Abstract
IgG glycosylation is currently at the forefront of both immunology and glycobiology, likely due in part to the widespread and growing use of antibodies as drugs. For over four decades, it has been recognized that the conserved N-linked glycan on asparagine 297 found within the second Ig domain of the heavy chain (CH2) that helps to comprise Fc region of IgG plays a special role in IgG structure and function. Changes in galactosylation, fucosylation and sialylation are now well-established factors, which drive differential IgG function, ranging from inhibitory/anti-inflammatory to activating complement and promoting antibody-dependent cellular cytotoxicity. Thus, if we are to truly understand how to design and deploy antibody-based drugs with maximal efficacy and evaluate proper vaccine responses from a protective and functional perspective, a deep understanding of IgG glycosylation is essential. This article is intended to provide a comprehensive review of the IgG glycosylation field and the impact glycans have on IgG function, beginning with the earliest findings over 40 years ago, in order to provide a robust foundation for moving forward.
Keywords: glycan, glycosylation, IgG, sialylation
The historical context
The earliest paper clearly linking IgG glycosylation with IgG biology was published in 1976, in which it was reported that four patients with variable agammaglobulinemia could not glycosylate their IgG heavy chains, leading to very low circulatory IgG concentrations (Ciccimarra et al. 1976). Although those findings were later refuted by another study that reported these patients could glycosylate their IgG (Schwaber and Rosen 1984), this initial study appears to have drawn attention to an otherwise overlooked aspect of IgG structure and function.
A thorough review of the literature on IgG glycosylation clearly shows two overlapping eras, with the first beginning in the 1970s. During this period, the focus was primarily on the impact of inflammation and disease on IgG glycosylation, and the functional impact of those changes. Indeed, most of what we know about the proinflammatory glycoforms of IgG came from this era, and thus these studies represent many of the fundamentals we now understand. Not only does inflammation correlate with decreased galactosylation, increased fucosylation and increased bisecting GlcNAc, but changes in Fc glycans also change both the structural integrity and conformation of the Fc domain, as well as the affinity to Fcγ receptors (FcγRs). As such, it was during this period that the relationship between IgG glycosylation, inflammation and function was largely mapped.
The second era of IgG glycosylation work remains ongoing and can be roughly traced back to a Science article in 2006 (Kaneko et al. 2006). In that study, Jeffrey Ravetch’s laboratory reported that terminal α2,6-sialylation of IgG N-glycans accounted for the anti-inflammatory activity of high-dose intravenous immunoglobulin (i.e., IgG) therapy for rheumatoid arthritis (RA) (Kaneko et al. 2006). Despite many of the fundamentals on the proinflammatory effects of IgG glycosylation having been worked out prior to this article, it is clear that the discovery of IgG sialylation being a key to the inhibitory effects of IgG has generated much interest. Indeed, approximately one-third of articles on IgG glycosylation date from 1976 to 2006, a 30-year span, while the other two-thirds have been published since 2006.
In this review, both eras of research are discussed in an effort to provide a detailed summary of where the field was, is and potentially will be, with an eye toward laying the foundation for new paths forward in exploring the potential of using the glycome as the next medicinal frontier.
Antibody structure and function
Antibodies are at the heart of adaptive immunity, biomedical research and modern pharmaceutical development. In vivo, their activity can be broken into a number of categories, which include antigen neutralization, promotion of phagocytosis and microbial killing via opsonization and macrophage activation, fixation of complement and induction of antibody-dependent cellular cytotoxicity. The combination of these activities with a high level of antigen specificity is why IgG is the desired outcome and measure of vaccine efficacy. Antibodies are also ubiquitous laboratory tools found across all biomedical fields through their use in immunohistochemistry, confocal microscopy, western blotting, immunoprecipitations, ELISA and other detection technologies, as well as tools for in vivo cellular depletion, cell sorting and purification. Since the release of the first monoclonal antibody (mAb) drug Orthoclone OKT3 in 1986 (Liu 2014), and the subsequent success of Rituxan (rituximab) released in 1997 (Scott 1998), there has been an explosion of antibodies as drugs both in the clinic and the current pharmaceutical pipeline. The reason for this is manifold, but these drugs are now among the top revenue generators for the industry. For example, Humira, a tumor necrosis factor α (TNFα)-neutralizing antibody therapy for autoimmunity, has earned AbbVie approximately $115 billion dollars to date (https://www.axios.com/abbvie-humira-2018-sales-20-billion-e4039176-baeb-44ff-b4fe-1b63005283b9.html). With this central place in biomedical science, understanding the variables underlying IgG function is not only big business, it is absolutely critical for efficacy.
Conventional immunology states that the function of IgG is driven by the fragment crystallizable (Fc) domain, which is encoded solely by the γ heavy chain, and comprised of two constant Ig domains, CH1 and CH2. While this domain is not responsible for antigen specificity—that is under the purview of the Fab domain, which includes the variable regions—it is the point of contact for all of the FcγR molecules. As a result, changes in structure of the Fc domain would likely have a profound impact on receptor interactions. Within CH2, there is a universally conserved site of N-linked glycosylation, asparagine 297 (N297). This site predominantly carries complex-type biantennary glycans with varied amounts of core fucose (Fuc), galactose (Gal), bisecting GlcNAc and terminal N-acetylneuraminic acid (Neu5Ac/sialic acid) residues (Figure 1). Importantly, these glycans fill a large pocket between the two copies of the CH2 domains, as described later and shown in Figure 2.
Fig. 1.
