Abstract
BACKGROUND
In esophageal squamous carcinoma, lymphadenectomy along the left recurrent laryngeal nerve (RLN) is recommended owing to its highly metastatic potential. However, this procedure is difficult due to limited working space in the left upper mediastinum, and increases postoperative complications.
AIM
To present a novel method for lymphadenectomy along the left RLN during thoracoscopic esophagectomy in the semi-prone position.
METHODS
The fundamental concept of this novel method is to exfoliate a bilateral pedicled nerve flap, which is a two-dimensional membrane, which includes the left RLN, lymph nodes (LNs) along the left RLN, and tracheoesophageal vessels, by suspending the esophagus to the dorsal side and pushing the trachea to the ventral side (named “bilateral exposure method”). Then, the hollow-out method is performed to transform the two-dimensional membrane to a three-dimensional structure, in which the left RLN and tracheoesophageal vessels are easily distinguished and preserved during lymphadenectomy along the left RLN. This novel method was retrospectively evaluated in 116 consecutive patients with esophageal squamous carcinoma from August 2016 to February 2018.
RESULTS
There were 58 patients in each group. No significant difference was found between the two groups in terms of age, gender, postoperative pneumonia, anastomotic fistula, and postoperative hospitalization. However, the number of dissected LNs along the left RLN in this novel method was significantly higher than that in the conventional method (4.17 ± 0.359 vs 2.93 ± 0.463, P = 0.0447). Moreover, the operative time and the rate of postoperative hoarseness in the novel method were significantly lower than those in the conventional method (306.0 ± 6.774 vs 335.2 ± 7.750, P = 0.0054; 4/58 vs 12/58, P = 0.0312).
CONCLUSION
This novel method for lymphadenectomy along the left RLN during thoracoscopic esophagectomy in the semi-prone position is much safer and more effective.
Keywords: Bilateral pedicled nerve flap, Bilateral exposure method, Hollow-out method, Left recurrent laryngeal nerve, Lymphadenectomy, Thoracoscopic esophagectomy
Core tip: Lymph nodes (LNs) along recurrent laryngeal nerves (RLNs) are highly involved in esophageal carcinoma. Due to limited working space in the superior mediastinum, lymphadenectomy along RLNs is very difficult, especially the left RLN. Our study provides a novel method for lymphadenectomy along the left RLN. The fundamental concept of this novel method was to exfoliate a bilateral pedicled nerve flap, which is a two-dimensional membrane that includes the left RLN, surrounding LNs, and tracheoesophageal vessels. Then, the hollow-out method was performed to transform it to a three-dimensional structure, in which the left RLN and tracheoesophageal vessels could be easily distinguished and preserved during lymphadenectomy. Compared with the conventional method, this novel method not only increased the number of dissected LNs, but also shortened the operative time and reduced the rate of postoperative hoarseness.
INTRODUCTION
Esophageal cancer is one of the most common malignant tumors with poor prognosis throughout the world[1,2]. It has become the sixth most common malignant tumor and the fourth leading cause of cancer deaths in China[3]. For resectable esophageal carcinoma, surgical resection with radical lymphadenectomy is still the most effective approach[4]. According to the 8th edition of the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) cancer staging manual, the number of metastatic lymph nodes (LNs) affects accurate nodal staging and prognosis, irrespective of the metastatic region[5]. It has been reported that LNs along recurrent laryngeal nerves (RLNs) are highly involved in esophageal carcinoma; in addition, LNs along RLNs are considered to be associated with cervical nodal metastasis and even an indication for cervical LN dissection[6-8]. Therefore, LNs along RLNs should be completely dissected.
Due to limited working space in the superior mediastinum and sensitivity of the nerve to thermal damage, lymphadenectomy along RLNs is very difficult and increases the risk of complications, especially RLN paralysis[9,10]. RLN paralysis after esophagectomy is associated with an increased pneumonia rate, longer hospital stay, and longer recovery[11]. Therefore, how to dissect these LNs safely and effectively has become a hotspot for thoracic surgeons. Compared with lymphadenectomy along the right RLN, lymphadenectomy along the left RLN is much more difficult because of the deeper location and longer path.
With the development of minimally invasive technologies, thoracoscopic esophagectomy has been widely applied in our country. We herein present a novel method, called bilateral pedicled nerve flap (BPNF) method, for en bloc lymphadenectomy along the left RLN during thoracoscopic esophagectomy in the semi-prone position for esophageal carcinoma.
MATERIALS AND METHODS
Patients
From August 2016 to February 2018, a single-institution nonrandomized study was performed in our hospital. A total of 116 consecutive patients were collected. According to the date of operation and different methods of lymphadenectomy along the left RLN, the patients were alternately allocated into two groups: 58 cases underwent the conventional method, while the other 58 cases underwent the novel method. They were all served by a single medical team and treated with thoraco-laparoscopic esophagectomy with cervical anastomosis in the semi-prone position. All patients were pre-operatively confirmed to have esophageal squamous carcinoma by both esophagogastroscopy and pathology. Clinical stages were assessed by enhanced chest and abdomen computed tomography (CT), as well as by cervical ultrasound, according to the 7th edition of the AJCC/UICC cancer staging manual[12]. PET-CT was performed in patients with good economic conditions who were willing to pay themselves. The patients’ physical conditions were evaluated by cardio-pulmonary, liver and renal function. Cigarette smokers were required to quit smoking for 2 wk with the help of medical staff to reduce the incidence of postoperative pneumonia[13].
