Cystic echinococcosis, which is caused by larval infection of Echinococcus granulosus (E. granulosus) sensu lato, is a widespread chronic cyst-forming helminthic disease that affects up to 3 million people.1 E. granulosus can actively divert the host immune response towards anergy and anti-inflammatory pathways.2 Previous studies have shown that E. granulosus can escape host immunosurveillance by modulating the maturation of dendritic cells (DCs).3,4 Nevertheless, how E. granulosus, an extracellular pathogen, modulates the inflammatory response in DCs remains poorly understood. In this study, we investigated the effects of E. granulosus cyst fluid (EgCF) on bone marrow-derived DCs (BMDCs) exposed to LPS, an inflammatory stimulus, and the mechanisms involved in the EgCF–DCs immune modulation.
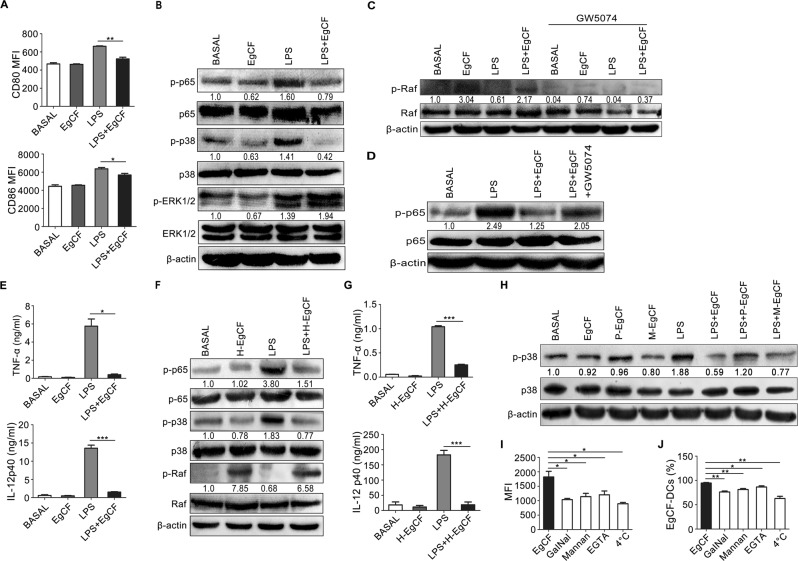
We initially treated BMDCs with LPS in combination with graded concentrations of EgCF.5 Flow cytometry analysis showed that the expression rate of the cell surface costimulatory marker CD80 and the mean fluorescence intensity (MFI) of CD80 and CD86, but not MHC class II, on BMDCs significantly decreased following EgCF (2.15 mg/ml) and LPS costimulation compared with LPS treatment alone (Fig. 1a, and Supplementary information, Fig. S1), suggesting that EgCF could affect the LPS-induced maturation of BMDCs. Exposure of BMDCs to EgCF abolished the LPS-induced phosphorylation of p38 MAPK and NF-κB p65, while no such effect was observed for ERK1/2 (Fig. 1b). Furthermore, EgCF treatment strikingly attenuated LPS-induced production of TNF-α and IL-12 at the mRNA and protein levels but did not inhibit IL-10 expression (Fig. 1e and Supplementary information, Fig. S2a, b).
Fig. 1.
EgCF inhibited LPS-induced DCs maturation and the TLR4 inflammatory signaling pathway. a BMDCs were stimulated with EgCF (2.15 mg/ml) and LPS (1 μg/ml) for 24 h. The mean fluorescence intensity (MFI) of the costimulatory markers CD80 and CD86 were analyzed by flow cytometry. b BMDCs were treated with EgCF and LPS for 30 min. Activation of NF-κB p65, p38, and ERK1/2 signaling was detected via Western blotting. The expression levels were quantified using ImageJ software and were normalized with β-actin. Data are representative of four independent experiments. c, d BMDCs were treated with GW5704 (5 μM) for 2 h before stimulation with LPS and/or EgCF. After 30 min, activation of c-Raf (c) and NF-κB (d) signaling was analyzed by Western blotting. β-actin was used as a loading control. Data are representative of three independent experiments. e BMDCs were incubated with the c-RAF inhibitor GW5074 (5 μM) or DMSO as a negative control for 2 h before stimulation with LPS in the presence or absence of EgCF for 24 h. Secretion of TNF-α and IL-12 p40 in cell culture supernatants was measured by ELISA. f BMDCs were stimulated with heat-denatured EgCF (H-EgCF) and/or LPS for 30 min. The phosphorylation levels of NF-κB p65, p38, and c-Raf were detected by Western blotting. The expression levels were normalized with β-actin. Data are representative of three independent experiments. g Secretion of TNF-a and IL-12 p40 from BMDCs treated with H-EgCF and/or LPS for 24 h was detected by ELISA. h BMDCs were stimulated with LPS in the presence of periodate-treated EgCF (P-EgCF) or mock periodate-treated EgCF (M-EgCF) for 30 min. p38 activation was detected by Western blotting. β-actin served as a loading control, and the data are representative of three independent experiments. i, j BMDCs were pretreated with mannan, GalNac, or EGTA or at 4 °C before incubation with fluorescently labeled EgCF. The rates of FITC-positive DCs (i) and MFI (j) were analyzed via flow cytometry. *P < 0.05, **P < 0.01, and ***P < 0.001
Some pathogens modulate Toll-like receptor (TLR) signaling via the c-Raf-acetylation-dependent pathway.6 Here, we found that BMDCs exposed to EgCF exhibited enhanced c-Raf phosphorylation, which could be decreased by GW5074,7 a selective c-Raf inhibitor (Fig. 1c). Following GW5074 treatment, the attenuated NF-κB p65 phosphorylation induced by EgCF was mostly restored to that of the LPS alone group (Fig. 1d). In addition, the TNF-α and IL-12 protein and mRNA levels were also restored to those observed under LPS single stimulation (Fig. 1e and Supplementary information, Fig. S2c), suggesting that EgCF impairs the TLR4-mediated inflammatory signaling pathway through c-Raf phosphorylation in the BMDCs.
