Abstract
In recent years, immune checkpoint inhibitors have become the most important drugs for treating renal cell carcinoma. In combination with performing nephrectomies, tyrosine kinase inhibitors have been used as neoadjuvant therapy, as they reduce the size of a primary renal mass and cause the disappearance of metastatic lesions. However, there are only a few reports on immune checkpoint inhibitors as neoadjuvant therapy. Herein, we report a case of renal cell carcinoma with multiple lung metastases and an inferior vena cava tumor thrombus that showed a complete response via radical nephrectomy after nivolumab plus ipilimumab. A 47-year-old man was diagnosed with renal cell carcinoma with multiple lung metastases and inferior vena cava tumor thrombus. After four treatment cycles of nivolumab plus ipilimumab and five cycles of nivolumab, we performed radical nephrectomy and resection of the thrombus tumor by excising a part of the inferior vena cava. The pathological diagnosis had no residual tumor. To our knowledge, this is the first case of complete disappearance of all malignant cells. Immunostaining of the primary renal mass revealed strong positivity for CD4 and CD8. The patient has been followed up without additional treatment for 8 months, but no recurrence has been observed. We suggest the use of nivolumab plus ipilimumab as neoadjuvant therapy. However, physicians should consider the possibilities of immune-related adverse events.
Keywords: Immune checkpoint inhibitors, Neoadjuvant therapy, Renal cell carcinoma
Introduction
The management of patients with renal cell carcinoma (RCC) has changed significantly in recent years [1, 2]. Radical or partial nephrectomy is currently an important intervention for localized RCC. However, before 2005, treatment for metastatic RCC included only cytokine therapy, which had a limited effect [3]. Subsequently, the number of drug options has greatly increased with the discovery of tyrosine kinase inhibitors (TKIs) [4]. In recent times, immune checkpoint inhibitors are the most important drugs for treating RCC [5]. Nivolumab plus ipilimumab was approved as a first-line treatment for unresectable or metastatic RCC by the Food and Drug Administration (FDA) in 2018. To date, TKIs have been used as a pre-surgical therapy to enable performing nephrectomy, as they reduce the primary renal mass [6]. However, there are only a few reports of immune checkpoint inhibitors as pre-surgical therapy. Herein, we report a case of left RCC with multiple lung metastases and an inferior vena cava (IVC) tumor thrombus that showed a complete response (CR) via radical nephrectomy after nivolumab plus ipilimumab.
Case report
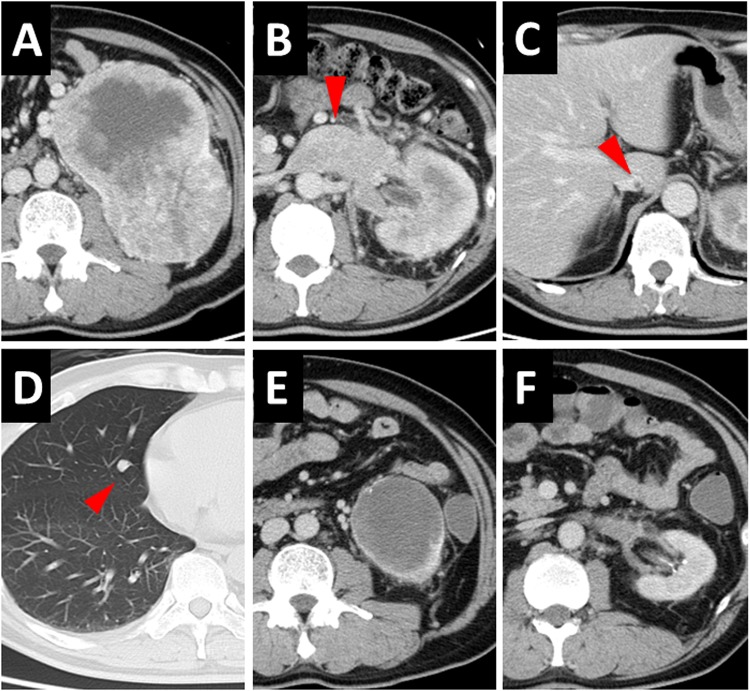
A 47-year-old man had hematuria and visited a nearby hospital in August 2018. He was diagnosed with a tumor in the left kidney with multiple lung metastases and a vena cava tumor thrombus and presented to our hospital for treatment. His performance status was 0. On physical examination, a mass was palpated in the left abdomen. Blood examination showed normal levels of C-reactive protein, lactate dehydrogenase, creatine, and calcium, as well as normal white blood cell and platelet counts. Computed tomography revealed a 15.0-cm left renal mass (Fig. 1A) and a thrombus extending within the renal vein (Fig. 1B) and the IVC to below the diaphragm (Fig. 1C). In addition, collateral circulation was observed around the left kidney and left varicocele. Moreover, nodules suspected to be metastases were observed in the lungs (Fig. 1D). We, therefore, performed percutaneous tumor biopsy, and the pathological diagnosis was a clear cell RCC. The classification was cT3bN0M1, stage IV, with intermediate risk according to the International mRCC Database Consortium.
