Abstract
Direct‐acting antivirals for hepatitis C virus (HCV) are highly effective and well‐tolerated. However, only a small percentage of HCV‐infected individuals globally have received therapy. Reducing the complexity of monitoring during HCV therapy, if shown to be safe, could facilitate greater access to HCV services, particularly in resource‐limited settings such as sub‐Saharan Africa. We enrolled a total of 300 patients who were chronically infected with genotype 4 HCV in Rwanda and treated them with fixed‐dose ledispasvir/sofosbuvir for 12 weeks. For 60 consecutive participants enrolled, we blinded the study clinician to on‐treatment laboratory results. We compared the efficacy, safety, and tolerability in those with blinded laboratory results to those with standard laboratory monitoring. Baseline characteristics among those with blinded laboratory values were comparable to those with standard monitoring. Among both groups, the median age was 63 years, and the median HCV viral load was 5.9 log (versus 64 years and 6.0 log, respectively). Sustained virologic response rates at 12 weeks after treatment completion were similar in those with blinded laboratories (87%) compared to those with standard laboratory monitoring (87%). There was no increase in adverse events in those with blinded laboratory results, and no participants discontinued the study medication because of an adverse event. Conclusion: On‐treatment laboratory monitoring did not improve patient outcomes in those treated with ledispasvir/sofosbuvir. Eliminating this monitoring in treatment programs in resource‐limited settings may facilitate and accelerate scale‐up of HCV therapy.
With DAA therapy for HCV‐infected individuals in Rwanda, eliminating on‐treatment laboratory monitoring was safe. There was no difference in safety, tolerability, or efficacy in those participants who did not receive on‐treatment monitoring than in those who did.

Abbreviations
- AE
adverse event
- AST
aspartate aminotransferase
- CBC
complete blood count
- CI
confidence interval
- DAA
direct‐acting antiviral
- EASL
European Association for the Study of Liver
- GT
genotype
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- LDV
ledipasvir
- LMIC
low‐middle‐income country
- SOF
sofosbuvir
- SSA
sub‐Saharan Africa
- SVR
sustained virologic response
An estimated 10 of the 71 million hepatitis C virus (HCV)‐infected individuals globally live in sub‐Saharan Africa (SSA).1 Despite significant price reductions and increased availability of direct‐acting antiviral (DAA) medications, fewer than 1% of those infected with HCV in SSA have received antiviral treatment.2 Several factors account for the very low treatment rate, including low rates of HCV case findings, limited funding for diagnostics and treatment, undertrained personnel, and the lack of simple, evidence‐based treatment protocols that can be implemented in non‐specialty centers.
Clinical trials and real‐life experiences in regions such as North America and Europe demonstrate high rates of efficacy and low rates of treatment‐related adverse effects with DAA‐containing regimens.3, 4, 5, 6, 7 In clinical trials investigating ledipasvir (LDV)/sofosbuvir (SOF), grazoprevir/elbasvir, glecaprevir/pibrentasvir, or velpatasvir/SOF, sustained virologic response (SVR) rates are in excess of 95%, and treatment discontinuations due to adverse events are less than 1%, albeit in individuals carefully screened for clinical trials.3, 4, 5, 6 This includes very low rates of clinically significant renal insufficiency, anemia, and transaminitis. These findings provide impetus for streamlining HCV treatment in order to scale up therapy in low‐income and low‐middle‐income countries (LMICs).
Despite these high levels of safety and efficacy with DAA therapy, on‐treatment laboratory monitoring to assess response to therapy and detect toxicity is frequently performed in routine clinical practice. On‐treatment laboratory testing increases the need for human resources (i.e., clinical, phlebotomy, and laboratory staff) and costs of health care. It was recently estimated that the cost of recommended on‐treatment lab monitoring is approximately equivalent to the cost of 1 month of DAA therapy at local prices in LMICs.8 In addition, repeat laboratory evaluations lead to increased patient discomfort and could decrease adherence to clinic visits, particularly among hard‐to‐reach and marginalized patient groups.