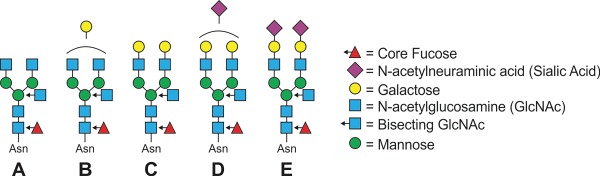
IgG glycans. Schematic showing common glycoforms found on mouse and human IgG molecules. These are typically biantennary with (A) terminal GlcNAcs, (B) monogalactosylated, (C) digalactosylated, (D) one sialic acid or (E) two sialic acids, and each of these with or without core fucose and bisecting GlcNAc. Other glycoforms are known to exist, but these are the major classes. (This figure is available in black and white in print and in colour at Glycobiology online.)
Fig. 2.
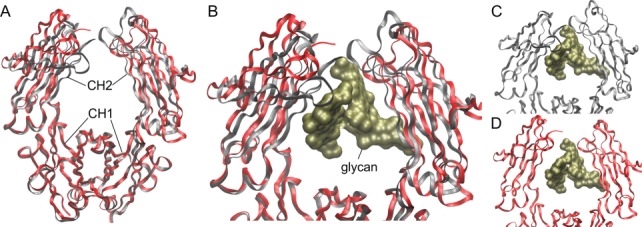
Structural impact of α2,6-sialylation on IgG Fc. Crystal structures of a normally glycosylated (nonenriched) wild-type Fc domain [PDB: 4Q7D] (red ribbons) compared to the structure of the same Fc carrying fully sialylated N-glycans [PDB: 4Q6Y] (gray ribbons), demonstrating the difference in conformation. (A) A broad view of the full structure highlights the close similarity of the CH1 domains but the divergence in conformation and position of the CH2 domains. (B) A closer view of the two structures, including a molecular surface rendering of the glycans (gold) from the disialylated Fc structure (gray). (C) The complete structure of the Fc with disialylated glycans. (D) The structure of the wild-type Fc with the glycan from the disialylated Fc structure to illustrate the change in the CH2 domain position relative to the glycan. (This figure is available in black and white in print and in colour at Glycobiology online.)
In the beginning
In 1976, an article appeared in the Journal of Clinical Investigation in which four patients with variable agammaglobulinemia were described (Ciccimarra et al. 1976). Their low IgG concentrations were attributed to an inability to glycosylate IgG, as measured by ex vivo culturing primary B cells from each patient in the presence of tritiated mannose and glucosamine. Released IgG was reportedly absent any carbohydrate label, leading to the conclusion that these patients did not glycosylate IgG at all. Eight years later, it was found that these patients were not deficient in IgG glycosylation (Schwaber and Rosen 1984), but this early study appears to have sparked interest in the role of IgG glycans on function.
Not long after this initial publication, it was reported that tunicamycin, an inhibitor of the oligosaccharyltransferase (OST) responsible for initiating glycosylation on nascent glycoproteins in the endoplasmic reticulum, reduced IgG plasma cell secretion of IgG by 28% and IgM by 81% (Hickman and Kornfeld 1978). Several years later, it was discovered that while tunicamycin eliminated IgG glycosylation, tyrosine sulfation was dramatically increased on the heavy chain, perhaps as a functional compensatory posttranslational mechanism to promote secretion (Baeuerle and Huttner 1984).
In the same year, Dr. Thomas Rademacher and team discovered that changes in glycosylation of IgG were associated with rheumatoid and osteoarthritis arthritis (RA and OA, respectively) (Parekh et al. 1985). In a cohort of patients from both Oxford and Tokyo, they reported glycan changes that continue to hold up to modern day scrutiny. Importantly, they did not find novel N-glycans appearing in RA/OA samples, but instead found that the relative abundance of the glycans present changed with disease compared to the healthy controls. The primary difference was a reduction in glycans with galactose and a concomitant increase in glycans terminating in GlcNAc, although reductions in sialylated species were also apparent within the Oxford portion of the cohort (Parekh et al. 1985). Galactosylation changes were also found in juvenile onset RA (Parekh et al. 1988a). These data are the foundation upon which the general, widely accepted and often repeated notion that decreased IgG galactosylation is a sign of inflammation is founded.
Consistent with this report, in 1987, IgG from RA patients was analyzed using lectins. It was found that IgG from RA patients bound more to ConA compared to normal patients, indicating a greater prevalence of hybrid and high mannose N-glycans associated with inflammatory disease (Malaise et al. 1987). In that same year, the galactosyltransferase activity from circulating B cells was found to be significantly reduced in RA patients (Axford et al. 1987). This was later replicated in RA-prone MRL-lpr/lpr mice, in that the galactosyltransferase B4GalT1 was decreased in association with RA development (Axford et al. 1994; Jeddi et al. 1994) and IgG glycans had reduced galactosylation (Bond et al. 1990; Mizuochi et al. 1990).
Another major step forward for the field occurred in 1988. The basic foundations of the study of another RA cohort duplicated prior findings, including the RA association with a relative loss of galactose and gain of terminal GlcNAc, without change in fucosylation (Pekelharing et al. 1988). However, during pregnancy, they found a reversal of this trend, whereby the IgG contained more galactose and sialic acid than IgG from nonpregnant females, while fucose, GlcNAc and total carbohydrate content was unchanged. This is significant because during pregnancy, women with RA often go into remission, and this study was the first to suggest that not only does pregnancy shift IgG glycosylation but that a shift to more galactose and sialic acid may be immunosuppressive, thereby temporarily limiting autoimmunity (Pekelharing et al. 1988). This idea was supported a few years later in a study with RA women and DBA/1 mice (Rook et al. 1991), which revealed loss of galactosylation with disease, reversal of those changes during pregnancy and recurrence after pregnancy. And much like the MRL-lpr/lpr mouse studies that came later (Axford et al. 1994; Jeddi et al. 1994), these glycan alterations were present in DBA/1 mice with collagen-induced arthritis (CIA). Mice with CIA had reduced galactosylation and pregnant females showed increased IgG galactosylation. Remarkably, this increased galactosylation was not observed in pseudo-pregnant mice (Rook et al. 1991). Collectively, these data demonstrate that IgG glycosylation is dynamic and regulated with respect to inflammation and autoimmunity.
