Abstract
Mammalian mitochondrial permeability transition pore (MPTP), across the inner and outer membranes of mitochondria, is a nonspecific channel for signal transduction or material transfer between mitochondrial matrix and cytoplasm such as maintenance of Ca2+ homeostasis, regulation of oxidative stress signals, and protein translocation evoked by some of stimuli. Continuous MPTP opening has been proved to stimulate neuronal apoptosis in ischemic stroke. Meanwhile, inhibition of MPTP overopening-induced apoptosis has shown excellent efficacy in the treatment of ischemic stroke. Among of which, the potential molecular mechanisms of drug therapy for stroke has also been gradually revealed by researchers. The characteristics of multi-components or multi-targets for ethnic drugs also provide the possibility to treat stroke from the perspective of mitochondrial MPTP. The advantages mentioned above make it necessary for us to explore and clarify the new perspective of ethnic medicine in treating stroke and to determine the specific molecular mechanisms through advanced technologies as much as possible. In this review, we attempt to uncover the relationship between abnormal MPTP opening and neuronal apoptosis in ischemic stroke. We further summarized currently authorized drugs, ethnic medicine prescriptions, herbs, and identified monomer compounds for inhibition of MPTP overopening-induced ischemic neuron apoptosis. Finally, we strive to provide a new perspective and enlightenment for ethnic medicine in the prevention and treatment of stroke by inhibition of MPTP overopening-induced neuronal apoptosis.
Keywords: ischemic stroke, mammalian mitochondrial permeability transition pore, mitochondrial apoptosis, ethnic medicine, prescription, monomer composition
Introduction
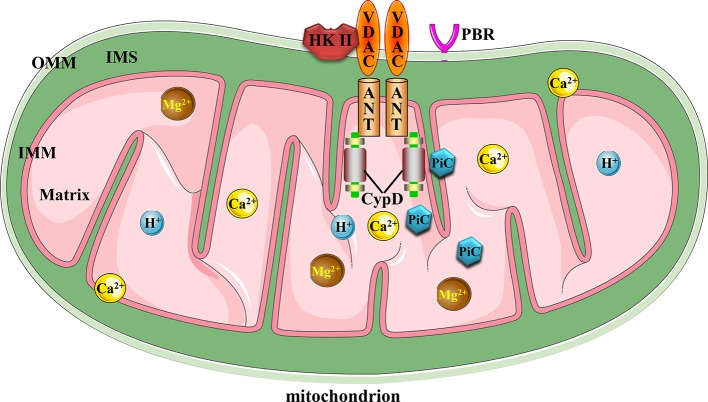
The mitochondrial permeability transition pore (MPTP) complex is a non-specific and -selective channel composed of multiple proteins, which is voltage-dependent and spans cytoplasm, outer mitochondrial membrane (OMM), inner mitochondrial membrane (IMM), and mitochondrial matrix. Excessive MPTP opening has been reported in relation to myocardial ischemia reperfusion injury (Morciano et al., 2017), hepatic ischemia-reperfusion injury (Panel et al., 2019), traumatic brain injury (Hånell et al., 2015), premature aging (Zhou et al., 2019), and Parkinson’s disease (Ludtmann et al., 2018). However, its structural composition of MPTP (Baines and Gutiérrez-Aguilar, 2018) and detailed regulatory mechanism in ischemic stroke are still poorly understood. To our knowledge, current evidences support the fact that MPTP is composed of voltage-dependent anion channel (VDAC) across the OMM, adenine nucleotide translocator (ANT) in the IMM, and cyclophilin D (CypD) in the mitochondrial matrix, which is responsible for sensing intracellular environmental oxidative stress injury, inflammatory cascade, pH imbalance, and ion disorders such as Ca2+ and Mg2+ ions in response to tissue ischemia (Kalani et al., 2018; Briston et al., 2019). These adverse factors, alone or together, can force persistent and irreversible MPTP opening beyond the range of physiological regulation, and thus inducing mitochondria-dependent apoptotic events. In addition, cytoplasmic hexokinase II (HK II) attached to VADC, the peripheral benzodiazepine receptor (PBR) on OMM and creatine kinase responsible for ATP production may be involved in the formation or regulation of MPTP (Zamzami and Kroemer, 2001). Possibly as a component of IMM and binding partner of CypD, the phosphate carrier (PiC) of mitochondria is responsible for the supply of inorganic phosphates required by ATP synthesis during oxidative phosphorylation of mitochondria (Brenner and Moulin, 2012; Bernardi et al., 2015; Solesio et al., 2016). However, whether PiC has a positive or negative effect on the structure and function of MPTP, it is still a matter of debate and disagreement. Figure 1 illustrates the canonical molecular composition of MPTP.
Figure 1.
Canonical mitochondrial MPTP molecular structure. Conventional MPTP complex is composed of VDAC, ANT, and CypD. Other factors could also stimulate MPTP opening.
In recent years, several other members involved in MPTP regulation responsible for cell fate decision have also been identified in succession. As one of the core components of IMM, RNAi-targeted silencing of the spastic paraplegia 7 gene blocked signal transmission between OMM and mitochondrial matrix by indirectly associating VDAC with CypD in the matrix, thereby abrogating overloaded Ca2+ and immoderate ROS evoked mitochondrial membrane potential (MMP) decline and MPTP-dependent cell death (Shanmughapriya et al., 2015). Except for the re-confirmation that ANT was the basic intimal component of MPTP, researchers had also found that other CypD-dependent components were involved in the composition of MPTP. Although the species destined for existence had not yet been identified, the authors suggested that PiC such as the Slc25a3 or F1FO ATP synthase may be involved, which still needed to be explored in a reasonable and rigorous in vivo and in vitro experiment (Karch et al., 2019). Further encouraging evidence suggested that F-ATP synthase was involved in the formation of MPTP, sensing Ca2+ concentration and subsequently mediating MPTP opening (Urbani et al., 2019). As the structure and functions of MPTP are gradually clarified, great quantities of studies have declared that abnormal MPTP conditions play a critical role in regulating cell fate in a variety of diseases. The VDAC has virtually no barrier effect on small molecules with molecular weights less than 5 kDa to circulate freely in the cytoplasm and mitochondrial matrix (Bonora and Pinton, 2019). As an intermediate bridge, ANT can interact directly with VDAC and CypD. And that is, ANT can alter OMM and IMM permeability by regulating VDAC and CypD, thus mediating the exchange of substances in the cytoplasmic matrix and the mitochondrial matrix (Chinopoulos, 2018). In a mouse model of heart failure, it had been substantiated that increased mitochondrial matrix Ca2+ caused by Ppif gene (encoding the synthesis of CypD protein) deficiency contributed to the remission of heart failure symptoms (Elrod et al., 2010). By further silencing CypD gene with in vitro siRNA and shRNA techniques on primary human pulmonary artery endothelial cells, and in vivo CypD knockout mice, evidence of CypD deficiency had been shown to promote angiogenesis, which may be partly due to increased mitochondrial matrix Ca2+ and nicotinamide adenine dinucleotide (NADH), activation of NAD+-dependent deacetylase sirtuin 1 (SIRT1) and serine-threonine kinase Akt signaling (Marcu et al., 2015). Evidence suggested that induced pluripotent stem cells (iPSCs) derived hepatocyte toxicity caused by valproic acid was associated with MPTP opening dependent mitochondrial apoptotic pathway (Li et al., 2015).
Causality Between Abnormal MPTP Opening and Apoptosis in Ischemic Stroke
Abnormalities of MPTP state are bound to trigger cellular dysfunction in ischemic stroke. We will briefly summarize the factors and related molecular mechanisms of MPTP opening-induced apoptosis after ischemic stroke. A large number of previous reports have shown that stroke-evoked decreased MMP, excessive mitochondrial reactive oxygen species (mtROS) (Zorov et al., 2014), endoplasmic reticulum stress (ERS), and excitatory amino acid toxicity all stimulated MPTP opening (Prentice et al., 2015), leading to mitochondrial edema, increased membrane permeability, corrupted cristae structure of IMM, and neuronal apoptosis. Notably, as the second messenger, Ca2+ is a stimulus of MPTP opening and also could be a landmark event after MPTP opening. However, from the actual effect, increased Ca2+ and depressed matrix Mg2+ and Mn2+ could all contribute to MPTP opening. In turn, evidence had announced that instantaneously MPTP opening could cause increased Ca2+ in microdomain of astrocytes, which was closely related to maintaining mitochondrial energy supply and stress response (Agarwal et al., 2017). The otherwise MPTP opening-prone factors are as following. Declined matrix PH, caused by protonation of histidine residues or loss of ANT and CypD signaling, could trigger MPTP to tend to shut down. Conversely, the increased matrix PH forces MPTP opening with its maximum openness at about 7.3 (Wang et al., 2016; Šileikytė and Forte, 2019). The formation of disulfide by oxidation on ANT dimer, oxidized pyridine nucleotides such as NAD+ and NADP+ all favor MPTP openness. Conversely, all the factors that inhibit MPTP opening may have a promising future in treating ischemic stroke. Ligands targeting VADC, ANT, CypD (Matsumoto et al., 1999), and TSPO/PBR targets have shown better inhibition of MPTP opening. Moreover, antioxidants such as propofol, metabolites such as glucose and creatine, coenzyme Q, glutamate, or Ca2+ chelators could limit MPTP opening (Zamzami and Kroemer, 2001; Brenner and Moulin, 2012).
It is well known that onset of ischemic stroke causes neurons to produce exorbitant mtROS, ERS, Ca2+ overload, and neuronal toxicity induced by excitatory amino acids. After that, neurons would raise the alarm of MMP decline, mitochondrial edema, elevated MMP and other signs of MPTP opening, which will eventually drive mitochondrial contents such as Cyto-c to be discharged into the cytoplasm and trigger apoptotic events. The results of in vivo animal evaluation have intimated that both transient and permanent cerebral ischemic insults can cause damage to mitochondrial ultrastructure of neuron, such as the appearance of swollen and condensate mitochondria, as well elevated matrix density caused by deposition of electron-dense material (Solenski et al., 2002). An ischemia-induced ROS elevation can favor MPTP opening, which in turn can lead to a subsequent surge in ROS production and a vicious cycle (Zorov et al., 2014). Therefore, inhibition of neuronal apoptosis by blocking MPTP opening would be a potential and promising strategy in the treatment of ischemic stroke. Further extensive in vivo and in vitro experimental evidence also suggested a positive effect of this therapy. In rat models of ischemic stroke, blocking MPTP opening by cyclosporine A had been shown to reduce infarcted volume of ischemic brain tissue (Matsumoto et al., 1999). As a ligand targeting CypD, pre-administration of cyclosporine A can protect primary rat neurons from OGD/R injury, involved mechanisms may be related to maintain mitochondrial integrity and inhibit MPTP opening-induced apoptosis by up-regulating Parkinson’s disease-associated protein DJ-1 (Tajiri et al., 2016). Further, the water-soluble coenzyme Q10 had been shown to protect the accumulation of glutamate-induced HT22 hippocampal neuron damage by inhibiting mitochondrial fragmentation and MPTP opening-induced apoptosis (Kumari et al., 2016). Furthermore, evidence had shown that intervention of MPTP opening inhibitor can reduce the expression of VDAC, manifesting by increased MMP, ATP supply, and improved cerebral ischemia injury symptoms in an in vitro rat model of MCAO (Wang et al., 2019a). The above evidence all conveys that ischemic stroke induced MPTP opening may be a factoid of neuronal apoptosis. Any measures to inhibit MPTP opening could repress cell apoptosis, thus exhibiting the role of anti-ischemic brain protection.
Explosive evidence corroborated that a sudden insult of ischemic stroke may break the balance between the anti-apoptotic and pro-apoptotic members of B-cell lymphoma-2 (Bcl-2) family, which may aggravate stroke condition. The results of ischemic stroke models with Bax gene knockout in vivo and in vitro showed that the improved ischemic neuron injury and decreased neuronal apoptosis were related to the decreased cytoplasmic Ca2+, which was a relatively upstream signal regulating the apoptosis of ischemic neurons (D’Orsi et al., 2015). A great deal of evidence has declassified such a fact that anti-apoptotic Bcl-2 and Bcl-xL can inhibit MPTP opening, whereas pro-apoptotic Bax and Bak proteins can stimulate MPTP opening (D’Orsi et al., 2017). Results from in vitro model of ischemic stroke in rats have shown that increased Bax/Bcl-2 ratio in ischemic insult could irritate MPTP opening, which may cause increased neuronal apoptosis (Andrabi et al., 2017; Andrabi et al., 2019). Actually, members in Bcl-2 family could also regulate two potential MPTP opening stimuli: Ca2+ homeostasis and energy metabolism of neurons (D’Orsi et al., 2017; Peña-Blanco and García-Sáez, 2018). The increased permeability caused by the formed Bax/Bak dimer on OMM contributed to the release or transfer of pro-apoptotic Cyto-c, Smac/Diablo and HtrA2/Omi from the mitochondrial matrix to cytoplasm (Arnoult et al., 2003). Further rat models of focal cerebral ischemia also demonstrated that overexpressed Bcl-2 protein could inhibit the rise of Cyto-c in cytoplasm, thereby preventing the occurrence of apoptotic DNA fragmentation events mediated by the transfer of AIF from mitochondria to nucleus (Zhao et al., 2004). While pro-apoptotic Bcl-xS-induced apoptosis via Bak also induced the exudation of mitochondrial Cyto-c, the formation of apoptosome composed of Cyto-c, Apaf-1 and Caspase-9, and the Caspase apoptotic cascade (Lindenboim et al., 2005; Zhang Q. et al., 2019). Visually breaking news, Bak/Bax macropores contribute to the outflow of mitochondrial contents such as Cyto-c and mitochondrial DNA into the cytoplasm, and thereafter inducing caspase-dependent cell apoptosis (McArthur et al., 2018). Amusingly, evidence also suggested that ROS and ERS could directly activate Bak/Bax-dependent apoptosis, showing condensed and hyperchromatic nucleus, loss of MMP, reduced Bcl-2, increased activation of Caspase-3/-9, PARP, and overexpressed Bak and Bax proteins (Seervi et al., 2018). Therefore, the formation of Bak/Bax macropores in mitochondrial OMM may serve as a hub for MPTP opening-induced mitochondrial apoptosis. Other factors involved in the regulation of MPTP opening after cerebral ischemia have also been reported. Accumulation of p53 in mitochondria has been corroborated to target CypD, leading to MPTP opening and neuronal apoptosis, which is independent of the formation of Bak/Bax macropores (Vaseva et al., 2012). It has been reported that activation of neuron mitochondrial cannabinoid receptor 1 after cerebral ischemia can help inhibit Ca2+ overload-induced MPTP opening and apoptosis (Cai et al., 2017). Another potential target involved in regulating mitochondrial MPTP in ischemic stroke was mitochondrial uncoupling protein 2 (UCP-2). Highly expressed UCP-2 has been demonstrated to inhibit apoptosis by activating redox signaling, evidenced by decreased ROS production, increased MMP and cleaved Caspase-3 protein expression (Mattiasson et al., 2003; Mehta and Li, 2009). The above analysis indicates that ischemic stroke is accompanied by an inevitable event of MPTP over-opening and apoptosis. Although the basic structure of MPTP has not yet been drastically uncovered and recognized. But a number of factors that regulate MPTP opening during the course of ischemic stroke have been exposed in the public eye. In the future, plenty of basic studies should be conducted to elucidate the molecular composition of MPTP and its relationship with ischemic neuron apoptosis. Meanwhile, natural product inhibitors targeting MPTP opening-evoked neuronal apoptosis are also worthy of further research in the treatment of ischemic stroke.
Progress in Stroke Prevention and Treatment by Regulating Mitochondrial MPTP Status in Ethnic Medicine
Ischemic stroke, which accounts for 71% of stroke, is the second and the first leading cause of death and disability worldwide and in China, respectively (Wu S. et al., 2019). In 2016, there were 9.5 million ischemic stroke patients worldwide, while in 2017, 2.7 million people died of ischemic stroke (Campbell et al., 2019). Although intravenous thrombolysis, antiplatelet aggregation, and anticoagulant therapy (Smith et al., 2019; Stoll and Nieswandt, 2019) could be used for the delivery of stroke therapies, but many apoplexy sequelae, characterized by ischemic contralateral or bilateral limb behavior disorders, memory decay, logopathy, dysphagia, and mood irritability (Zhao et al., 2016; Hou et al., 2020), have not yet cure. However, the ethnic medicine has manifested significant clinical efficacy in alleviating above unbearable symptoms or sequelae of stroke. In recent years, the mechanisms of action of drugs have also been gradually revealed. Remarkably, some of them, such as Danhong injection and Naoxintong capsule (Haiyu et al., 2016; Liu M. et al., 2016; Xu et al., 2020), have been officially approved by the China Food and Drug Administration (CFDA) and are bringing good news to stroke patients around the world. Next, we summarized the current officially authorized products, clinically effective traditional Chinese medicine (TCM) prescriptions, ethnic drugs, and effective monomer components based on literature review, trying to clarify the molecular mechanisms of natural products inhibiting neuronal apoptosis and improving ischemic brain from the perspective of mitochondrial MPTP.
Authorized Products for Stroke Improvement by Regulating Mitochondrial MPTP
With the policy guidance and inclination, as well as the accelerated modernization of TCM, tens of thousands of individuals dedicated to clinical and scientific research positions are gradually devoting themselves to the drug development and mechanism exploration of traditional medicine to prevent major diseases, such as stroke. Most ethnic drugs for treatment of ischemic stroke have the function of activating blood circulation to remove blood stasis or clear collaterals. NaoShuanTong capsule (Zhang H. et al., 2019), ShenQi Fuzheng injection (Cai et al., 2016), ShengMai injection (Yang et al., 2016), and PeiYuan TongNao capsule (Bai J. et al., 2019) have been reported to significantly improve the symptoms of ischemic stroke with few adverse events. In recent years, some antiapoptotic protective effects of cerebral ischemia have also been reported, such as XueShuanTong injection (Li et al., 2009) and QianCao NaoMaiTong mixture (Lu et al., 2016).