Here, we report the results of a prospective blinded study investigating the safety and efficacy of limited lab monitoring during HCV treatment conducted in an adult population in Rwanda, a country of 12 million inhabitants in Central Africa with an adult HCV antibody seroprevalence of approximately 3% and predominance of HCV genotype (GT) 4.9, 10
Patients and Methods
Study Design and Participants
The SHARED study (“Simplifying Hepatitis C Antiviral Treatment in Rwanda for Elsewhere in the Developing World”) was a single‐arm prospective study (n = 300) evaluating the antiviral efficacy, safety, and tolerability of LDV/SOF in adults with chronic HCV infection GT 1 or 4 in Rwanda.11 SHARED‐2 was a study embedded within SHARED that evaluated the safety and efficacy of a limited lab‐monitoring schedule in 60 consecutively enrolled participants (Fig. 1). Here we report the results of SHARED‐2 and compare the results to the 240 SHARED‐1 participants who had guideline‐based on‐treatment lab monitoring during the study.
Figure 1.
SHARED study design: Allocation of participants to SHARED‐1 Versus SHARED‐2.
SHARED participants were recruited from four HCV treatment centers in Rwanda and treated at a single study site (Rwanda Military Hospital, Kigali, Rwanda). Eligible participants were 18 years or older with chronic HCV GT 1 or 4 infection with a serum HCV RNA ≥ 1,000 IU/mL. Participants were required to have a baseline hemoglobin ≥ 8.0 g/dL; platelets ≥ 40,000/mm3; aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase ≤ 10 times the upper limit of normal; and a calculated creatinine clearance ≥ 30 mL/minute, as estimated by the Cockcroft‐Gault equation.
Exclusion criteria included current or a history of decompensated liver disease, active tuberculosis, positive hepatitis B surface antigen, hepatocellular carcinoma, prior DAA therapy (prior interferon‐based therapy allowed), active drug or alcohol abuse, and pregnancy. Individuals with human immunodeficiency virus (HIV) co‐infection were eligible for the study if the participant was on stable antiretroviral therapy compatible with LDV/SOF and the HIV RNA level was < 200 copies/mL with a clusters of differentiation 4 (CD4) + T‐cell count ≥ 100 cells/µL.
Study Procedures
Screening evaluations included testing for plasma HCV‐RNA level and GT, HIV antibody, hepatitis B surface antigen, a right upper quadrant ultrasound, a complete blood count (CBC), a comprehensive metabolic panel (CMP), and a complete physical examination.
At entry, participants received a fixed‐dose combination tablet containing 90 mg of LDV and 400 mg of SOF administered orally once daily for 12 weeks. Social workers provided pretreatment adherence counseling and directly observed the administration of the first study drug dose.
During on‐treatment visits at weeks 1, 4, 8, and 12, participants had a clinical evaluation including a targeted physical examination based on participant symptom report. At weeks 4 and 8, pill counts were performed and adherence counseling was conducted, as needed, at all study visits.
We collected blood for HCV‐RNA levels at weeks 4, 12, and 24 and a CBC and CMP at weeks 4, 8, and 12, consistent with the American Association for the Study of Liver Disease (AASLD) guidelines at the time of study initiation in 2015.12 However, study personnel involved in the care of SHARED‐2 participants did not have access to any of the on‐treatment laboratory results (i.e., laboratory results from weeks 4, 8, and 12). The on‐treatment laboratory results were only accessible by the independent lab monitor. The results were then entered into a limited‐access field of the study database for later analysis.
To ensure participant safety, the independent lab monitor released laboratory results to the study clinician in the event of a new grade 3 or 4 laboratory adverse event (AE), according to the DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (version 2.1),13 if the week‐4 HCV viral load declined less than 2 log10 from entry, or if the study clinician requested a laboratory result based on participant signs or symptoms. For new grade‐3 or grade‐4 laboratory AEs, the laboratory test was repeated as soon as possible and preferably within 10 days of the initial blood draw.
