Abstract
Introduction: Alcohol is a carcinogen for human cancer. This contribution summarizes the relationships between alcohol use and gastrointestinal cancers, and implications for prevention.
Methods: Comparative risk assessment and narrative literature review.
Results: The following gastrointestinal cancer sites were found to be causally impacted by alcohol use: lip and oral cavity, pharynx other than nasopharynx, esophagus, colon and rectum, and liver. Globally, 368,000 deaths (304,000 men and 64,000 women) and more than 10 million disability-adjusted life years (DALYs) lost (10.1 million; 8.4 million men and 1.6 million women) in 2016 were attributable to alcohol use, making up about 10% of all deaths and DALYs lost due to these cancers, respectively. There are effective and cost-effective alcohol control policies available to reduce this burden, namely the best buys of increasing taxation, reducing availability, and banning advertisement. In addition, public knowledge about the alcohol-cancer link should be increased.
Discussion: There are a number of assumptions underlying these estimates, but overall all of them seem to be conservative.
Keywords: alcohol, gastrointestinal tract, liver cancer, colorectal cancer, esophagus cancer, mortality, burden of disease, prevention
Introduction
Alcohol use is one of the major risk factors for cancer (1, 2), with about 5% of all cancers being caused by alcohol (3). Gastrointestinal cancers, which contributed about one third to all cancer burden of disease and more than 40% to all cancer deaths in 2017 (4), are no exception. On the contrary, alcohol-attributable gastrointestinal cancer types made up the majority of all alcohol-attributable cancers [more than 80% of both mortality and burden of disease (3)].
For most of the cancers in the gastrointestinal tract, alcohol use has been established as a causal factor [for criteria see (5, 6)], and the categorizations converge between the International Agency for Research on Cancer (7, 8) and the Continuous Update Project of the World Cancer Research Fund (9) (see Table 1 for details).
Table 1.
Alcohol-attributable mortality and burden of disease for cancers of the gastrointestinal tract in 2016.
| Cancer type | Deaths (in thousands) | DALYs (in millions) | PAF in % |
|---|---|---|---|
| Lip and oral cavity cancer | 52.2 | 1.7 | 31.3 |
| Pharynx other than nasopharynx | 38.6 | 1.2 | 34.9 |
| Esophagus | 82.6 | 2.2 | 19.3 |
| Colon and rectum | 92.6 | 2.2 | 11.7 |
| Liver | 101.4 | 2.8 | 12.2 |
| All cancers of the gastrointestinal tract | 367.7 | 10.1 | 9.9 |
PAF, population-attributable fraction.
It is the aim of this contribution to give epidemiological indicators for alcohol-attributable cancer burden for organs of the gastrointestinal tract globally, and to look into the best prevention efforts. As a basis, we used a recent publication on alcohol use and disease burden (3), from which we aggregated the alcohol-attributable death and burden of disease [in disability-adjusted life-years—DALYs—lost (10)], and the recommendations of the World Health Organization (WHO) (11) to prevent premature mortality from non-communicable disease, namely the so-called “best buys,” which constitute a particular set of alcohol control mechanism, which are not only highly cost-effective (12), but also very affordable and easy to implement (13).
Materials and Methods
Based on available global data (14), the following cancer categories were considered to be in part caused by alcohol use: lip and oral cavity (C00–C08), other pharynx than nasopharynx (C12–C14), esophagus (C15), colon and rectum (C18–C21), and liver (C22). Thus, our estimates exclude cancers of small intestine (C17) and cancer of the intestinal tract, part unspecified (C26), which has been determined to be potentially alcohol-attributable (7, 8).
The mortality and disease burden of alcohol-attributable gastrointestinal cancer for 2016 was calculated based on the recent publication of Shield et al. (3), who based their estimates on the exposure estimates of Manthey et al. (15) combined with cancer-site specific risk relations of Turati et al. (16) for liver cancer, and Bagnardi et al. (17) for all other cancer categories. A 10-year lag between exposure and outcomes was assumed (18).
Shield and colleagues performed a comparative risk assessment for alcohol (19) based on the tradition of the Global Burden of Disease Studies [last assessment published: (20) and the WHO Global Status Reports on Alcohol and Health [last: (21)]. The usual formula for attributable risk was used (22), with exposure being continuous, with a specific term for former drinkers added (23, 24) to adjust for the “sick quitter” phenomenon (25)]. For a general discussion of the assumptions of comparative risk assessments, see the overview by Murray et al. (26), and for further detail on the assumptions for comparative risk assessments for alcohol, [see Rehm et al. (27)]. All dose-response curves show a monotonous exponential course (3), with cancers of the upper digestive tract showing steeper slopes. Further details on the risk relation formulas can be found in Shield et al. (3).
