excessive daytime sleepiness in children. Sleep Breath. 10.1007/s11325-017-1545-y (2017). [DOI] [PubMed]
- 130.Postiglione, E. et al. The clinical spectrum of childhood narcolepsy. Sleep Med. Rev. 10.1016/j.smrv.2017.04.003 (2017). [DOI] [PubMed]
- 131.Partinen M, et al. Narcolepsy as an autoimmune disease: the role of H1N1 infection and vaccination. Lancet Neurol. 2014;13:600–613. doi: 10.1016/S1474-4422(14)70075-4. [DOI] [PubMed] [Google Scholar]
- 132.Thorpy M. Current concepts in the etiology, diagnosis and treatment of narcolepsy. Sleep. Med. 2001;2:5–17. doi: 10.1016/S1389-9457(00)00081-2. [DOI] [PubMed] [Google Scholar]
- 133.Lin L, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/S0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 134.Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients. Sleep. 1997;20:1012–1020. [PubMed] [Google Scholar]
- 135.Bernard-Valnet R, et al. CD8 T cell-mediated killing of orexinergic neurons induces a narcolepsy-like phenotype in mice. Proc. Natl Acad. Sci. USA. 2016;113:10956–10961. doi: 10.1073/pnas.1603325113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nohynek H, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS ONE. 2012;7:e33536. doi: 10.1371/journal.pone.0033536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Saariaho AH, et al. Autoantibodies against ganglioside GM3 are associated with narcolepsy-cataplexy developing after Pandemrix vaccination against 2009 pandemic H1N1 type influenza virus. J. Autoimmun. 2015;63:68–75. doi: 10.1016/j.jaut.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 138.Sturkenboom MCJM. The narcolepsy-pandemic influenza story: can the truth ever be unraveled? Vaccine. 2015;33:B6–B13. doi: 10.1016/j.vaccine.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 139.Han F, et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in china. Ann. Neurol. 2011;70:410–417. doi: 10.1002/ana.22587. [DOI] [PubMed] [Google Scholar]
- 140.Luo G, et al. Autoimmunity to hypocretin and molecular mimicry to flu in type 1 narcolepsy. Proc. Natl Acad. Sci. USA. 2018;115:E12323–E12332. doi: 10.1073/pnas.1818150116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Masoudi S, Ploen D, Kunz K, Hildt E. The adjuvant component α-tocopherol triggers via modulation of Nrf2 the expression and turnover of hypocretin in vitro and its implication to the development of narcolepsy. Vaccine. 2014;32:2980–2988. doi: 10.1016/j.vaccine.2014.03.085. [DOI] [PubMed] [Google Scholar]
- 142.Kim WJ, et al. Incidence of narcolepsy before and after MF59-adjuvanted influenza A(H1N1)pdm09 vaccination in South Korean soldiers. Vaccine. 2015;33:4868–4872. doi: 10.1016/j.vaccine.2015.07.055. [DOI] [PubMed] [Google Scholar]
- 143.Canelle Q, Innis BL, Most RVanDer. Evaluation of potential immunogenicity differences between Pandemrix™ and Arepanrix™. Human Vaccines Immunotherapeut. 2016;12:2289–2298. doi: 10.1080/21645515.2016.1168954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jacob L, et al. Comparison of Pandemrix and Arepanrix, two pH1N1 AS03-adjuvanted vaccines differentially associated with narcolepsy development. Brain. Behav. Immun. 2015;47:44–57. doi: 10.1016/j.bbi.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 145.Vaarala O, et al. Antigenic differences between AS03 adjuvanted influenza A (H1N1) pandemic vaccines: Implications for pandemrix-associated narcolepsy risk. PLoS ONE. 2014;9:1–23. doi: 10.1371/journal.pone.0114361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kinashi T. Overview of integrin signaling in the immune system. Methods Mol. Biol. 2011;757:261–278. doi: 10.1007/978-1-61779-166-6_17. [DOI] [PubMed] [Google Scholar]
- 147.Ohkawa Y, et al. Ganglioside GD3 enhances adhesion signals and augments malignant properties of melanoma cells by recruiting integrins to glycolipid-enriched microdomains. J. Biol. Chem. 2010;285:27213–27223. doi: 10.1074/jbc.M109.087791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yu RK, Tsai YT, Ariga T, Yanagisawa M. Structures, biosynthesis, and functions of gangliosides-an overview. J. Oleo Sci. 2011;60:537–544. doi: 10.5650/jos.60.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Alaedini A, Latov N. Transglutaminase-independent binding of gliadin ton intestinal brush border membrane and GM1 ganglioside. J. Neuroimmunol. 2006;177:167–172. doi: 10.1016/j.jneuroim.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 150.Hakomori S. The glycosynapse. Proc. Natl Acad. Sci. USA. 2002;99:225–232. doi: 10.1073/pnas.012540899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rodella U, et al. Schwann cells are activated by ATP released from neurons in an in vitro cellular model of Miller Fisher syndrome. Dis. Model. Mech. 2017;10:597–603. doi: 10.1242/dmm.027870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Costallat LT, de Oliveira RM, Santiago MB, CossermelliW, Samara AM. Neuropsychiatric manifestations of systemic lupus erythematosus: the value of anticardiolipi, antigangliosides, anticerebrosides antibodies. Clin. Rheumatol. 1990;9:489–497. doi: 10.1007/BF02030510. [DOI] [PubMed] [Google Scholar]
- 153.Nicolae I, Nicolae CDE, Ceausu E. INvestigation on antiganglioside antibodies in asymptomatic HIV patients. BMC Infect. Dis. 2014;14(Suppl 4):P25. doi: 10.1186/1471-2334-14-S4-P25. [DOI] [Google Scholar]
- 154.Degroote SG, Gu Y, Julien S, Delannoy P. Gangliosides in breast cancer: new perspectives. Biochemistry. 2015;80:808–819. doi: 10.1134/S0006297915070020. [DOI] [PubMed] [Google Scholar]