Programmed death-ligand-1 (PD-L1) plays a crucial role in the suppression of the antitumor immune response upon interaction with programmed cell death protein-1 (PD-1) on cytotoxic T cells. PD-L1 is constitutively expressed on antigen-presenting cells or tumor cells. Indeed, PD-L1 has been detected by immunohistochemistry in a variety of tumors, including malignant pleural mesothelioma (MPM), in which it has emerged as a predictive biomarker for PD-1/PD-L1 immune checkpoint blockers (ICBs).1,2
PD-L1 expression on tumor cells can be upregulated by several factors including the interferon gamma (IFN-γ) cytokine produced by tumor-infiltrating lymphocytes (TILs).3
In MPM cells, it has been reported that IFN-γ upregulates PD-L1 mRNA expression due to the activation of the interferon regulatory factor 1 (IRF1) transcription factor,4 but no data have been shown regarding cell surface PD-L1 that is functionally relevant for its contact with PD-1-positive cells.
Herein, we analyzed the ability of IFN-γ to upregulate the cell surface expression and release of PD-L1 in MPM cells. Both membranous and soluble PD-L1 may have implications in the tumor immune microenvironment and consequently in the clinical response of MPM to PD-1/PD-L1 ICBs.
Our study was conducted on the three MPM cell lines, MPP89, IST-MES1 and IST-MES2, all of which express a basal level of cell membranous PD-L1.5
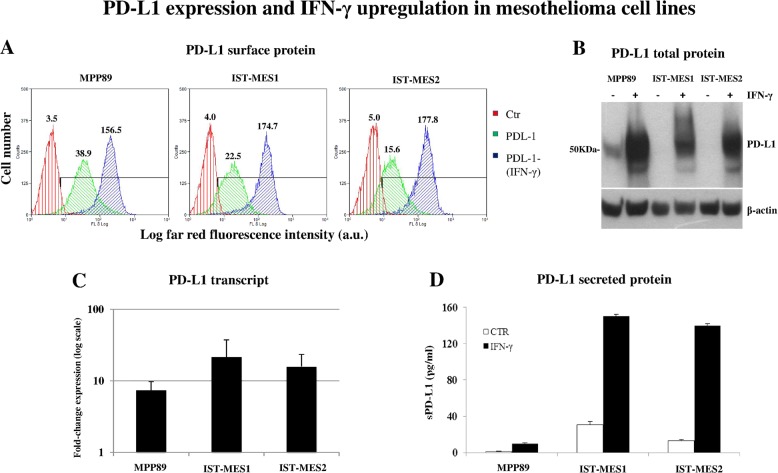
Flow cytometry assessment showed that incubation of these cell lines with IFN-γ markedly increased membrane PD-L1 expression (by 4.02-, 7.76- and 11.40-fold in MPP89, IST-MES1 and IST-MES2, respectively) (Fig. 1a). This increase appeared to be dependent on the increase in the synthesis of PD-L1 protein, as shown by Western blot analysis of cell lysates (Fig. 1b). In turn, the synthesis of PD-L1 was associated with a strong IFN-γ-induced upregulation of the full-length transcript coding for membranous PD-L1 (mean expression fold change: 7.41, 21.61 and 15.84 in MPP89, IST-MES1 and IST-MES2 cells, respectively) (Fig. 1c). In addition, we found by ELISA that all cell lines were able to spontaneously secrete low amounts of the soluble form of PD-L1 (sPD-L1); more importantly, IFN-γ strongly increased the amount of sPD-L1 found in the culture supernatants (by 9.29-, 4.87- and 10.53-fold in MPP89, IST-MES1 and IST-MES2 cells, respectively) (Fig. 1d).
Fig. 1.