Prevention suggestions were based on the WHO's recommendations for prevention and control of non-communicable disease burden (11), with special emphasis on the cost-effectiveness of models.
Results
Epidemiology
Globally, 368,000 deaths (304,000 men and 64,000 women) and more than 10 million DALYs lost (10.1 million; 8.4 million men and 1.6 million women) in 2016 were attributable to alcohol use, making up about 10% of all deaths and DALYs lost due to these cancers, respectively. These deaths and years of life lost could have been avoided if no alcohol had been consumed. Table 2 gives an overview of alcohol-attributable mortality and burden of disease by subcategory of gastrointestinal cancer.
Table 2.
Alcohol-attributable mortality and burden of disease for cancers of the gastrointestinal tract in 2016.
| Cancer type | Deaths thousands (95% CI) | DALYs lost millions (95% CI) | PAF in % (95% CI) |
|---|---|---|---|
| Lip and oral cavity cancer | 52.2 (35.2, 53.1) |
1.7 (1.1, 1.7)* |
31.3 (21.1, 31.8) |
| Pharynx other than nasopharynx | 38.6 (26.6, 40.1) |
1.2 (0.8, 1.2)* |
34.9 (24.1, 36.3) |
| Esophagus | 82.6 (55.3, 85.7) |
2.2 (1.5, 2.2)* |
19.3 (12.9, 20.0) |
| Colon and rectum | 92.6 (73.7, 109.1) |
2.2 (1.8, 2.6) |
11.7 (9.3, 13.7) |
| Liver | 101.4 (54.1, 140.4) |
2.8 (1.5, 3.9) |
12.2 (6.5, 16.9) |
| All cancers of the gastrointestinal tract | 367.7 (278.6, 394.1) |
10.1 (7.5, 10.7) |
9.9 (7.5, 10.7) |
CI, Confidence interval; DALYs, disability-adjusted life years; PAF, population-attributable fraction of burden of disease in DALYs lost.
Due to the dose-response function, the 95% confidence intervals are asymmetric.
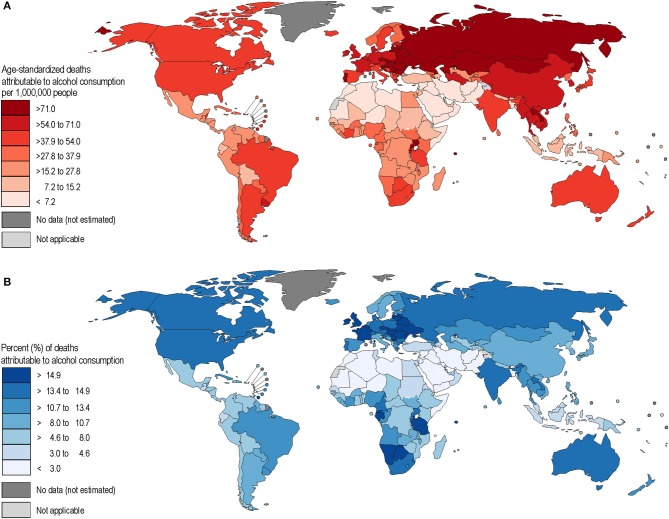
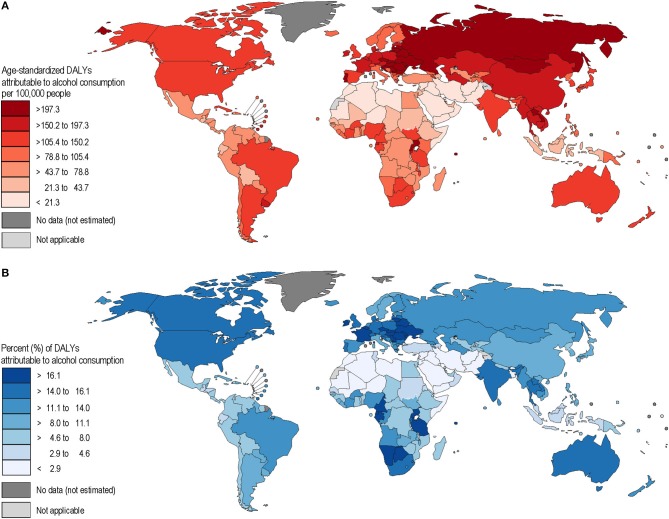
Alcohol-attributable liver cancer had the largest share of mortality and burden of disease out of all the alcohol-attributable cancer categories, followed by colorectal cancers. Burden showed a high variation in different parts of the world, mainly based on the distribution of alcohol use (Figures 1A,B, 2A,B). It should be noted that all figures show point estimates only, and some of these estimates have wide confidence intervals (see also Table 2).