While this early work laid the foundation, a focus on RA continues even today. With increasing technological innovations in glycomics and a greater understanding of the immune system, more and more detail has been revealed. For example, using two strains of mice, one susceptible to pristane-induced arthritis (DBA/1) and one resistant (CBA/Igb), it was discovered that both strains had strongly elevated IL-6 and both showed increased agalactosyl IgG (Hitsumoto et al. 1992). These data suggested that IL-6 may be a player in the mechanism leading to the change in glycoforms of IgG. Consistent with this interpretation, the kinetics of IL-6 production parallels the kinetics of agalactosyl IgG release in this system (Thompson et al. 1992).
Large cohort studies have also robustly confirmed that during pregnancy, when many female RA patients enter remission, galactosylation and sialylation are increased and are highly correlated, but then decrease significantly during postpartum relapse (van de Geijn et al. 2009). Interestingly, a study in 1995 showed that while women with RA have increased IgG galactosylation associated with pregnancy-associated remission, the ratio of agalactosyl-IgG to galactosylated IgG was notably higher in the fetus, suggesting that galactosylated (presumably noninflammatory) IgG is particularly good at crossing the placental barrier (Williams et al. 1995). However, nearly 20 years later, a study comparing fetal and maternal Fc glycans found similar levels of galactosylation, sialylation, bisecting GlcNAc and fucosylation (Einarsdottir et al. 2013). The reason for this discrepancy remains unclear.
In a rather curious study in 1996, IgG glycosylation was monitored in RA patients who fasted for 7–10 days and then followed a strict vegetarian diet for the subsequent 3.5 months. The levels of agalactosylated IgG went down during the fasting, and this was correlated with improved disease presentation (Kjeldsen-Kragh et al. 1996). This observation is interesting because it appears to link the overall metabolic state of the individual to IgG glycosylation.
Finally, it is important to note that it is clear that IgG fucosylation can also change in RA patients. Initially, individuals with juvenile chronic arthritis were studied, and it was found that IgG fucosylation was increased in those with disease (Flogel et al. 1998). This was extended the following year to adult RA patients, showing a 40% increase in IgG fucosylation compared to healthy controls (Gornik et al. 1999).
Galactosylation outside of RA
In 1988, the RA founded observation that IgG losses galactosylation with disease was expanded to include systemic lupus erythematosus (SLE) and Crohn’s disease (Tomana et al. 1988). This was the first time that changes in IgG glycosylation were implicated as a general feature of inflammation, rather than a peculiar change seen only in RA. Since then, a long list of circumstances, be they inflammation, infection, cancer or aging, has been correlated with decreased IgG galactosylation.
From an infection perspective, reduced IgG galactosylation has been found in Mycobacterium tuberculosis and leprosy patients (McCulloch et al. 1995). More recently, loss of galactosylated IgG was seen in both HIV+ (Moore et al. 2005) and HCV+ (Mehta et al. 2008) patients. From the autoimmune and inflammation perspective, IgG α2,6-sialylation and galactosylation is reduced among anti-PR3 (anti-proteinase 3) antibodies in Wegener’s granulomatosis with polyangiitis (GPA) and is correlated with disease severity (Espy et al. 2011), while Lambert-Eaton myasthenic syndrome and myasthenia gravis patients showed reductions in IgG galactosylation without changes in total sialylation (Selman et al. 2011). Nonalcoholic fatty liver disease patients also show similar changes, with decreased IgG galactosylation and increased fucose and bisecting GlcNAc (Zhao et al. 2018).
Cancer is another area in which IgG glycosylation is altered. In prostate cancer, the decrease in IgG galactosylation was found to strongly correlate with serum prostate-specific antigen (PSA) levels (Kanoh et al. 2004). Decreases in IgG galactosylation also occur in multiple myeloma (Aurer et al. 2007), stomach adenocarcinoma (Bones et al. 2010) and gastric cancer (Kodar et al. 2012).
In a study of 151 individuals ranging in age from 1 to 70 years, it was reported that the amount of GlcNAc-terminating IgG glycans decreased from birth to approximately 25 years of age, but then increased slowly thereafter (Parekh et al. 1988b). This change was mirrored in the degree of galactosylation, with increases up to 25 years of age and decreases after. This study was the first to suggest that the changes in galactosylation associated with chronic inflammatory conditions could potentially represent a premature aging of the immune system. In a somewhat more recent study, individuals over 65 years of age were found to have reduced α2,6-sialylation in IgG1 and IgG2 compared to young (0–19 years old) and adult (20–39 years old) groups (Keusch et al. 1996). Mirroring RA data, the geriatric group also had increased agalactosyl glycoforms in IgG2, 3 and 4 compared to healthy adults. This was supported 2 years later in women, which showed decreased galactosylation with age (Shikata et al. 1998).
The glycan influence on function
Thus far, our discussion of IgG glycosylation has focused upon the early identified changes, particularly galactosylation, seen associated with a variety of biological circumstances. While interesting, these trends leave open the possibility that they are nothing more than the consequence of the biological context and are therefore little more than broad inflammatory biomarkers. However, given the physical placement and conserved nature of the Fc N-glycan at N297, it is not difficult to imagine that changes in the size, composition and charge of the glycans could have a profound impact on structure and function of the Fc domain of IgG and therefore play an active role in determining optimal interaction partners and function.
Credit for the fundamental discovery that glycans actually change IgG function appears to go to Dr. Hans Wigzell. In 1983, Dr. Wigzell published an article in the PNAS in which a mAb was functionally compared to a nonglycosylated version of itself (Nose and Wigzell 1983). As with other early work, the nonglycosylated mAb was produced by culturing the hybridoma cells in tunicamycin. They found no change in antigen or protein A binding properties, but the ability to activate complement, associated with FcγR on cells, and the ability to induce antibody-dependent cellular cytotoxicity (ADCC) was lost. In addition, clearance of immune complexes in vivo was significantly reduced. Two years later, it was reported that mAbs known to be suppressive in vivo failed to suppress responses when they lacked glycosylation (Heyman et al. 1985), and that this function was independent of complement (Heyman et al. 1988), suggesting that the glycans can also promote anti-inflammatory functionality via FcγR engagement.