IFN-γ induces the upregulation and secretion of PD-L1 in mesothelioma cell lines. All analyses were performed on the mesothelioma cell lines MPP89, IST-MES1 and IST-MES2, untreated or treated with 10 ng/ml IFN-γ for 48 h. Cell lines were obtained from the Interlab Cell Line Collection (ICLC, IRCCS Ospedale Policlinico San Martino, Genoa, Italy), a member of the ICLAC (International Cell Line Authentication Committee), on 05 July 2017 and kept frozen until use from 27 December 2018 to 12 April 2019. ICLC verified the authenticity of the cell lines by using STR loci plus amelogenin (Cell IDTM System, Promega, Madison, WI, USA) and tested the cell lines for mycoplasma contamination by using bisbenzimide (Hoechst 33258) DNA fluorescence staining and PCR analysis with oligonucleotide primer pairs (TIB Molbiol S.r.l., Genoa, Italy). a Cytofluorimetric analysis of PD-L1 expression on the surface of mesothelioma cell lines performed with the antibody MIH1 (eBioscience Thermo Fisher Scientific, Milan, Italy). Green histograms: basal expression of PD-L1; blue histograms: PD-L1 expression upon incubation with IFN-γ; red histograms: negative control. Data are shown as the number of cells vs. log far red fluorescence intensity in arbitrary units. The numbers inside the panels indicate the mean fluorescence intensity (MFI) of each histogram. The MFI of the negative control (cells stained with the second reagent alone) is shown for comparison (red histograms). The black horizontal bar indicates the gate of each histogram used to calculate the PD-L1-positive cells. b Western blot analysis of PD-L1 total cell protein from lysates of the three cell lines upon incubation with IFN-γ (+), or the basal content of PD-L1 (−). Western blotting was performed with the anti-PD-L1 antibody E1L3N (Cell Signaling Technology, Milan, Italy) also used for PD-L1 immunohistochemistry in mesothelioma tissues (not shown). The β-Actin content of each lysate is shown for comparison. c mRNA levels of transcripts coding for the full-length PD-L1 molecule in the indicated mesothelioma cell lines incubated with IFN-γ. Data are shown as the expression fold change (log scale) relative to the basal mRNA levels of PD-L1 in each cell line. d ELISA of culture supernatants harvested from cultures of the mesothelioma cell lines incubated with IFN-γ (black columns) or in the absence of IFN-γ (white columns, CTR)
Thus, we provide the novel information that MPM cells can secrete sPD-L1 and that the levels of both membranous and soluble PD-L1 are upregulated by IFN-γ.
These findings may have a role in the clinical trials targeting either PD-1 or PD-L1 with ICBs that are currently underway in MPM in order to promote the antitumor immune response.2
In particular, since tumor cell-derived sPD-L1 is able to bind to the PD-1 receptor and to trigger pro-apoptotic signals into activated T cells,6 its binding to PD-1 may further contribute to an immunosuppressive microenvironment in MPM. Moreover, sPD-L1 might interfere with the efficacy of anti-PD-L1 antibodies by trapping them, as has recently been shown for circulating PD-L1 in non-small cell lung cancer patients.7 Consequently, sPD-L1 might also impair the anti-PD-L1 antibody-dependent cellular cytotoxicity (ADCC) of MPM cells induced by natural killer (NK) cells.8
In conclusion, we hypothesize that the high levels of IFN-γ that are released by TILs in MPM, via increased tumor cell surface expression and release of PD-L1, might contribute to the resistance to ICBs targeting PD-1 and/or PD-L1. Future investigations will be required to understand whether IFN-γ signaling blockade could increase the efficacy of immunotherapy with ICBs in MPM patients.
Acknowledgements
This work was supported by grants from the Italian Ministry of Health (5x1000 funds 2014 and 2015; Ricerca Corrente 2017).
Competing interests
The authors declare no competing interests.
References
- 1.Li X, Song W, Shao C, Shi Y, Han W. Emerging predictors of the response to the blockade of immune checkpoints in cancer therapy. Cell Mol. Immunol. 2019;16:28–39. doi: 10.1038/s41423-018-0086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lantuejoul S, Le Stang N, Damiola F, Scherpereel A, Galateau-Sallé F. PD-L1 testing for immune checkpoint inhibitors in mesothelioma: for want of anything better? J. Thorac. Oncol. 2017;12:778–781. doi: 10.1016/j.jtho.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Mimura K, et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018;109:43–53. doi: 10.1111/cas.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kao SC, et al. Tumor suppressor microRNAs contribute to the regulation of PD-L1 expression in malignant pleural mesothelioma. J. Thorac. Oncol. 2017;12:1421–1433. doi: 10.1016/j.jtho.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Calabrò L, et al. CTLA4 blockade in mesothelioma: finally a competing strategy over cytotoxic/target therapy? Cancer Immunol. Immunother. 2015;64:105–112. doi: 10.1007/s00262-014-1609-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frigola X, et al. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin. Cancer Res. 2011;17:1915–1923. doi: 10.1158/1078-0432.CCR-10-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong B, et al. Secreted PD-L1 variants mediate resistance to PD-L1 blockade therapy in non-small cell lung cancer. J. Exp. Med. 2019;216:982–1000. doi: 10.1084/jem.20180870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khanna S, et al. Malignant mesothelioma effusions are infiltrated by CD3+T cells highly expressing PD-L1 and the PD-L1+tumor cells within these effusions are susceptible to ADCC by the anti-PD-L1 antibody avelumab. J. Thorac. Oncol. 2016;11:1993–2005. doi: 10.1016/j.jtho.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]