Fig. 1.
CT of A pre- and E post-treatment primary renal mass, B pre- and F post-treatment renal vein tumor thrombus, C pre-treatment IVC tumor thrombus, and D pre-treatment lung metastases. Nivolumab plus ipilimumab was the employed pre-surgical therapy
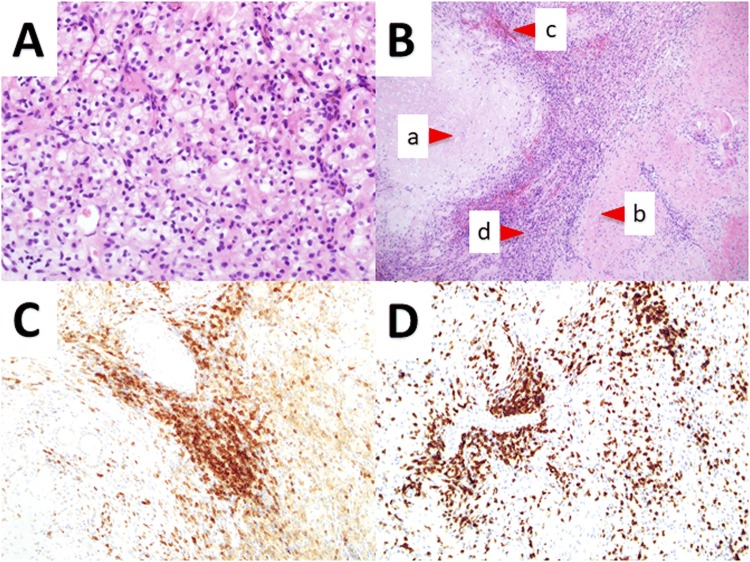
We started treating the patient with nivolumab (240 mg) plus ipilimumab (1 mg/kg). After two treatment cycles, the patient had diarrhea (grade 2 as per the Common Terminology Criteria for Adverse Events), but it resolved without any treatment. Computed tomography revealed that the primary renal mass reduced to 9.0 cm and that lung metastases had reduced or disappeared. After four treatment cycles, the primary renal mass reduced to 7.0 cm (Fig. 1E), the thrombus decreased (Fig. 1F), and lung metastases had completely disappeared as observed using computed tomography. The patient then received five cycles of nivolumab, after which we performed radical nephrectomy and resection of the thrombus tumor by excising a part of the IVC. Intraoperative findings indicated that the adhesions around the tumor were tight, and we confirmed the tip of the tumor thrombus with ultrasound and removed the tumor. The pathological diagnosis was a CR (i.e., no residual tumor) in which both the primary renal mass and the tumor thrombus were completely replaced by necrosis, fibrosis, hyalinization, hemorrhage, and macrophages (Fig. 2A, B). Immunostaining of the primary renal mass revealed strong positivity for CD4 and CD8 (Fig. 2C, D). The patient has been followed up without additional treatment for 6 months, but no recurrence has been observed.
Fig. 2.
A HE staining of renal cell carcinoma, B biopsy imaging showing no residual tumor of the primary renal mass, and C immunostaining of CD4 and D CD8. The primary renal mass was completely replaced by a necrosis, b fibrosis, hyalinization, c hemorrhage, and d macrophages. (a × 400, b × 100, c × 200, d × 200)
Discussion
In 2018, the FDA approved nivolumab plus ipilimumab as a first-line treatment for unresectable or metastatic RCC, based on the results of the CheckMate 214 trial that showed that overall survival and objective response rates were significantly higher after nivolumab plus ipilimumab than after sunitinib among patients with intermediate- and poor-risk disease with previously untreated advanced RCC [7]. Moreover, nivolumab plus ipilimumab was effective for clear cell carcinoma and papillary RCC with sarcomatoid dedifferentiation [8], similar to the efficacy observed in the current case.
Nivolumab and ipilimumab are immune checkpoint inhibitors. As such, the possibility of immune-related adverse events (irAEs) should be kept in mind, owing to their unique mechanisms of action [9]. However, among patients with malignant melanoma and non-small cell lung cancer, those with irAEs tended to have prolonged overall survival and progression-free survival [10, 11]. Fortunately, in the current case, the irAE was only diarrhea (grade 2). When diarrhea appeared, we considered discontinuing nivolumab plus ipilimumab. However, the patient was followed up without treatment, and the symptoms were improving with no bloody stool observed. Therefore, we considered the higher possibility of infectious enteritis and continued the treatment. In fact, diarrhea had disappeared in the next cycle. Accordingly, early detection and early treatment, if needed, are required to prevent the exacerbation of irAEs [12]. Even in the current case, possible occurrence of other irAEs in the future cannot be ruled out, so strict follow-up must be continued.
