Abstract
A 48-year-old male was referred to our hospital for evaluation of motor speech disorders and difficulty in the movement of both the hands. The clinical diagnosis was Trousseau’s syndrome due to advanced gallbladder cancer (cT3aN1M0). The patient received anticoagulation therapy and systemic chemotherapy (gemcitabine and cisplatin). Motor speech disorders and difficulty in movement of both hands were recovered. After 2 courses of chemotherapy, the primary tumor developed a massive hepatic invasion and the peripheral blood eosinophils increased gradually. The patient was admitted to our hospital for abdominal distension, fever, right upper quadrant pain, systemic edema, loss of appetite, and general malaise. The peripheral blood eosinophil count was markedly elevated to 45,900/μl (90.3%). The serum level of GM-CSF was high and there was no evidence of leukemia, allergic status and other diseases. The patient was diagnosed as paraneoplastic eosinophilia with advanced gallbladder cancer, which was suspected to produce GM-CSF. The patient received palliative support and died of disseminated intravascular coagulation 15 days after admission. We herein reported a fatal case of gallbladder cancer suspected of producing GM-CSF.
Keywords: Eosinophilia, Gallbladder cancer, GM-CSF
Introduction
Eosinophilia is defined as an abnormal accumulation of eosinophils in the blood or tissue. Marked eosinophilia occurs in several disorders such as parasitic infections, allergic disease, collagen disease, vascular disorders, and malignant disease [1]. There are some reports on eosinophilia in various types of cancer patients, including breast, rectum, lung, thyroid, cervix, liver, pancreas, and stomach [2]. We herein report an advanced case of gallbladder cancer suspected of producing GM-CSF, which caused an uncontrollable peripheral blood eosinophilia.
Case report
A 48-year-old male was referred to our hospital for evaluation of motor speech disorders and difficulty in movement of both the hands. Magnetic resonance imaging indicated multiple cerebral microinfarcts and computed tomography indicated wall thickening of the gallbladder fundus with lymphadenopathy (Fig. 1a). Endoscopic ultrasound-guided fine-needle aspiration revealed malignant cells in gallbladder wall. Magnetic resonance cholangiopancreatography showed pancreaticobiliary maljunction without biliary dilatation [pancreatic duct (junction) type] (Fig. 2). The patient was diagnosed with Trousseau’s syndrome due to advanced gallbladder cancer with lymph node metastases (cT3aN1M0, Stage IIIB, according to the Japanese classification system, 6th edition). The patient received anticoagulation therapy and systemic chemotherapy [gemcitabine (1000 mg/m2) and cisplatin (25 mg/m2) given on days 1 and 8]. This regimen was repeated at a 21-day interval. Motor speech disorders and difficulty in movement of both hands improved immediately after starting the anticoagulation therapy. Although two courses of chemotherapy with gemcitabine and cisplatin (GC) were given, the primary tumor developed massive hepatic invasion (Fig. 1b). Complete blood count analysis revealed marked eosinophil elevation from 200/μl (5.8%) to 3900/μl (14%). GC were switched to gemcitabine and S-1 combination chemotherapy. However, the patients developed tumor progression and further increase in peripheral blood eosinophilia (Fig. 3).
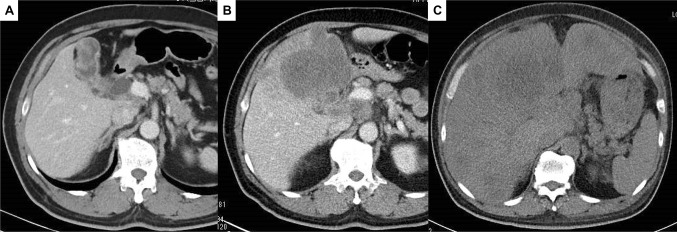
Fig. 1.
Enhanced computed tomography findings. a A thickening of the gallbladder wall. b Following two courses of chemotherapy with GC, the primary tumor developed massive hepatic invasion. c On admission, tumor had spread to the liver, which resulted in hepatomegaly
Fig. 2.