Most, such as Cerebralcare Granule® (Sun et al., 2010), DanHong injection (Xu J. et al., 2017; Li M. et al., 2018), and AnGong NiuHuang wan (Wang G. et al., 2014; Tsoi et al., 2019), can inhibit ischemia-evoked neuronal apoptosis by regulating bcl-2 family members. As a prescription commonly used in Tibetan medicine to treat ischemic sequelae, our research group proved that the anti-cerebral ischemia effect of ErShiWei ChenXiang pills may be related to its regulation of Bcl-2 family, inhibition of apoptosis, and increase of energy supply (Hou et al., 2020). While regulating Bcl-2 family members, AnNao tablets (Zhang et al., 2020) and YiQi FuMai powder injection (Cao et al., 2016; Xu Y. et al., 2017) may also be involved in inflammation and mitochondrial autophagy to maintain mitochondrial MMP and energy production. In addition, both TongXinLuo's regulation of AKT/ERK signaling (Yu et al., 2016; Cheng et al., 2017) and XingNaoJing injection's regulation of the PI3K-AKT pathway (Zhang Y. et al., 2018) ultimately contributed to the regulation of Bcl-2 and the inhibition of ischemic neuron apoptosis. In addition to the Bcl-2 family, it was reported that Zhenlong Xingnao capsule (Wei X. et al., 2019) and NaoLuoTong capsule (Bai M. et al., 2019) could also be through the regulation of NF-кB to confine ischemia induced inflammatory cascade process. Of course, multiple mechanisms of drugs have also been reported against ischemic neuron apoptosis. QingKaiLing injection could simultaneously inhibit oxidative stress, activation of NLRP3 inflamosome and AMPK signaling pathway, and thus inhibiting neuronal apoptosis (Cheng et al., 2012). PienTzeHuang capsule suppressed the inflammatory and apoptotic cascade of ischemia by regulating AKT/GSK-3β and the Bcl-2 family (Zhang X. et al., 2018). As a fatal blow to the body, disregardful ischemic stroke induced hypoimmunity was also one of the main culprits of exacerbating stroke. Noteworthy, XueSaiTong (Li et al., 2019) and Danggui-Jakyak-San (Kim et al., 2016) may mediate inflammatory responses by regulating STAT3 signaling pathway, and enhance immune function of the body, which were helpful to reduce symptoms of brain injury after ischemia. The above officially certified drugs’ information and specific mechanisms of action are shown in Supplementary Table 1 , and Tables 1 and 2 . Through in-depth comparative analysis, we found that although the above drugs prevailing in the market have good clinical efficacy, most of their active ingredients, in vivo pharmacokinetic parameters, and potential targeted organ toxicity have not been well evaluated. Importantly, the further regulation of apoptosis still has good research value and prospect. Although there is no direct evidence that they regulate MPTP to inhibit ischemic neuron apoptosis, their effect on members of the Bcl-2 family makes MPTP a potential target for anti-stroke drugs.
Table 1.
The in vivo mechanism underlying the inhibition of MPTP opening-induced neuronal apoptosis by authorized drugs in the treatment of ischemic stroke.
| Agents | Objects | Gender | Weight (g) | Animal model | Dose | Time periods | Mechanisms | References |
|---|---|---|---|---|---|---|---|---|
| XueShuanTong injection | SD | Both | 270–320 | MCAO (2 h)/R (46 h) | 25 mg/kg, i.p. | Pretreatment for 5 min and 12/24/36 h after MCAO | Caspase-1/3↓, TUNEL-positive neurons↓ | Li et al., 2009 |
| Cerebralcare granule | Mongolian gerbils | Male | 65–90 | MCAO (0.5 h)/R (5 d) | 0.4 and 0.8 g/kg, i.g. | 3 h after the reperfusion, 5 d, q.d. | Bcl-2↑; leukocyte adhesion↓, fluorescence intensity of DHR↓, albumin leakage↓, Caspase-3↓, Bax↓, TUNEL-positive neurons↓ | Sun et al., 2010 |
| AnGong NiuHuang wan. | SD | Male | 250–280 | MCAO (1.5 h)/R (24 h) | 0.065, 0.125, and 0.25 g/kg, i.g. | Pretreatment 3 d, q.d., and 1 d, q.d. after reperfusion | Bcl-2↑; Bax↓, Caspase-3↓, TUNEL-positive neurons↓ | Wang G. et al., 2014 |
| SD | Male | 260–280 | MCAO (2 h)/R (22 h) | 257 mg/kg, i.g. | Single dose before reperfusion | Bcl-2↑, ZO-1↑, claudin-5↑, eNOS↑; Bax↓, p47phox↓, iNOS↓, 3-NT↓, MMP-2↓, MMP-9↓, iNOS↓ | Tsoi et al., 2019 | |
| QingKaiLing injection | KM/C57BL/6 | Male | 25–28/25–30 | MCAO (1.5 h)/R (28 h) | 3 ml/kg, i.v. | 4 h after reperfusion, and once every 12 h, three times | Procaspase-12↑; Caspase-3↓, p-eIF2α↓, ROS↓, Ca2+↓, TUNEL-positive neurons↓ | Cheng et al., 2012 |
| DangGui Jakyak san | SD | Male | —— | pMCAO (28 d) | 50, 100, and 200 mg/kg, i.g. | 24 h after surgery, 28 d, q.d. | STAT3↑, Pim-1↑, GSH↑, SOD↑, CAT↑; MDA↓, Caspase-3↓, PARP↓, NT↓, 4-HNE↓ | Kim et al., 2016 |
| YiQi FuMai powder injection | C57BL/6J | Male | 18–22 | pMCAO (24 h) | 1.342 g/kg, i.p. | Single dose after pMCAO onset | cerebral blood flow↑, Bcl-2↑; Caspase-12↓, GRP78↓, CHOP↓, ATF-4/6↓, p-eIF2α/eIF2α↓, XBP-1↓ | Cao et al., 2016 |
| SD | Male | 280–300 | tMCAO (1.5 h)/R (24 h) | 0.957 g/kg, i.p. | Single dose after tMCAO onset | Bcl-2↑, cytosolic Drp1↑; Bax↓, cleaved Caspase-9↓, mtDrp1↓, total p-Drp1 and Drp1↓ | Xu Y. et al., 2017 | |
| TongXinLuo | SD | Male | 200–220 | MCAO (1.5 h)/R (24 h) | 0.4, 0.8, and 1.6 g/kg, i.g. | Pretreatment for 3 d, b.i.d., and after MCAO for 1 d, b.i.d. | p-PTEN/PTEN↑, p-PDK1/PDK1↑, p-AKT/AKT↑, p-Bad/Bad↑, p-c-Raf/c-Raf↑; cleaved Caspase-3↓, TUNEL-positive neurons↓ | Yu et al., 2016 |
| SD | Male | 240–270 | MCAO (1.5 h)/R (14 d) | 0.1 g/kg, i.g. | Pretreatment for 5 d and 14 d after MCAO, q.d. | Connexin 43↓, Calpain II↓, Bax↓, cleaved Caspase-3↓, TUNEL-positive neurons↓ | Cheng et al., 2017 | |
| QianCao NaoMaiTong mixture | SD | 180–200 | MCAO (2 h)/R | 2.7, 5.4, and 10.8 ml/kg | Pretreatment for 28 d | Bcl-2/Bax↑, SOD↑, CAT↑, BDNF↑, ICAM-1↑, NGF↑, MDA↓, IL-6↓ | Lu et al., 2016 | |
| DanHong injection | SD | Male | 250–280 | MCAO (1 h)/R (24 h) | 4 ml/kg, i.p. | 4 h after MCAO | claudin-5↑, occludin↑, ZO-1↑, Bcl-2↑; Bax↓, Caspase-3↓, MMP-9↓, PAI-1↓, P-selectin↓ | Li M. et al., 2018 |
| XingNaoJing injection | SD | Male | 250–280 | MCAO (2 h)/R (24 h) | 5, 10, and 15 ml/kg, i.p. | 24 h after reperfusion | Bcl2/Bax↑, p-PI3K/PI3K↑, p-AKT (308 and 473)/AKT↑, p-eNOS/ eNOS↑, NO↑, p-PI3K/AKT↑ | Zhang Y. et al., 2018 |
| PienTzeHuang capsule | SD | Male | 240–260 | MCAO (1.5 h)/R (24 h) | 180 mg/kg, i.g. | Pretreatment 4 d before MCAO |
NeuN↑, mtCyto-c↑, Bcl-xl↑, p-AKT↑, p-GSK-3β↑; IL-1β↓, IL-6↓, TNF-α↓, cytosolic Cyto-c↓, Bax↓, p53↓, cleaved Caspase-3/9↓, TUNEL-positive neurons↓ | Zhang X. et al., 2018 |
| XueSaiTong | C57BL/6 | Male | 20–25 | MCAO (45 min)/R (14 d) | 15 µg/g, i.v. | Immediately after reperfusion, 14 d, q.d. | arginase-1↑, CD206↑, CD206/Iba-1↑, IL-10↑, TGF-β1↑; IL-1β↓, p-STAT3/STAT3↓, CD16↓, CD16/Iba-1↓, iNOS↓, TUNEL-positive neurons↓ | Li et al., 2019 |
| NaoLuoTong capsule | Wistar | Male | 250–280 | MCAO (2 h)/R (22 h) | 75, 150, and 300 mg/kg, i.g. | Pretreatment for 7 d, q.d. | Bcl-2↑, NGF↑; TNF-α↓, IL-1β↓, IL-6↓, Bax↓, Caspase-3↓, ICAM-1↓, NF-κBp65↓ | Bai M. et al., 2019 |
| ZhenLong XingNao capsule | Wistar | Male | 200–250 | MCAO (1.5 h)/R (24 h) | 125 and 250 mg/kg, i.g. | Pretreatment 14 d, q.d. | T-AOC↑, T-SOD↑, Bcl-2↑, Bcl-2/Bax↑; Caspase-3↓, NF-кB↓, p38↓, Bax↓, MDA↓, GABA↓, Glu↓, Tau↓ | Wei X. et al., 2019 |
| ErShiWei ChenXiang pills | SD | Male | 260–300 | MCAO (2 h)/R (24h) | 1.33 and 2.00 g/kg, i.g. | Pretreatment 14 d, q.d. | Bcl-2↑, CaMK II↑; Bax↓, cleaved Caspase-3↓, Cyto-c↓, ATF4↓, c-Jun↓, TUNEL-positive neurons↓ | Hou et al., 2020 |
| AnNao tablets | SD | Male | 250–270 | MCAO (2 h)/R (7 d) | 300, 600, and 1,200 mg/kg, i.g. | 1 h after reperfusion, 1 d or 7 d, q.d. | Drp1↑, OPA1↑, PINK1↑, Parkin↑, Bcl-2↑, Bcl-2/Bax↑; Bax↓ | Zhang et al., 2020 |
↑, upgrade; ↓, downgrade.
Table 2.
The in vitro mechanism underlying the inhibition of MPTP opening-induced neuronal apoptosis by authorized drugs in the treatment of ischemic stroke.
| Agents | Cell lines | Model | Dose | Time periods | Mechanisms | References |
|---|---|---|---|---|---|---|
| TongLuo JiuNao injection | BMECs of SD rats | OGD (95% N2 and 5% CO2 6 h)/R (74% N2, 21% O2, and 5% CO2, 6 h) | 2 μl/ml | Before OGD, the neurons were incubated 6 h in drug treatment and then equilibrated OGD | VEGF↑, MMP↑; LDH↓, Ca2+↓, cytosolic Cyto-c↓, NMDAR1↓, PAF↓ | Li et al., 2014 |
| QianCao NaoMaiTong mixture | SH-SY5Y | OGD (N2, 1 h)/R (24 h) | 0.5, 1, 5, 10, 50, 100 and 200 mg/ml | Pretreatment for 2 h and during reperfusion period | Caspase-3/8↓, neuronal apoptosis under flow cytometry↓ | Lu et al., 2016 |
| YiQi FuMai powder injection | PC12 | OGD (5% CO2, 94% N2, and 1% O2, 12 h) | 100, 200, and 400 μg/ml | during OGD period | Bcl-2↑; neuronal apoptosis under flow cytometry↓, Caspase-3↓, cleaved Caspase-3↓, Caspase-12↓, CHOP↓, GRP78↓, ATF-4/6↓, p-eIF2α/eIF2α↓, XBP-1↓, Hoechst 33342 positive neurons↓ | Cao et al., 2016 |
| PCN of embryonic, 16–18-d SD rats | 100 μM H2O2 for 12 h | 100, 200, and 400 μg/ml | 6 h before and during H2O2 treatment | ATP↑, MMP↑; Bcl-2↑, Bcl-xl↑, cytosolic Drp1↑, cytosolic PKCδ↑; Bax↓, Bak↓, Caspase-3↓, cleaved Caspase-3↓, mtROS↓, PKCδ↓, neuronal apoptosis under flow cytometry↓, intracellular ROS↓, p-Drp1/Drp1↓, mtDrp1↓, mtPKCδ↓ | Xu Y. et al., 2017 | |
| DanHong injection | PCN of embryonic, 14-d C57 BL/6 mice | OGD (95% N2 and 5% CO2, 6 h) | 0.01, 0.03, 0.1, 0.3, and 1 μl/ml | During OGD period | LDH↓, ROS↓, Ca2+↓, neuronal apoptosis under flow cytometry↓ | Xu J. et al., 2017 |
| XingNaoJing injection | HBMECs | OGD (5% CO2, 85% N2, and 10% H2, 3 h)/R (24 h) | 1.5 and 2.5 μl/ml | Pretreatment for 1 h and during reperfusion period | p-eNOS/eNOS↑, MMP↑, NO↑; cleaved Caspase-3/Caspase-3↓, neuronal apoptosis under flow cytometry↓ | Zhang Y. et al., 2018 |
↑, upgrade; ↓, downgrade.
Prescription and Molecular Mechanisms in Regulating MPTP Openness of Ischemic Stroke
Clinical experience has proved that TCM has excellent efficacy in treating stroke, which can be seen in Huangdi Neijing. But at bottom it is the cold, hot, warm, cool, and other characteristics of drugs to balance the imbalance of Yin and Yang in the body under the condition of disease. In ischemic stroke, a variety of exogenous pathogens and dysfunction of the viscera can lead to poor blood flow or blood stasis, resulting in cerebral ischemia or hypoxia (Hou et al., 2020). Therefore, the clinic mainly focuses on promoting blood circulation to remove stasis, replenishing Qi to nourish blood, and nursing viscera. Extensive clinical and in vivo and in vitro studies have confirmed that prescriptions SiJunZi decoction (Yang et al., 2019), ShengMai san (Li et al., 2013), and YangYin TongNao granules (Wang et al., 2019f) have a significant effect on ischemic stroke. Of course, the regulation of oxidative stress and inflammatory response are also common mechanisms of prescription in the treatment of ischemic brain injury. The antioxidant and anti-inflammatory activities of ShengNaoKang decoction (Chen et al., 2014) could contribute to the inhibition of apoptosis and the alleviation of ischemic brain injury. Other studies have reported that HuangLian JieDu decoction (HJD) could inhibit ischemic neuron apoptosis by regulating PI3K/AKT and HIF-1α/VEGF (Zhang Q. et al., 2014). Further metabolomics (Zhu et al., 2018) and systemic pharmacology (Wang P. et al., 2019) studies have revealed that its anti-ischemic protective effect may also involve the Bcl-2 family such as Bak. Regulating vascular function and increasing cerebral blood flow supply is another effective strategy for stroke treatment. Abundant evidence demonstrated that BuYang HuanWu decoction (BHD) could increase cerebral blood by regulating HIF-1α/VEGF-related signaling pathways (Chen et al., 2019). Improving the mitochondrial ATP supply has also been shown to be an effective treatment for stroke. BHD has been reported to improve ischemic brain injury by reducing glutamate-mediated excitatory amino acid toxicity, resulting in enhanced ATP supply and weakened apoptosis (Wang et al., 2011). At the same time, the improved synaptic ultrastructure by BHD also contributed to the recovery of cerebral ischemia sequelae (Pan et al., 2017). Similarly, ShenGui SanSheng san could also improve the efficiency of citric acid cycle to improve the brain energy deficit after ischemia (Luo et al., 2019). Interestingly, as a cell-sensing oxygen sensor, most studies have also reported evidence of other TCM prescriptions regulating HIF-1α to inhibit apoptosis and inflammation in treatment of stroke, such as XueFu ZhuYu decoction (Lee et al., 2011) and TaoHong SiWu decoction (Yen et al., 2014). Members of the Bcl-2 family are also potential targets for prescription inhibition of apoptosis to improve ischemic brain injury. XiaoXuMing decoction (Lan et al., 2014), ShuanTongLing (Mei et al., 2017), and GuaLou Guizhi decoction (Zhang Y. et al., 2014) all have the potential to regulate the Bcl-2 family and inhibit caspase-dependent mitochondrial apoptosis, which has a similar mechanism to that of MuXiang You fang (Zhao et al., 2016) reported in our previous study. In addition to the Bcl-2 family, DiHuang YinZi (Hu et al., 2009) and DiDang tang (Huang et al., 2018) could also inhibit the generation of Ca2+ and improve MMP to inhibit the apoptosis of ischemic neurons by regulating the ERK signaling pathway. It has also been reported that HouShiHei san (Chang J. et al., 2016) could regulate PI3K/Akt signaling to inhibit the apoptosis of ischemic neurons. The specific mechanisms in vivo and in vitro of the above prescriptions are shown in Table 3 .
Table 3.
The in vivo and in vitro mechanism underlying the inhibition of MPTP opening-induced neuronal apoptosis by TCM prescriptions in the treatment of ischemic stroke.
| In vivo study | ||||||||
|---|---|---|---|---|---|---|---|---|
| Agents | Objects | Gender | Weight (g) | Animal model | Dose | Time periods | Mechanisms | References |
| DiHuang YinZi | Wistar | Both | 320–350 | MCAO (1 h)/R (10 d) | 6 and 12 g/kg, i.g. | 30 min after MCAO, 10 d, q.d. | synaptophysin↑, ERK↑; LDH↓, TUNEL-positive neurons↓ | Hu et al., 2009 |
| XueFu ZhuYu decoction | Wistar | Male | 250–300 | MCAO (1 h)/R (24 h) | 1.5 and 3.0 g/kg, i.g. | Pretreatment for 14 d, q.d. | cleaved Caspase-3↓, HIF-1α↓, TNF-α↓, iNOS↓ | Lee et al., 2011 |
| BuYang HuanWu decoction | ICR | Male | 17–22 | MCAO (30 min)/R (14 d) | 0.5 and 1.0 g/kg, i.g. | 2 h after reperfusion, 14 d, b.i.d. | glucose metabolism↑, BrdU↑; ROS↓, TUNEL-positive neurons↓, CD11b↓ | Wang et al., 2011 |
| ShengNaoKang decoction | SD | Male | 280–320 | MCAO (2 h)/R (24 h) | 0.7, 1.4, and 2.8 g/kg, i.g. | Pretreatment for 6 d and 1 d after reperfusion, q.d. | SOD↑; GSH-Px↑, Caspase-3↓, MDA↓, iNOS↓, TNOS↓ | Chen et al., 2014 |
| TaoHong SiWu decoction | Wistar | Male | 250–300 | MCAO (1 h)/R (24 h) | 0.7 g/kg, i.g. | Pretreatment for 14 d, q.d. | cleaved Caspase-3↓, HIF-1α↓, iNOS↓, TNF-α↓ | Yen et al., 2014 |
| HuangLian JieDu tang | SD | Male | 300–350 | MCAO (2 h)/R (72 h) | 2.7 g/kg, i.g. | Single dose and pretreatment for 24 h | p-PI3K/PI3K↑, p-AKT/AKT↑, HIF-1α↑, EPO↑, VEGF↑, BrdU↑; LDH↓, TUNEL-positive neurons↓ | Zhang Q. et al., 2014 |
| XiaoXuMing decoction | SD | Male | 250–280 | MCAO (1.5 H)/R (24 h) | 60 g/kg, i.g. | Pretreatment for 3 d, t.i.d. | mtBcl-2↑, mtCyto-c↑, cytoplasmic Bax↑, cytoplasmic c-IAP1↑; mtbroken cristae↓, cleaved Caspase-3/9↓, p53↓, mtp53↓, mtBax↓, cytoplasmic Smac↓, cytoplasmic Cyto-c↓, TUNEL-positive neurons↓ | Lan et al., 2014 |
| GuaLou Guizhi decoction | SD | Male | 280–300 | MCAO (2 h)/R (7 d) | 14.4 g/kg, i.g. | Posttreatment for 7 d, q.d. | NeuN↑, MAP-2↑, Bcl-2↑; GFAP↓, Bax↓, TUNEL-positive neurons↓ | Zhang Y. et al., 2014 |
| MuXiang You fang | SD | Male | 260–300 | MCAO (2 h)/R (48 h) | 58, 116, and 232 mg/kg, i.g. | Posttreatment for 3 d, q.d. | Bcl-2↑, Bcl-2/Bax↑; Bax↓, Cyto-c↓, Caspase-3/7/9↓ | Zhao et al., 2016 |
| ShuanTongLing | SD | Male | 250–280 | MCAO (1.5 h)/R (24 h) | 5.7 and 17.2 ml/kg, i.g. | Pretreatment for 7 d, q.d. | SIRT1↑, Bcl-2↑; TNF-α↓, IL-1β↓, Ac-p53↓, Bax↓ | Mei et al., 2017 |
| In vivo study | ||||||||
| Agents | Cell lines | Model | Dose | Time periods | Mechanisms | References | ||
| DiDang tang | PC12 | OGD (95% N2 and 5% CO2, 0.5–2.5 or 2–10 h) | 12.5, 25, and 50 mg/ml | After the OGD induced PC12 cell model for 24 or 48 h | Bcl-2/Bax↑; Ca2+↓, MMP↓, GRP78↓, p-IRE1/IRE1↓, p-PERK/PERK↓, p-eIF2α/eIF2α↓, p-Bad/Bad↓, ATF-6↓, Cyto-c↓, cleaved PARP↓, neuronal apoptosis under flow cytometry↓ | Huang et al., 2018 | ||
↑, upgrade; ↓, downgrade.