The preoperative inclusion criteria were as follows: (1) Diagnosed with squamous cell carcinoma; (2) The depth of tumor invasion no more than T3; (3) LN metastasis no more than N1; and (4) Physical condition that was tolerable for minimally invasive esophagectomy (MIE). The study was approved by the institutional review board and the Ethics Committee of Fujian Provincial Hospital (#K2019-06-028), and registered in the Chinese Clinic Trial Registry Center (#ChiCTR1900026287). Informed consent was obtained from all patients who met the inclusion criteria.
Position
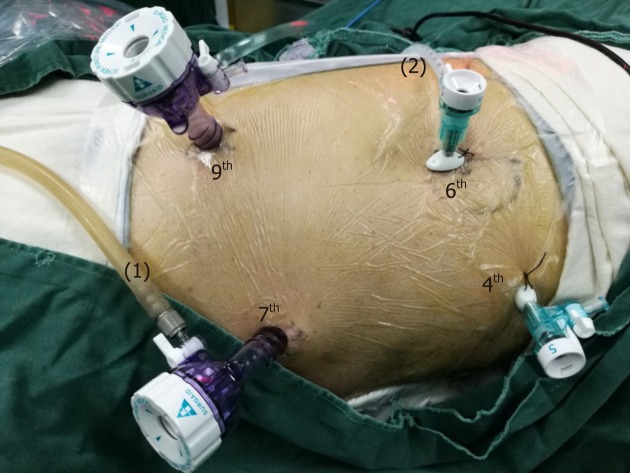
All patients were placed in the left semi-prone position during thoracic surgery. The surgeon stood on the patient’s ventral side. The camera port was placed at the 7th intercostal space (ICS) along the mid-axillary line. The other three ports were placed at the 4th ICS along the anterior axillary line, the 6th ICS along the posterior axillary line, and the 9th ICS along the subscapular line (Figure 1).
Figure 1.
The position and operation ports of the patient during thoracic surgery. The patient was placed in the semi-prone position. The camera port was placed at the 7th intercostal space (ICS) along the mid-axillary line. The other three ports were located at the 4th ICS along the anterior axillary line, the 6th ICS along the posterior axillary line, and the 9th ICS along the subscapular line. (1) Air intake duct of artificial pneumothorax; (2) Air outlet duct of artificial pneumothorax. ICS: Intercostal space.
Thoracic surgery procedures
For better retraction of the trachea, a single-lumen tracheal intubation combined with artificial pneumothorax was performed in all patients. General anesthesia was performed by bilateral ventilation during the thoracic procedure. The chest cavity was inflated with a CO2 insufflation pressure of 8 cm H2O. All procedures were performed by a surgeon with MIE experience in more than 300 cases.
The procedures were divided into four parts, adopting the way of modularization. The steps were as follows:
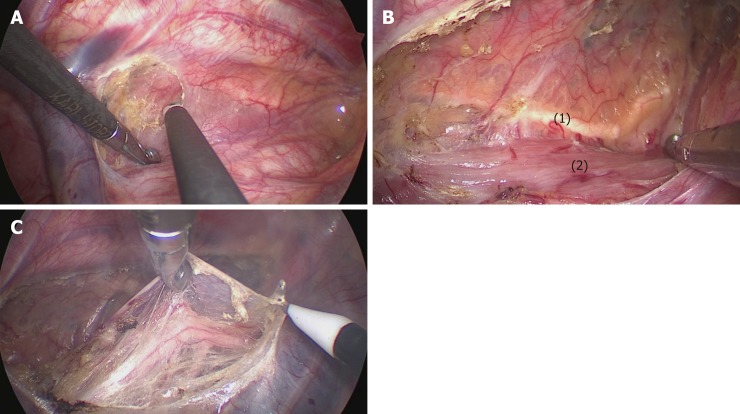
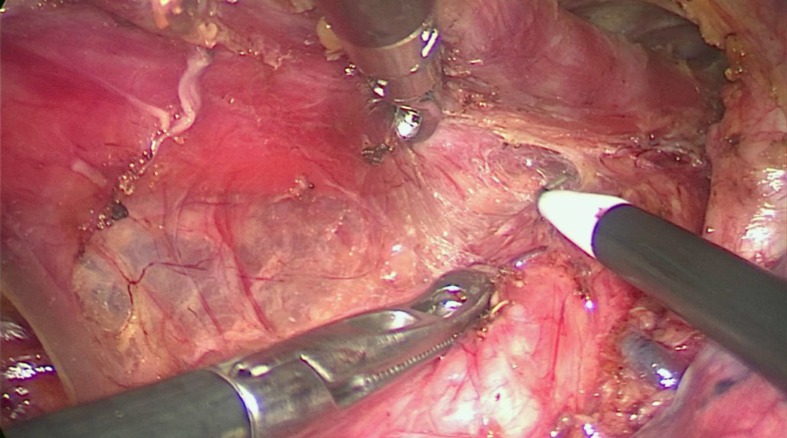
Process 1 - Mobilization of the upper thoracic esophagus and lymphadenectomy along the right RLN (common to both methods). (1) The upper thoracic esophagus was mobilized from the trachea on both sides of the mediastinal pleura. Firstly, the posterior mediastinal pleura above the arch of the azygos vein was dissected along the spine (Figure 2A) until the thoracic duct was fully exposed and carefully preserved (Figure 2B), protecting the esophageal arteries on the dorsal side. Then, the pleura was dissected on the dorsal side of the right vagus nerve toward the right subclavian artery (Figure 2C). And (2) The initial part of the right RLN was revealed at the level of the right subclavian artery. The hollow-out method was performed so that the right RLN, LNs along the nerve, and the tracheoesophageal vessels could be easily identified (Figure 3A). Then, lymphadenectomy along the right RLN was performed, and the right inferior thyroid artery was used as a landmark for the cranial boundary[14] (Figure 3B). During this procedure, esophageal vessels were cut with a harmonic scalpel, avoiding thermal injury to the right RLN and membranous wall of the trachea.