Figure 1.
(A,B) Age-standardized mortality rates and population-attributable fractions of alcohol-attributable gastrointestinal cancers in 2016.
Figure 2.
(A,B) Age-standardized disability-adjusted life years (DALY) rates and population-attributable fractions of alcohol-attributable gastrointestinal cancers in 2016.
The highest alcohol-attributable cancer mortality and burden rates are found in eastern Europe, and parts of Central Asia, whereas countries in the Muslim belt from North Africa to Pakistan were found to have the lowest rates of mortality and burden (see Figures 1A,B, 2A,B).
Prevention
As shown above, the burden of alcohol-attributable gastrointestinal cancer is large, with more than 360,000 deaths and more than one million DALYs lost. What are the best prevention strategies, based on the risk relations between alcohol use and gastrointestinal cancer? First, unlike other alcohol-attributable disease outcomes with complex risk curves for the average level of drinking and interactions with the pattern of drinking—such as ischemic heart disease (28, 29)—the relationship between gastrointestinal cancer and alcohol is simple: the more alcohol consumed, the higher the risk (3). Prevention efforts should therefore mainly focus on a reduction in the level of drinking (30). There also do not seem to be specific effects for different beverage types (31). Thus, the best ways to reduce overall level of consumption would be those that are effective in the prevention of any type of cancer, and for those which work to prevent gastrointestinal cancer specifically (32). Any reduction in alcohol consumption will be associated reductions in cancer risks, albeit with lag times [e.g., (33, 34)].
Given this situation, and the fact that many alcohol-attributable cancers are found light to moderate drinkers (35, 36), the most effective and cost-effective prevention method available to us is the alcohol control policy outlined in the WHO “best buys” [e.g., (12, 13): an increase in the price of alcoholic beverages via an increase in alcohol taxation (37)], and restrictions on their availability, such as a restriction on sale hours, and a ban on marketing (38). In addition, there may be two interventions to prevent alcohol-attributable gastrointestinal cancer burden specifically: educating the public and container labeling. Both of these interventions build on the fact that knowledge that alcohol is a carcinogen has still not entered into the mainstream in most countries (39). Even those who indicated an awareness of the impact alcohol use has on cancer, did not know whether the risk was specific to alcohol; rather, they viewed it as being part of an overall belief that “everything causes cancer” (40). This indicates an urgent need to inform the general public regarding the alcohol-cancer link. Given the marked risk of usual alcohol intake, one common way of disseminating this information would be via the use of specific cancer-warning labels on alcoholic-beverage packaging (41). While most evidence available regarding the effectiveness of warning labels placed on packaging is not specific to alcohol, we see no reason why such an intervention would not work for alcohol as well. There have, in fact, been some reports of positive effects from the use of warning labels for alcohol containers as well [e.g., (42)].
Discussion
We would first like to highlight the potential limitations of our study. As for the epidemiology, almost all single studies have found a relationship between alcohol use and the risk of gastrointestinal cancers. Biological evidence corroborates these findings. While knowledge about the biological pathways of cancer is certainly not complete, acetaldehyde—the first derivative of alcohol metabolism in humans and an ingredient in some alcoholic beverages (43)—seems to be the major pathway between alcohol use and esophagus and other gastrointestinal cancers (8): Acetaldehyde associated with consumption of alcoholic beverages has been classified as a Group 1 carcinogen for humans [i.e., sufficient evidence exists that it is carcinogenic; see also (44, 45)].
Part of the evidence for acetaldehyde serving as a biological pathway in the development of cancer came from studies in populations having genetic constellations (Aldehyde Dehydrogenase 2 gene) that slowed the breakdown of acetaldehyde, thereby creating a situation in which acetaldehyde was active for longer periods of time (46). People with this genetic constellation had a markedly increased risk of esophagus and other gastrointestinal cancers (7).