To date, neoadjuvant therapy for RCC has involved the use of TKIs [6], and its safety and effectiveness have been reported [13]. However, the number of studies on neoadjuvant immunotherapy is still limited. Only one previous report showed the efficacy of neoadjuvant nivolumab plus ipilimumab for RCC with an IVC tumor thrombus [14]. In that case, radical nephrectomy and combined resection of the IVC were performed, similar to those performed for the current case. The tumor size on computed tomography was 10.4 cm, and the size of the malignant tumor on pathological evaluation was 6.3 cm. Moreover, malignant cells had disappeared in other lesions [14]. There has been no previous report of the disappearance of all malignant cells, even in the primary lesion with a pathological diagnosis of CR, as observed in the current case. After immunotherapy, even if a tumor is observed on computed tomography, malignant cells may reduce and disappear on pathological evaluation, possibly owing to edema and tumor-infiltrating immune cells [15].
In the current case, the tumor was positive for CD4 and CD8 on immunostaining. This result indicates that T cells may play a major role in the disappearance of malignant cells owing to the action of nivolumab [16].
Immediate radical nephrectomy without nivolumab plus ipilimumab was an option. However, the risk of blood loss was considered because the left renal vein was markedly swollen to the IVC junction, and the collateral circulation was very developed around the kidney. According to the previous report, in RCC with IVC thrombus, blood loss and operative duration were significantly lower and shorter, respectively, in the pre-surgical axitinib therapy than upfront nephrectomy [17]. Furthermore, CheckMate 214 has shown that nivolumab plus ipilimumab has a higher objective response rate than sunitinib [7]. Therefore, we considered pre-surgical nivolumab plus ipilimumab for a safer and more effective surgery. In the end, because there was an interval until the surgery date desired by the patient, we administered pre-surgical nivolumab plus ipilimumab to reduce tumor size with patient selection.
There were no evaluable lesions after complete surgical resection in the current patient, who was followed up without any additional treatment because nivolumab remained effective even after treatment discontinuation. However, to date, there is no information regarding the follow-up protocol for RCC that has shown a CR after treatment with nivolumab. Therefore, it is necessary to establish an appropriate method for the management of irAEs and follow-up by accumulating more cases in the future.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
- CR
Complete response
- FDA
Food and Drug Administration
- IVC
Inferior vena cava
- RCC
Renal cell carcinoma
- TKI
Tyrosine kinase inhibitor
- irAE
Immune-related adverse event
Funding
None.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 2.de Velasco G, Bex A, Albiges L, et al. Sequencing and combination of systemic therapy in metastatic renal cell carcinoma. Eur Urol Oncol. 2019;2:505–514. doi: 10.1016/j.euo.2019.06.022. [DOI] [PubMed] [Google Scholar]
- 3.Coppin C, Porzsolt F, Awa A, et al. Immunotherapy for advanced renal cell cancer. Cochrane Database Syst Rev. 2005;25:CD001425. doi: 10.1002/14651858.CD001425.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther. 2005;315:971–979. doi: 10.1124/jpet.105.084145. [DOI] [PubMed] [Google Scholar]
- 5.Atkins MB, Tannir NM. Current and emerging therapies for first-line treatment of metastatic clear cell renal cell carcinoma. Cancer Treat Rev. 2018;70:127–137. doi: 10.1016/j.ctrv.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Borregales LD, Adibi M, Thomas AZ, et al. The role of neoadjuvant therapy in the management of locally advanced renal cell carcinoma. Ther Adv Urol. 2016;8:130–141. doi: 10.1177/1756287215612962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schvartsman G, Carneiro AP, Filippi RZ, et al. Rapid deep responses with nivolumab plus ipilimumab in papillary renal cell carcinoma with sarcomatoid dedifferentiation. Clin Genitourin Cancer. 2019;17:315–318. doi: 10.1016/j.clgc.2019.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4:374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886–894. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapin BF, Delacroix SE, Jr, Culp SH, et al. Safety of pre-surgical targeted therapy in the setting of metastatic renal cell carcinoma. Eur Urol. 2011;60:964–971. doi: 10.1016/j.eururo.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labbate C, Hatogai K, Werntz R, et al. Complete response of renal cell carcinoma vena cava tumor thrombus to neoadjuvant immunotherapy. J Immunother Cancer. 2019;7:66. doi: 10.1186/s40425-019-0546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol. 2015;33:3541–3543. doi: 10.1200/JCO.2015.61.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choueiri TK, Fishman MN, Escudier B, et al. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clin Cancer Res. 2016;22:5461–5471. doi: 10.1158/1078-0432.CCR-15-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka Y, Hatakeyama S, Hosogoe S, et al. Pre-surgical axitinib therapy increases fibrotic reactions within tumor thrombus in renal cell carcinoma with thrombus extending to the inferior vena cava. Int J Clin Oncol. 2018;23:134–141. doi: 10.1007/s10147-017-1169-z. [DOI] [PubMed] [Google Scholar]