Figure 2.
The mediastinal pleura was dissected on both sides. A: The posterior mediastinal pleura above the arch of the azygos vein was dissected along the spine; B: The thoracic duct was fully exposed and carefully preserved, protecting the esophageal arteries on the dorsal side; C: The right vagus nerve was exposed by cutting the pleura toward the right subclavian artery. (1) Thoracic duct; (2) Esophagus.
Figure 3.
Lymphadenectomy along the right RLN. A: The right RLN was revealed at the level of the right subclavian artery. The hollow-out method was performed to identify the right RLN, LNs along the nerve, and the tracheoesophageal vessels; B: The right inferior thyroid artery was the landmark for the cranial boundary of lymphadenectomy along the right RLN. (1) Right RLN; (2) Right vagus nerve; (3-5) Esophageal branches of the right vagus nerve and RLN; (6) Mediastinal pleura; (7) Right subclavian artery; (8) Right inferior thyroid artery.
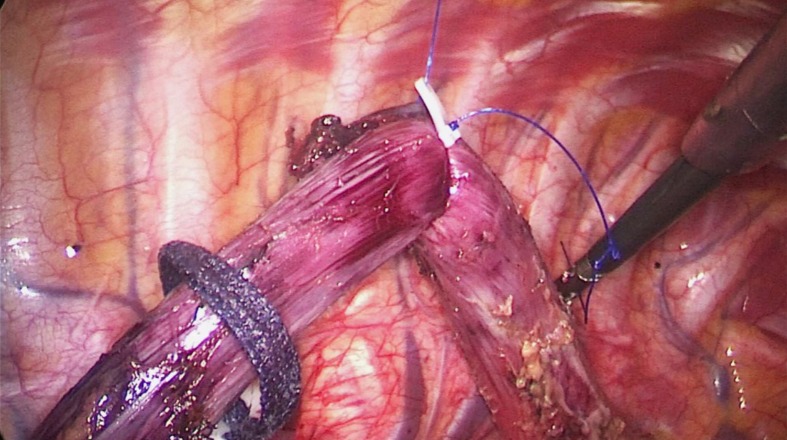
Process 2 - Mobilization of the middle and lower esophagus (common to both methods). (1) The arch of the azygos vein was double ligated and transected. (2) The middle and lower esophagus was mobilized from the posterior to anterior wall, until the bilateral crura of the diaphragm, pericardium, bilateral main bronchus, and carina were fully exposed (Figure 4). The surrounding LNs, mediastinal pleura and peri-esophageal tissues were dissected. And (3) Using a traction suture, the middle and lower esophagus was suspended at the level of the carina and drawn away from the trachea. The traction line was punctured into the thoracic cavity via the 5th ICS between the right scapula and the vertebral body, and pulled out via the trocar in the 6th ICS along the posterior axillary line (Figure 5).
Figure 4.
The middle and lower esophagus was mobilized from the posterior to anterior wall, until the bilateral crura of the diaphragm, pericardium, bilateral main bronchus, and carina were fully exposed. During the procedure, the surrounding lymph nodes, mediastinal pleura and peri-esophageal tissues were dissected.
Figure 5.
The upper thoracic esophagus was suspended to the dorsal side with a traction line.