The increased risk for people with different genetic constellations in some Asian populations has not yet been incorporated into the comparative risk assessments like those noted above. Comparative risk assessments to date have been based on risk relations found mainly in Western high-income countries, which makes the above analysis conservative, and suggests that the gastrointestinal cancer risks posed by alcohol use may be markedly underestimated in Asian countries (47).
All of these findings assumed a lag time between alcohol use and cancer of 10 years (18). Clearly, this is an average across individuals and cancer subtypes, which may lead to biases that are not quantified in the current analyses.
The most contested is that the finding that there is no lower threshold for the impact of alcohol use on cancer (7). While there are empirical studies which seem to show protective effects for light drinking, most of these studies cannot exclude the “sick quitter” hypothesis—that abstainers are former drinkers who gave up alcohol due to health problems [(25); see also (48)]. Indeed, there are significantly elevated risks for former drinkers for all gastrointestinal cancers (3).
Finally, focusing only on the prevention of gastrointestinal cancer may lead to biased recommendations since alcohol use has been linked to over 200 diseases and injuries with different risk relations (6, 49). While it is true that some of these diseases may require additional alcohol control measures specifically directed at certain patterns of drinking [e.g., (6)], reducing the overall volume of drinking will yield at least some benefit for all of these disease and injury categories.
In conclusion, alcohol use is a major contributor to gastrointestinal cancers globally, with the exception of some Muslim-majority countries. There are effective and cost-effective alcohol control policies to reduce alcohol-attributable burden available: most importantly, the WHO's three best buys (increase in taxation, restriction on availability, and ban on advertisement), in addition to public education efforts, including the placement of warning labels on alcoholic beverage containers to increase public awareness that alcohol is a carcinogen.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
JR and KS are equally responsible for all parts of the article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Ms. Astrid Otto for copy-editing and referencing assistance.
Footnotes
Funding. The authors acknowledge funding from the Canadian Institutes of Health Research's Institute of Neurosciences, Mental Health and Addiction (Canadian Research Initiative on Substance Misuse Ontario Node Grant SMN-13950).
References
- 1.National Institutes of Health - National Cancer Institute Risk Factors for Cancer. (2015). Available online at: https://www.cancer.gov/about-cancer/causes-prevention/risk
- 2.Rehm J, Shield KD, Weiderpass E. Alcohol consumption: a leading risk factor for cancer. In: Wild CP WE, Stewart BW, editors. World Cancer Report: Cancer Research for Cancer Prevention. Lyon: International Agency for Research on Cancer; (2020). p. 68–76. [Google Scholar]
- 3.Shield K, Manthey J, Rylett M, Probst C, Wettlaufer A, Parry CD, et al. National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: a comparative risk assessment study. Lancet Public Health. (2020) 5:e51–61. 10.1016/S2468-2667(19)30231-2 [DOI] [PubMed] [Google Scholar]
- 4.Global Health Data Exchange (GHDx) GBD Results Tool. (2020). Available online at: http://ghdx.healthdata.org/gbd-results-tool (accessed January 10, 2020).
- 5.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology, 3rd ed Philadelphia, PA: Lippincott Williams & Wilkins; (2012). [Google Scholar]
- 6.Rehm J, Gmel GE, Sr, Gmel G, Hasan OS, Imtiaz S, Popova S, et al. The relationship between different dimensions of alcohol use and the burden of disease—an update. Addiction. (2017) 112:968–1001. 10.1111/add.13757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Alcohol Consumption and Ethyl Carbamate. Vol. 96 Lyon: International Agency for Research on Cancer; (2010). [PMC free article] [PubMed] [Google Scholar]
- 8.International Agency for Research on Cancer IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 100E. Lyon: International Agency for Research on Cancer; (2012). [PMC free article] [PubMed] [Google Scholar]
- 9.World Cancer Research Fund International, American Institute for Cancer Research About the Continuous Update Project. (2019). Available online at: https://www.wcrf.org/int/continuous-update-project (accessed January 10, 2020).
- 10.World Health Organization Metrics: Disability-Adjusted Life Year (DALY): Quantifying the Burden of Disease from Mortality and Morbidity. (2019). Available online at: https://www.who.int/healthinfo/global_burden_disease/metrics_daly/en/ (accessed Febuary 13, 2019).
- 11.World Health Organization . Preventing Noncommunicable Diseases. (2019). Available online at: https://www.who.int/activities/preventing-noncommunicable-diseases (accessed January 10, 2020).