The above evidence indicates that most TCM prescriptions could more or less improve mitochondrial morphology and respiratory function by inhibiting neuronal Ca2+ overload through anti-oxidative stress and anti-inflammatory. Meanwhile, we note that most of them also regulates many members of the Bcl-2 family to inhibit ischemic neuron apoptosis. We, therefore, see the potential of drugs to indirectly inhibit MPTP opening to improve ischemic neuron apoptosis. Nevertheless, the unclear drug distribution of target organs and the intricate network of interactive targets should still drive us to further study.
Herbal Extracts and Molecular Mechanisms in Regulating MPTP Openness of Ischemic Stroke
The overall concept of TCM and the characteristics of treatment based on syndrome differentiation of ethnic medicine determine that prescriptions from diversified drug sources are mainly used in the treatment of diseases. The purpose is to comprehensively consider the functions of viscera to exorcize evil spirits while strengthening the body, and finally cure diseases. However, in addition to conventional prescriptions mentioned above, people have also discovered that the individual application of certain herbs also has the potential to treat diseases. Based on recent literature reports, most of them exhibit outstanding antioxidant effects, such as methanol extract of Artemisia absinthium (Bora and Sharma, 2010) and Colebrookea oppositifolia Smith (Viswanatha et al., 2018). As the most sensitive hippocampal neuron to ischemic invasion, studies have shown that Moringa oleifera seed extract could promote hippocampal nerve regeneration, enhance synaptic plasticity and cholinergic function to treat ischemic stroke (Zeng et al., 2019). More interestingly, Gynostemma pentaphyllum extract could protect OGD/R-induced rats isolated hippocampal slices damage by inhibiting neuronal Ca2+ overload and mitochondrial oxidative stress-induced MPTP opening (Schild et al., 2009), which may help to inhibit the MPTP opening-activated mitochondrial apoptotic cascade event. At the same time, herbs could regulate the expression level of anti-apoptotic and pro-apoptotic proteins of Bcl-2 family and inhibit mitochondrial apoptosis in the treatment of hypoxia brain injury. The specific in vivo and in vitro mechanisms of reported herbs for ischemic stroke treatment by inhibiting mitochondrial MPTP opening-induced neuronal apoptosis are shown in Tables 4 and 5 . Figure 2 shows pictures of 16 representative herbs. It is world-renowned that superior immune enhancement of plant polysaccharides could prevent and cure many diseases. Previous investigations reported the anti-ischemic effects of Ganoderma lucidum polysaccharides (GLP) (Zhou et al., 2010), Lycium barbarum polysaccharide (LBP) (Wang T. et al., 2014; Zhao et al., 2017b), Panax notoginseng polysaccharides (PNP) (Dong et al., 2014), and Cistanche deserticola polysaccharides (CDP) (Liu et al., 2018) were associated with anti-oxidant activity and the regulation of Bcl-2 family members to maintain mitochondrial function and morphology. Furthermore, Achyranthes bidentata polypeptides (ABP) (Shen et al., 2010), astragalosides (Chiu et al., 2014), and phenolic acid extracts derived from Sargentodoxa cuneata (Bai M. et al., 2019) and Salvia miltiorrhiza (Hou et al., 2016; Yang et al., 2018; Wei Y. et al., 2016) also have potential anti-ischemic stroke effects. In conclusion, although the clinical treatment of ischemic stroke with a single herb is rare, a large number of definitive in vitro and in vivo and clinical reports are sufficient to support further studies. However, the mechanism of some herbs with better efficacy proved by experiments is still in the preliminary stage, and the ischemic brain protection mechanism of anti-neuronal apoptosis is worthy of further exploration. More promisingly, some ethnic herbs for stroke prevention, such as Tibetan medicine saffron (Ochiai et al., 2007) and Mongolian medicine Eerdun Wurile (Gaowa et al., 2018), have also been gradually reported in recent years. In the early stage, our research group also revealed that the anti-hypoxia brain protection effect of the Tibetan medicine Rhodiola crenulata was related to the regulation of the HIF-1α/microRNA 210/ISCU1/2(COX10) signal pathway to improve mitochondrial energy metabolism, inhibit oxidative stress and mitochondrial apoptosis (Wang et al., 2019c). Although the medication law of ethnic medicine for prevention and treatment of ischemic stroke is bound to limit the scope of effective single herbal medicine. But optimistically taking the long view, such a gradual herbal medicine research model should be warranted.
Table 4.
The in vivo mechanism underlying the inhibition of MPTP opening-induced neuronal apoptosis by herbal medicine in the treatment of ischemic stroke.
| Agents | Objects | Gender | Weight (g) | Animal model | Dose | Time periods | Mechanisms | References |
|---|---|---|---|---|---|---|---|---|
| Curcuma oil | SD | Male | 200–225 | MCAO (1 h)/R (24 h) | 250 mg/kg, i.p. | Single dose and pretreatment for 0.5 h | Bcl-2↑, MMP↑; MPO↓, nitrite↓, nitrate↓, iNOS↓, nNOS↓, e NOS↓, peroxynitrite↓, ROS↓, Ca2+↓, Cyto-c↓, p53↓, cleaved Caspase-3, Bax↓, TUNEL-positive neurons↓, neuronal apoptosis under flow cytometry↓ | Dohare et al., 2008 |
| Hawthorn extract | SD | Male | 300–320 | MCAO (1.25 h)/R (3 or 24 h) | 100 mg/kg, i.g. | Pretreatment for 15 d, q.d. | Bcl-xL↑, Foxp3↑, pSTAT-3/STAT-3↑; IL-10↑; MPO↓, TNF-α↓, IL-6↓, IL-1β↓, ICAM-1↓, CD3+ & CD8+ positive cells↓, TUNEL-positive neurons↓ | Elango and Devaraj, 2010 |
| Rosa laevigata Michx | SD | Male | 250–300 | MCAO (2 h)/R (24 h) | 50, 100, and 200 mg/kg, i.g. | Pretreatment for 7 d, q.d. | Bcl-2↑, SOD↑, GSH↑, MMP↑; MDA↓, T-NOS↓, NO↓, iNOS↓, MMP-9↓, p53↓, Apaf1↓,Fas↓, Fasl↓, Bax↓, Bid↓, Cyto-c↓, cleaved Caspases-3/8/9↓, NF-κB↓, COX-2↓, TNF-α↓, IL-1β↓, IL-4↓, IL-6↓, p-JNK↓, p-ERK↓, p-p38↓, TUNEL-positive neurons↓ | Zhang et al., 2013 |
| PNP | Wistar | Male | 250–300 | MCAO (2 h)/R (22 h) | 50, 100, and 200 mg/kg, i.g. | Pretreatment for 7 d, q.d. | Bcl-2/Bax↑; cleaved Caspase-3↓, TUNEL-positive neurons↓ | Dong et al., 2014 |
| LBP | ICR | Male | 20–25 | MCAO (2 h)/R (24 h) | 10, 20, and 40 mg/kg, i.g. | Pretreatment for 7 d, q.d. | Bcl-2↑; Bax↓, Cyto-c↓, Caspases-3/9↓, cleaved PARP-1↓, TUNEL-positive neurons↓ | Wang T. et al., 2014 |
| Rhizoma Pinelliae Pedatisectae | SD | Male | 250–300 | MCAO (2 h)/R (24 h) | 5, 10, and 20 mg/kg, i.g. | Pretreatment for 7 d, b.i.d. | SOD↑, Bcl-2↑; Bax↓, MDA↓, TNF-α↓, IL-1 β↓, TUNEL-positive neurons↓ | Ye et al., 2016 |
| Clinacanthus nutans Lindau | Long-Evans | Male | —— | MCAO (0.5 h)/R (24 h) | 10–60 pg, icv; 24 mg/kg, i.p. |
Single dose and 30 min after MCAO; pretreatment for 1 h or posttreatment for 3–24 h |
PPAR-γ↑, C/EBPβ↑, 14-3-3ϵ↑, p-Bad↑, Bad↑, Bcl-2↑; cleaved Caspase-3↓, PARP↓ | Wu J. et al., 2017 |
| Spatholobi Caulis extract | SD | Male | 240–260 | MCAO (0.75 h)/R (7 d) | 100 and 200 mg/kg, i.g. | Pretreatment for 3 d and posttreatment for 7 d, q.d. | BDNF↑, β-III-tubulin↑, ROS↓, GFAP↓, cleaved PARP↓, cleaved Caspases-3↓, p-p38↓, p-JNK↓, TUNEL-positive neurons↓ | Park et al., 2018 |
| Radix Scrophulariae aqueous extract | KunMing mice | Male | 18–22 | MCAO (2 h)/R (22 h) | 2.4 g/kg, i.g. | Pretreatment for 7 d; q.d. | Bcl-2↑, SOD↑, MDA↓, NO↓, Bax↓, p-ERK1/2↓, p-P38↓ | Meng et al., 2018 |
↑, upgrade; ↓, downgrade.
Table 5.
The in vitro mechanism underlying the inhibition of MPTP opening-induced neuronal apoptosis by herbal medicine in the treatment of ischemic stroke.
| Agents | Cell lines | Model | Dose | Time periods | Mechanisms | References |
|---|---|---|---|---|---|---|
| ABP | PHN of embryonic, 18-d SD rats | OGD (NMDA insult, 0.5 h)/R (24 h) | 1 µg/ml | Pretreatment for 12 h and during OGD/R period | MMP↑; Bax↓, Caspase-3↓, ROS↓ | Shen et al., 2010 |
| GLP | PCN of neonatal SD rats (<24 h) | OGD (5% CO2 and 95% N2, 2 h)/R (24 h) | 0.1, 1.0, and 10.0 μg/ml | Pretreatment for 0.5 h and during OGD/R period | Bcl-2↑; cleaved Caspases-3/8/9↓, Bax↓ | Zhou et al., 2010 |
| Astragalosides | PC12 | OGD (5% CO2 and 95% N2, 5 h)/R (24 h) | 1, 100, and 200 g/ml | During reperfusion period | MMP↑, p-p38/p38↑,; fragmented DNA↓, LDH↓, Caspase-3/9/12↓, cleaved Caspases-3/9↓, ROS↓, LC3–11↓, Bip↓, neuronal apoptosis under flow cytometry↓, | Chiu et al., 2014 |
| Clinacanthus nutans Lindau | PCN of embryonic, 15.5-d Balb/c mouse | OGD (0.02–0.1% O2, 5% CO2, 10% H2, and 85% N2, 0.5 h)/R (4–24 h) | 6.25 μg/ml | Pretreatment for 1 h and during OGD/R period | 14-3-3ϵ↑, C/EBPβ↑, PPAR-γ↑, p-Bad↑, Bcl-2↑, MMP↑; cleaved Caspase-3↓, PARP-1↓ | Wu J. et al., 2017 |
| LBP | PHN of neonatal SD rats (<24 h) | OGD (5% CO2 and 95% N2, 2 h)/R (24 h) | 10, 20, and 40 mg/L | During reperfusion period | MMP↑, IκB-α↑, LDH↓, ROS↓, Ca2 +↓, IL-6↓, TLR4↓, NF-κB↓, Hoechst 33342 positive neurons↓, TUNEL-positive cells↓ | Zhao et al., 2017b |
| Spatholobi Caulis extract |
SH-SY5Y | A-24 h etoposide insult | 25 and 50 μg/ml | Pretreatment for 6 h and co-culture with etoposide for 24 h | MMP↑; cleaved PARP↓, p-p53↓, cleaved Caspase-3↓, Caspase-3/7↓, p-JNK/JNK↓, p-p38 /p38↓, TUNEL-positive neurons↓, neuronal apoptosis under flow cytometry↓ | Park et al., 2018 |
| Radix Scrophulariae aqueous extract | PC12 | OGD (5% CO2 and 95% N2, 2 h)/R (24 h) | 6.25, 12.50, 25.00, and 50.00 µg/ml | Pretreatment for 24 h | Bcl-2↑, SOD↑, GSH-Px↑, CAT↑, MMP↑; LDH↓, MDA↓, NO↓, Bax↓ | Meng et al., 2018 |
| CDP | PC12 | OGD (5% CO2 and 95% N2, 4 h)/R (24 h) | 0.05, 0.50, and 5.00 μg/ml | During reperfusion period | CAT↑, GSH-Px↑, T-AOC↑, MMP↑, DJ-1↑; ROS↓, LDH↓, MDA↓, Ca2+↓, neuronal apoptosis under flow cytometry↓, Hoechst33342 positive neurons↓ | Liu et al., 2018 |
| Scutellaria barbata D. Don extract | PC12 | OGD (1% O2, 5% CO2, and 94% N2, 6 h)/R (18 h) | 0.1–0.8 mg/ml | Pretreatment for 12–48 h | p-PI3K/ PI3K↑, p-AKT/AKT↑, p-PI3K↑, p-AKT↑, Nrf2↑, SOD↑, GSH↑, MMP↑, Ki67 positive cells↑,Cycin D1↑, Cyclin E↑; MDA↓, Bax↓, cleaved Caspase-3↓, Bid↓, neuronal apoptosis under flow cytometry↓ | Wang et al., 2019e |
| Aglaia odorata Lour. extract | PC12 | OGD (5% CO2 and 95% N2, 4 h)/R (24 h) | 5, 10, and 50 ng/ml | During OGD/R period | MMP↑, Caspase-3↑, PARP↑, Bcl-2↑; LDH↓, cleaved PARP↓, cleaved Caspase-3/9↓, Bax↓, p53↓, Puma↓, mtROS↓, AO/EB and Hoechst 33258 positive neurons↓ | Wang et al., 2020 |
↑, upgrade; ↓, downgrade.
Figure 2.
Representative herbal images that may inhibit ischemic neuron apoptosis by regulating MPTP. Sixteen herbs are shown here.
Monomers and Molecular Mechanisms in Regulating MPTP Openness of Ischemic Stroke
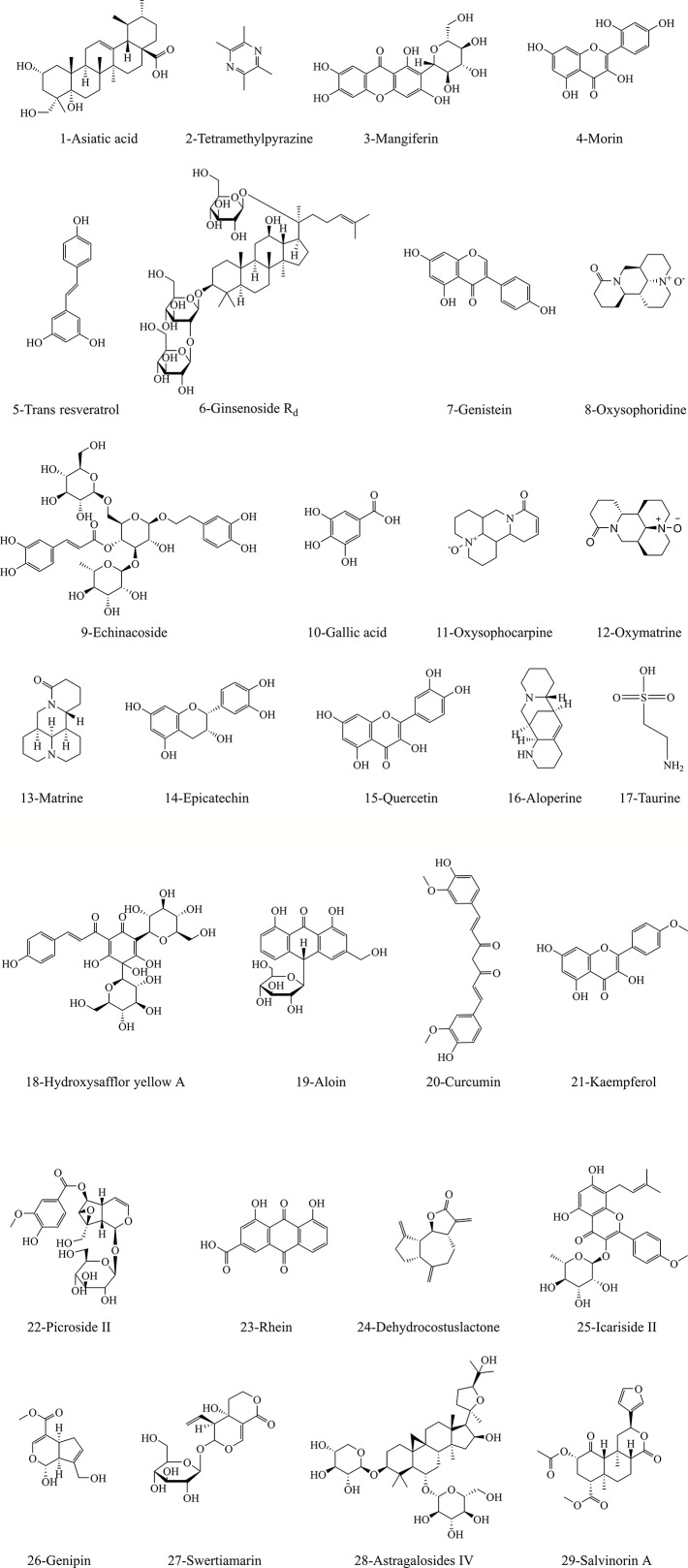
As research continues, massive active ingredients for treating stroke have been identified from herbal medicines. According to literature reports, we summarized 29 monomer compounds that may target to inhibit mitochondrial MPTP overopening-induced neuronal apoptosis, including alkaloids, flavonoids, terpenoids, and phenolic acids. Figure 3 shows the structure information of these potential compounds. Tables 6 and 7 list the specific brain protective mechanisms of monomer compounds against ischemia-induced neuronal apoptosis. Notably, some of these compounds have been shown to regulate MPTP to improve ischemic stroke. The anti-oxidant and anti-inflammatory effects of hydroxy safflor yellow A (HSYA) and carboxyatractyloside could help to inhibit ischemia-induced MPTP opening and play a protective role against cerebral ischemia (Ramagiri and Taliyan, 2016). The anti-hypoxic effect of kaempferol was related to inhibition of mitochondrial fission, maintenance of mitochondrial integrity and function, and therefore repressing MPTP opening-induced apoptosis (Wu B. et al., 2017). In vivo and in vitro experiments showed that the protective effect of gallic acid (GA) on cerebral ischemia against apoptosis might be related to inhibition of oxidative stress response, Ca2+ and ROS overproduction-evoked MPTP opening, and the transfer of mitochondrial Cyto-c to the cytoplasm, and thus increasing mitochondrial ATP supply and MMP (Sun et al., 2014). The authors further illuminated that GA could inhibit MPTP-induced apoptosis by regulating ERK-CypD axis, which may make GA a natural MPTP opening inhibitor for treating ischemic stroke (Sun et al., 2017). Earlier studies have reported that the anti-oxidative stress and apoptotic properties of trans resveratrol (Agrawal et al., 2011) and resveratrol (Narayanan et al., 2015) may lead to a protective effect against ischemia. Subsequently, studies confirmed that OGD/R induced bEND3 cerebrovascular endothelial cell edema was associated with monocyte chemoattractant protein and intracellular Ca2+ overload, while resveratrol could maintain mitochondrial MMP by inhibiting ROS and elevated Ca2+ ions, thus improving hypoxic brain edema (Panickar et al., 2015). Excitingly, recent study has further demonstrated that the anti-anoxic brain protection of preadministrated resveratrol may be related to consolidating mitochondrial tolerance to anoxia and increasing VDAC level and energy synthesis (Khoury et al., 2019). However, on the contrary, picroside II could interdict release of pro-apoptotic factor Endo G into the nucleus driven by MPTP opening, ROS production and VDAC1 protein expression (Li S. et al., 2018). Therefore, it is worth further focus on VDAC, one of the main components of MPTP, as an interesting target for stroke treatment.