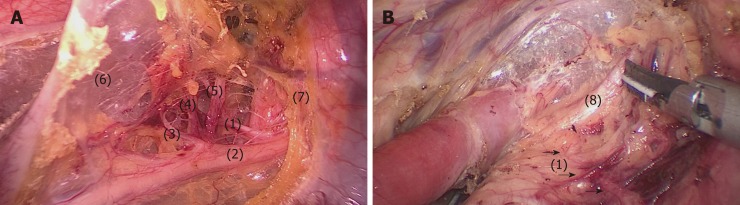
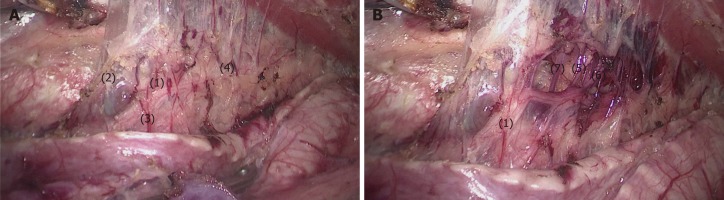
Process 3 - Lymphadenectomy along the left RLN: The bilateral pedicled nerve flap method (BPNF, new method). (1) When the upper esophagus was drawn to the vertebral body by a traction suture, the trachea was pushed to the ventral side by an assistant (Figure 6). For a better retraction of the trachea, the endotracheal tube cuff was deflated during this process. Therefore, the working space in the upper mediastinum was increased, and the tracheoesophageal groove was better exposed. This method was named as “bilateral exposure method”. (2) Subsequently, an electric hook was used to dissociate the tissues close to the left main bronchus and the left edge of the trachea. The anterior wall of the upper esophagus was completely mobilized from the trachea. The left RLN and lymph nodes remained attaching to the esophagus, so that a bilateral pedicled nerve flap (a two-dimensional membrane) including the left RLN, the peripheral LNs, and the tracheoesophageal arteries, was exfoliated from the trachea up to the cervical root (Figure 7A). Since esophageal vessels in the dorsal side were also preserved in Process 1, we named the membrane as a “bilateral pedicled nerve flap”. (3) The “hollow-out method” was performed to separate the LNs from the left RLN with isolating forceps. With this method, the bilateral pedicled nerve flap was transformed into a three-dimensional structure, in which the left RLN, esophageal branches of the left RLN, LNs and tracheoesophageal vessels could be easily identified (Figure 7B). The esophageal vessels were transected with a harmonic scalpel, while the esophageal branches of the left RLN were cut off sharply. (4) The LNs beneath the left RLN were harvested, keeping the remaining LNs attached to the esophagus. Lymphadenectomy was performed from the lower edge of the aortic arch to the level of the left inferior thyroid artery. And (5) During this process, the esophagus was suspended with a traction line and not severed in the thoracic cavity. The tension of the suspension line could be adjusted for a better surgical field, especially in the dissection of the cranial side of the left RLN lymph nodes.
Figure 6.
Bi-directional exposure method. When the upper esophagus was suspended with a traction line and drawn to the vertebral body, the trachea was pushed to the ventral side by an assistant. Meanwhile, the surgeon pushed the esophagus more delicately using grasping forceps to increase the working space in the upper mediastinum.
Figure 7.
The bilateral pedicled nerve flap method. After the tissues were released from the edge of the trachea and left main bronchus, the anterior wall of the upper esophagus was completely mobilized from the trachea, with the left recurrent laryngeal nerve (RLN) and lymph nodes attaching to the upper esophagus. A: Then, a bilateral pedicled nerve flap, which was a two-dimensional membrane including the left RLN, peripheral LNs, and tracheoesophageal arteries, was exfoliated from the trachea; B: Subsequently, the “hollow-out” method was performed to transform the two-dimensional membrane into a three-dimensional structure, in which the left RLN, esophageal branches of the left RLN, lymph nodes and tracheoesophageal vessels could be easily identified. (1) Left RLN; (2) Lymph nodes along the left RLN; (3,4,7) Tracheoesophageal vessels; (5-6) Esophageal branches of the left RLN. RLN: Recurrent laryngeal nerve.
Conventional method
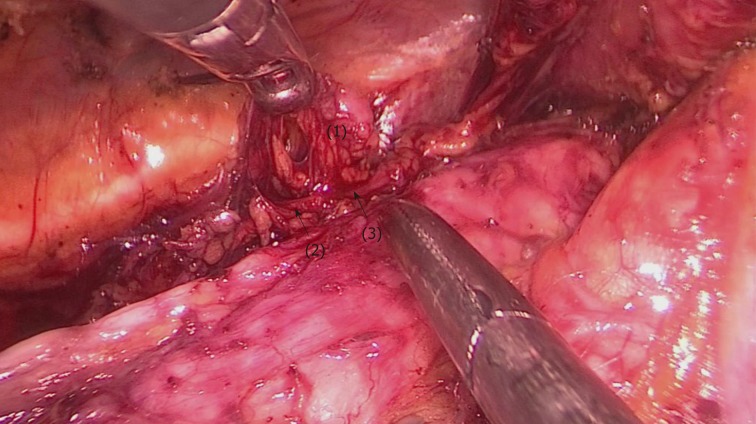
After the middle and lower esophagus was mobilized and suspended with a traction suture, the upper esophagus was further mobilized towards the neck. The tissues including the left RLN and LNs were released from the upper esophagus, and maintained in the tracheoesophageal groove. Subsequently, LNs and peripheral tissues were held by the left hand of the surgeon to separate them from the left RLN (Figure 8). To better expose the tracheoesophageal groove, the endotracheal tube cuff was deflated, and the trachea was pushed to the ventral side by an assistant. Finally, the left RLN was sharply isolated from the explored tissues.
Figure 8.
The upper esophagus was suspended with a traction line after dissociation from the trachea. The left RLN and lymph nodes remained adjacent to the trachea in situ. Then, lymph nodes and peripheral tissues (marked 1) were held by the left hand of the surgeon to separate them from the left RLN (arrows 2-3).
Process 4 (common to both methods): LNs in the initial part of the left RLN and on the surface of the trunk of the pulmonary artery were dissected, respectively.
Abdominal procedure
The abdominal procedure was performed laparoscopically, which was common in both groups. The surgeon was on the patient’s right side, and the assistant was on the left side. After gastric mobilization and abdominal lymphadenectomy around the left gastric artery, common hepatic artery, splenic artery and cardia, the entire thoracic esophagus with dissected LNs were pulled into the abdomen through an esophageal hiatus. The stomach was pulled out from a 4-5 cm incision in the upper abdomen to make a gastric tube 3-4 cm in width. Then, the gastric tube was drawn to the left neck via an esophageal bed.