Plasma HCV‐RNA levels were determined by the RealTime HCV Assay (Abbott, Chicago, IL) with a lower limit of detection of 30 IU/mL. For entry purposes, HCV GT was determined by the HCV Genotype II Amplification Reagent Kit (Abbott). To assess for baseline fibrosis, we calculated the AST‐to‐platelet ratio index (APRI) score using screening AST and platelet values, and Fibrosis‐4 using values for AST, age, platelets, and ALT.14 We performed PCR amplification of the NS3/4A, NS5A, or/and NS5B regions to assign the HCV GT and subtype.15, 16
Study Endpoints
The primary efficacy outcome was the proportion of participants achieving SVR12 (i.e., those with no quantifiable HCV RNA in plasma 12 weeks after the end of study treatment). The primary safety outcome was the proportion of participants with a grade 3 or 4 clinical AE. The primary tolerability outcome was the proportion of participants discontinuing study medication due to an AE. We determined the proportion of participants experiencing a serious AE (i.e., death or an event leading to hospitalization or long‐term disability) and those experiencing a clinical AE of any grade. We also determined the percentage of participants who required unblinding of laboratory results by the independent laboratory monitor due to a laboratory AE or inadequate HCV viral load decline, and the percentage of participants who the study clinician requested unblinding of laboratory results and the subsequent course of the clinical event.
Statistical Analysis
The target sample size of 60 for SHARED‐2 was based on feasibility. We calculated the proportion of SHARED‐2 participants meeting primary efficacy, tolerability, and safety endpoints and requiring laboratory result unblinding and the 95% confidence around these estimates using the Wald approximation. We performed univariate analysis to determine the association between GT‐4 subtype and lack of SVR12. We compared the proportions of SHARED‐2 participants meeting efficacy, tolerability, and safety endpoints to those proportions in SHARED‐1 participants. We conducted all statistical analyses in STATA 15.1 (College Station, TX). The study is registered at http://ClinicalTrials.gov (NCT02964091).
Ethics
The study was approved by the Rwanda National Ethics Committee (Protocol No. 172/RNEC/2017; Kigali, Rwanda), National Health Research Committee (Kigali, Rwanda), Inshuti Mu Buzima Research Committee (Rwinkwavu, Rwanda), Partners Human Research Committee (Boston, MA), and Stanford Institutional Review Board (Stanford, CA). All participants provided written informed consent, and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Role of the Funding Source
Gilead Sciences provided funding and the study medication. They were allowed to provide input on the study design, protocol, and manuscript, but had no role in data analysis or interpretation. All authors had full access to all study data; the first author drafted the first version of the manuscript and had final responsibility for submission.
Results
Baseline Characteristics
A total of 60 participants were enrolled during April and May 2017 into SHARED‐2. Baseline characteristics of those participants enrolled in SHARED‐2 were similar to the 240 participants enrolled in SHARED‐1 (Table 1). Seventy‐two percent of SHARED‐2 participants were female, and the median age was 63 years. A total of 4 participants were co‐infected with HIV. Thirteen participants had cirrhosis, as indicated by an APRI score of less than 1.0.
Table 1.