Figure 3.
The structural information of underlying compounds for regulation of MPTP opening to inhibit apoptosis in ischemic neurons. The structural formulae of 29 monomer compounds are shown in the figure.
Table 6.
The in vivo mechanism underlying the inhibition of MPTP opening-induced neuronal apoptosis by monomeric compounds in the treatment of ischemic stroke.
| Agents | Objects | Gender | Weight (g) | Animal model | Dose | Time periods | Mechanisms | References |
|---|---|---|---|---|---|---|---|---|
| Asiatic acid | C57BL/6 | male | 22–27 | pMCAO | 30, 75, and 165 mg/kg, i.g. | 1 h before and 3, 10, and 20 h after pMCAO | Cyto-c↓, BBB permeability (IgG)↓ | Krishnamurthy et al., 2009 |
| Ginsenoside Rd | SD | male | 270–320 | MCAO | 50 mg/kg, i.p. | 30 min before MCAO | MMP↑, aconitase↑, mitochondrial complexes I-IV↑; ROS↓, lactate/pyruvate ratio↓, cleaved Caspase-3, Cyto-c↓, AIF↓ | Ye et al., 2011b |
| Genistein | C57/BL6J | male | 24–28 | MCAO (1 h)/R (24 h) | 2.5, 5, and 10 mg/kg, i.g. | Pretreatment once daily for 2 w | SOD↑, GSH-Px↑, mitochondrial Cyto-c↑; MDA↓, mtROS↓, cytosolic Cyto-c↓, Caspase-3↓, TUNEL-positive neurons↓, p-NF-κB p65 subunit↓, p-IκBα↓ | Qian et al., 2012 |
| Oxysophoridine | ICR | male | 20–25 | MCAO (2 h)/R (24 h) | 62.5, 125, and 250 mg/kg, i.p. | Pretreatment once daily for 1 w | SOD↑, GSH-Px↑, Bcl-2↑; MDA↓, Caspase-3↓, Bax↓, TUNEL-positive neurons↓ | Wang et al., 2013 |
| Echinacoside | SD | both | 12–17 | Permanent ligation of the left CCA plus low oxygen atmosphere (8% O2, 92% N2) for 2.5 h | 40, 80, and 160 mg/kg, i.p. | Every 12 h after operation, a total of 4 times | SOD↑, GSH-Px↑, CAT↑, T-AOC↑, Bcl-2/Bax↑; MDA↓, Caspase-3↓, TUNEL-positive neurons↓ | Wei W. et al., 2019 |
| Gallic acid | SD | male | 250–300 | MCAO (2 h)/R (24 h) | 25 and 50 mg/kg, i.v. | 20 min before MCAO | MMP↑, mitochondrial Cyto-c↑; MDA↓, ROS↓, cytosolic Cyto-c↓, TUNEL-positive neurons↓ | Sun et al., 2014 |
| SD | male | 250–300 | MCAO | 50 mg/kg | binding capacity of CypD and ANT-1↓, MPTP openinng↓, p-ERK↓, Cyto-c↓, cleaved Caspase-3/8/9↓ | Sun et al., 2017 | ||
| Oxymatrine | SD | both | —— | Permanent ligation of the left CCA plus low oxygen atmosphere (8% O2, 92% N2) for 2.5 h | 30, 60, and 120 mg/kg, i.p. | Every 12 h after operation, a total of 2 times | SOD↑, GSH-Px↑, CAT↑, T-AOC↑, Bcl-2/Bax↑, MDA↓, Caspase-3↓, neuronal apoptosis under flow cytometry↓ | Zhao et al., 2015a |
| Matrine | ICR | male | 20–25 | MCAO (2 h)/R (24 h) | 7.5, 15, and 30 mg/kg, i.p. | Pretreatment once daily for 1 w | SOD↑, GSH-Px↑, CAT↑, T-AOC↑, Bcl-2/Bax↑, MDA↓, Caspase-3↓, neuronal apoptosis under flow cytometry↓ | Zhao et al., 2015b |
| Taurine | SD | both | —— | Permanent ligation of the left CCA plus low oxygen atmosphere (8% O2, 92% N2) for 2 h | 30, 60, and 120 mg/kg, i.p. | Every 12 h after operation, a total of 2 times | SOD↑, GSH-Px↑, T-AOC↑, Bcl-2/Bax↑, ATP↑; LA↓; MPO↓, MDA↓, AIF↓, Cyto-c↓ | Zhu et al., 2016 |
| HSYA | Wistar | male | 220–250 | MCAO (1 h)/R (24 h) | 8 mg/kg, i.v. | After reperfusion | GSH↑, CAT↑; MDA↓, TNF-α↓, MPTP opening↓ | Ramagiri and Taliyan, 2016 |
| Curcumin | Wistar | male | 180–200 | MCAO/R | 25 mg/kg, i.p. | Bcl-↑, Sirt1↑, MMP ↑; p53↓, Bax↓, IL-6↓, TNF-α↓, ROS↓ | Zhang et al., 2017 | |
| Picroside II | Wistar | male | 240–260 | MCAO (2 h)/R (24 h) | 20 mg/kg, i.p. | 15 min before MCAO/R. | VDAC1↓, cytoplasmic and nuclear EndoG↓, ROS↓, MPTP opening↓, TUNEL-positive neurons↓ | Li S. et al., 2018 |
| Rhein | SD | male | 260–300 | MCAO (2 h)/R (72 h) | 25, 50, and 100 mg/kg, i.g. | 3 days following MCAO/R | SOD↑, GSH-Px↑, CAT↑, Bcl-2/Bax ratio↑; MDA↓, Caspase-3/9↓, cleaved Caspase-3↓ | Zhao et al., 2018b |
| Genipin | C57BL/6 | male | 25–30 | MCAO (1 h)/R (24 h) | 50 mg/kg, i.g. | Pretreatment once daily for 3 d | ATP↑, SOD↑, GSH↑; UCP2↓, SIRT3↓, NAD+/NADH↓, LDH↓, cleaved Caspase-3↓, TUNEL-positive neurons↓ | Zhao B. et al., 2019 |
| Swertiamain | ICR | male | 20–25 | MCAO (2 h)/R (24 h) | 25, 100, and 400 mg/kg, i.p. | Pretreatment once daily for 1 w | Bcl-2/Bax↑, SOD↑, GSH-Px↑, CAT↑, GSH↑, nulcear Nrf2↑, HO-1↑, NQO1↑; MDA↓, Keap1↓, cytoplasmic Nrf2↓, TUNEL-positive neurons↓ | Wang et al., 2019b |
↑, upgrade; ↓, downgrade.
Table 7.
The in vitro mechanism underlying the inhibition of MPTP opening-induced neuronal apoptosis by monomeric compounds in the treatment of ischemic stroke.
| Agents | Cell lines | Model | Dose | Time periods | Mechanisms | References |
|---|---|---|---|---|---|---|
| Asiatic acid | HT-22 | OGD (5 h)/R (24 h) | 1 and 10 µg/ml | Posttreatment for 24 h | MMP↑, Cyto-c↓ | Krishnamurthy et al., 2009 |
| Tetrahydroxystilbene glucoside | PCN of neonatal SD rats | OGD (5% CO2 and 95% N2, 2 h)/R (24 h) | 25 µM | Pretreatment for 24 h | MMP↑, SIRT1↑, Bcl-2/Bax↑; LDH↓, ROS↓, p-JNK↓, iNOS↓, nuclear p65↓, Ca2+↓, Hoechst 33258 positive staining↓ | Wang et al., 2009 |
| Mangiferin and Morin |
PCN of embryonic SD rats | 50 μM glutamate plus 10 μM glycine | 1–104 nM | During and after glutamate exposure |
SOD↑, CAT↑, p-Akt↑, cytoplasmic p65↑, MMP↑; Calpain↓, p-Erk1/2↓, nuclear p65↓, AIF↓, Bax↓, ROS↓ |
Campos-Esparza et al., 2009 |
| Trans resveratrol | PC12 | OGD (5% CO2, 94% N2, and 1% O2, 6 h)/R (24 h) | 5, 10, and 25 μM | 24 h before/post OGD | Bcl-2↑, GSH↑; Bax↓, HIF-1α↓, Caspase-3↓, ROS↓, LPO↓ | Agrawal et al., 2011 |
| Oxysophoridine | PHN of neonatal SD rats | OGD (2 h)/R (24 h) | 5, 20, and 80 μM | OGD (2 h)/R (24 h) | Bcl-2/Bax↑; Caspase-3/8/9↓, Cyto-c↓, Hoechst-33342 fluorescence intensity↓ SOD↑, CAT↑, GSH-Px↑, MMP↑; NOS↓, glutamate↓, Ca2+↓, MDA↓, NO↓ |
Chen et al., 2013; Zhao et al., 2013 |
| Gallic acid | SH-SY5Y | Hypoxia (Na2S2O4, 2 h)/R (2 h) | 0.1, 1, and 10 μM | Pretreatment for 24 h | MMP↑, ATP↑, oxygen consumption↑; MDA↓, intracellular ROS↓, mtROS↓, MPTP opening↓ | Sun et al., 2014 |
| SH-SY5Y | / | 0.1, 1, and 10 mM | 24 h before H2O2-induced MPTP opening | binding capacity of CypD and ANT-1↓, MPTP openinng↓, p-ERK↓, Cyto-c↓, cleaved Caspase-3/8/9↓ | Sun et al., 2017 | |
| Oxysophocarpine | PHN of neonatal SD rats | OGD (5% CO2, and 95% N2, 2 h)/R (24 h) | 1, 2, and 5 μmol/L | During reperfusion period | MMP↑; LDH↓, Ca2+↓, Caspase-3/12↓ | Zhu et al., 2014 |
| Epicatechin and Quercetin | PCN of embryonic CD1 mice | OGD (5% CO2, 5% H2, and 90% N2, 5 min)/R (1.5 h) | 0.1–10 μM | Pretreatment for 24 h | OCRs↑, p-Akt/Akt↑, p-CREB/CREB↑, Bcl-2↑, PGC-1a↑, MT-ND2 (complex I)↑, MT-ATP6 (complex V)↑, MMP↑; Ca2+↓, NOS↓ | Nichols et al., 2015 |
| Aloperine | PHN of neonatal SD rats | OGD (5% CO2, and 95% N2, 2 h)/R (24 h) | 25, 50, and 100 mg/L | During reperfusion period | CAT↑, SOD↑, GSH-Px↑, T-AOC↑, MMP↑; LDH↓, Ca2+↓, MDA↓, ROS↓, Hoechst 33342 positive staining↓ | Ma et al., 2015 |
| Aloin | PC12 | OGD (5% CO2, and 95% N2, 4 h)/R (24 h) | 10, 20, and 40 μg/ml | During OGD/R period | MMP↑, Bcl-2↑, SOD↑; LDH↓, MDA↓, ROS↓, Ca2+↓, Bax↓, Caspase-3↓, Hoechst 33342 positive staining↓, apoptosis under flow cytometry↓ | Chang R. et al., 2016 |
| Kaempferol | PCN of 17-d embryonic rats | OGD (2 h) | 10 μM | Before OGD | OCRs↑, p-Akt/Akt↑, MMP↑, p-Drp1/Drp1↑, ATG5↑, ATP↑, HK-II↑, LC3 II/I ratios↑, mitochondrial Cyto-c/cytosolic Cyto-c↑; ROS↓, Ca2+↓, SDH↓, apoptosis under flow cytometry↓, MPTP openinng↓ | Wu B. et al., 2017 |
| Dehydrocostuslactone | hippocampal slices of SD rats | OGD (5% CO2, and 95% N2, 0.5 h)/R (1 h) | 1, 5, and 10 µM | During OGD/R period | LC3 II/I ratios↑, Bcl-2↑; LDH↓, Bax↓, Cyto-c↓, Apaf-1↓, Caspase-3/7/9↓, p62↓ | Zhao et al., 2018a |
| Icariside II | PC12 | OGD (5% CO2, and 95% N2, 2 h)/R (24 h) | 12.5, 25, and 50 μM | Posttreatment for 24 h | nuclear Nrf2↑, NQO-1↑, HO-1↑, Bcl-2/Bax↑, SIRT3↑, IDH2↑, MMP↑; LDH↓, ROS↓, cytoplasmic Nrf2↓, Keap1↓, cleaved Caspase-3↓, TUNEL-positive neurons↓ | Feng et al., 2018 |
| Astragaloside IV | PCN of 18-d embryonic SD rats | OGD (1% O2, 5% CO2, 3 h)/R (24 h) | 6.25, 12.5, and 25 μmol/L | During OGD/R period | p-PKA/PKA and p-CREB/CREB↑, ATP↑, MMP↑; LDH↓, cleaved Caspase-3↓, ROS↓ | Xue et al., 2019 |
| Oxymatrine | PHN of newborn SD rats | OGD (5% CO2 and 95% N2, 2 h)/R (24 h) | 0.2, 1, and 5 µg/ml | During reperfusion period | MCL-1↑, Bcl-2↑, p-Akt↑, p-PI3K↑, p-GSK3β↑, MMP↑; LDH↓, Ca2+↓, Caspase-3↓, NR2B↓ (NMDAR1), TUNEL-positive neurons↓, neuronal apoptosis under flow cytometry↓ | Liu Y. et al., 2019 |
| Salvinorin A | HBMECs | OGD (5% CO2 and 95% N2, 6 h)/R (24 h) | 5 uM | During reperfusion period | p-AMPK↑, Mfn2↑, ATP↑, MMP↑; ROS↓, Ca2+↓, apoptosis under flow cytometry↓ | Dong et al., 2019 |
↑, upgrade; ↓, downgrade.
In vivo and in vitro studies have shown that oxysophoridine could regulate Bcl-2 family members, and thereby counteracting mitochondria-mediated apoptosis. Meanwhile, it was possible to suppress Ca2+ overload of neurons and maintain mitochondrial MMP by anti-oxidative stress and inhibiting the toxicity of neuronal excitatory amino acids (Chen et al., 2013; Wang et al., 2013; Zhao et al., 2013). Oxysophocarpine could also limit hypoxia-induced neuronal apoptosis by inhibiting Ca2+ and increasing MMP (Zhu et al., 2014). Further studies have shown that the inhibitory effect of apoptosis was related to anti-inflammatory and down-regulation of MAPK signaling pathway (Zhao et al., 2017a). Similarly, aloperine (Ma et al., 2015), matrine, and oxymatrine (Zhao et al., 2015a; Zhao et al., 2015b; Liu Y. et al., 2019) may have the same protective effect against ischemic neuron apoptosis. As a reversible selective inhibitor of true cholinesterase, huperzine A has been shown to inhibit mitochondrial complexes I–IV, a-ketoglutarate dehydrogenase, and MMP decline after ischemia, which helps to eliminating excessive ROS and Ca2+ (Zheng et al., 2008). Considering the short in vivo half-life of tetramethylpyrazine, a novel compound containing tetramethylpyrazine and carnitine structures was synthesized. Further in vivo and in vitro results also confirmed that its anti-hypoxic brain protective effect was related to anti-oxidative stress and anti-inflammatory, ultimately maintaining the morphology and function of neurons and inhibiting neuronal apoptosis (Wang et al., 2017). Of course, there are other natural compounds that antagonize ischemia-infuriated morphological and functional disorders of brain mitochondria by regulating oxidative stress signals such as leonurine (Loh et al., 2010) and neferine (Wu C. et al., 2019).
Flavonoids resisting oxidative stress may drive the recovery of ischemia attacked neuron mitochondrial function, evidenced by increased mitochondrial biosynthesis and respiration, dampened Ca2+ production, and mitochondria edema, such as icariside II (Feng et al., 2018), as well quercetin and epicatechin in flavonols (Nichols et al., 2015). As an Nrf2 activator, mangiferin inhibited the nuclear translocation of two subunits of NF-κB, p65 and p50, and the superior antioxidant properties of mangiferin and morin inhibited Ca2+ overload and improved mitochondrial MMP, thus counteracting the lethal post-ischemic neuronal excitatory toxic damage and cascade apoptosis (Campos-Esparza et al., 2009). Other reports suggested that the protective effects of genistein (Qian et al., 2012), isorhamnetin (Li et al., 2016), and vitexin (Cui et al., 2019) against ischemia may involve both inflammation and inhibition of neuronal apoptosis. Most terpenoids also have antioxidant properties similar to those of alkaloids and flavonoids, which helped maintain mitochondrial morphology and respiratory function as well as ischemia-induced neuronal apoptosis, such as bilobalide (Schwarzkopf et al., 2013) and Swertiamain (Wang et al., 2019b). Studies have shown that the treatment time window of asiatic acid can be maintained for at least 12 h, which is related to the improvement of MMP and the inhibition of mitochondrial Cyto-c release (Krishnamurthy et al., 2009). The balanced redox effect of ginsenoside Rd may contribute to the improvement of cerebral injury symptoms (Ye et al., 2011a). Further evidence showed that Rd could improve mitochondrial respiratory function and increase ATP production by reducing ROS production, thereby maintaining MMP and inhibiting neuronal apoptosis (Ye et al., 2011b), which was similar to dehydrocostuslactone’s protection of rat hippocampal slices from OGD/R-induced damage (Zhao et al., 2018a). Astragalosides IV may maintain mitochondrial function and inhibit OGD/R-induced cortical neuronal apoptosis by regulating PKA/CREB signaling pathway (Xue et al., 2019). In vivo and in vitro evidence suggested that Salvinorin A played an anti-apoptotic and anti-hypoxia protective role in brain involving the reduction of ROS and Ca2+ production in cerebrovascular endothelial cells, the activation of AMPK/Mfn2 signaling pathway, and ultimately maintenance of mitochondrial morphology and MMP (Dong et al., 2019). As an excellent natural biological cross-linking agent and a specific inhibitor of mitochondrial uncoupling protein 2 (UCP2), in vivo studies have shown that genipin could improve mitochondrial energy metabolism by inhibiting UCP2-SIRT3 signaling pathway to mitigate oxidative stress injury and neuronal apoptosis after hypoxic brain injury (Zhao B. et al., 2019).