Cervical procedure
The cervical esophagus was dissociated and cut in the left neck. Anastomosis was performed in the left neck. Cervical lymphadenectomy should be performed in patients with high suspicion of cervical LN metastasis based on preoperative cervical ultrasound, enhanced CT or PET-CT.
Data collection and statistical analysis
Data were collected in both groups, including age, gender, operative time, postoperative pneumonia, anastomotic fistula, postoperative hospitalization, the number of dissected LNs, and the rate of postoperative recurrent laryngeal nerve injury, which was confirmed by hoarseness and laryngoscopy. Values are presented as mean ± SD.
Data were compared between the two groups. All data were analyzed using the statistical program SPSS version 18.0. t-tests were used to compare mean values. Chi-square tests were used to compare ratios. A P value of less than 0.05 was considered to be statistically significant.
Follow-up protocol
A follow-up visit was performed after the operation every 3 mo for the first 2 years. All patients were monitored with thoracic and abdominal CT, cervical lymph node ultrasound, and tumor markers in the peripheral serum.
RESULTS
There were 58 patients in each group. The clinicopathological characteristics of patients and tumors are shown in Table 1. No significant differences were found between the two groups in terms of age, gender, tumor location, depth of tumor invasion, lymph node metastasis, AJCC pathological stage, and preoperative neoadjuvant therapy. Conversion to open surgery and intraoperative morbidity related to the left RLN did not occur in either group.
Table 1.
Clinicopathological characteristics of patients and tumors from two groups
| NM group, n = 58 | CM group, n = 58 | P value | |
| Age in yr | 60.34 ± 1.089 | 60.26 ± 1.029 | 0.954 |
| Gender | 0.221 | ||
| Male | 38 | 44 | |
| Female | 20 | 14 | |
| Tumor location | 0.884 | ||
| Upper | 12 | 10 | |
| Middle | 37 | 38 | |
| Lower | 9 | 10 | |
| Depth of tumor invasion | 0.616 | ||
| pT1 | 22 | 17 | |
| pT2 | 7 | 7 | |
| pT3 | 28 | 31 | |
| pT4 | 1 | 3 | |
| Lymph node metastasis | 0.484 | ||
| pN0 | 32 | 35 | |
| pN1 | 18 | 16 | |
| pN2 | 4 | 6 | |
| pN3 | 4 | 1 | |
| AJCC pathological stage | 0.326 | ||
| I | 21 | 18 | |
| II | 14 | 21 | |
| III | 19 | 18 | |
| IV | 4 | 1 | |
| Preoperative neoadjuvant chemotherapy/radiotherapy | 0.242 | ||
| Yes | 2 | 5 | |
| No | 56 | 53 |
CM: Conventional method; NM: Novel method.
Surgical outcomes are shown in Table 2. The number of dissected lymph nodes along the left RLN was significantly higher than that of the CM group (4.17 ± 0.359 vs 2.93 ± 0.463, P = 0.0447). The operative time and the rate of postoperative recurrent laryngeal nerve injury in the NM group were significantly lower than those of the CM group (306.0 ± 6.774 vs 335.2 ± 7.750, P = 0.0054; 4/58 vs 12/58, P = 0.0312). There were no significant differences between the two groups in postoperative pneumonia, chylothorax, anastomatic fistula, and postoperative hospitalization. Patients with postoperative recurrent laryngeal nerve injury in both groups restored normal function 3 mo after operation. Recurrent laryngeal nerve injury was confirmed by electronic laryngoscope. One patient in the CM group died of tumor recurrence 1 year after the operation, while one patient in the NM group died without a definitive reason 3 mo after the operation.
Table 2.
Surgical outcomes of patients from the two groups
| NM group, n = 58 | CM group, n = 58 | P value | |
| Operative time in min | 306.0 ± 6.774 | 335.2 ± 7.750 | 0.0054 |
| Number of dissected lymph nodes along the left RLN | 4.172 ± 0.359 | 2.983 ± 0.463 | 0.0447 |
| Postoperative complication | |||
| Hoarseness | 4 | 12 | 0.0312 |
| Pneumonia | 3 | 8 | 0.113 |
| Anastomatic fistula | 4 | 9 | 0.141 |
| Chylothorax | 3 | 1 | 0.309 |
| Postoperative hospitalization in d | 15.52 ± 0.905 | 17.72 ± 1.571 | 0.226 |
DISCUSSION
Lymph node metastasis is the most common type of esophageal squamous cell carcinoma, which often represents as bi-directional or skips metastasis[15]. According to the 8th edition of the AJCC/UICC cancer staging manual, accurate assessment of the N stage plays a decisive role in the long-term survival of esophageal and esophagogastric junction cancers[5]. The number of harvested LNs affects accurate N staging and long-term prognosis. It has been reported that the overall survival is better in three-field lymphadenectomy than in two-field lymphadenectomy for esophageal carcinoma with cervical and/or upper mediastinal LN metastasis[16,17]. Due to high involvement and better prognosis after radical dissection, LN dissection along the RLN is recommended[4,18,19].