Mirroring this initial discovery, most of this early period focused on the impact of completely removing the N-glycans on Fc. For example, human IgG1 and IgG3 lacking N-glycans were found to not bind FcγRI and FcγRII (Walker et al. 1989). Similarly, rat IgG from tunicamycin-treated hybridomas showed Fc receptor binding deficiencies and reduced transportation from the gut lumen to blood (Peppard et al. 1989), and mutagenesis of N297 in a mouse-human chimeric IgG yielded greater sensitivity to proteases, failed to bind Fc gamma RI, failed to activate complement, and IgG3 showed a shorter serum half-life (Tao and Morrison 1989). Surface plasmon resonance (SPR) studies also confirmed alterations in affinity between deglycosylated Fc and FcγRs (Radaev and Sun 2001).
In more detailed studies in which differential glycoforms, not just the presence or absence of a glycan, were compared, other features began to emerge. IgG lacking either sialic acids or galactoses were reported to have weaker binding to FcγR (Adler et al. 1995). In 2001, it was reported that removal of galactose residues on the Fc glycan did not alter binding to FcγRIIB, but that removal of the GlcNAcs and mannoses significantly reduced binding (Mimura et al. 2001).
Since many IgG functions, albeit not all, are dependent upon FcγR binding and signaling, it is not surprising that these changes in binding affinity translate into functional differences (Nose and Wigzell 1983). IgG secreted from hybridomas treated with the glucosidase inhibitor castanospermine (which limits N-glycan processing to yield predominantly high mannose forms), but not the mannosidase inhibitor swainsonine (which permits N-glycan process into hybrid structures), had enhanced lymphocyte and NK cell-mediated ADCC activity. Yet, ADCC mediated by polymorphonuclear cells (PMNs) was reduced in IgG from swainsonine and tunicamycin, but not castanospermine-treated hybridomas (Rothman et al. 1989). These data suggested that IgG Fc glycans can differentially influence FcγR binding, and that the outcome may depend upon which receptor is present.
Recombinant mouse-human chimeric IgG1 produced in CHO Lec1 cells, which lack the ability to process high mannose N-glycans and therefore cannot synthesize complex-type N-glycans (Stanley and Chaney 1985), showed an inability to support complement-mediated hemolysis and was incapable of complement consumption, C1q binding and C1 activation (Wright and Morrison 1994). Moreover, this IgG1 molecule showed reduced FcγRI binding affinity and had a shorter half-life in vivo. Interestingly, the clearance enhancement could be overcome by injection with mannan, suggesting that the increased clearance was due to binding the mannosylated N-glycans on the IgG molecule. Then in the following year, exciting findings based on a combination of X-ray and NMR data were published showing that the agalactosylated IgG glycoforms with terminal GlcNAc residues are able to bind the mannose-binding lectin (MBP), and thus selectively activate the lectin-arm of complement (Malhotra et al. 1995).
These findings not only suggested that FcγR binding is dependent upon the conformation of the Fc domain, and that the glycans are important contributors to the structural integrity of IgG, but also revealed a potential mechanistic connection between the loss of galactose and inflammation through differential FcγR ligation and stimulation of the MBL/lectin pathway of complement.
Glycans and the Fc domain
With evidence pointing toward a potent structure–function effect of the N297 glycan on IgG, structural biologists began entering the field. It appears that the first structural detail of the Fc domain with the glycans resolved was in 1983 (Sutton and Phillips 1983). In that article, it was reported that the N-glycans at N297 formed a number of noncovalent bonds between amino acids within the CH2 domain and the glycans themselves. The mere fact that the glycans could be resolved at all is testimony to their structural confinement within the Fc domain. Indeed, many years later, it had become quite clear that the structural integrity (Krapp et al. 2003; Yamaguchi et al. 2006) and thermostability (Mimura et al. 2000) of the Fc domain were dependent upon the N-glycans.
Over a decade later, mutagenesis helped to further define the key carbohydrate–protein contacts, which appear to drive conformational states preferred by various FcγRs (Lund et al. 1995). Specifically, mutagenesis of K246, D249 and E258 in human IgG3, all of which form direct bonds with a GlcNAc and Gal on one branch of the glycan (Sutton and Phillips 1983), did not alter FcγRI or FcγRII binding. In contrast, mutagenesis of D265, which contacts the first GlcNAc within the N-glycan core, abolished binding. These observations were also duplicated in IgG2b (Lund et al. 1995), suggesting a conserved mechanism influencing overall Fc structure.
In a follow-up study, the same group showed that other mutations in the Fc CH2 region resulted in increased galactosylation and sialylation (Lund et al. 1996). As an example, mutagenesis of F243 to alanine allowed for dramatic increases in total sialylation, going from 4% to 73% sialylated. These data suggested that the ability to sialylate may be driven by accessibility of the glycans and the intramolecular interactions within the Fc domain, while also providing a potential way to increase IgG sialylation in antibody-based drugs.
Another study repeated some of these observations by showing that wild-type IgG3 mAb expressed in CHO cells had very little sialic acid (~3%), and that all of it was α2,3-linked, but mutagenesis of F243 resulted in robust α2,3-linked sialylation (~53%). The key advance, however, came with recombinant expression of ST6Gal1 in the CHO cells, which converted half of the sialic acid to α2,6-linkage. Remarkably, the α2,3-linked sialylated IgG3 had reduced recognition by FcγRI and FcγRII compared to wild-type IgG3, but the introduction of α2,6-linked sialic acids fully restored FcγR recognition and activation (Jassal et al. 2001). These data demonstrate that α2,3- and α2,6-linked sialic acids are not equivalent in terms of Fc structure and must be separated in order to understand the impact of IgG sialylation on function. Moreover, the degree of sialylation appears to involve accessibility to the termini of the glycans, which is directly impacted by the local protein conformation.