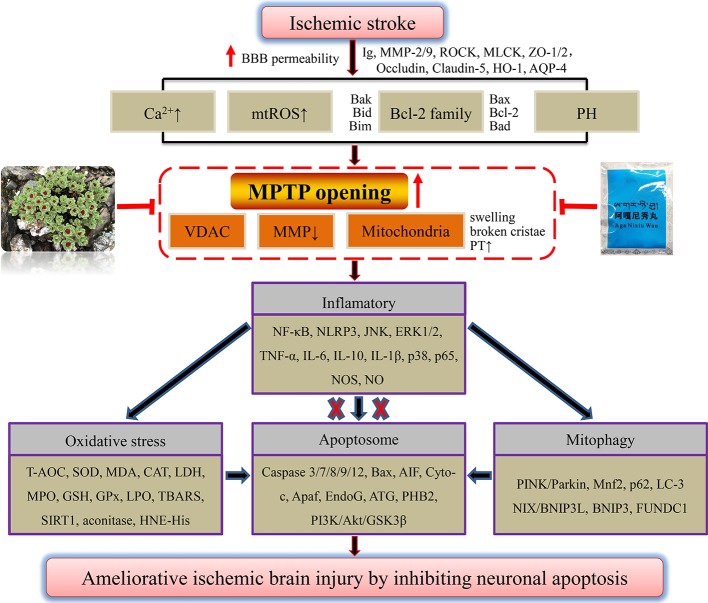
Other compounds such as taurine (Zhu et al., 2016) and echinacoside (Wei W. et al., 2019) could also regulate Bcl-2 family members through antioxidant stress, and inhibit mitochondrial apoptosis to improve hypoxic brain injury. Ischemic brain protection against neuronal apoptosis of phenolic acid compounds tetrahydroxystilbene glucoside (Wang et al., 2009), vanillin (Lan et al., 2019), curcumin (Zhang et al., 2017), and apocynin (Connell et al., 2012) may also further involved in the mechanism of anti-inflammatory, such as regulating the NF-κB and JNK, or targeting SIRT1. The antioxidant activity of quinones shikonin (Wang et al., 2010) and aloin (Chang R. et al., 2016), with a similar anti-cerebral ischemia action of rhein in our previous study (Zhao et al., 2018b), as well as phenylpropanoid compounds cinnamtannin D1 and trans-cinnamaldehyde (Panickar et al., 2015; Qi et al., 2016) from cinnamon might reduce the accumulation of Ca2+ and ROS, thus improving MMP to exert anti-ischemic neuron apoptosis. Through the above analysis of officially authorized drugs for the treatment of ischemic stroke, ethnic drug prescription, herbs, and monomer components, we found that most of them have the effect of anti-oxidative stress. The inhibition of overloaded Ca2+ and overproduced mtROS is the premise of drugs to reverse the decline of MMP after ischemia, improve mitochondrial respiratory function, and maintain the ATP supply of neurons. Although apoptosis might be the ultimate destination of neurons after ischemic stroke, we are pleasantly surprised to find that many adverse factors after ischemia might drive mitochondrial MPTP overopening. Meanwhile, we have previously discussed some potential proteins or oligomers that may be involved in regulating MPTP opening after cellular hypoxia, such as Bcl-2, Bax, Bcl-xL, and oligomer Bax/Bak of the Bcl-2 family. Through reviewing literatures, we also found that the above natural products could directly or indirectly inhibit MPTP overopening after ischemia. Furthermore, increased OMM permeability and collapsed mitochondrial membrane structures are inhibited. Ultimately, the integrity of the mitochondrial membrane and MMP are rescued, thus inhibiting the vicious cycle of excessive Ca2+ and mtROS production. As seen from the end results, caspase-dependent apoptosis triggered by the release of mitochondrial contents such as Cyto-c and AIF was blocked. Collectively, we have reason to believe that mitochondrial MPTP may be a potential target of natural products to inhibit neuronal apoptosis in treatment of ischemic stroke. Among the mechanisms, there may also be inflammation and oxidative stress signaling involved in MPTP opening and apoptosis. We summarized the mechanisms by which ethnic drugs may regulate MPTP to inhibit apoptosis of ischemic neurons, as shown in Figure 4 . Among them, the mechanisms that have not been reported and elucidated still need to be further probed.
Figure 4.
A panoramic view of natural products inhibiting MPTP opening-induced neuronal apoptosis in the treatment of ischemic stroke. Any adverse stimuli after ischemic stroke could favor MPTP opening. However, natural products that inhibit MPTP opening could further prevent neuronal inflammation after ischemia, oxidative stress injury, and mitophagy, and finally repress ischemic neuron apoptosis.
Conclusion and Future Prospects
The literatures on targeted improvement of mitochondrial MPTP by ethnic medicine were reviewed systematically and purposefully. We were ecstatic to accept the trend that balanced mitochondrial MPTP was becoming a novel strategy for drug treatment of stroke (Briston et al., 2019). First, we identified that the process of stroke was associated with an abnormal over-opening of mitochondrial MPTP. Any factors that induced insufficient blood supply to the brain may lead to robust ROS, unbalanced intracellular Ca2+ homeostasis, decrease MMP, inflammation, and ERS. These detrimental events were doomed to be fatal to mitochondria and initiate changes in the three-dimensional conformation of mitochondrial MPTP, which would in turn aggravate the production of mitochondrial ROS, mitochondrial edema, the booming cytoplasmic Ca2+, the decline of MMP, and the reduction of ATP synthesis. To sum up, all these adverse biological events that caused the loss of the function of mitochondrial bilayer barrier would inevitably disrupt the material transfer between mitochondrial matrix and cytoplasm. Consequently, the activated mitochondrial dependent apoptosis was triggered according to an inherent set of biological procedures. And this process was regularly and strictly executed by mitochondria-emitted apoptosis signal, and delivered step by step. For instance, ischemia-induced MPTP opening leaded to the translocation of Cyto-c from mitochondrial matrix into cytoplasm, and binding with Apaf-1 and Caspase-9 to form apoptosome, thereby activating caspase-dependent programmed cell death pathways in ischemic/anoxic neurons. Secondly, we found piece by piece that ethnic drug prescriptions, herbal medicine, and monomer components could participate in regulation of excessive MPTP opening induced-ischemic neuronal apoptosis from different perspectives. We therefore concluded that mitochondrial MPTP, a very considerably intermediate link in apoptosis signaling, might be a novel target for natural products in treatment of stroke.
However, by weighing the pros and cons, the following aspects should be worthy to further optimization considering the anti-apoptotic brain protection effect of ethnic drugs through regulation of mitochondrial MPTP. First, the complexity and uncertainty of active ingredients penetrating blood brain barrier (BBB). Current methods for identifying active ingredients included high performance liquid chromatography (HPLC), mass spectrum, gas chromatography-mass spectrometer (GC-MS), or liquid chromatograph-mass spectrometer (LC-MS). However, the key problem lay in the selection and preprocessing of samples for content determination: the original herbs or prescription extracted by simple decoction, ultrasound or different proportions of organic reagents, animal serum or brain tissue homogenate after administration. Any test based on those ideas would simply identify specific monomer compounds contained in certain prescriptions or extracts. However, the premise of drug efficacy was to achieve a certain concentration in target organs or tissues such as specific brain regions to stimulate the transmission of anti-apoptotic protective signals. Slightly regretfully, the qualitative or quantitative identification methods mentioned above cannot completely represent the concentration of drug enrichment in cerebral ischemic regions. For this existing and confronting problems, we proposed that a microdialysis device coupled HPLC/MS would be a potential platform for screening active ingredients (Reyes-Garcés et al., 2019) or changeable pH value of brain microenvironment (Su and Ho, 2019). Moreover, distribution concentrations of different small molecule drugs targeting distinct brain regions could be dynamically presented in real time and in vivo by an integrated platform of high resolution laser confocal microimaging coupled with brain MS imaging (He et al., 2019; Liu C. et al., 2019). Finally, a multi-dimensional image of drug distribution in brain tissue was visually and stereoscopically constructed. Second, the rationality of in vivo and in vitro simulation of clinical stroke model in light of the complexity of BBB tissue structure (Sweeney et al., 2019). Currently, diverse in vivo stroke models for cerebral ischemia, or in vitro OGD/R-induced hippocampal slices or different neuron injury models, which were widely accepted and acquiescent, cannot reproduce the scene of changes in brain tissue structure and the specific molecular-mediated damage mechanisms yet. Therefore, the above existing stroke models needed to be further discussed. However, it was encouraging to note that our research group had successfully established in vitro co-culture models of cerebrovascular endothelial cell, astrocytes, and pericytes to simulate BBB (the data have not yet been published), referring to the organ-like models of multiple neurons co-culture or BBB previously reported (Bergmann et al., 2018). Of course, through establishment of in vitro neurovascular unit (NVU), we also strived to achieve real-time and rapid evaluation of natural small molecule compounds passing BBB, and to screen the quality markers of ethnic drugs and functional protein targets on a coupled microfluidic chip-mass spectrometry (MC-MS) platform (Wang et al., 2019d).
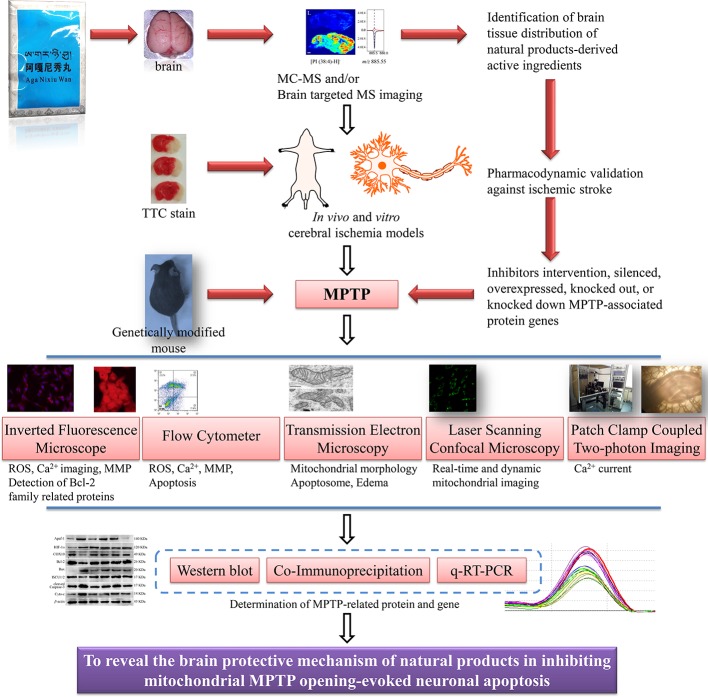
Third, the mechanisms of small molecular compounds acting on mitochondrial MPTP to inhibit apoptosis after ischemic stroke were unsophisticated. According to what we have learned, the conventional means demonstrating the interrelationship between drugs and MPTP were limited to the following. After intervention with MPTP inhibitors or agonists, conventional western blot, immunohistochemistry/fluorescence (Bonora et al., 2016), flow cytometry, and qRT-PCR were employed to evaluate the effect of drugs on changes in protein and gene expression that made up MPTP, such as VDAC and ANT. In addition, gene editing such as plasmids or viruses transfection of target gene vectors to overexpress or silence the target gene, or to completely knock out or down the target gene and observe the effect of drugs on MPTP were also some popular molecular biology methods. Certain proteins or protein complexes such as Bax/Bak dimerization, mtROS, oxidative stress, and inflammatory factors could regulate MPTP opening-induced cell apoptosis, thus providing indirect evidence for drug regulation of MPTP. The more intuitive evidence might be to detect some of triggering hallmarks after MPTP opening, such as mitochondrial swelling, decreased MMP and ATP production, and detection of fluorescent labeled cytoplasmic Ca2+ surge. However, none of the above methods could provide direct evidence of drug-MPTP-apoptosis. That is, it cannot be visualized that drugs confined MPTP opening, and thus inhibiting cell apoptosis. The deficiencies of the above mechanisms investigation included the limited understanding of MPTP and the limitations of current molecular imaging technologies. Therefore, more efforts were needed to explore the molecular basis and regulatory mechanism of MPTP. We also had reason to believe that the laser confocal high intentionality live cell real-time imaging and analysis system would be a robust alternative for probing drug targeted regulation of MPTP. Moreover, patch-clamp combined with two-photon living cell imaging technology also had potential prospects for detection of prophylaxis and treatment of ethnic drugs on post-stroke mitochondrial MMP and Ca2+ or other ion levels (Kislin et al., 2017; Wilson et al., 2018; Zhang et al., 2019c). In conclusion, we were optimistic that abnormal opening of mitochondrial MPTP-induced apoptosis would become a potential target for stroke treatment by ethnic medicine. Further, we conceived and constructed the systematic process and program of drugs regulating mitochondrial MPTP to inhibit apoptosis in ischemic stroke, as shown in Figure 5 . However, objectively speaking, no matter how many preclinical investigations were merely paving the way for screening mitochondrial MPTP targeted candidates, clinical trials with large samples and multi-center joint evaluation of the clinical efficacy of candidates were still necessary to be carried out.
Figure 5.
Conceptual flowchart of combined multiple techniques for MPTP regulation by natural products on apoptosis of ischemic neurons. Mitochondrial MPTP is a novel target for the treatment of ischemic stroke. Determination of the distribution of natural products in distinct brain regions, reasonable in vivo and in vitro stroke models, and advanced MPTP imaging technologies will be conducive to the development of ethnic drugs targeting MPTP.
Author Contributions
XW conceived the study. YL, JS, RW, JB, YH, YZe, XM, and YZh reviewed and summarized the literatures. XW wrote the manuscript and drew all the figures. XW, ZW, and XM supervised the study and gave final approval of the version to be published. The final version of the manuscript was read and approved by all authors.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by the National Key R&D Program of China (2017YFC1703904), the National Natural Science Foundation of China (81973569), the first-class interdisciplinary project of Chengdu University of Traditional Chinese medicine (CZYJC-1903), the special scientific research fund for doctoral programs of higher education in China's ministry of education (20115132120007), and the key project of Sichuan province applied and basic research program (2016JY0017).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.00352/full#supplementary-material
Abbreviations
AIF, apoptosis-inducing factor; Apaf-1, apoptotic protease activating factor 1; ATF6, activating transcription factor-6; Bcl-2, B-cell lymphoma 2; BDNF, brain-derived neurotrophic factor; BrdU, 5-bromo-20-deoxyuridine; CHOP, C/EBP homologous protein; c-IAP1, cellular inhibitor of apoptosis 1; CREB, cyclic AMP response element binding protein; EndoG, endonuclease G; ERK, extracellular signal-regulated protein kinases; GFAP, glial fibrillary acidic protein; GluR1, glutamate receptor 1; GRP78, glucose regulator protein 78; HBMECs, human brain microvascular endothelial cells; HIF-1α, hypoxia-inducible factor-1 alpha; IκBα, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; ICAM-1, intercellular cell adhesion molecule-1; IDH2, isocitrate dehydrogenase 2; IRE1, inositol-requiring protein 1; Keap1, Kelch-like ECH-associated protein 1; MAP-2, microtubule-associated protein-2; MCAO, middle cerebral artery occlusion; Mfn2, mitofusin-2; MMP-2, matrix metalloproteinase-2; MPTP, mitochondrial permeability transition pore; NeuN, neuron-specific nuclear; NMDAR1, N-methyl-D-aspartate receptor 1; NQO1, NAD(P)H, quinone oxidoreductase-1; Nrf2, nuclear factor erythroid-2-related factor 2; OCRs, oxygen consumption rates; OGD/R, oxygen glucose deprivation/reperfusion; OMM, outer mitochondrial membrane; PAF, platelet activating factor; PARP, poly ADP-ribose polymerase; PCN, primary cortical neurons; p-eIF2α, phospho-eIF2α; PERK, protein kinase RNA-like ER kinase; p-GSK-3β, phospho-glycogen synthase kinase-3β; PHN, primary hippocampal neurons; Pim-1, proto-oncogene serine/threonine-protein kinase; PKA, protein kinase A; p-PDK1, phosphoinositol-dependent kinase-1; PTEN, phosphatase and tensin homolog deleted on chromosome 10; Puma, p53 up-regulated modulator of apoptosis; SIRT3, silent mating type information regulation 2 homolog 3; STAT3, signal transducer and activator of transcription 3; TBARS, thiobarbituric acid reactive substances; TGF-β1, transforming growth factor; β-III-tubulin, microtubule element of the tubulin family; XBP-1, X-box-binding protein-1; ZO-1, zonula occludens-1.