- 12.Chisholm D, Moro D, Bertram M, Pretorius C, Gmel G, Shield K, et al. Are the “best buys” for alcohol control still valid? an update on the comparative cost-effectiveness of alcohol control strategies at the global level. J Stud Alcohol Drugs. (2018) 79:514–22. 10.15288/jsad.2018.79.514 [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization Tackling NCDs: “Best Buys” and Other Recommended Interventions for the Prevention and Control of Noncommunicable Diseases. (2017). Available online at: https://apps.who.int/iris/handle/10665/259232 (accessed January 20, 2020).
- 14.World Health Organization . Global Health Estimates (GHE). (2020). Available online at: http://www.who.int/healthinfo/global_burden_disease/en/ (accessed January 10, 2020).
- 15.Manthey J, Shield KD, Rylett M, Hasan OSM, Probst C, Rehm J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet. (2019) 393:2493–502. 10.1016/S0140-6736(18)32744-2 [DOI] [PubMed] [Google Scholar]
- 16.Turati F, Galeone C, Rota M, Pelucchi C, Negri E, Bagnardi V, et al. Alcohol and liver cancer: a systematic review and meta-analysis of prospective studies. Ann Oncol. (2014) 25:1526–35. 10.1093/annonc/mdu020 [DOI] [PubMed] [Google Scholar]
- 17.Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. (2015) 112:580–93. 10.1038/bjc.2014.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grundy A, Poirier AE, Khandwala F, McFadden A, Friedenreich CM, Brenner DR. Cancer incidence attributable to insufficient fibre consumption in Alberta in 2012. CMAJ Open. (2017) 5:E7–13. 10.9778/cmajo.20160043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rehm J, Imtiaz S. A narrative review of alcohol consumption as a risk factor for global burden of disease. Subst Abuse Treat Prev Policy. (2016) 11:37. 10.1186/s13011-016-0081-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GBD 2017 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018). 392:1923–94. 10.1016/S0140-6736(18)32225-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization Global status report on alcohol and health 2018. (2018). Available online at: https://www.who.int/substance_abuse/publications/global_alcohol_report/en/ (accessed Febuary 05, 2019).
- 22.Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. (1953) 9:531–41. [PubMed] [Google Scholar]
- 23.Rehm J, Kehoe T, Gmel G, Stinson F, Grant B, Gmel G. Statistical modeling of volume of alcohol exposure for epidemiological studies of population health: the US example. Population Health Metrics. (2010) 8:3. 10.1186/1478-7954-8-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ezzati M, Lopez A, Rodgers A, Murray CJL. Comparative Quantification of Health Risks. Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva: World Health Organization; (2004). [Google Scholar]
- 25.Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: explaining the U-shaped curve. Lancet. (1988) 2:1267–73. 10.1016/S0140-6736(88)92890-5 [DOI] [PubMed] [Google Scholar]
- 26.Murray CJL, Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S. Comparative quantification of health risks: conceptual framework and methodological issues. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative Quantification of Health Risks: Global and regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva: World Health Organization. (2004) p. 1–38. [Google Scholar]
- 27.Rehm J, Monteiro M, Room R, Gmel G, Jernigan D, Frick U, et al. Steps towards constructing a global comparative risk analysis for alcohol consumption: determining indicators and empirical weights for patterns of drinking, deciding about theoretical minimum, and dealing with different consequences. Eur Addict Res. (2001) 7:138–47. 10.1159/000050731 [DOI] [PubMed] [Google Scholar]
- 28.Roerecke M, Rehm J. Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. (2014) 12:182. 10.1186/s12916-014-0182-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roerecke M, Rehm J. The cardioprotective association of average alcohol consumption and ischaemic heart disease: a systematic review and meta-analysis. Addiction. (2012) 107:1246–60. 10.1111/j.1360-0443.2012.03780.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rehm J, Crepault JF, Hasan OSM, Lachenmeier DW, Room R, Sornpaisarn B. Regulatory policies for alcohol, other psychoactive substances and addictive behaviours: the role of level of use and potency. A systematic review. Int J Environ Res Public Health. (2019) 16:3749. 10.3390/ijerph16193749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rehm J, Hasan OSM. Is burden of disease differentially linked to spirits? a systematic scoping review and implications for alcohol policy. Alcohol. (2019) 82:1–10. 10.1016/j.alcohol.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 32.Rehm J, Soerjomataram I, Ferreira-Borges C, Shield KD. Does alcohol use affect cancer risk? Curr Nutr Rep. (2019) 8:222–9. 10.1007/s13668-019-0267-0 [DOI] [PubMed] [Google Scholar]