Baseline Characteristics of SHARED‐2 Versus SHARED‐1 Participants
| SHARED‐2 (n = 60) | SHARED‐1 (n = 240) | |
|---|---|---|
| Median age, years (IQR) | 63 (54, 69) | 64 (55, 74) |
| Female, n (%) | 43 (72%) | 143 (60%) |
| Median body mass index, kg/m2 (IQR) | 23 (20, 26) | 23 (20, 26) |
| Primary education or less, n (%) | 36 (60%) | 150 (63%) |
| Unemployed, n (%) | 35 (58%) | 157 (65%) |
| Income < $120/month, n (%) | 53 (88%) | 193 (80%) |
| HIV co‐infection, n (%) | 4 (7%) | 25 (10%) |
| Previous HCV treatment, n (%) | 1 (1%) | 2 (1%) |
| Median HCV RNA, log10 IU/mL (IQR) | 5.9 (5.5, 6.2) | 6.0 (5.6, 6.4) |
| Albumin < 3.5 g/dL, n (%) | 6 (10%) | 17 (7%) |
| Platelet count < 90,000 mm3 | 3 (5%) | 10 (4%) |
| APRI > 1.0, n (%) | 13 (22%) | 68 (23%) |
| Fibrosis‐4 > 3.25, n (%) | 10 (17%) | 60 (25%) |
| HCV GT 4, n (%) | 60 (100%) | 240 (100%) |
| 4k | 31(52%) | 103 (43%) |
| 4r | 6 (10%) | 42 (18%) |
| 4v | 5 (8%) | 21 (9%) |
| 4q | 4 (7%) | 37 (15%) |
| Untypeable | 5 (8%) | 18 (8%) |
| Others (4a, 4b, 4c, 4d, 4g, 4l, mixed) | 9 (15%) | 19 (8%) |
Abbreviation: IQR, interquartile range.
The median HCV viral load was 5.9 log10. Viral sequencing indicated that all participants had GT 4. The predominant subtypes were 4k (n = 31; 52%), 4r (n = 6; 10%), and 4v (n = 5; 8%). Five isolates (8%) were unable to be subtyped (Table 1).
Efficacy
All SHARED‐2 participants completed the study and were evaluable for the primary efficacy endpoint. A total of 52 participants (87%; 95% confidence interval [CI]: 80, 96; Fig. 2) achieved SVR12, similar to the SVR12 rate in the participants who had routine on‐treatment laboratory monitoring in SHARED‐1 (209 of 240; 87%; 95% CI: 82, 91).
Figure 2.
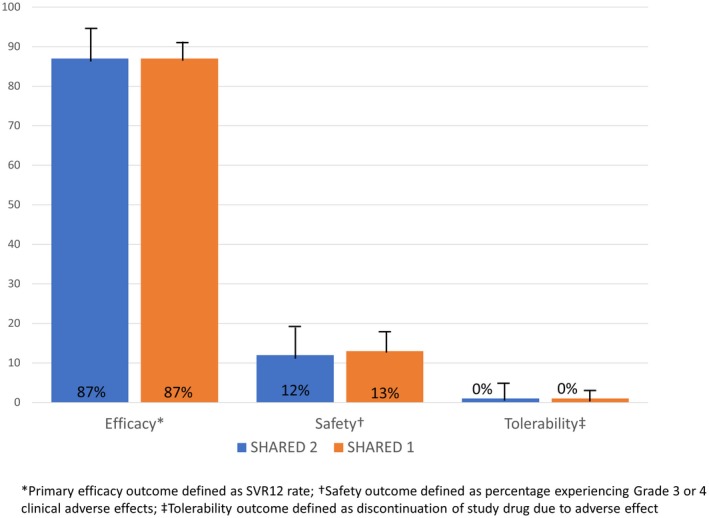
Primary efficacy, tolerability, and safety outcomes for SHARED‐2 Versus SHARED‐1 participants.
All eight failures in SHARED‐2 participants represented relapses (i.e., an unquantifiable viral load at the end of treatment followed by a quantifiable viral load at week 24). SHARED‐2 participants with subtype 4r had a lower SVR12 rate compared to those without subtype 4r (50% vs. 91%; P = 0.027). All 8 participants who failed to achieve SVR12 had adherence levels of over 95%, based on pill count.