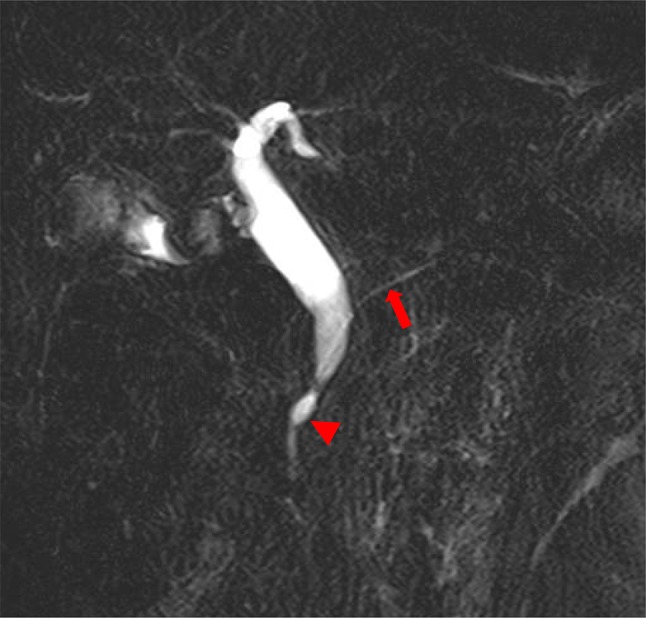
Magnetic resonance cholangiopancreatography findings. Main pancreatic duct (arrow) and bile duct join anatomically outside (arrowhead) the duodenal wall
Fig. 3.
Clinical course of the patient. White blood cell counts, eosinophil counts and serum CA19-9 levels elevated logarithmically. These comments [CT (A), (B) and (C)] in this image correspond to Fig. 1
When the patient was admitted to our hospital, he complained of various symptoms including abdominal distension due to hepatomegaly, right upper quadrant pain, fever, systemic edema, loss of appetite, and general malaise (Fig. 1c). The total white blood cell count was 50,800/μl, of which the absolute eosinophil count was markedly elevated at 45,900/μl (90.3%). No blast or immature cells were found in the peripheral blood. The patient did not have any history of smoking, allergic disease, parasitic infections, or exposure to tuberculosis. Stool examination was negative for ova and parasites. Although bone marrow aspiration was not performed due to his poor general condition, flow cytometry CD45 gating revealed no evidence of myeloid leukemia. The serum levels of Interleukin (IL)-5 were normal, while serum granulocyte macrophage-colony stimulating factor (GM-CSF) was elevated to 410 pg/ml. The patient was diagnosed as paraneoplastic eosinophilia with advanced gallbladder cancer, which was suspected to produce GM-CSF. The patient received palliative support with prednisolone, diuretics, ascites puncture, and physical therapy, and died of disseminated intravascular coagulation (DIC) 15 days after admission.
Discussion
Peripheral eosinophilia is classified into two major pathways: cytokine-mediated increased differentiation and prolonged survival of eosinophils (extrinsic eosinophilic disorders), and mutation-mediated clonal expansion of eosinophils (intrinsic eosinophilic disorders). Paraneoplastic eosinophilia is caused by extrinsic eosinophilic disorders. Approximately 0.5–7.0% cases of eosinophilia are associated with malignancies [3, 4]. Generally, paraneoplastic eosinophilia represents a late manifestation, and is considered to be a threatening sign reflecting dissemination of the malignancies and poor prognosis [5–7].
Several mechanisms of the paraneoplastic eosinophilia have been proposed [2]. First, release of protein material following necrotic changes of the tumor causes an eosinophilotactic response. The second is stimulation of eosinophil production as a result of seeding of metastatic neoplastic cells to bone marrow. The third is production of an eosinophiliopoietic factor by the tumor cells, which stimulates the bone marrow resulting in the production of eosinophils. The fourth is release of an eosinophilic chemotactic factor by the tumor cells. IL-3 and IL-5, and GM-CSF are involved in the selective activation and accumulation of eosinophils [1]. These cytokines also inhibit eosinophil apoptosis for at least 12–14 days in vitro [8] and eosinophils cannot survive for more than 48 h without these cytokines [2]. Of these, GM-CSF contributes to proliferation of human eosinophils in culture, and administration of human recombinant GM-CSF causes eosinophilia in humans [8]. In the present case, the patient had elevated serum GM-CSF level, which may have caused differentiation of eosinophils and their release to the peripheral circulation. Although immunohistochemical analysis of GM-CSF was not performed due to sample unavailability, we suspected this case of GM-CSF producing gallbladder cancer, considering elevated serum GM-CSF. Cytokine overpopulation, which caused eosinophilia, may involve malignant expansion of T-cell clones [9]. In addition, hypereosinophilia is often associated with DIC [10]. As for paraneoplastic eosinophilia with DIC, there are some case reports on the clinical utility of systemic chemotherapy, radical resection, and palliative therapy including anticoagulation therapy and corticosteroid [1, 9–11]. In the current case, extreme aggressive eosinophilia and rapid progression of DIC made conducting life-saving and symptom relief measures difficult. We need to acknowledge another possible reason of eosinophilia. Gemcitabine has been reported to increase GM-CSF production, resulting in differentiation of myeloid-derived suppressor cells [12]. Since gemcitabine was given to the patients, gemcitabine-induced GM-CSF needs to be considered.