- 33.Grundy A, Poirier AE, Khandwala F, McFadden A, Friedenreich CM, Brenner DR. Cancer incidence attributable to alcohol consumption in Alberta in 2012). CMAJ Open. (2016) 4:E507–14. 10.9778/cmajo.20160070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rehm J, Patra J, Popova S. Alcohol drinking cessation and its effect on esophageal and head and neck cancers: a pooled analysis. Int J Cancer. (2007) 121:1132–7. 10.1002/ijc.22798 [DOI] [PubMed] [Google Scholar]
- 35.Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, et al. Light alcohol drinking and cancer: a meta-analysis. Ann Oncol. (2013) 24:301–8. 10.1093/annonc/mds337 [DOI] [PubMed] [Google Scholar]
- 36.Rehm J, Shield KD. Alcohol Use and Cancer in the European Union. A Review. European Addiction Research; (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sornpaisarn B, Shield KD, Österberg E, Rehm J. Resource Tool on Alcohol Taxation and Pricing Policies. Geneva: World Health Organization and others; (2017). [Google Scholar]
- 38.Babor TF, Caetano R, Casswell S, Edwards G, Giesbrecht N, Graham K, et al. Alcohol: No Ordinary Commodity. Research and public policy, 2nd ed Oxford; London: Oxford University Press; (2010). [Google Scholar]
- 39.Scheideler JK, Klein WMP. Awareness of the Link between Alcohol Consumption and Cancer across the World: A Review. Cancer Epidemiol Biomarkers Prev. (2018) 27:429–37. 10.1158/1055-9965.EPI-17-0645 [DOI] [PubMed] [Google Scholar]
- 40.Wiseman KP, Klein WMP. Evaluating Correlates of Awareness of the Association between Drinking Too Much Alcohol and Cancer Risk in the United States. Cancer Epidemiol Biomarkers Prev. (2019) 28:1195–201. 10.1158/1055-9965.EPI-18-1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Llopis EJ, Kokole D, Neufeld M, Hasan OSM, Rehm J. Evidence Synthesis Report on Alcohol Labelling. Hen Review prepared for WHO Health Evidence Network. Barcelona: ESADE; (2019). [Google Scholar]
- 42.Weerasinghe A, Schoueri-Mychasiw N, Vallance K, Stockwell T, Hammond D, McGavock J, et al. Improving knowledge that alcohol can cause cancer is associated with consumer support for alcohol policies: findings from a real-world alcohol labelling study. Int J Environ Res Public Health. (2020) 17:398. 10.3390/ijerph17020398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lachenmeier DW, Kanteres F, Rehm J. Carcinogenicity of acetaldehyde in alcoholic beverages: Risk assessment outside ethanol metabolism. Addiction. (2009) 104:533–50. 10.1111/j.1360-0443.2009.02516.x [DOI] [PubMed] [Google Scholar]
- 44.Kanteres F, Lachenmeier DW, Rehm J. The contribution of acetaldehyde from alcohol to cancer: a review and exposure estimate for Germany. Sucht. (2009) 55:111–7. 10.1024/2009.02.07 [DOI] [Google Scholar]
- 45.Balbo S, Brooks PJ. Implications of acetaldehyde-derived DNA adducts for understanding alcohol-related carcinogenesis. Adv Exp Med Biol. (2015) 815:71–88. 10.1007/978-3-319-09614-8_5 [DOI] [PubMed] [Google Scholar]
- 46.Brooks PJ, Enoch MA, Goldman D, Li TK, Yokoyama A. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. (2009) 6:258–63. 10.1371/journal.pmed.1000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roerecke M, Shield KD, Higuchi S, Yoshimura A, Larsen E, Rehm MX, et al. Estimates of alcohol-related oesophageal cancer burden in Japan: systematic review and meta-analyses. Bull World Health Organ. (2015) 93:329–38c. 10.2471/BLT.14.142141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rehm J, Irving H, Ye Y, Kerr WC, Bond J, Greenfield TK. Are lifetime abstainers the best control group in alcohol epidemiology? on the stability and validity of reported lifetime abstention. Am J Epidemiol. (2008) 168:866–71. 10.1093/aje/kwn093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. (2009) 373:2223–33. 10.1016/S0140-6736(09)60746-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.