To determine which component of EgCF is critical for the EgCF–DCs immune modulation, we subjected EgCF to heat denaturation (H-EgCF) of proteins or periodate treatment for modification of carbohydrates.8 The results showed that H-EgCF retained the ability to induce c-Raf phosphorylation and inhibited LPS-induced NF-κB and p38 activation (Fig. 1f). Consistently, the production of TNF-α and IL-12, but not IL-10, upon LPS stimulation was diminished in the H-EgCF treatment costimulatory group (Fig. 1g and Supplementary information, Fig. S2d). Moreover, periodate-treated EgCF lost the ability to impair LPS-induced p38 phosphorylation compared with EgCF or mock periodate-treated EgCF (Fig. 1h), suggesting that glycomolecules in EgCF contributed to c-Raf activation and the inhibition of the inflammatory response.
Carbohydrates are recognized by C-type lectin receptors (CLRs), such as mannose receptor (MR) and macrophage galactose C-type lectin (MGL).9 BMDCs were incubated with FITC-labeled EgCF at 37 °C. Flow cytometry analysis revealed that ~94.3% of the BMDCs were FITC positive, indicating that EgCF was recognized by DCs (Fig. 1i and Supplementary information, Fig. S3). However, the addition of exogenous mannan (blocking MR), GalNac (blocking MGL), and EGTA (impairing all CLR binding),10 as well as 4 °C incubation, led to significantly reduced rates and MFI of EgCF-FITC binding to the BMDCs (Fig. 1i, j), suggesting that multiple CLRs, including MR and MGL, on the BMDCs were involved in EgCF recognition.
In summary, these findings provide evidence that glycomolecules in EgCF could interfere with the TLR4-mediated activation of DCs, including downregulation of CD80 and CD86, NF-κB, and p38 MAPK signaling, and the downstream inflammatory factors TNF-α and IL-12. c-Raf phosphorylation is a critical contributor to and a potential therapeutic target against the immune escape of E. granulosus.
Supplementary information
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81760371, 81760570, 81602810, 81703174), the XPCC Youth Science and Technology Innovation Leading Talent Program (2018CB017) and Key Areas of Science and Technology Projects (2019AB031).
Author contributions
X.C., J.H., L.L., and X.W. conceived the project. L.L., D.D., X.W., K.Y., X.X., and C.C. performed the experiments; L.L., L.W., J.H., X.W., and X.C. wrote and revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Jun Hou, Linlin Li, Dan Dong
Contributor Information
Xiangwei Wu, Email: wxwshz@126.com.
Xueling Chen, Email: chenxueling@shzu.edu.cn.
Supplementary information
The online version of this article (10.1038/s41423-019-0314-1) contains supplementary material.
References
- 1.Zheng H, et al. The genome of the hydatid tapeworm Echinococcus granulosus. Nat. Genet. 2013;45:1168–1175. doi: 10.1038/ng.2757. [DOI] [PubMed] [Google Scholar]
- 2.Gottstein B, et al. Immunology of Alveolar and Cystic Echinococcosis (AE and CE) Adv. Parasitol. 2017;96:1–54. doi: 10.1016/bs.apar.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Rigano R, et al. Echinococcus granulosus antigen B impairs human dendritic cell differentiation and polarizes immature dendritic cell maturation towards a Th2 cell response. Infect. Immun. 2007;75:1667–1678. doi: 10.1128/IAI.01156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, et al. Impairment of dendritic cell function and induction of CD4(+)CD25(+)Foxp3(+) T cells by excretory-secretory products: a potential mechanism of immune evasion adopted by Echinococcus granulosus. BMC Immunol. 2015;16:44. doi: 10.1186/s12865-015-0110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghislat G, Lawrence T. Autophagy in dendritic cells. Cell Mol. Immunol. 2018;15:944–952. doi: 10.1038/cmi.2018.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gringhuis SI, et al. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity. 2007;26:605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Chin PC, et al. The c-Raf inhibitor GW5074 provides neuroprotection in vitro and in an animal model of neurodegeneration through a MEK-ERK and Akt-independent mechanism. J. Neurochem. 2004;90:595–608. doi: 10.1111/j.1471-4159.2004.02530.x. [DOI] [PubMed] [Google Scholar]
- 8.Pan W, et al. The excretory-secretory products of Echinococcus granulosus protoscoleces directly regulate the differentiation of B10, B17 and Th17 cells. Parasit. Vectors. 2017;10:348. doi: 10.1186/s13071-017-2263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terrazas CA, Alcantara-Hernandez M, Bonifaz L, Terrazas LI, Satoskar AR. Helminth-excreted/secreted products are recognized by multiple receptors on DCs to block the TLR response and bias Th2 polarization in a cRAF dependent pathway. FASEB J. 2013;27:4547–4560. doi: 10.1096/fj.13-228932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Liempt E, et al. Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation. Mol. Immunol. 2007;44:2605–2615. doi: 10.1016/j.molimm.2006.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.