Safety, Tolerability, and Adherence
A total of 7 SHARED‐2 participants (12%; 95% CI: 4, 20; Fig. 2) experienced grade 3 AEs, similar to the 13% (95% CI: 9, 18) of SHARED‐1 participants who experienced grade 3 or 4 AEs. There were no grade 4 AEs or deaths in SHARED‐2 participants during the study. The grade 3 AEs among SHARED‐2 participants consisted of hypertension (6), insomnia (1), weakness (1), and hyperglycemia (1) with none of the AEs judged to be related to the study drug.
AEs of any grade were common, with 90% of SHARED‐2 participants reporting at least one AE during the study period (similar to the 89% of SHARED‐1 participants with an AE of any grade). The most common AEs of any grade reported by SHARED‐2 participants were fatigue/weakness (n = 25), abdominal pain (n = 24), headache (n = 23), hypertension (n = 20), and dizziness (n = 15).
LDV/SOF was well‐tolerated with no treatment discontinuations due to AEs in SHARED‐2 participants (0%; 95% CI: 0, 6; Fig. 2) or in SHARED‐1 participants (0%; 95% CI: 0, 2).
Among the 58 SHARED‐2 participants who returned pill bottles at week 4 and week 8, 51 (88%) had adherence levels of 100%, 3 (5%) had adherence levels between 95% and 100%, and 4 (7%) between 90% and 95%. There was no association between adherence levels and achieving SVR12 (P = 0.56).
Requirements for Unblinding and Requests from Study Clinician for Lab Results
Among the 60 SHARED‐2 participants, 5 participants (8%; 95% CI: 1, 15) had abnormal laboratory results that met the criteria for release to the study clinician. For three of the unblinding events, the grade 3 hypernatremia results were reported on the same day and none of the participants exhibited signs or symptoms of dehydration. Without intervention, the sodium levels in these 3 participants were within normal levels on redraw.
One participant had her week‐4 HCV viral‐load result released due to a lack of a 2 log decrease. The participant did not bring in her pill bottle for the study visit, but the participant reported poor adherence. The importance of adherence was reinforced prior to the availability of the laboratory result, and the participant subsequently achieved SVR12.
One participant had asymptomatic grade 3 hyponatremia at week 4 with weight gain and increased lower extremity edema. The participant continued her routine diuretic medication, and the hyponatremia resolved at the next study visit without additional intervention.
For 1 participant (2%; 95% CI: 0, 9), the study clinician asked for the release of the week‐4 CBC due to a clinical suspicion of abdominal infection. The white blood cell count was found to be normal and the participant's symptoms resolved without specific intervention.
Discussion
The high overall safety and efficacy profile of DAAs has dramatically improved HCV treatment outcomes and offers the potential for the public health implementation of HCV therapy globally, including in resource‐poor settings with widespread epidemics. The pace and progress of this global scale‐up, however, may be limited by the remaining complexities of treatment delivery in poorly equipped health systems with limited experience with DAA management. This study prospectively evaluates the safety and efficacy of DAAs with a simplified laboratory‐monitoring schedule in SSA. We found similar safety, efficacy, and tolerability results with LDV/SOF with the simplified laboratory monitoring compared to the results in those participants treated and monitored with a more intensive monitoring regimen.
Specifically, in SHARED‐2, the SVR12 rate was 87% compared with an 87% rate in the SHARED‐1 participants. Similarly, the rate of grade 3 and grade 4 AEs was similar in SHARED‐2 and SHARED‐1 participants (12% and 13%, respectively), with no participants in either study group discontinuing therapy due to AEs. None of the grade 3 AEs in SHARED‐2 participants were judged to be related to study medication, and most represented exacerbations of chronic medical conditions in individuals with limited prior access to medical care. Five of 60 participants had laboratory results that met study‐defined criteria for unblinding. However, the availability of these laboratory results did not appear to improve outcomes in these participants. In three of the cases, the results were likely lab errors and led to unnecessary evaluations and clinic visits.