References
- Agarwal A., Wu P. H., Hughes E. G., Fukaya M., Tischfield M. A., Langseth A. J., et al. (2017). Transient Opening of the Mitochondrial Permeability Transition Pore Induces Microdomain Calcium Transients in Astrocyte Processes. Neuron 93, 587–605.e7. 10.1016/j.neuron.2016.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal M., Kumar V., Kashyap M. P., Khanna V. K., Randhawa G. S., Pant A. B. (2011). Ischemic insult induced apoptotic changes in PC12 cells: protection by trans resveratrol. Eur. J. Pharmacol. 666, 5–11. 10.1016/j.ejphar.2011.05.015 [DOI] [PubMed] [Google Scholar]
- Andrabi S. S., Parvez S., Tabassum H. (2017). Progesterone induces neuroprotection following reperfusion-promoted mitochondrial dysfunction after focal cerebral ischemia in rats. Dis. Model. Mech. 10, 787–796. 10.1242/dmm.025692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrabi S. S., Ali M., Tabassum H., Parveen S., Parvez S. (2019). Pramipexole prevents ischemic cell death via mitochondrial pathways in ischemic stroke. Dis. Model. Mech. 12, 1–11. 10.1242/dmm.033860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoult D., Gaume B., Karbowski M., Sharpe J. C., Cecconi F., Youle R. J. (2003). Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J. 22, 4385–4399. 10.1093/emboj/cdg423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J., Gao Y., Gao Y. H. (2019). Effect of Pei Yuan Tong Nao capsules on neuronal function and metabolism in cerebral ischemic rats. J. Ethnopharmacol. 238, 111837. 10.1016/j.jep.2019.111837 [DOI] [PubMed] [Google Scholar]
- Bai M., Liu B., Peng M., Jia J., Fang X., Miao M. (2019). Effect of Sargentodoxa cuneata total phenolic acids on focal cerebral ischemia reperfusion injury rats model. Saudi J. Biol. Sci. 26, 569–576. 10.1016/j.sjbs.2018.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines C. P., Gutiérrez-Aguilar M. (2018). The still uncertain identity of the channel-forming unit(s) of the mitochondrial permeability transition pore. Cell Calcium 73, 121–130. 10.1016/j.ceca.2018.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann S., Lawler S. E., Qu Y., Fadzen C. M., Wolfe J. M., Regan M. S., et al. (2018). Blood-brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat. Protoc. 13, 2827–2843. 10.1038/s41596-018-0066-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P., Rasola A., Forte M., Lippe G. (2015). The Mitochondrial Permeability Transition Pore: Channel Formation by F-ATP Synthase, Integration in Signal Transduction, and Role in Pathophysiology. Physiol. Rev. 95, 1111–1155. 10.1152/physrev.00001.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonora M., Pinton P. (2019). A New Current for the Mitochondrial Permeability Transition. Trends Biochem. Sci. 44, 559–561. 10.1016/j.tibs.2019.04.009 [DOI] [PubMed] [Google Scholar]
- Bonora M., Morganti C., Morciano G., Giorgi C., Wieckowski M. R., Pinton P. (2016). Comprehensive analysis of mitochondrial permeability transition pore activity in living cells using fluorescence-imaging-based techniques. Nat. Protoc. 11, 1067–1080. 10.1038/nprot.2016.064 [DOI] [PubMed] [Google Scholar]
- Bora K. S., Sharma A. (2010). Neuroprotective effect of Artemisia absinthium L. @ on focal ischemia and reperfusion-induced cerebral injury. J. Ethnopharmacol. 129, 403–409. 10.1016/j.jep.2010.04.030 [DOI] [PubMed] [Google Scholar]
- Brenner C., Moulin M. (2012). Physiological roles of the permeability transition pore. Circ. Res. 111, 1237–1247. 10.1161/CIRCRESAHA.112.265942 [DOI] [PubMed] [Google Scholar]
- Briston T., Selwood D. L., Szabadkai G., Duchen M. R. (2019). Mitochondrial Permeability Transition: A Molecular Lesion with Multiple Drug Targets. Trends Pharmacol. Sci. 40, 50–70. 10.1016/j.tips.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Cai Y. M., Zhang Y., Zhang P. B., Zhen L. M., Sun X. J., Wang Z. L., et al. (2016). Neuroprotective effect of Shenqi Fuzheng injection pretreatment in aged rats with cerebral ischemia/reperfusion injury. Neural Regen. Res. 11, 94–100. 10.4103/1673-5374.175052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M., Yang Q., Li G., Sun S., Chen Y., Tian L., et al. (2017). Activation of cannabinoid receptor 1 is involved in protection against mitochondrial dysfunction and cerebral ischaemic tolerance induced by isoflurane preconditioning. Br. J. Anaesth. 119, 1213–1223. 10.1093/bja/aex267 [DOI] [PubMed] [Google Scholar]
- Campbell B. C. V., De Silva D. A., Macleod M. R., Coutts S. B., Schwamm L. H., Davis S. M., et al. (2019). Ischaemic stroke. Nat. Rev. Dis. Primers 5, 70. 10.1038/s41572-019-0118-8 [DOI] [PubMed] [Google Scholar]
- Campos-Esparza M. R., Sánchez-Gómez M. V., Matute C. (2009). Molecular mechanisms of neuroprotection by two natural antioxidant polyphenols. Cell Calcium 45, 358–368. 10.1016/j.ceca.2008.12.007 [DOI] [PubMed] [Google Scholar]
- Cao G., Zhou H., Jiang N., Han Y., Hu Y., Zhang Y., et al. (2016). YiQiFuMai Powder Injection Ameliorates Cerebral Ischemia by Inhibiting Endoplasmic Reticulum Stress-Mediated Neuronal Apoptosis. Oxid. Med. Cell Longev. 2016, 1–14. 10.1155/2016/5493279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Yao X., Zou H., Wang L., Lu Y., Zhang Q., et al. (2016). BDNF/PI3K/Akt and Nogo-A/RhoA/ROCK signaling pathways contribute to neurorestorative effect of Houshiheisan against cerebral ischemia injury in rats. J. Ethnopharmacol. 194, 1032–1042. 10.1016/j.jep.2016.11.005 [DOI] [PubMed] [Google Scholar]
- Chang R., Zhou R., Qi X., Wang J., Wu F., Yang W., et al. (2016). Protective effects of aloin on oxygen and glucose deprivation-induced injury in PC12 cells. Brain Res. Bull. 121, 75–83. 10.1016/j.brainresbull.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Chen R., Li Y. X., Jiang N., Ma N. T., Zhu Q. L., Hao Y. J., et al. (2013). Anti-apoptotic and neuroprotective effects of oxysophoridine on cerebral ischemia both in vivo and in vitro. Plant. Med. 79, 916–923. 10.1055/s-0032-1328705 [DOI] [PubMed] [Google Scholar]
- Chen L., Zhao Y., Zhang T., Dang X., Xie R., Li Z., et al. (2014). Protective effect of Sheng-Nao-Kang decoction on focal cerebral ischemia-reperfusion injury in rats. J. Ethnopharmacol. 151, 228–236. 10.1016/j.jep.2013.10.015 [DOI] [PubMed] [Google Scholar]
- Chen Z. Z., Gong X., Guo Q., Zhao H., Wang L. (2019). Bu Yang Huan Wu decoction prevents reperfusion injury following ischemic stroke in rats via inhibition of HIF-1α, VEGF and promotion β-ENaC expression. J. Ethnopharmacol. 228, 70–81. 10.1016/j.jep.2018.09.017 [DOI] [PubMed] [Google Scholar]
- Cheng F., Zhong X., Lu Y., Wang X., Song W., Guo S., et al. (2012). Refined Qingkailing Protects MCAO Mice from Endoplasmic Reticulum Stress-Induced Apoptosis with a Broad Time Window. Evid. Based Complement. Alternat. Med. 2012, 1–12. 10.1155/2012/567872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Hou Z., Sun J., Huang Y., Wang L., Zhou Z., et al. (2017). Protective effects of Tongxinluo on cerebral ischemia/reperfusion injury related to Connexin 43/Calpain II/Bax/Caspase-3 pathway in rat. J. Ethnopharmacol. 198, 148–157. 10.1016/j.jep.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Chinopoulos C. (2018). Mitochondrial permeability transition pore: Back to the drawing board. Neurochem. Int. 117, 49–54. 10.1016/j.neuint.2017.06.010 [DOI] [PubMed] [Google Scholar]
- Chiu B. Y., Chang C. P., Lin J. W., Yu J. S., Liu W. P., Hsu Y. C., et al. (2014). Beneficial effect of astragalosides on stroke condition using PC12 cells under oxygen glucose deprivation and reperfusion. Cell. Mol. Neurobiol. 34, 825–837. 10.1007/s10571-014-0059-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell B. J., Saleh M. C., Khan B. V., Rajagopal D., Saleh T. M. (2012). UPEI-100, a conjugate of lipoic acid and apocynin, mediates neuroprotection in a rat model of ischemia/reperfusion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R886–R895. 10.1152/ajpregu.00644.2011 [DOI] [PubMed] [Google Scholar]
- Cui Y. H., Zhang X. Q., Wang N. D., Zheng M. D., Yan J. (2019). Vitexin protects against ischemia/reperfusion-induced brain endothelial permeability. Eur. J. Pharmacol. 853, 210–219. 10.1016/j.ejphar.2019.03.015 [DOI] [PubMed] [Google Scholar]
- D’Orsi B., Kilbride S. M., Chen G., Perez Alvarez S., Bonner H. P., Pfeiffer S., et al. (2015). Bax regulates neuronal Ca2+ homeostasis. J. Neurosci. 35, 1706–1722. 10.1523/JNEUROSCI.2453-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Orsi B., Mateyka J., Prehn J. H. M. (2017). Control of mitochondrial physiology and cell death by the Bcl-2 family proteins Bax and Bok. Neurochem. Int. 109, 162–170. 10.1016/j.neuint.2017.03.010 [DOI] [PubMed] [Google Scholar]
- Dohare P., Varma S., Ray M. (2008). Curcuma oil modulates the nitric oxide system response to cerebral ischemia/reperfusion injury. Nitric. Oxide 19, 1–11. 10.1016/j.niox.2008.04.020 [DOI] [PubMed] [Google Scholar]
- Dong J., Deng Y., Gao J., Liu X., Chu J., Shu Y. (2014). Neuroprotective effect of Panax notoginseng plysaccharides against focal cerebral ischemia reperfusion injury in rats. Int. J. Biol. Macromol. 63, 177–180. 10.1016/j.ijbiomac.2013.10.034 [DOI] [PubMed] [Google Scholar]
- Dong H., Zhou W., Xin J., Shi H., Yao X., He Z., et al. (2019). Salvinorin A moderates postischemic brain injury by preserving endothelial mitochondrial function via AMPK/Mfn2 activation. Exp. Neurol. 322, 113045. 10.1016/j.expneurol.2019.113045 [DOI] [PubMed] [Google Scholar]
- Elango C., Devaraj S. N. (2010). Immunomodulatory effect of Hawthorn extract in an experimental stroke model. J. Neuroinflammation 7, 97. 10.1186/1742-2094-7-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod J. W., Wong R., Mishra S., Vagnozzi R. J., Sakthievel B., Goonasekera S. A., et al. (2010). Cyclophilin D controls mitochondrial pore-dependent Ca2+ exchange, metabolic flexibility, and propensity for heart failure in mice. J. Clin. Invest. 120, 3680–3687. 10.1172/JCI43171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Gao J., Liu Y., Shi J., Gong Q. (2018). Icariside II alleviates oxygen-glucose deprivation and reoxygenation-induced PC12 cell oxidative injury by activating Nrf2/SIRT3 signaling pathway. Biomed. Pharmacother. 103, 9–17. 10.1016/j.biopha.2018.04.005 [DOI] [PubMed] [Google Scholar]
- Gaowa S., Bao N., Da M., Qiburi Q., Ganbold T., Chen L., et al. (2018). Traditional Mongolian medicine Eerdun Wurile improves stroke recovery through regulation of gene expression in rat brain. J. Ethnopharmacol. 222, 249–260. 10.1016/j.jep.2018.05.011 [DOI] [PubMed] [Google Scholar]
- Hånell A., Greer J. E., McGinn M. J., Povlishock J. T. (2015). Traumatic brain injury-induced axonal phenotypes react differently to treatment. Acta Neuropathol. 129, 317–332. 10.1007/s00401-014-1376-x [DOI] [PubMed] [Google Scholar]
- Haiyu X., Yang S., Yanqiong Z., Qiang J., Defeng L., Yi Z., et al. (2016). Identification of key active constituents of Buchang Naoxintong capsules with therapeutic effects against ischemic stroke by using an integrative pharmacology-based approach. Mol. Biosyst. 12, 233–245. 10.1039/c5mb00460h [DOI] [PubMed] [Google Scholar]
- He H., Qin L., Zhang Y., Han M., Li J., Liu Y., et al. (2019). 3,4-Dimethoxycinnamic Acid as a Novel Matrix for Enhanced In Situ Detection and Imaging of Low-Molecular-Weight Compounds in Biological Tissues by MALDI-MSI. Anal. Chem. 91, 2634–2643. 10.1021/acs.analchem.8b03522 [DOI] [PubMed] [Google Scholar]
- Hou S., Zhao M. M., Shen P. P., Liu X. P., Sun Y., Feng J. C. (2016). Neuroprotective Effect of Salvianolic Acids against Cerebral Ischemia/Reperfusion Injury. Int. J. Mol. Sci. 17, 1190. 10.3390/ijms17071190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Qieni X., Li N., Bai J., Li R., Gongbao D., et al. (2020). Longzhibu disease and its therapeutic effects by traditional Tibetan medicine: Ershi-wei Chenxiang pills. J. Ethnopharmacol. 249, 112426. 10.1016/j.jep.2019.112426 [DOI] [PubMed] [Google Scholar]
- Hu R., Yin C. L., Wu N., Cui G. Y., Meng H., Wu X. G., et al. (2009). Traditional Chinese herb Dihuang Yinzi (DY) plays neuroprotective and anti-dementia role in rats of ischemic brain injury. J. Ethnopharmacol. 121, 444–450. 10.1016/j.jep.2008.09.035 [DOI] [PubMed] [Google Scholar]
- Huang Q., Lan T., Lu J., Zhang H., Zhang D., Lou T., et al. (2018). DiDang Tang Inhibits Endoplasmic Reticulum Stress-Mediated Apoptosis Induced by Oxygen Glucose Deprivation and Intracerebral Hemorrhage Through Blockade of the GRP78-IRE1/PERK Pathways. Front. Pharmacol. 9, 14–23. 10.3389/fphar.2018.01423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani K., Yan S. F., Yan S. S. (2018). Mitochondrial permeability transition pore: a potential drug target for neurodegeneration. Drug Discovery Today 23, 1983–1989. 10.1016/j.drudis.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch J., Bround M. J., Khalil H., Sargent M. A., Latchman N., Terada N., et al. (2019). Inhibition of mitochondrial permeability transition by deletion of the ANT family and CypD. Sci. Adv. 5, eaaw4597. 10.1126/sciadv.aaw4597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury N., Xu J., Stegelmann S. D., Jackson C. W., Koronowski K. B., Dave K. R., et al. (2019). Resveratrol Preconditioning Induces Genomic and Metabolic Adaptations within the Long-Term Window of Cerebral Ischemic Tolerance Leading to Bioenergetic Efficiency. Mol. Neurobiol. 56, 4549–4565. 10.1007/s12035-018-1380-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Chung D. K., Lee Y. J., Song C. H., Ku S. K. (2016). Neuroprotective effects of Danggui-Jakyak-San on rat stroke model through antioxidant/antiapoptotic pathway. J. Ethnopharmacol. 188, 123–133. 10.1016/j.jep.2016.04.060 [DOI] [PubMed] [Google Scholar]
- Kislin M., Sword J., Fomitcheva I. V., Croom D., Pryazhnikov E., Lihavainen E., et al. (2017). Reversible Disruption of Neuronal Mitochondria by Ischemic and Traumatic Injury Revealed by Quantitative Two-Photon Imaging in the Neocortex of Anesthetized Mice. J. Neurosci. 37, 333–348. 10.1523/JNEUROSCI.1510-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy R. G., Senut M. C., Zemke D., Min J., Frenkel M. B., Greenberg E. J., et al. (2009). Asiatic acid, a pentacyclic triterpene from Centella asiatica, is neuroprotective in a mouse model of focal cerebral ischemia. J. Neurosci. Res. 87, 2541–2550. 10.1002/jnr.22071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S., Mehta S. L., Milledge G. Z., Huang X., Li H., Li P. A. (2016). Ubisol-Q10 Prevents Glutamate-Induced Cell Death by Blocking Mitochondrial Fragmentation and Permeability Transition Pore Opening. Int. J. Biol. Sci. 12, 688–700. 10.7150/ijbs.13589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan R., Zhang Y., Xiang J., Zhang W., Wang G. H., Li W. W., et al. (2014). Xiao-Xu-Ming decoction preserves mitochondrial integrity and reduces apoptosis after focal cerebral ischemia and reperfusion via the mitochondrial p53 pathway. J. Ethnopharmacol. 151, 307–316. 10.1016/j.jep.2013.10.042 [DOI] [PubMed] [Google Scholar]
- Lan X. B., Wang Q., Yang J. M., Ma L., Zhang W. J., Zheng P., et al. (2019). Neuroprotective effect of Vanillin on hypoxic-ischemic brain damage in neonatal rats. Biomed. Pharmacother. 118, 109–196. 10.1016/j.biopha.2019.109196 [DOI] [PubMed] [Google Scholar]
- Lee J. J., Hsu W. H., Yen T. L., Chang N. C., Luo Y. J., Hsiao G., et al. (2011). Traditional Chinese medicine, Xue-Fu-Zhu-Yu decoction, potentiates tissue plasminogen activator against thromboembolic stroke in rats. J. Ethnopharmacol. 134, 824–830. 10.1016/j.jep.2011.01.033 [DOI] [PubMed] [Google Scholar]
- Li H., Deng C. Q., Chen B. Y., Zhang S. P., Liang Y., Luo X. G. (2009). Total saponins of Panax notoginseng modulate the expression of caspases and attenuate apoptosis in rats following focal cerebral ischemia-reperfusion. J. Ethnopharmacol. 121, 412–418. 10.1016/j.jep.2008.10.042 [DOI] [PubMed] [Google Scholar]
- Li L. H., Wang J. S., Kong L. Y. (2013). Protective effects of shengmai san and its three fractions on cerebral ischemia-reperfusion injury. Chin. J. Nat. Med. 11, 222–230. 10.1016/S1875-5364(13)60020-5 [DOI] [PubMed] [Google Scholar]
- Li W., Li P., Liu Z., Du Q., Steinmetz A., Wang N., et al. (2014). A Chinese medicine preparation induces neuroprotection by regulating paracrine signaling of brain microvascular endothelial cells. J. Ethnopharmacol. 151, 686–693. 10.1016/j.jep.2013.11.035 [DOI] [PubMed] [Google Scholar]
- Li S., Guo J., Ying Z., Chen S., Yang L., Chen K., et al. (2015). Valproic acid-induced hepatotoxicity in Alpers syndrome is associated with mitochondrial permeability transition pore opening-dependent apoptotic sensitivity in an induced pluripotent stem cell model. Hepatology 61, 1730–1739. 10.1002/hep.27712 [DOI] [PubMed] [Google Scholar]