Translating the glycomics
If the IgG glycans change under inflammatory and other circumstances, and these changes alter the conformation and function of the Fc domain in a way that has a direct influence over their binding to receptors and immunologic environment, it stands to reason that targeting those glycans should alter the inflammatory course. In support of such a notion, and using a model of collagen antibody-induced arthritis, it was discovered that normally nonpathogenic autoantibodies could be converted into pathogenic antibodies through selective isolation of the IgG glycoforms lacking galactose (Rademacher et al. 1994), an IgG modification contemporaneously shown to have indistinguishable antigen binding and specificity (Donadel et al. 1994). This 1994 study was the first to directly demonstrate that specific glycoforms are not only associated with disease, but can be directly causative, thereby laying the ground work for things to come.
IgG glycans and antigen exposure/immunization
Inflammatory disease is not the only venue in which IgG is a central player. In an interesting 1998 study, it was found that moving mice reared in specific pathogen-free (SPF) environmental conditions into a more conventional environment elicited not only increases in total IgG but also in the amount of agalactosylated IgG. This suggests that the sudden exposure to new environmental/microbial antigens, potentially during vaccination, causes the same type of inflammatory shift in IgG glycosylation previously described in RA and other diseases (Parekh et al. 1985).
More directly relevant to vaccination, when mice were immunized with BSA, BSA-specific IgG titers dramatically increased, and these antibodies were predominantly agalactosylated (Lastra et al. 1998). During the immunization period, the other IgG (i.e., not BSA-specific) present in the mouse did not shift in glycoform. However, weeks later, the BSA-specific IgG glycoforms shifted to be more like the non-BSA-specific IgG glycans as the animals returned to a steady state weeks later (Lastra et al. 1998). These data demonstrate that antigen-specific IgG glycosylation can differ from the bulk IgG present in circulation during times of strong antigen-driven antibody production, but that this may simply be a function of what is being made at the moment in that environment.
A number of years later, a study was published in which the levels of fucose, galactose, mannose and sialic acids were measured on antigen-specific IgG after immunization and subsequent boosts.
It was reported that with each additional immunization boost, the resulting IgG molecules were increasingly fucosylated, but that the galactose, mannose and α2,6-linked sialic acid content were unchanged after serial boosting (Guo et al. 2005). It would be interesting to know the fate of these IgG molecules long after the final boost, or how they compared to the bulk IgG over time.
A new era
It should be clear at this point that a plurality of IgG glycosylation data focused on RA, in humans and mouse models, in which alterations in IgG glycans had been consistently seen across time and cohort demographics. These generally included the increase in agalactosylated and fucosylated glycans, as well as changes in the level of bisecting GlcNAc and sialylation. As such, it should not be surprising that the next major step in the field occurred in a mouse model of arthritis—the K/BxN serum-transfer model.
In 2006, the IgG glycosylation story remained focused on galactose and the proinflammatory nature of the agalactosyl glycoforms, but a report in Science significantly altered that narrative. In the laboratory of Dr. Jeffrey Ravetch, it was found that α2,6-sialylation of IgG was responsible for the anti-inflammatory activity of IVIg/IgG in autoimmune disease therapy of the K/BxN model of RA (Kaneko et al. 2006). This was recapitulated 2 years later, with the demonstration that the IVIg effect could be duplicated with isolated Fc domains preferentially harboring α2,6-sialylated glycans (Anthony et al. 2008a). This was the first time the IVIg effect had been shown to work without fully intact IgG, robustly demonstrating independence from antigen binding and immune complex formation.
Although changes in IgG glycosylation associated with disease, function, FcγR binding and structure were all reported previously, as reviewed here, these two initial IVIg studies (Anthony et al. 2008b; Kaneko et al. 2006) led to an explosion of work aimed at further understanding the relationship between IgG glycans and human health, as well as how to harness those observations in the design and deployment of antibody-based drugs.
Much of the IVIg data stems from the use of SNA, the Sambucus nigra agglutinin lectin, which binds to α2,6- but not α2,3-linked sialic acid-containing glycans, to enrich for sialylated IgG. This led to several studies focused upon the IgG glycoforms found in such preparations. In 2009, it was reported that most SNA binding to IgG was due to sialylation of Fab fragment-localized glycans, and that Fc-localized glycans required at least 2 sialic acids in order to associate with SNA (Stadlmann et al. 2009). Another report claimed that SNA enriched specifically for sialylated Fab-glycans, not sialylated Fc glycans (Guhr et al. 2011). However, the isolated Fc fragment study (Anthony et al. 2008a) combined with the observation that only about 30% of IgG molecules carry sites of N-glycosylation in the Fab domain argues that Fc glycan α2,6-linked sialylation, especially highly sialylated glycoforms, is potently anti-inflammatory in the K/BxN setting.
Outside of the K/BxN model, however, results have been mixed. IVIg therapy was effective at reducing platelet loss in a model of passive immune thrombocytopenia (PIT), but the SNA-enriched IgG had no protective effect at all, indicating that IgG α2,6-linked sialylation is not a protective determinant within IVIg in the PIT setting (Guhr et al. 2011). In another murine immune thrombocytopenia (ITP) model, α2,6-sialylated and desialylated IgG were found to work equally well in ameliorating the disease (Leontyev et al. 2012). IVIg-mediated reduction in autoimmune hemolytic anemia also was found to be dependent upon the galactosylation of IgG1, but not total sialylation (Yamada et al. 2013), and a recent study suggested that the clinical outcome in antibody-mediated transplant rejection patients was functionally independent of IgG α2,6-sialylation (Barba et al. 2019).