Given the clinical data suggesting the high efficacy and safety of DAA therapy, international organizations have recently recommended more streamlined approaches to the laboratory monitoring of HCV treatment.14, 17, 18 The 2018 European Association for the Study of Liver (EASL) guideline update recommends measurement of HCV viral load, renal function (specifically for SOF‐containing regimens), and a hepatic function panel only prior to therapy and at 12 weeks following completion of therapy. In 2018, the World Health Organization (WHO) recommended a similar approach as EASL for LMICs with HCV viral load, renal function, hepatic function panel, and CBC prior to therapy and at 12 weeks following completion of treatment. It should be noted that EASL also endorses a simplified version of monitoring when using pan‐genotypic regimens, which recommends only a pretreatment assessment and considers the 12‐week end‐of‐treatment HCV viral load as optional. Similarly, the AASLD/Infectious Diseases of America guidance in their November 2019 update published a simplified algorithm for treatment‐naïve patients without cirrhosis, which no longer recommends on‐treatment laboratory monitoring in uncomplicated patients.
Consistent with the clinical trial data,19 the results of our study suggest a limited role for on‐treatment laboratory monitoring. Although 1 participant in our study was noted to have a suboptimal HCV viral load decline at week 4, poor adherence to therapy was detected on routine patient interview without the need for an HCV viral‐load measurement. Additionally, our findings support a limited role for monitoring of renal function, liver enzymes, and CBC during or after completion of the treatment course, given the absence of clinically significant AEs, including renal insufficiency, transaminitis, and anemia. Our lower‐than‐expected SVR12 response rate was found in both SHARED‐2 and SHARED‐1 participants and appeared to be caused by the prevalence of subtype 4r isolates, which have been found to have higher half‐maximal effective concentration to ledipasvir in vitro compared with other GT‐4 subtypes.20
A well‐accepted, simplified laboratory‐monitoring algorithm for DAA‐based HCV treatment could substantially lower the overall cost of HCV management and improve overall efficiencies for resource‐constrained health systems.21 These cost savings would further enhance the return on investment for HCV treatment, which has already been shown to be cost‐effective, and even cost‐saving when using generic DAAs.22 In LMICs where many direct costs of medications and laboratory exams are borne directly by the patient, such savings are significant and may improve affordability, access, adherence, and patient willingness to test and/or treat. Our study provides valuable evidence for a streamlined, safe, and effective protocol for HCV treatment tailored for LMICs.
Although more challenging to quantify, simplification of monitoring algorithms would also decrease requirements on laboratory infrastructure and health system staffing. Such efficiencies would facilitate decentralization of HCV management to lower‐level facilities (i.e., primary hospitals or health centers) and away from urban tertiary centers, which is a key step to promote access and coverage for HCV services and improving the HCV care cascade.23 There is evidence for improved cost‐effectiveness and delivery improvements with simplification of disease‐monitoring protocols, such as was the case with reduced CD4 monitoring in HIV in LMICs.24
Several clinical investigations used in this study, according to guidelines at the time of study initiation or due to study entry criteria, may warrant further adaptation or investigation. HCV genotype was assessed for all participants as entry criteria in the study for treatment with LDV/SOF, which is approved as a first‐line treatment only for GT 1 and GT 4. However, since the initiation of our study, several pan‐genotypic regimens have been approved and are now recommended for use in LMIC without consistent access to routine pretreatment genotyping, including SOF/daclatasvir, SOF/velpatasvir, and glecaprevir/pibrentasvir.15 Although affordability and access to some of these regimens remain limited, ongoing prequalification and generic manufacturing for several of these drugs is encouraging for increased access, thereby reducing the need for genotyping and further decreasing the cost of HCV treatment. In this study, abdominal ultrasound was performed prior to treatment initiation to exclude HCC. However, in clinical practice, it may be warranted to reserve abdominal ultrasound for patients with evidence of advanced fibrosis based on noninvasive markers. Algorithms for HCC screening may warrant specific investigation, particularly in highly endemic regions with limited treatment options for HCC, such as SSA.25 Additionally, participants in this study had research visits every 4 weeks while on treatment, which may have contributed to the high level of adherence seen in our study. The optimal frequency of clinic visits while on DAA therapy will likely need to be tailored depending on the clinical setting.