- Li W., Chen Z., Yan M., He P., Chen Z., Dai H. (2016). The protective role of isorhamnetin on human brain microvascular endothelial cells from cytotoxicity induced by methylglyoxal and oxygen-glucose deprivation. J. Neurochem. 136, 651–659. 10.1111/jnc.13436 [DOI] [PubMed] [Google Scholar]
- Li M., Zhou J., Jin W., Li X., Zhang Y. (2018). Danhong Injection Combined With t-PA Improves Thrombolytic Therapy in Focal Embolic Stroke. Front. Pharmacol. 9, 308. 10.3389/fphar.2018.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Wang T., Zhai L., Ge K., Zhao J., Cong W., et al. (2018). Picroside II Exerts a Neuroprotective Effect by Inhibiting mPTP Permeability and EndoG Release after Cerebral Ischemia/Reperfusion Injury in Rats. J. Mol. Neurosci. 64, 144–155. 10.1007/s12031-017-1012-z [DOI] [PubMed] [Google Scholar]
- Li F., Zhao H., Han Z., Wang R., Tao Z., Fan Z., et al. (2019). Xuesaitong May Protect Against Ischemic Stroke by Modulating Microglial Phenotypes and Inhibiting Neuronal Cell Apoptosis via the STAT3 Signaling Pathway. CNS Neurol. Disord. Drug Targets. 18, 115–123. 10.2174/1871527317666181114140340 [DOI] [PubMed] [Google Scholar]
- Lindenboim L., Kringel S., Braun T., Borner C., Stein R. (2005). Bak but not Bax is essential for Bcl-xS-induced apoptosis. Cell Death Differ. 12, 713–723. 10.1038/sj.cdd.4401638 [DOI] [PubMed] [Google Scholar]
- Liu M., Liu X., Wang H., Xiao H., Jing F., Tang L., et al. (2016). Metabolomics study on the effects of Buchang Naoxintong capsules for treating cerebral ischemia in rats using UPLC-Q/TOF-MS. J. Ethnopharmacol. 180, 1–11. 10.1016/j.jep.2016.01.016 [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang H., Yang M., Liu N., Zhao Y., Qi X., et al. (2018). Cistanche deserticola polysaccharides protects PC12 cells against OGD/RP-induced injury. Biomed. Pharmacother. 99, 671–680. 10.1016/j.biopha.2018.01.114 [DOI] [PubMed] [Google Scholar]
- Liu C., Qi K., Yao L., Xiong Y., Zhang X., Zang J., et al. (2019). Imaging of Polar and Nonpolar Species Using Compact Desorption Electrospray Ionization/Postphotoionization Mass Spectrometry. Anal. Chem. 91, 6616–6623. 10.1021/acs.analchem.9b00520 [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang H., Liu N., Du J., Lan X., Qi X., et al. (2019). Oxymatrine protects neonatal rat against hypoxic-ischemic brain damage via PI3K/Akt/GSK3β pathway. Life Sci. 10.1016/j.lfs.2019.04.070 [DOI] [PubMed] [Google Scholar]
- Loh K. P., Qi J., Tan B. K., Liu X. H., Wei B. G., Zhu Y. Z. (2010). Leonurine protects middle cerebral artery occluded rats through antioxidant effect and regulation of mitochondrial function. Stroke 41, 2661–2668. 10.1161/STROKEAHA.110.589895 [DOI] [PubMed] [Google Scholar]
- Lu J., Li Y. H., Zhan X., Li G., Chen Z., Chen X. (2016). The protective effect of qiancao naomaitong mixture on neuronal damage and cerebral ischemia/reperfusion injury. Pharm. Biol. 54, 2304–2311. 10.3109/13880209.2016.1155627 [DOI] [PubMed] [Google Scholar]
- Ludtmann M. H. R., Angelova P. R., Horrocks M. H., Choi M. L., Rodrigues M., Baev A. Y., et al. (2018). α-synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease. Nat. Commun. 9, 2293. 10.1038/s41467-018-04422-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., Bian X., Zhang Q., Xia Z., Liu B., Chen Q., et al. (2019). Shengui Sansheng San Ameliorates Cerebral Energy Deficiency via Citrate Cycle After Ischemic Stroke. Front. Pharmacol. 10, 386. 10.3389/fphar.2019.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N. T., Zhou R., Chang R. Y., Hao Y. J., Ma L., Jin S. J., et al. (2015). Protective effects of aloperine on neonatal rat primary cultured hippocampal neurons injured by oxygen–glucose deprivation and reperfusion. J. . Nat. Med. 69, 575–583. 10.1007/s11418-015-0928-2 [DOI] [PubMed] [Google Scholar]
- Marcu R., Kotha S., Zhi Z., Qin W., Neeley C. K., Wang R. K., et al. (2015). The mitochondrial permeability transition pore regulates endothelial bioenergetics and angiogenesis. Circ. Res. 116, 1336–1345. 10.1161/CIRCRESAHA.116.304881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S., Friberg H., Ferrand-Drake M., Wieloch T. (1999). Blockade of the mitochondrial permeability transition pore diminishes infarct size in the rat after transient middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 19, 736–741. 10.1097/00004647-199907000-00002 [DOI] [PubMed] [Google Scholar]
- Mattiasson G., Shamloo M., Gido G., Mathi K., Tomasevic G., Yi S., et al. (2003). Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat. Med. 9, 1062–1068. 10.1038/nm903 [DOI] [PubMed] [Google Scholar]
- McArthur K., Whitehead L. W., Heddleston J. M., Li L., Padman B. S., Oorschot V., et al. (2018). BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 359, 883, eaao6047. 10.1126/science.aao6047 [DOI] [PubMed] [Google Scholar]
- Mehta S. L., Li P. A. (2009). Neuroprotective role of mitochondrial uncoupling protein 2 in cerebral stroke. J. Cereb. Blood Flow Metab. 29, 1069–1078. 10.1038/jcbfm.2009.4 [DOI] [PubMed] [Google Scholar]
- Mei Z. G., Tan L. J., Wang J. F., Li X. L., Huang W. F., Zhou H. J. (2017). Shuan-Tong-Ling Fermented Chinese formula attenuates ischemic stroke by inhibiting inflammation and apoptosis. Neural Regen. Res. 12, 425–432. 10.4103/1673-5374.202946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X., Xie W., Xu Q., Liang T., Xu X., Sun G., et al. (2018). Neuroprotective Effects of Radix Scrophulariae on Cerebral Ischemia and Reperfusion Injury via MAPK Pathways. Molecules 23, 2401. 10.3390/molecules23092401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morciano G., Bonora M., Campo G., Aquila G., Rizzo P., Giorgi C., et al. (2017). Mechanistic Role of mPTP in Ischemia-Reperfusion Injury. Adv. Exp. Med. Biol. 982, 169–189. 10.1007/978-3-319-55330-6_9 [DOI] [PubMed] [Google Scholar]
- Narayanan S. V., Dave K. R., Saul I., Perez-Pinzon M. A. (2015). Resveratrol Preconditioning Protects Against Cerebral Ischemic Injury via Nuclear Erythroid 2-Related Factor 2. Stroke 46, 1626–1632. 10.1161/STROKEAHA.115.008921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols M., Zhang J., Polster B. M., Elustondo P. A., Thirumaran A., Pavlov E. V., et al. (2015). Synergistic neuroprotection by epicatechin and quercetin: Activation of convergent mitochondrial signaling pathways. Neuroscience 308, 75–94. 10.1016/j.neuroscience.2015.09.012 [DOI] [PubMed] [Google Scholar]
- Ochiai T., Shimeno H., Mishima K., Iwasaki K., Fujiwara M., Tanaka H., et al. (2007). Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim. Biophys. Acta 1770, 578–584. 10.1016/j.bbagen.2006.11.012 [DOI] [PubMed] [Google Scholar]
- Pan R., Cai J., Zhan L., Guo Y., Huang R. Y., Li X., et al. (2017). Buyang Huanwu decoction facilitates neurorehabilitation through an improvement of synaptic plasticity in cerebral ischemic rats. BMC Complement. Altern. Med. 17, 173. 10.1186/s12906-017-1680-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panel M., Ruiz I., Brillet R., Lafdil F., Teixeira-Clerc F., Nguyen C. T., et al. (2019). Small-Molecule Inhibitors of Cyclophilins Block Opening of the Mitochondrial Permeability Transition Pore and Protect Mice From Hepatic Ischemia/Reperfusion Injury. Gastroenterology 157, 1368–1382. 10.1053/j.gastro.2019.07.026 [DOI] [PubMed] [Google Scholar]
- Panickar K. S., Qin B., Anderson R. A. (2015). Ischemia-induced endothelial cell swelling and mitochondrial dysfunction are attenuated by cinnamtannin D1, green tea extract, and resveratrol in vitro. Nutr. Neurosci. 18, 297–306. 10.1179/1476830514Y.0000000127 [DOI] [PubMed] [Google Scholar]
- Park H. R., Lee H., Lee J. J., Yim N. H., Gu M. J., Ma J. Y. (2018). Protective Effects of Spatholobi Caulis Extract on Neuronal Damage and Focal Ischemic Stroke/Reperfusion Injury. Mol. Neurobiol. 55, 4650–4666. 10.1007/s12035-017-0652-x [DOI] [PubMed] [Google Scholar]
- Peña-Blanco A., García-Sáez A. J. (2018). Bax, Bak and beyond-mitochondrial performance in apoptosis. FEBS J. 285, 416–431. 10.1111/febs.14186 [DOI] [PubMed] [Google Scholar]
- Prentice H., Modi J. P., Wu J. Y. (2015). Mechanisms of Neuronal Protection against Excitotoxicity, Endoplasmic Reticulum Stress, and Mitochondrial Dysfunction in Stroke and Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2015, 964518. 10.1155/2015/964518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X., Zhou R., Liu Y., Wang J., Zhang W. N., Tan H. R., et al. (2016). Trans-cinnamaldehyde protected PC12 cells against oxygen and glucose deprivation/reperfusion (OGD/R)-induced injury via anti-apoptosis and anti-oxidative stress. Mol. Cell. Biochem. 421, 67–74. 10.1007/s11010-016-2785-z [DOI] [PubMed] [Google Scholar]
- Qian Y., Guan T., Huang M., Cao L., Li Y., Cheng H., et al. (2012). Neuroprotection by the soy isoflavone, genistein, via inhibition of mitochondria-dependent apoptosis pathways and reactive oxygen induced-NF-κB activation in a cerebral ischemia mouse model. Neurochem. Int. 60, 759–767. 10.1016/j.neuint.2012.03.011 [DOI] [PubMed] [Google Scholar]
- Ramagiri S., Taliyan R. (2016). Neuroprotective effect of hydroxy safflor yellow A against cerebral ischemia-reperfusion injury in rats: putative role of mPTP. J. Basic Clin. Physiol. Pharmacol. 27, 1–8. 10.1515/jbcpp-2015-0021 [DOI] [PubMed] [Google Scholar]
- Reyes-Garcés N., Diwan M., Boyacı E., Gómez-Ríos G. A., Bojko B., Nobrega J. N., et al. (2019). In Vivo Brain Sampling Using a Microextraction Probe Reveals Metabolic Changes in Rodents after Deep Brain Stimulation. Anal. Chem. 91, 9875–9884. 10.1021/acs.analchem.9b01540 [DOI] [PubMed] [Google Scholar]
- Schild L., Roth A., Keilhoff G., Gardemann A., Brödemann R. (2009). Protection of hippocampal slices against hypoxia/hypoglycemia injury by a Gynostemma pentaphyllum extract. Phytomedicine 16, 734–743. 10.1016/j.phymed.2009.03.006 [DOI] [PubMed] [Google Scholar]
- Schwarzkopf T. M., Koch K. A., Klein J. (2013). Neurodegeneration after transient brain ischemia in aged mice: beneficial effects of bilobalide. Brain Res. 1529, 178–187. 10.1016/j.brainres.2013.07.003 [DOI] [PubMed] [Google Scholar]
- Seervi M., Rani A., Sharma A. K., Santhosh, Kumar T. R. (2018). ROS mediated ER stress induces Bax-Bak dependent and independent apoptosis in response to Thioridazine. Biomed. Pharmacother. 106, 200–209. 10.1016/j.biopha.2018.06.123 [DOI] [PubMed] [Google Scholar]
- Shanmughapriya S., Rajan S., Hoffman N. E., Higgins A. M., Tomar D., Nemani N., et al. (2015). SPG7 Is an Essential and Conserved Component of the Mitochondrial Permeability Transition Pore. Mol. Cell 60, 47–62. 10.1016/j.molcel.2015.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Yuan Y., Ding F., Hu N., Liu J., Gu X. (2010). Achyranthes bidentata polypeptides confer neuroprotection through inhibition of reactive oxygen species production, Bax expression, and mitochondrial dysfunction induced by overstimulation of N-methyl-D-aspartate receptors. J. Neurosci. Res. 88, 669–676. 10.1002/jnr.22221 [DOI] [PubMed] [Google Scholar]
- Šileikytė J., Forte M. (2019). The Mitochondrial Permeability Transition in Mitochondrial Disorders. Oxid. Med. Cell. Longev. 2019, 3403075. 10.1155/2019/3403075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., Reddy U., Robba C., Sharma D., Citerio G. (2019). Acute ischaemic stroke: challenges for the intensivist. Intensive Care Med. 45, 1177–1189. 10.1007/s00134-019-05705-y [DOI] [PubMed] [Google Scholar]
- Solenski N. J., diPierro C. G., Trimmer P. A., Kwan A. L., Helm G. A., Helms G. A. (2002). Ultrastructural changes of neuronal mitochondria after transient and permanent cerebral ischemia. Stroke 33, 816–824. 10.1161/hs0302.104541 [DOI] [PubMed] [Google Scholar]
- Solesio M. E., Elustondo P. A., Zakharian E., Pavlov E. V. (2016). Inorganic polyphosphate (polyP) as an activator and structural component of the mitochondrial permeability transition pore. Biochem. Soc Trans. 44, 7–12. 10.1042/BST20150206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll G., Nieswandt B. (2019). Thrombo-inflammation in acute ischaemic stroke-implications for treatment. Nat. Rev. Neurol. 15, 473–481. 10.1038/s41582-019-0221-1 [DOI] [PubMed] [Google Scholar]
- Su C. K., Ho C. C. (2019). Online profiling of living rat brain extracellular pH using a pH-Dependent solid phase extraction scheme coupled with microdialysis sampling and inductively coupled plasma mass spectrometry. Anal. Chim. Acta 1055, 36–43. 10.1016/j.aca.2018.12.020 [DOI] [PubMed] [Google Scholar]
- Sun K., Hu Q., Zhou C. M., Xu X. S., Wang F., Hu B. H., et al. (2010). Cerebralcare Granule, a Chinese herb compound preparation, improves cerebral microcirculatory disorder and hippocampal CA1 neuron injury in gerbils after ischemia-reperfusion. J. Ethnopharmacol. 130, 398–406. 10.1016/j.jep.2010.05.030 [DOI] [PubMed] [Google Scholar]
- Sun J., Li Y. Z., Ding Y. H., Wang J., Geng J., Yang H., et al. (2014). Neuroprotective effects of gallic acid against hypoxia/reoxygenation-induced mitochondrial dysfunctions in vitro and cerebral ischemia/reperfusion injury in vivo. Brain Res. 1589, 126–139. 10.1016/j.brainres.2014.09.039 [DOI] [PubMed] [Google Scholar]
- Sun J., Ren D. D., Wan J. Y., Chen C., Chen D., Yang H., et al. (2017). Desensitizing Mitochondrial Permeability Transition by ERK-Cyclophilin D Axis Contributes to the Neuroprotective Effect of Gallic Acid against Cerebral Ischemia/Reperfusion Injury. Front. Pharmacol. 8, 184. 10.3389/fphar.2017.00184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M. D., Zhao Z., Montagne A., Nelson A. R., Zlokovic B. V. (2019). Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 99, 21–78. 10.1152/physrev.00050.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri N., Borlongan C. V., Kaneko Y. (2016). Cyclosporine A Treatment Abrogates Ischemia-Induced Neuronal Cell Death by Preserving Mitochondrial Integrity through Upregulation of the Parkinson’s Disease-Associated Protein DJ-1. CNS Neurosci. Ther. 22, 602–610. 10.1111/cns.12546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi B., Chen X., Gao C., Wang S., Yuen S. C., Yang D., et al. (2019). Neuroprotective Effects and Hepatorenal Toxicity of Angong Niuhuang Wan Against Ischemia-Reperfusion Brain Injury in Rats. Front. Pharmacol. 10, 593. 10.3389/fphar.2019.00593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani A., Giorgio V., Carrer A., Franchin C., Arrigoni G., Jiko C., et al. (2019). Purified F-ATP synthase forms a Ca2+-dependent high-conductance channel matching the mitochondrial permeability transition pore. Nat. Commun. 10, 4341. 10.1038/s41467-019-12331-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaseva A. V., Marchenko N. D., Ji K., Tsirka S. E., Holzmann S., Moll U. M. (2012). p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 149, 1536–1548. 10.1016/j.cell.2012.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanatha G. L., Venkataranganna M. V., Prasad N. B. L., Hanumanthappa S. (2018). Chemical characterization and cerebroprotective effect of methanolic root extract of Colebrookea oppositifolia in rats. J. Ethnopharmacol. 223, 63–75. 10.1016/j.jep.2018.05.009 [DOI] [PubMed] [Google Scholar]
- Wang T., Gu J., Wu P. F., Wang F., Xiong Z., Yang Y. J., et al. (2009). Protection by tetrahydroxystilbene glucoside against cerebral ischemia: involvement of JNK, SIRT1, and NF-κB pathways and inhibition of intracellular ROS/RNS generation. Free Radic. Biol. Med. 47, 229–240. 10.1016/j.freeradbiomed.2009.02.027 [DOI] [PubMed] [Google Scholar]
- Wang Z., Liu T., Gan L., Wang T., Yuan X., Zhang B., et al. (2010). Shikonin protects mouse brain against cerebral ischemia/reperfusion injury through its antioxidant activity. Eur. J. Pharmacol. 643, 211–217. 10.1016/j.ejphar.2010.06.027 [DOI] [PubMed] [Google Scholar]
- Wang H. W., Liou K. T., Wang Y. H., Lu C. K., Lin Y. L., Lee I. J., et al. (2011). Deciphering the neuroprotective mechanisms of Bu-yang Huan-wu decoction by an integrative neurofunctional and genomic approach in ischemic stroke mice. J. Ethnopharmacol. 138, 22–33. 10.1016/j.jep.2011.06.033 [DOI] [PubMed] [Google Scholar]
- Wang T. F., Lei Z., Li Y. X., Wang Y. S., Wang J., Wang S. J., et al. (2013). Oxysophoridine protects against focal cerebral ischemic injury by inhibiting oxidative stress and apoptosis in mice. Neurochem. Res. 38, 2408–2417. 10.1007/s11064-013-1153-6 [DOI] [PubMed] [Google Scholar]
- Wang G. H., Lan R., Zhen X. D., Zhang W., Xiang J., Cai D. F. (2014). An-Gong-Niu-Huang Wan protects against cerebral ischemia induced apoptosis in rats: up-regulation of Bcl-2 and down-regulation of Bax and caspase-3. J. Ethnopharmacol. 154, 156–162. 10.1016/j.jep.2014.03.057 [DOI] [PubMed] [Google Scholar]