Conversely, in a study focused upon Guillain-Barre syndrome, which is often treated with IVIg, investigators reported that sialylated and galactosylated IgG were better at inhibiting C3 deposition compared to the original IVIg preparation or IgG lacking galactose (Sudo et al. 2014). It was also reported that desialylated immune complexes enhance osteoclastogenesis in RA, whereas sialylated IgG did not (Harre et al. 2015). This was extended in mice receiving N-acetylmannosamine (ManNAc), which is known to drive the production of the nucleotide-sugar CMP-sialic acid required for all sialyltransferases (Gu and Wang 1998). These mice showed increased total IgG sialylation and were less susceptible to inflammatory bone loss (Harre et al. 2015). Finally, in an SLE study, it was found that anti-histone IgG was poorly sialylated, but that the α2,6-sialylated fraction displayed reduced ability to promote phagocytosis of secondary necrotic cells by monocytes (Magorivska et al. 2016).
It was published in 2014 that increased galactosylation and total sialylation induces more robust antibody-dependent cellular phagocytosis (ADCP) (Chung et al. 2014). Analysis of antituberculosis IgG molecules from latently Mtb-infected individuals showed increased galactosylation and enhanced macrophage activation compared to actively infected individuals. However, inconsistencies with respect to the impact of sialylation of IgG exist for ADCC activity. In a 2015 PNAS article, it was reported that IgG with enhanced α2,6-sialylation was optimized for ADCC (Lin et al. 2015). However, later in that same year, it was shown that α2,6-IgG sialylation reduced complement-mediated cytotoxicity (CDC) (Quast et al. 2015) and that α2,6-sialylation of IgG1 had no significant impact on ADCC activity (Thomann et al. 2015).
Given the varied outcomes with sialylated IgG, it is not surprising that the mechanism driving immune inhibition remains a debate. Two reports proposed that the mechanism in K/BxN arthritis depends upon the sialylated Fc domain binding to the C-type lectin receptor DC-SIGN (Anthony et al. 2008a, 2011). This ligation was reported to induce the release of IL-33, triggering IL-4 production in basophils and the upregulation of the inhibitory FcγRIIB receptor on macrophages (Anthony et al. 2011). However, this mechanism remains somewhat controversial in that the ability of sialylated IgG/Fc to bind DC-SIGN has been directly challenged in two subsequent studies (Temming et al. 2019; Yu et al. 2013). The nature of the discrepancy remains unclear.
Another explanation for the α2,6-sialylation effect, which is not mutually exclusive with the proposed DC-SIGN pathway, is that α2,6-sialylation appears to substantially reduce the binding affinity to all of the FcγRs (Anthony et al. 2012). Also, structural studies comparing fully α2,6-sialylated Fc to singly and asialo-Fc revealed that the conformation of the CH2 domain is significantly different with sialylation (Ahmed et al. 2014) (Figure 2), supporting the differential FcγR binding properties. Indeed, Fc sialylation increases circulatory half-life of antibodies (Bas et al. 2019), likely by avoiding ligation by both the asialoglycoprotein receptor (ASGPR) in the liver and the neonatal Fc receptor FcRn.
Despite the progress in the field since 2006, the manner in which α2,6-sialylated IgG/Fc actively inhibits inflammation remains a topic of great interest and investigation. One reason is that sialylation clearly does not always inhibit inflammation or disease. The effect probably depends upon the context, the underlying disease mechanisms and other metabolic factors that we can only speculate about at the present time.
Core fucose and ADCC
In contrast to the sialylation effect, it is now well established that core fucosylation of IgG N-glycans, in which fucose is added α1,6 to the protein-adjacent GlcNAc of N-glycans, directly impacts IgG ADCC activity. The initial discovery was the demonstration that IgG molecules lacking fucose had enhanced ADCC via increased FcγRIIIA binding and signaling (Okazaki et al. 2004; Shields et al. 2002; Shinkawa et al. 2003). These data are receiving much more attention now that the IgG glycan field has grown.
In 2008, it was shown that low fucose levels on IgG enhanced mononuclear cell (e.g., NK cell)-mediated ADCC (Peipp et al. 2008). However, the same study also showed the opposite for PMN-mediated ADCC. For PMNs, high fucose on IgG enhanced ADCC (Peipp et al. 2008). These data suggest that core fucosylation impacts the association of the Fc domain with various FcγRs differently, raising the possibility of selectively targeting different effector cells for ADCC using engineered antibodies. Indeed, it is clear that decreasing core fucosylation on IgG increases the binding affinity between the Fc domain and FcγRIIIA. Comparing the crystal structures of fucosylated vs. nonfucosylated IgG in complex with FcγRIIIA revealed that the core fucose causes steric hindrance at the point of interaction (Mizushima et al. 2011). With these data, there is growing realization that fucose could significantly impact antibody-based cancer therapies, such as rituximab and trastuzumab, which are believed to rely upon ADCC for efficacy (Cartron et al. 2002; Weng and Levy 2003).
A move to high throughput
One of the limitations in the field of glycobiology has been the relative difficulty in determining glycan structure, which limits the number of samples and therefore statistical power associated with any trend being measured. If one desires a high level of detail, mass spectroscopy on many micrograms of isolated material remains the only way forward; however, there have been major steps taken to make hydrophilic interaction liquid chromatography (HILIC), a high-throughput platform to analyze glycans. This is particularly useful with IgG glycans since they tend to be no more than biantennary.