During lymphadenectomy along RLNs in esophageal carcinoma, RLN injury often occurs due to thermal injury, compression, stretching, and nourishing vessel injury, which further leads to RLN paralysis. RLN paralysis increases the rate of postoperative respiratory complication (including dyspnea, pneumonia, aspiration, and acute respiratory distress syndrome), as well as esophagogastric anastomatic leakage, and prolongs postoperative hospitalization[9-11,20]. Despite the development of surgical techniques and instruments, the incidence of RLN paralysis still remains at a high level, ranging from 2-59.5%[9-11,20,21]. Left RLN paralysis is much more common than right because of the longer path and deeper location. Therefore, sufficient exposure and precise operations are necessary during lymphadenectomy in this area.
Due to magnified and clear surgical vision, video-assisted thoracoscopic minimally invasive surgery has been widely used for early and mid-stage esophageal carcinoma. However, lymphadenectomy along the left RLN is still thought to be a tedious step because of difficult exposure in the left upper mediastinum. Compared to the left lateral decubitus position, the prone position provides better surgeon ergonomics and better operative exposure during lymphadenectomy along the left RLN[21]. Nevertheless, disadvantages of the prone position include emergent conversion to open surgery and the need for an assistant to expose the operative field or retract adjacent organs[21]. Therefore, the prone position is not suitable for patients in the advanced stage or receiving neoadjuvant radiochemotherapy. In the semi-prone position, the right lung prolapses to the ventral side, and exudates accumulate in the right lower thoracic cavity. The semi-prone position is also convenient for conversion to thoracotomy when thoracoscopic surgery is difficult. Moreover, artificial pneumothorax contributes to right lung collapse and mediastinal inflation, which results in better exposure for the operative field.
In the left upper mediastinum, there are three important anatomical structures: The left RLN, LNs, and the left tracheoesophageal vessels. In the novel method, the three anatomical structures are integrated into a two-dimensional membrane during lymphadenectomy along the left RLN, which is a bilateral pedicled nerve flap. For more elaborate manipulation in subsequent steps, the two-dimensional membrane should be substantially thin. On the dorsal side of the membrane, the posterior wall of the upper esophagus should be mobilized until the thoracic duct is fully exposed. On the ventral side of the membrane, the endotracheal tube cuff is deflated so that the trachea can be easily pushed to the ventral side by an assistant. Then, in the novel method, the working space between the esophagus and trachea is extended. The upper esophagus, including these three structures, is exfoliated from the left side of the trachea. The two-dimensional membrane is then transformed into a three-dimensional structure by blunt dissection, so that LNs, the esophageal branches of the left RLN, and esophageal vessels can be easily distinguished. Subsequently, LNs are en-bloc dissected, while the left RLN and tracheal vessels can be precisely preserved. In the conventional method, the esophagus is completely freed and separated from these three structures. Without traction of the upper esophagus, LNs should be lifted by the left hand of the surgeon during lymphadenectomy along the left RLN. Lymphadenectomy is performed one by one. The exposure of the left RLN and tracheoesophageal arteries is not sufficient. These may be the reasons for the decreased number of harvested LNs and increased incidence of left RLN paralysis.
During the past few years, many methods have been reported to protect the left RLN during lymphadenectomy, however, some limitations remain. Zheng et al[22] applied the esophageal suspension method to decrease the postoperative hoarseness rate. However, the number of scavenged peripheral LNs in the esophageal suspension group did not increase compared to the non-suspension group. Oshikiri et al[23] reported the “Bascule Method” for lymphadenectomy along the left RLN during prone esophagectomy. In the “Bascule Method”, the esophagus was divided into two sections at the level of the aortic arch, and the proximal portion of the divided esophagus was suspended using a traction suture. The left RLN, LNs along the left RLN, as well as the tracheoesophageal and primary esophageal arteries were then integrated into a two-dimensional membrane. The operative time during LN dissection along the left RLN, estimated blood loss, and hoarseness rates were significantly lower owing to the “Bascule Method”. However, it was not suitable for patients with tumors across the aortic arch.
In this study, the novel method not only significantly increased the number of dissected LNs along the left RLN, but also shortened the operative time and decreased the rate of postoperative recurrent laryngeal nerve injury. The main reason for these advantages was better operative exposure in the novel method. Therefore, it was easier to identify these anatomical structures, and the surgical procedure was much smoother and more efficient. Although there was no statistical difference, a lower incidence of RLN injury also appeared to reduced postoperative hospitalization. Since it is not required to divide the esophagus, the novel method is applicable to all esophageal carcinomas in the early and middle stages. However, since no definitive conclusions can be drawn from such a retrospective study, a randomized controlled trial is needed to further assess the novel method.
In conclusion, the novel method reported herein is safe and feasible for lymphadenectomy along the left RLN during thoracoscopic esophagectomy in the semi-prone position. It not only increases the number of harvested LNs, but also decreases the operative time and incidence of postoperative recurrent laryngeal nerve injury. Although there are limitations in this study, further research is still needed to verify the validity. This study provides a new idea to solve this problem. We believe that the “bilateral pedicled nerve flap” method will be a useful technique for en bloc lymphadenectomy along the left RLN during thoracoscopic esophagectomy in the semi-prone position.
ARTICLE HIGHLIGHTS
Research background
LNs along recurrent laryngeal nerves (RLNs) are highly involved in esophageal carcinoma. Due to limited working space in the superior mediastinum, lymphadenectomy along RLNs is very difficult and increases the risk of complications, especially RLN paralysis. Compared with lymphadenectomy along the right RLN, lymphadenectomy along the left RLN is much more difficult because of the deeper location and longer path.