We herein reported a rare case of gallbladder cancer suspected of producing GM-CSF with poor prognosis. The clinician should start optimal treatment immediately, either a radical or palliative one, keeping in mind that solid tumor with eosinophilia signifies a poor prognosis.
Author contributions
MT wrote the manuscript. KH and HS contributed to writing the manuscript. TU and KY supervised the study and initially revised the manuscript. MT, KH, HS and TU served as attending physicians of the present patients. All authors read and approved the final manuscript.
Funding
None.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interest.
Informed consent
Written informed consent was obtained from the patients for publication of this case report and any accompanying images.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Butt NM, Lambert J, Ali S, et al. Guideline for the investigation and management of eosinophilia. Br J Haematol. 2017;176:553–572. doi: 10.1111/bjh.14488. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberg ME. Eosinophilia. N Engl J Med. 1998;338:1592–1600. doi: 10.1056/NEJM199805283382206. [DOI] [PubMed] [Google Scholar]
- 3.Ackerman SJ, Bochner BS. Mechanisms of eosinophilia in the pathogenesis of hypereosinophilic disorders. Immunol Allergy Clin N Am. 2007;27:357–375. doi: 10.1016/j.iac.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bain BJ. Hypereosinophilia. Curr Opin Hematol. 2000;7:21–25. doi: 10.1097/00062752-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Zhou WW, Guan YY, Liu XM. Paraneoplastic eosinophilia in clear cell renal cell carcinoma. Chin Med J. 2015;128:2271–2272. doi: 10.4103/0366-6999.162501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abali H, Altundag MK, Engin H, et al. Hypereosinophilia and metastatic anaplastic carcinoma of unknown primary. Med Oncol. 2001;18:285–288. doi: 10.1385/MO:18:4:285. [DOI] [PubMed] [Google Scholar]
- 7.Anagnostopoulos GK, Sakorafas GH, Kostopoulos P. Disseminated colon cancer with severe peripheral blood eosinophilia and elevated serum levels of interleukin-2, interleukin-3, interleukin-5, and GM-CSF. J Surg Oncol. 2005;89:273–275. doi: 10.1002/jso.20173. [DOI] [PubMed] [Google Scholar]
- 8.Simon HU, Yousefi S, Schranz C, et al. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol. 1997;158:3902–3908. [PubMed] [Google Scholar]
- 9.Sonoda Y, Arai N, Ogawa M. Humoral regulation of eosinophilopoiesis in vitro: analysis of the targets of interleukin-3, granulocyte/macrophage colony-stimulating factor (GM-CSF), and interleukin-5. Leukemia. 1989;3:14–18. [PubMed] [Google Scholar]
- 10.Takeda H, Nishikawa H, Tsumura T, et al. Prominent hypereosinophilia with disseminated intravascular coagulation as an unusual presentation of advanced gastric cancer. Intern Med. 2014;53:563–569. doi: 10.2169/internalmedicine.53.1483. [DOI] [PubMed] [Google Scholar]
- 11.Vassilatou E, Fisfis M, Morphopoulos G, et al. Papillary thyroid carcinoma producing granulocyte-macrophage colony-stimulating factor is associated with neutrophilia and eosinophilia. Hormones. 2006;5:303–309. doi: 10.14310/horm.2002.11196. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi S, Baghdadi M, Tsuchikawa T, et al. Chemotherapy-derived inflammatory responses accelerate the formation of immunosuppressive myeloid cells in the tissue microenvironment of human pancreatic cancer. Cancer Res. 2015;75:2629–2640. doi: 10.1158/0008-5472.CAN-14-2921. [DOI] [PubMed] [Google Scholar]