Relying largely on the HILIC approach, there has been a significant investment in Croatia, under the leadership of Gordon Lauc at Genos, to analyze IgG from many thousands of samples in an effort to better define the variability and trends seen with IgG glycosylation. This has generated many publications—too many to adequately review here—aligning glycan structure with variables such as sex, age, disease status and country of origin. For example, a 2011 report using IgG isolated from the plasma of nearly 2300 individuals from either Vis or Korčula (both Croatian Adriatic islands) or the Northern Scottish Orkney Islands was published (Pucic et al. 2011). They reported that disialylated N-glycans represented only about 3% of the total glycans, and that nearly all (96%) of all neutral glycans were core fucosylated. Within this cohort, the number of agalactosylated IgG glycans was approximately 40% of all neutral glycans, with glycans harboring one or two galactose residues being 40% and 20%, respectively. It was found that 11.6% of the mono-galactosylated structures carried a sialic acid and over 50% of the digalactosylated molecules were sialylated. Importantly, there was a lot of variability within these values. For example, the agalactosylated structures ranged from 14% to 70% of the total neutral glycans, while the number of neutral glycans without core fucosylation ranged from 1.3% to 19%.
In terms of trends, age was found to be the best predictor of the variance within the proportion of agalactosylated structures, in that agalactosylation increased parallel to the increases in bisecting GlcNAc. Interestingly, sex was not associated with glycan changes in this study (Pucic et al. 2011). However, these trends do not hold up in all populations or in all studies. For example, in a study involving 735 controls and 138 thyroid cancer patients, the authors report differences in IgG glycosylation among healthy control females, particularly at puberty and menopause (Chen et al. 2012). Consistent with differences between males and females, a 2017 study demonstrated that menopause was associated with increased agalactosylated glycoforms, but that hormone replacement therapy reversed this effect (Ercan et al. 2017). Likewise, males deprived of gonadal hormones with goserelin had decreased galactosylated IgG glycoforms, which was reversible with testosterone treatment. Finally, in a recent study of Mtb-infected patients from two populations—South Africa and Texas/Mexico—there were large differences in IgG glycan composition reported (Lu et al. 2016). In the South Africa cohort, patients were found to have 90% or greater of their IgG glycans fucosylated and 15–20% sialylated. Yet, in the Texas/Mexico cohort, fucosylation was closer to 75–80% and sialylation was between 30% and 45%. Even the amount of digalactosylated glycans was distinct, with higher levels in South Africa.
The overall theme of these results is that IgG glycosylation is quite variable within and across populations, though it follows general trends. The sialylation and bisecting GlcNAc rates tend to be relatively low, fucosylation relatively high and galactosylation somewhere in the middle, making the interpretation of small changes as a function of any variable within a population difficult. In addition, the HILIC method, which has made a major positive impact on the field, has limitations in the amount of compositional detail. For example, the linkage of sialic acid, which has an impact on function (Jassal et al. 2001), is not typically reported. Also, the number of peaks analyzed is lower than the total number of glycoforms identified on IgG using more stringent, albeit much slower and laborious, methods such as mass spectrometry.
Nonetheless, it is reassuring that many of the trends in IgG glycosylation can be associated with genome-wide association study (GWAS) results, which identified four genes closely linked to the expected changes (Lauc et al. 2013). These were ST6GAL1, B4GALT1, FUT8 and MGAT3, which encode the enzymes responsible for α2,6-linked sialylation, galactosylation, core fucosylation and bisecting GlcNAc addition within typical N-glycans, respectively. Another report from 2018 suggested an association between IgG glycosylation and DNA methylation among smokers (Wahl et al. 2018), although a causal relationship was not established. Many other recent articles fall into a similar category, aligning GWAS results and/or some characteristic of the subjects with the changes in IgG glycoforms: Smoking and longevity are two such correlations (Wahl et al. 2018); depression is another (Park et al. 2018). IBD (Klasic et al. 2018); hypertension (Liu et al. 2018b); dyslipidemia (Liu et al. 2018a); COPD (Pavic et al. 2018); metabolic syndrome, hypertriglyceridemic waist phenotype and abdominal obesity (Wang et al. 2019); and so on.
Whether these associations are functional or causally related to disease requires further mechanistic studies. Moreover, the source of nucleic acid for the GWAS studies is, in most cases, not the B-cell compartment. As a specific example, promoter methylation of the MGAT3 gene, which produces the enzyme responsible for bisecting GlcNAc, was reported as being associated with IgG glycans and IBD (Klasic et al. 2018). Yet, the epigenetics was performed with whole peripheral blood, making it difficult to know the extent of epigenetic changes in the B-cell compartment. Collectively, these studies are exciting because they form the foundation for new hypotheses and fuel much needed mechanistic research to fully understand the biology.
IgG glycans and regulation
Considering the impact of IgG glycosylation on the function of an IgG antibody, renewed interest in vaccination is palpable from the perspective that a protective antibody response to a vaccine may hinge upon the glycoforms of the antibodies generated. However, there is substantial debate about how IgG glycosylation is regulated, and whether it is possible that IgG glycoforms can be selectively and permanently elicited at all.
A recent study showed that immunization of patients with the seasonal influenza vaccine resulted in shifting glycoforms over time (Wang et al. 2015), similar to what was reported previously (Lastra et al. 1998). Interestingly, this was extended using mouse models in which immunization with immune complexes comprised of human IgG and hemagglutinin, where the IgG was either partially sialylated or enzymatically desialylated, resulted in higher affinity IgG when α2,6-sialylated IgG was used (Wang et al. 2015). It was proposed that this effect was through upregulation of FcγRIIB, but the precise mechanism remains unclear given that increased FcγRIIB signaling on the B cell was previously reported to induce apoptosis (Pearse et al. 1999).
In healthy human samples collected from vaccinated individuals at varied time points following exposure, it was found that the amount of galactosylation and total sialylation on vaccine-specific IgG varied over time. Both were low at first, increased later, and then established a baseline at the latest time point. Interestingly, the nonvaccine-specific IgG did not change over the same period (Selman et al. 2012). Similarly, in 2016, it was reported that despite the large variability in IgG glycosylation across multiple geographical locations, that immunization “overcomes” these differences and yields antigen-specific antibodies with similar antibody glycosylation patterns, and that these patterns were influenced by the vaccination regimen (Mahan et al. 2016). In support of this interpretation, previous work using primary human B cells harvested and stimulated to produce IgG with and without other stimuli demonstrated that CpG (TLR9 agonist) and IL-21 increased galactosylation and reduced bisecting GlcNAc, whereas all-trans retinoic acid decreased galactosylation and total sialylation levels (Wang et al. 2011).