Research motivation
In this study, we present a novel protocol, a “bilateral pedicled nerve flap” method, for lymphadenectomy along the left RLN during thoracoscopic esophagectomy in the semi-prone position for esophageal carcinoma. To our knowledge, this is the first report of applying this technique for the treatment of esophageal tumors.
Research objectives
To search for a novel safe and effective method for lymphadenectomy along the left RLN during thoracoscopic esophagectomy for esophageal carcinoma.
Research methods
From August 2016 to February 2018, a single-institution nonrandomized study was performed in our hospital. According to the date of operation and different methods of lymphadenectomy along the left RLN, a total of 116 consecutive patients were collected and alternately allocated into two groups: 58 cases underwent the conventional method, while the other 58 cases underwent the novel method. They were all served by a single medical team and treated with thoraco-laparoscopic esophagectomy with cervical anastomosis in the semi-prone position. Postoperative complications, operative time, postoperative hospitalization, and the number of dissected LNs were compared between the two groups.
Research results
No significant difference was found between the two groups in terms of age, gender, postoperative pneumonia, anastomotic fistula, and postoperative hospitalization. The number of dissected LNs along the left RLN in the novel method was significantly higher than that of the conventional method (4.17 ± 0.359 vs 2.93 ± 0.463, P = 0.0447). Moreover, the operative time and the rate of postoperative hoarseness in the novel method were significantly lower than those of the conventional method (306.0 ± 6.774 vs 335.2 ± 7.750, P = 0.0054; 4/58 vs 12/58, P = 0.0312).
Research conclusions
The novel method for lymphadenectomy along the left RLN during thoracoscopic esophagectomy in the semi-prone position is much safer and more effective. The theories of this technique lie in better exposure of the operative field and protection of neurotrophic vessels. In the novel method, the bilateral exposure method and hollow-out method were successively performed. These not only increased the operative field of the tracheoesophageal groove, but also made the left RLN and microvessels easier to identify. Therefore, the number of dissected LNs was significantly higher in the novel group. Moreover, the operative time and the rate of postoperative hoarseness in the novel method were significantly lower than the conventional group. These findings will not only improve short-term prognosis, but also have a positive impact on long-term prognosis.
ACKNOWLEDGEMENTS
The authors thank Professor Guo-Zhang Zhu, PhD (Department of Biological Sciences at Marshall University) for English revision.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the institutional review board and the Ethics Committee of Fujian Provincial Hospital (#K2019-06-028).
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Data sharing statement: No additional data are available.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Peer-review started: November 9, 2010
First decision: December 23, 2019
Article in press: March 9, 2020
P-Reviewer: Hosoda K S-Editor: Wang J L-Editor: Filipodia E-Editor: Zhang YL
Contributor Information
Wen-Shu Chen, Department of Thoracic Surgery, Shengli Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, Fuzhou 350001, Fujian Province, China. doctorcws@163.com.
Li-Huan Zhu, Department of Thoracic Surgery, Shengli Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, Fuzhou 350001, Fujian Province, China.
Wu-Jin Li, Department of Thoracic Surgery, Shengli Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, Fuzhou 350001, Fujian Province, China.
Peng-Jie Tu, Department of Thoracic Surgery, Shengli Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, Fuzhou 350001, Fujian Province, China.
Jian-Yuan Huang, Department of Thoracic Surgery, Shengli Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, Fuzhou 350001, Fujian Province, China.
Pei-Lin You, Department of Thoracic Surgery, Shengli Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, Fuzhou 350001, Fujian Province, China.
Xiao-Jie Pan, Department of Thoracic Surgery, Shengli Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, Fuzhou 350001, Fujian Province, China.
Data sharing statement
No additional data are available.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology. 2018;154:360–373. doi: 10.1053/j.gastro.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng R, Zeng H, Zhang S, Chen W. Estimates of cancer incidence and mortality in China, 2013. Chin J Cancer. 2017;36:66. doi: 10.1186/s40880-017-0234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin CS, Cheng CT, Liu CY, Lee MY, Hsiao MC, Shih CH, Liu CC. Radical Lymph Node Dissection in Primary Esophagectomy for Esophageal Squamous Cell Carcinoma. Ann Thorac Surg. 2015;100:278–286. doi: 10.1016/j.athoracsur.2015.02.053. [DOI] [PubMed] [Google Scholar]