- Wang T., Li Y., Wang Y., Zhou R., Ma L., Hao Y., et al. (2014). Lycium barbarum polysaccharide prevents focal cerebral ischemic injury by inhibiting neuronal apoptosis in mice. PloS One 9, e90780. 10.1371/journal.pone.0090780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Gong G., Wang X., Wei-LaPierre L., Cheng H., Dirksen R., et al. (2016). Mitochondrial Flash: Integrative Reactive Oxygen Species and pH Signals in Cell and Organelle Biology. Antioxid. Redox Signal. 25, 534–549. 10.1089/ars.2016.6739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zhou Z., Wei X., Wang M., Wang B. O., Zhang Y., et al. (2017). Therapeutic Potential of Novel Twin Compounds Containing Tetramethylpyrazine and Carnitine Substructures in Experimental Ischemic Stroke. Oxid. Med. Cell. Longev. 2017, 7191856. 10.1155/2017/7191856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Chen S., Zhang Y., Xu H., Sun H. (2019. a). Electroacupuncture ameliorates neuronal injury by Pink1/Parkin-mediated mitophagy clearance in cerebral ischemia-reperfusion. Nitric. Oxide 91, 23–34. 10.1016/j.niox.2019.07.004 [DOI] [PubMed] [Google Scholar]
- Wang H., Wei W., Lan X., Liu N., Li Y., Ma H., et al. (2019. b). Neuroprotective Effect of Swertiamain on Cerebral Ischemia/Reperfusion Injury by Inducing the Nrf2 Protective Pathway. ACS Chem. Neurosci. 10, 2276–2286. 10.1021/acschemneuro.8b00605 [DOI] [PubMed] [Google Scholar]
- Wang P., Dai L., Zhou W., Meng J., Zhang M., Wu Y., et al. (2019). Intermodule Coupling Analysis of Huang-Lian-Jie-Du Decoction on Stroke. Front. Pharmacol. 10, 1288. 10.3389/fphar.2019.01288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Hou Y., Li Q., Li X., Wang W., Ai X., et al. (2019. c). Rhodiola crenulata attenuates apoptosis and mitochondrial energy metabolism disorder in rats with hypobaric hypoxia-induced brain injury by regulating the HIF-1α/microRNA 210/ISCU1/2(COX10) signaling pathway. J. Ethnopharmacol. 241, 111801. 10.1016/j.jep.2019.03.028 [DOI] [PubMed] [Google Scholar]
- Wang X., Liu Z., Fan F., Hou Y., Yang H., Meng X., et al. (2019. d). Microfluidic chip and its application in autophagy detection. Trends Analyt. Chem. 117, 300–315. 10.1016/j.trac.2019.05.043 [DOI] [Google Scholar]
- Wang Y., Li B., Zhang X. (2019. e). Scutellaria barbata D. Don (SBD) protects oxygen glucose deprivation/reperfusion-induced injuries of PC12 cells by up-regulating Nrf2. Artif. Cells Nanomed. Biotechnol. 47, 1797–1807. 10.1080/21691401.2019.1610413 [DOI] [PubMed] [Google Scholar]
- Wang Y., Yang J., Du H., Zhang H., Wan H., He Y. (2019. f). Yangyin Tongnao granules enhance neurogenesis in the peri-infarct area and upregulate brain-derived neurotrophic factor and vascular endothelial growth factor after focal cerebral ischemic infarction in rats. Mol. Biol. Rep. 46, 3817–3826. 10.1007/s11033-019-04824-5 [DOI] [PubMed] [Google Scholar]
- Wang J. K., Guo Q., Zhang X. W., Wang L. C., Liu Q., Tu P. F., et al. (2020). Aglaia odorata Lour. extract inhibit ischemic neuronal injury potentially via suppressing p53/Puma-mediated mitochondrial apoptosis pathway. J. Ethnopharmacol. 248, 112336. 10.1016/j.jep.2019.112336 [DOI] [PubMed] [Google Scholar]
- Wei W., Lan X. B., Liu N., Yang J. M., Du J., Ma L., et al. (2019). Echinacoside Alleviates Hypoxic-Ischemic Brain Injury in Neonatal Rat by Enhancing Antioxidant Capacity and Inhibiting Apoptosis. Neurochem. Res. 44, 1582–1592. 10.1007/s11064-019-02782-9 [DOI] [PubMed] [Google Scholar]
- Wei X., Zhu Q., Liu N., Xu L., Wei S., Fan Z., et al. (2019). In Vivo Neuroprotective Effects and Mechanisms of Zhenlong Xingnao Capsule in Vivo and in Vitro Models of Hypoxia. Front. Pharmacol. 10, 1096. 10.3389/fphar.2019.01096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Zhu L., Huang X., Zhang Y. (2019). Lipidomics study of protective effect of Salvia miltiorrhiza and Ligusticum chuanxiong on rats with focal cerebral ischemia injury based on UPLC-Q/TOF-MS. Chin. Traditional Herbal Drugs 50, 408–417. 10.7501/j.issn.0253-2670.2019.02.020 [DOI] [Google Scholar]
- Wilson D. E., Scholl B., Fitzpatrick D. (2018). Differential tuning of excitation and inhibition shapes direction selectivity in ferret visual cortex. Nature 560, 97–101. 10.1038/s41586-018-0354-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Luo H., Zhou X., Cheng C. Y., Lin L., Liu B. L., et al. (2017). Succinate-induced neuronal mitochondrial fission and hexokinase II malfunction in ischemic stroke: Therapeutical effects of kaempferol. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 2307–2318. 10.1016/j.bbadis.2017.06.011 [DOI] [PubMed] [Google Scholar]
- Wu J. S., Kao M. H., Tsai H. D., Cheung W. M., Chen J. J., Ong W. Y., et al. (2017. b). Clinacanthus nutans Mitigates Neuronal Apoptosis and Ischemic Brain Damage Through Augmenting the C/EBPβ-Driven PPAR-γ Transcription. Mol. Neurobiol. 55, 5425–5438. 10.1007/s12035-017-0776-z [DOI] [PubMed] [Google Scholar]
- Wu C., Chen J., Yang R., Duan F., Li S., Chen X. (2019). Mitochondrial protective effect of neferine through the modulation of nuclear factor erythroid 2-related factor 2 signalling in ischaemic stroke. Br. J. Pharmacol. 176, 400–415. 10.1111/bph.14537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Wu B., Liu M., Chen Z., Wang W., Anderson C. S., et al. (2019). Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 18, 394–405. 10.1016/S1474-4422(18)30500-3 [DOI] [PubMed] [Google Scholar]
- Xu J., Tang L., Zhang Q., Wei J., Xian M., Zhao Y., et al. (2017). Relative quantification of neuronal polar lipids by UPLC-MS reveals the brain protection mechanism of Danhong injection. RSC Adv. 7, 45746. 10.1039/c7ra09245h [DOI] [Google Scholar]
- Xu Y., Wang Y., Wang G., Ye X., Zhang J., Cao G., et al. (2017). YiQiFuMai Powder Injection Protects against Ischemic Stroke via Inhibiting Neuronal Apoptosis and PKCδ/Drp1-Mediated Excessive Mitochondrial Fission. Oxid. Med. Cell. Longev. 2017, 1–17. 10.1155/2017/1832093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Liu X., Luo L., Tang L., Guo N., Liu M., et al. (2019). A Metabonomics Investigation into the Therapeutic Effects of BuChang NaoXinTong Capsules on Reversing the Amino Acid-Protein Interaction Network of Cerebral Ischemia. Oxid. Med. Cell. Longev. 2019, 1–14. 10.1155/2019/7258624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B., Huang J., Ma B., Yang B., Chang D., Liu J. (2019). Astragaloside IV Protects Primary Cerebral Cortical Neurons from Oxygen and Glucose Deprivation/Reoxygenation by Activating the PKA/CREB Pathway. Neuroscience 404, 326–337. 10.1016/j.neuroscience.2019.01.040 [DOI] [PubMed] [Google Scholar]
- Yang H., Li L., Zhou K., Wang Y., Guan T., Chai C., et al. (2016). Shengmai injection attenuates the cerebral ischemia/reperfusion induced autophagy via modulation of the AMPK, mTOR and JNK pathways. Pharm. Biol. 54, 2288–2297. 10.3109/13880209.2016.1155625 [DOI] [PubMed] [Google Scholar]
- Yang Q., Lu L., Sun R. (2018). Mechanism analysis of Salviae Miltiorrhiza and Ligustrazine Hydrochloride Injection based on network pharmacology. Chin. Traditional Herbal Drugs 49, 2606–2613. 10.7501/j.issn.0253-2670.2018.11.018 [DOI] [Google Scholar]
- Yang P., Tian Y. M., Deng W. X., Cai X., Liu W. H., Li L., et al. (2019). Sijunzi decoction may decrease apoptosis via stabilization of the extracellular matrix following cerebral ischaemia-reperfusion in rats. Exp. Ther. Med. 18, 2805–2812. 10.3892/etm.2019.7878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R., Kong X., Yang Q., Zhang Y., Han J., Zhao G. (2011. a). Ginsenoside Rd attenuates redox imbalance and improves stroke outcome after focal cerebral ischemia in aged mice. Neuropharmacology 61, 815–824. 10.1016/j.neuropharm.2011.05.029 [DOI] [PubMed] [Google Scholar]
- Ye R., Zhang X., Kong X., Han J., Yang Q., Zhang Y., et al. (2011. b). Ginsenoside Rd attenuates mitochondrial dysfunction and sequential apoptosis after transient focal ischemia. Neuroscience 178, 169–180. 10.1016/j.neuroscience.2011.01.007 [DOI] [PubMed] [Google Scholar]
- Ye Y., Li J., Cao X., Chen Y., Ye C., Chen K. (2016). Protective effect of n-butyl alcohol extracts from Rhizoma Pinelliae Pedatisectae against cerebral ischemia-reperfusion injury in rats. J. Ethnopharmacol. 188, 259–265. 10.1016/j.jep.2016.04.046 [DOI] [PubMed] [Google Scholar]
- Yen T. L., Ong E. T., Lin K. H., Chang C. C., Jayakumar T., Lin S. C., et al. (2014). Potential advantages of Chinese medicine Taohong Siwu Decoction combined with tissue-plasminogen activator for alleviating middle cerebral artery occlusion-induced embolic stroke in rats. Chin. J. Integr. Med. 1–9. 10.1007/s11655-014-1847-x [DOI] [PubMed] [Google Scholar]
- Yu Z. H., Cai M., Xiang J., Zhang Z. N., Zhang J. S., Song X. L., et al. (2016). PI3K/Akt pathway contributes to neuroprotective effect of Tongxinluo against focal cerebral ischemia and reperfusion injury in rats. J. Ethnopharmacol. 181, 8–19. 10.1016/j.jep.2016.01.028 [DOI] [PubMed] [Google Scholar]
- Zamzami N., Kroemer G. (2001). The mitochondrion in apoptosis: how Pandora’s box opens. Nat. Rev. Mol. Cell Biol. 2, 67–71. 10.1038/35048073 [DOI] [PubMed] [Google Scholar]
- Zeng K., Li Y., Yang W., Ge Y., Xu L., Ren T., et al. (2019). Moringa oleifera seed extract protects against brain damage in both the acute and delayed stages of ischemic stroke. Exp. Gerontol. 122, 99–108. 10.1016/j.exger.2019.04.014 [DOI] [PubMed] [Google Scholar]
- Zhang S., Qi Y., Xu Y., Han X., Peng J., Liu K., et al. (2013). Protective effect of flavonoid-rich extract from Rosa laevigata Michx on cerebral ischemia-reperfusion injury through suppression of apoptosis and inflammation. Neurochem. Int. 63, 522–532. 10.1016/j.neuint.2013.08.00 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Bian H., Li Y., Guo L., Tang Y., Zhu H. (2014). Preconditioning with the traditional Chinese medicine Huang-Lian-Jie-Du-Tang initiates HIF-1α-dependent neuroprotection against cerebral ischemia in rats. J. Ethnopharmacol. 154, 443–452. 10.1016/j.jep.2014.04.022 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li H., Huang M., Chu K., Xu W., Zhang S., et al. (2014). Neuroprotective effects of Gualou Guizhi decoction in vivo and in vitro. J. Ethnopharmacol. 158, 76–84. 10.1016/j.jep.2014.10.020 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yan Y., Cao Y., Yang Y., Zhao Q., Jing R., et al. (2017). Potential therapeutic and protective effect of curcumin against stroke in the male albino stroke-induced model rats. Life Sci. 183, 45–49. 10.1016/j.lfs.2017.06.023 [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhang Y., Tang S., Yu L., Zhao Y., Ren Q., et al. (2018). Pien-Tze-Huang protects cerebral ischemic injury by inhibiting neuronal apoptosis in acute ischemic stroke rats. J. Ethnopharmacol. 219, 117–125. 10.1016/j.jep.2018.03.018 [DOI] [PubMed] [Google Scholar]
- Zhang Y. M., Qu X. Y., Zhai J. H., Tao L. N., Gao H., Song Y. Q., et al. (2018). Xingnaojing Injection Protects against Cerebral Ischemia Reperfusion Injury via PI3K/Akt-Mediated eNOS Phosphorylation. Evid. Based Complement. Alternat. Med. 2018, 1–13. 10.1155/2018/2361046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Xing Y., Chang J., Wang L., An N., Tian C., et al. (2019). Efficacy and Safety of NaoShuanTong Capsule in the Treatment of Ischemic Stroke: A Meta-Analysis. Front. Pharmacol. 10, 1133. 10.3389/fphar.2019.01133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Liu J., Zhang M., Wei S., Li R., Gao Y., et al. (2019). Apoptosis induction of fibroblast-like synoviocytes is an important molecular mechanism for herbal medicine and its active components to treat rheumatoid arthritis. Biomolecules 9, 795. 10.3390/biom9120795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Hernandez O., Chrapkiewicz R., Shai A., Wagner M. J., Zhang Y., et al. (2019). Kilohertz two-photon brain imaging in awake mice. Nat. Methods 16, 1119–1122. 10.1038/s41592-019-0597-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Cao M., Wu Y., Wang J., Zheng J., Liu N., et al. (2020). Improvement in mitochondrial function underlies the effects of ANNAO tablets on attenuating cerebral ischemia-reperfusion injuries. J. Ethnopharmacol. 246, 112–212. 10.1016/j.jep.2019.112212 [DOI] [PubMed] [Google Scholar]
- Zhao H., Yenari M. A., Cheng D., Barreto-Chang O. L., Sapolsky R. M., Steinberg G. K. (2004). Bcl-2 transfection via herpes simplex virus blocks apoptosis-inducing factor translocation after focal ischemia in the rat. J. Cereb. Blood Flow Metab. 24, 681–692. 10.1097/01.WCB.0000127161.89708.A5 [DOI] [PubMed] [Google Scholar]
- Zhao J., Li Y. X., Hao Y. J., Chen R., Zhang J. Z., Sun T., et al. (2013). Effects of oxysophoridine on rat hippocampal neurons sustained oxygen-glucose deprivation and reperfusion. CNS Neurosci. Ther. 19, 138–141. 10.1111/cns.12047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P., Zhou R., Li H. N., Yao W. X., Qiao H. Q., Wang S. J., et al. (2015. a). Oxymatrine attenuated hypoxic-ischemic brain damage in neonatal rats via improving antioxidant enzyme activities and inhibiting cell death. Neurochem. Int. 89, 17–27. 10.1016/j.neuint.2015.06.008 [DOI] [PubMed] [Google Scholar]
- Zhao P., Zhou R., Zhu X. Y., Hao Y. J., Li N., Wang J., et al. (2015. b). Matrine attenuates focal cerebral ischemic injury by improving antioxidant activity and inhibiting apoptosis in mice. Int. J. Mol. Med. 36, 633–644. 10.3892/ijmm.2015.2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Cheng X., Wang X., Wang J., Zhu Y., Ma X. (2016). Neuroprotective effect and mechanism of Mu-Xiang-You-Fang on cerebral ischemia-reperfusion injury in rats. J. Ethnopharmacol. 192, 140–147. 10.1016/j.jep.2016.07.016 [DOI] [PubMed] [Google Scholar]
- Zhao P., Chang R. Y., Liu N., Wang J., Zhou R., Qi X., et al. (2017). Neuroprotective Effect of Oxysophocarpine by Modulation of MAPK Pathway in Rat Hippocampal Neurons Subject to Oxygen-Glucose Deprivation and Reperfusion. Cell. Mol. Neurobiol. 38, 529–540. 10.1007/s10571-017-0501-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P., Zhou R., Zhu X. Y., Liu G., Zhao Y. P., Ma P. S., et al. (2017). Neuroprotective Effects of Lycium barbarum Polysaccharide on Focal Cerebral Ischemic Injury in Mice. Neurochem. Res. 42, 2798–2813. 10.1007/s11064-017-2293-x [DOI] [PubMed] [Google Scholar]
- Zhao Q., Chen A., Wang X., Zhang Z., Zhao Y., Huang Y., et al. (2018. a). Protective effects of dehydrocostuslactone on rat hippocampal slice injury induced by oxygen–glucose deprivation/reoxygenation. Int. J. Mol. Med. 42, 1190–1198. 10.3892/ijmm.2018.3691 [DOI] [PubMed] [Google Scholar]
- Zhao Q., Wang X., Chen A., Cheng X., Zhang G., Sun J., et al. (2018. b). Rhein protects against cerebral ischemic-/reperfusion-induced oxidative stress and apoptosis in rats. Int. J. Mol. Med. 41, 2802–2812. 10.3892/ijmm.2018.3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Sun L. K., Jiang X., Zhang Y., Kang J., Meng H., et al. (2019. a). Genipin protects against cerebral ischemia-reperfusion injury by regulating the UCP2-SIRT3 signaling pathway. Eur. J. Pharmacol. 845, 56–64. 10.1016/j.ejphar.2018.12.028 [DOI] [PubMed] [Google Scholar]
- Zheng C. Y., Zhang H. Y., Tang X. C. (2008). Huperzine A attenuates mitochondrial dysfunction after middle cerebral artery occlusion in rats. J. Neurosci. Res. 86, 2432–2440. 10.1002/jnr.21681 [DOI] [PubMed] [Google Scholar]
- Zhou Z. Y., Tang Y. P., Xiang J., Wua P., Jin H. M., Wang Z., et al. (2010). Neuroprotective effects of water-soluble Ganoderma lucidum polysaccharides on cerebral ischemic injury in rats. J. Ethnopharmacol. 131, 154–164. 10.1016/j.jep.2010.06.023 [DOI] [PubMed] [Google Scholar]
- Zhou B., Kreuzer J., Kumsta C., Wu L., Kamer K. J., Cedillo L., et al. (2019). Mitochondrial Permeability Uncouples Elevated Autophagy and Lifespan Extension. Cell 177, 299–314. e16. 10.1016/j.cell.2019.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q. L., Li Y. X., Zhou R., Ma N. T., Chang R. Y., Wang T. F., et al. (2014). Neuroprotective effects of oxysophocarpine on neonatal rat primary cultured hippocampal neurons injured by oxygen-glucose deprivation and reperfusion. Pharm. Biol. 52, 1052–1059. 10.3109/13880209.2013.877039 [DOI] [PubMed] [Google Scholar]
- Zhu X. Y., Ma P. S., Wu W., Zhou R., Hao Y. J., Niu Y., et al. (2016). Neuroprotective actions of taurine on hypoxic-ischemic brain damage in neonatal rats. Brain Res. Bull. 124, 295–305. 10.1016/j.brainresbull.2016.06.010 [DOI] [PubMed] [Google Scholar]
- Zhu B., Cao H., Sun L., Li B., Guo L., Duan J., et al. (2018). Metabolomics-based mechanisms exploration of Huang-Lian Jie-Du decoction on cerebral ischemia via UPLC-Q-TOF/MS analysis on rat serum. J. Ethnopharmacol. 216, 147–156. 10.1016/j.jep.2018.01.015 [DOI] [PubMed] [Google Scholar]
- Zorov D. B., Juhaszova M., Sollott S. J. (2014). Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 94, 909–950. 10.1152/physrev.00026.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.