The generalizability of this study to support large test‐and‐treat public health programs may be limited as a result of entry criteria excluding patients with decompensated liver disease, renal insufficiency, pregnant women, and HBV co‐infection. Special monitoring considerations as outlined in major guidelines for these groups may prove beneficial, and a simplified approach may not be sufficient. Our treatment group also did not include active injection drug users, who may require additional adherence support and may benefit from additional services and more frequent clinical visits. Furthermore, we did not attempt to quantify the impact or severity of individual AEs on disability or quality of life, and therefore cannot provide direct comparison of cost‐effectiveness of different monitoring algorithms.
Overall, this study provides high‐quality evidence supporting simplification of on‐treatment laboratory monitoring for HCV in a LMIC. Our findings support updated simplified algorithms suggested by several expert HCV guidelines committees, and may suggest even further simplification by obviating the need for follow‐up testing for renal insufficiency or liver enzymes in patients with normal baseline values. Simplification of these algorithms would likely result in direct financial savings and efficiency gains to both patients and health systems, and facilitate increased coverage of HCV treatment services and improvement in the overall HCV care cascade.
Potential conflict of interest: P.G. and N.G. have received grant support from Gilead Sciences.
Financial Support: Gilead Sciences.
Clinical Trials Registration: The study is registered at http://ClinicalTrials.gov (NCT02964091).
References
- 1. Blach S, Zeuzem S, Manns M, Altraif I, Duberg AS, Muljono DH, et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017;2:161‐176. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Global Hepatitis Report 2017. World Health Organization; Available at: http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/. Published 2017. Accessed March 25, 2019. [Google Scholar]
- 3. Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014;370:1889‐1898. [DOI] [PubMed] [Google Scholar]
- 4. Zeuzem S, Ghalib R, Reddy KR, Pockros PJ, Ben Ari Z, Zhao Y, et al. Grazoprevir‐elbasvir combination therapy for treatment‐naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med 2015;163:1‐13. [DOI] [PubMed] [Google Scholar]
- 5. Zeuzem S, Foster GR, Wang S, Asatryan A, Gane E, Feld JJ, et al. Glecaprevir‐pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med 2018;378:354‐369. [DOI] [PubMed] [Google Scholar]
- 6. Feld JJ, Jacobson IM, Hézode C, Asselah T, Ruane PJ, Gruener N, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med 2015;373:2599‐2607. [DOI] [PubMed] [Google Scholar]
- 7. Terrault NA, Zeuzem S, Di Bisceglie AM, Lim JK, Pockros PJ, Frazier LM, et al. Effectiveness of ledipasvir‐sofosbuvir combination in patients with hepatitis C virus infection and factors associated with sustained virologic response. Gastroenterology 2016;151:1131‐1140.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goel A, Chen Q, Chhatwal J, Aggarwal R. Cost‐effectiveness of generic pan‐genotypic sofosbuvir/velpatasvir versus genotype‐dependent direct‐acting antivirals for hepatitis C treatment. J Gastroenterol Hepatol 2018;33:2029‐2036. [DOI] [PubMed] [Google Scholar]
- 9. Riou J, Aït Ahmed M, Blake A, Vozlinsky S, Brichler S, Eholié S, et al. Hepatitis C virus seroprevalence in adults in Africa: a systematic review and meta‐analysis. J Viral Hepat 2016;23:244‐255. [DOI] [PubMed] [Google Scholar]