- 5.Donohoe CL, Phillips AW. Cancer of the esophagus and esophagogastric junction: an 8th edition staging primer. J Thorac Dis. 2017;9:E282–E284. doi: 10.21037/jtd.2017.03.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye K, Xu JH, Sun YF, Lin JA, Zheng ZG. Characteristics and clinical significance of lymph node metastases near the recurrent laryngeal nerve from thoracic esophageal carcinoma. Genet Mol Res. 2014;13:6411–6419. doi: 10.4238/2014.August.25.4. [DOI] [PubMed] [Google Scholar]
- 7.Tabira Y, Yasunaga M, Tanaka M, Nakano K, Sakaguchi T, Nagamoto N, Ogi S, Kitamura N. Recurrent nerve nodal involvement is associated with cervical nodal metastasis in thoracic esophageal carcinoma. J Am Coll Surg. 2000;191:232–237. doi: 10.1016/s1072-7515(00)00348-3. [DOI] [PubMed] [Google Scholar]
- 8.Shiozaki H, Yano M, Tsujinaka T, Inoue M, Tamura S, Doki Y, Yasuda T, Fujiwara Y, Monden M. Lymph node metastasis along the recurrent nerve chain is an indication for cervical lymph node dissection in thoracic esophageal cancer. Dis Esophagus. 2001;14:191–196. doi: 10.1046/j.1442-2050.2001.00206.x. [DOI] [PubMed] [Google Scholar]
- 9.Sato Y, Kosugi S, Aizawa N, Ishikawa T, Kano Y, Ichikawa H, Hanyu T, Hirashima K, Bamba T, Wakai T. Risk Factors and Clinical Outcomes of Recurrent Laryngeal Nerve Paralysis After Esophagectomy for Thoracic Esophageal Carcinoma. World J Surg. 2016;40:129–136. doi: 10.1007/s00268-015-3261-8. [DOI] [PubMed] [Google Scholar]
- 10.Koyanagi K, Igaki H, Iwabu J, Ochiai H, Tachimori Y. Recurrent Laryngeal Nerve Paralysis after Esophagectomy: Respiratory Complications and Role of Nerve Reconstruction. Tohoku J Exp Med. 2015;237:1–8. doi: 10.1620/tjem.237.1. [DOI] [PubMed] [Google Scholar]
- 11.Scholtemeijer MG, Seesing MFJ, Brenkman HJF, Janssen LM, van Hillegersberg R, Ruurda JP. Recurrent laryngeal nerve injury after esophagectomy for esophageal cancer: incidence, management, and impact on short- and long-term outcomes. J Thorac Dis. 2017;9:S868–S878. doi: 10.21037/jtd.2017.06.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 13.Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy for treatment of benign and malignant esophageal disease. World J Surg. 2001;25:196–203. [PubMed] [Google Scholar]
- 14.Oshikiri T, Nakamura T, Miura Y, Yamamoto M, Kanaji S, Yamashita K, Matsuda T, Sumi Y, Suzuki S, Kakeji Y. A new method (the "Pincers maneuver") for lymphadenectomy along the right recurrent laryngeal nerve during thoracoscopic esophagectomy in the prone position for esophageal cancer. Surg Endosc. 2017;31:1496–1504. doi: 10.1007/s00464-016-5124-2. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Wu S, Zheng X, Pan J, Zhu K, Chen Y, Li J, Liao L, Lin Y, Liao Z. Cervical lymph node metastasis classified as regional nodal staging in thoracic esophageal squamous cell carcinoma after radical esophagectomy and three-field lymph node dissection. BMC Surg. 2014;14:110. doi: 10.1186/1471-2482-14-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang QX, Chen LQ, Hu WP, Deng HY, Yuan Y, Cai J. Three-field lymph node dissection in treating the esophageal cancer. J Thorac Dis. 2016;8:E1136–E1149. doi: 10.21037/jtd.2016.10.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Igaki H, Tachimori Y, Kato H. Improved survival for patients with upper and/or middle mediastinal lymph node metastasis of squamous cell carcinoma of the lower thoracic esophagus treated with 3-field dissection. Ann Surg. 2004;239:483–490. doi: 10.1097/01.sla.0000118562.97742.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tachimori Y, Nagai Y, Kanamori N, Hokamura N, Igaki H. Pattern of lymph node metastases of esophageal squamous cell carcinoma based on the anatomical lymphatic drainage system. Dis Esophagus. 2011;24:33–38. doi: 10.1111/j.1442-2050.2010.01086.x. [DOI] [PubMed] [Google Scholar]
- 19.Wu SG, He ZY, Wang Y, Sun JY, Lin HX, Su GQ, Li Q. Lymph node dissection improved survival in patients with metastatic thoracic esophageal cancer: An analysis of 220 patients from the SEER database. Int J Surg. 2016;35:13–18. doi: 10.1016/j.ijsu.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Gockel I, Kneist W, Keilmann A, Junginger T. Recurrent laryngeal nerve paralysis (RLNP) following esophagectomy for carcinoma. Eur J Surg Oncol. 2005;31:277–281. doi: 10.1016/j.ejso.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Noshiro H, Iwasaki H, Kobayashi K, Uchiyama A, Miyasaka Y, Masatsugu T, Koike K, Miyazaki K. Lymphadenectomy along the left recurrent laryngeal nerve by a minimally invasive esophagectomy in the prone position for thoracic esophageal cancer. Surg Endosc. 2010;24:2965–2973. doi: 10.1007/s00464-010-1072-4. [DOI] [PubMed] [Google Scholar]
- 22.Zheng W, Zhu Y, Guo CH, Zheng B, Han ZY, Chen C. Esophageal suspension method in scavenging peripheral lymph nodes of the left recurrent laryngeal nerve in thoracic esophageal carcinoma through semi-prone-position thoracoscopy. J Cancer Res Ther. 2014;10:985–990. doi: 10.4103/0973-1482.144354. [DOI] [PubMed] [Google Scholar]
- 23.Oshikiri T, Yasuda T, Harada H, Goto H, Oyama M, Hasegawa H, Ohara T, Sendo H, Nakamura T, Fujino Y, Tominaga M, Kakeji Y. A new method (the "Bascule method") for lymphadenectomy along the left recurrent laryngeal nerve during prone esophagectomy for esophageal cancer. Surg Endosc. 2015;29:2442–2450. doi: 10.1007/s00464-014-3919-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.