- 10. Gupta N, Kabahizi J, Mukabatsinda C, Walker TD, Musabeyezu E, Kiromera A, et al. “Waiting for DAAs”: a retrospective chart review of patients with untreated hepatitis C in Rwanda. PLoS ONE 2017;12:e0174148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta N, Mbituyumuremyi A, Kabahizi J, Ntaganda F, Muvunyi CM, Shumbusho F, et al. Treatment of chronic hepatitis C virus infection in Rwanda with ledipasvir‐sofosbuvir (SHARED): a single‐arm trial. Lancet Gastroenterol Hepatol 2019;4:119‐126. [DOI] [PubMed] [Google Scholar]
- 12. American Association for the Study of Liver Diseases, Infectious Diseases Society of America . Recommendations for Testing, Managing, and Treating Hepatitis C. Available at: http://www.hcvguidelines.org/full-report-view. Published March 18, 2013. Accessed May 28, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Division of AIDS . Table for Grading the Severity of Adult and Pediatric Adverse Events. Available at: http://www.niaid.nih.gov/LabsAndResources/resources/DAIDSClinRsrch/Documents/daidsaegradingtable.pdf. Published December 28, 2004. Accessed February 2, 2015. [Google Scholar]
- 14. Guidelines for the Care and Treatment of Persons Diagnosed With Chronic Hepatitis C Virus Infection. Available at: http://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2018/en/. Published July, 2018. Accessed March 25, 2019. [PubMed] [Google Scholar]
- 15. Hedskog C, Chodavarapu K, Ku KS, Xu S, Martin R, Miller MD, et al. Genotype‐and subtype‐independent full‐genome sequencing assay for hepatitis C virus. J Clin Microbiol 2015;53:2049‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI‐BLAST: a new generation of protein database search programs. Nucleic Acids Res 1997;25:3389‐3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. AASLD HCV Treatment Guidelines. Available at http://www.hcvguidelines.org. Accessed March 25, 2019. [Google Scholar]
- 18. European Association for the Study of the Liver . EASL recommendations on treatment of Hepatitis C 2018. J Hepatol 2018;69:461‐511. [DOI] [PubMed] [Google Scholar]
- 19. Falade‐Nwulia O, Suarez‐Cuervo C, Nelson DR, Nelson DR, Fried MW, Segal JB, et al. Oral direct‐acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med 2017;166:637‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Camus G, Han B, Asselah T, Hsieh D, Dvory‐Sobol H, Lu J, et al. Resistance characterization of ledipasvir and velpatasvir in hepatitis C virus genotype 4. J Viral Hepat 2018;25:134‐143. [DOI] [PubMed] [Google Scholar]
- 21. Ford N, Singh K, Cooke GS, Mills EJ, von Schoen‐Angerer T, Kamarulzaman A, et al. Expanding access to treatment for hepatitis C in resource‐limited settings: lessons from HIV/AIDS. Clin Infect Dis 2012;54:1465‐1472. [DOI] [PubMed] [Google Scholar]
- 22. Aggarwal R, Chen Q, Goel A, Seguy N, Pendse R, Ayer T, et al. Cost‐effectiveness of hepatitis C treatment using generic direct‐acting antivirals available in India. PLoS One 2017;12:e0176503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clinton Health Access Initiative . HCV Diagnostics Market Intelligence Report 2017. Available at: https://clintonhealthaccess.org/content/uploads/2018/04/HCV-Diagnostics-Market-Intelligence-Report_2018.pdf. Published April 23, 2018. Accessed February 23, 2019. [Google Scholar]
- 24. Ouattara EN, Robine M, Eholié SP, MacLean RL, Moh R, Losina E, et al. Laboratory monitoring of antiretroviral therapy for HIV infection: cost‐effectiveness and budget impact of current and novel strategies. Clin Infect Dis 2016;62:1454‐1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zakharia K, Luther CA, Alsabbak H, Roberts LR. Hepatocellular carcinoma: epidemiology, pathogenesis and surveillance—implications for sub‐Saharan Africa. S Afr Med J 2018;108:35‐40. [DOI] [PubMed] [Google Scholar]