These data have been interpreted as suggesting that specific IgG glycoforms are permanently encoded within a B cell and can be specifically elicited, and that the associated glycoforms are optimized for the required protective function of that particular IgG target (Mahan et al. 2016). While the data are clear, the interpretation is debatable. In this study, all of the glycan analyses were performed at 2 weeks postfinal immunization, but it is clear that the glycan profiles change over time after immunization, with 2 weeks being a peak in that difference (Lastra et al. 1998, Wang et al. 2015). Moreover, since all IgG is antigen specific and produced by a clonal population of B-cell-derived cells, the changes in IgG glycoforms with age, sex, inflammation and disease reviewed herein runs counter to the notion of hardwired programming of IgG glycosylation.
Consistent with a dynamic glycoform model of IgG glycosylation whereby the glycan composition is driven by the immediate in vivo environment, patients with multiple sclerosis (MS) showed reduced fucose and galactose and increased bisecting GlcNAc in IgG localized within the central nervous system (CNS), yet IgG from serum did not reflect those same changes (Wuhrer et al. 2015). This could mean that the antibody-producing plasma cells localized within the inflamed CNS produce antibodies with divergent glycoforms compared to the plasma cells outside of the localized inflammation characteristic of MS. It would be interesting to know if this difference holds for clonal IgG with identical specificity produced in both anatomical/inflammatory environments.
Another line of evidence comes from studies in mice where the sialyltransferases responsible for IgG α2,6-sialylation (Hennet et al. 1998) was manipulated. First, in mice lacking the P1 region of the ST6Gal1 promoter, IgG α2,6-sialylation is reduced substantially (Jones et al. 2012). Since B-cell expression of ST6Gal1 is reportedly independent of the P1 region, these data suggested that B cells were not responsible for IgG α2,6-sialylation. Supporting those findings, mice in which ST6Gal1 was selectively deleted from B cells showed no change in IgG α2,6-sialylation (Jones et al. 2016). These data demonstrate that IgG α2,6-sialylation is not controlled by IgG-secreting plasma cells but is sialylated after release by circulatory ST6Gal1 in the plasma (Jones et al. 2012, 2016). Perhaps more importantly, the ability of IgG to be sialylated after release from an antibody-producing plasma cell directly supports a model in which IgG glycans can be dynamically remodeled long after secretion into the circulatory environment. Indeed, it is now known that Neu1 and Neu3, sialidases which remove sialic acids from glycans, are released into the circulation as well (Yang et al. 2015), though it is not yet clear whether this release directly alters IgG sialylation. Finally, while these studies were performed in mice, it is important to note that ST6Gal1 is also known to exist in human plasma.
Translating the glycomics, revisited
With these profound differences in function, the notion of manipulating the IgG glycome as a novel approach to disease therapy has matured rapidly. Using collagen antibody-induced arthritis as a model, it was demonstrated that disease-causing collagen-specific antibodies could be rendered nonpathogenic by removing the N-glycans with EndoS treatment (Nandakumar et al. 2007). Perhaps more important from a translational perspective, this was extended to direct in vivo injections of EndoS to remove most of the glycan. Treated mice were protected in the CIA model (Albert et al. 2008). Supporting these findings, EndoS deglycosylation of antiaquaporin-4 autoreactive IgG, which is causative for neuromyelitis optica, was also shown to not only eliminate its pathogenic efficacy but also converted the IgG into a robust therapeutic antibody that blocked disease (Tradtrantip et al. 2013).
In 2016, it was reported that sialylation of RA-associated antibodies converted them into potent inhibitors of arthritogenic activity and suppresses CIA development in treated mice (Ohmi et al. 2016). A recent and more directly translational extension of this work was published in Cell, wherein investigators administered engineered glycosyltransferases (B4GalT1 and ST6Gal1, which catalyze N-glycan galactosylation and α2,6-linked sialylation respectively) to mice. These increased galactosylation and sialylation of IgG and reduced severity in a Goodpasture Disease model of autoimmunity (Pagan et al. 2018).
Cancer is also a primary target for glycomic engineering of drugs. There is a recent and comprehensive review on the relationship between fucose and anticancer antibody drugs, which provides details that go far beyond the scope of this review (Pereira et al. 2018). However, it is important to recognize that there are a number of strategies being employed to translate the core fucosylation effect into pharmaceuticals. For example, recombinant systems producing anticancer IgG have been manipulated to delete Fut8, the fucosyltransferase responsible for core fucosylation. In this case, afucosylated anti-CD20 (the target of rituximab) showed a two-fold increase in ADCC (Yamane-Ohnuki et al. 2004). Others are working to delete the nucleotide-sugar transporter Slc35c1, which is responsible for providing GDP-fucose into the Golgi for all fucosylation reactions (Chan et al. 2016). And still others are attempting to make mutants that will mimic the fucose structural effect leading to enhanced FcγRIIIA binding and activation (Jung et al. 2010; Shields et al. 2001).
Finally, there are efforts to harness the sialylation effect to improve IVIg efficacy. As an example, researchers have produced an IVIg in which all IgG molecules carry fully α2,6-sialylated glycans at N297. This drug candidate was reported to have a 10-fold higher anti-inflammatory activity compared to conventional IVIg using a variety of animal models, including collagen antibody-induced arthritis, K/BxN arthritis, ITP and epidermolysis bullosa acquisita (Washburn et al. 2015).
Conclusions
In this article, a concerted effort has been made to provide a thorough historical perspective interlaced with an up to date review of what is known about IgG glycosylation. The field is broad, and the impact cannot be overstated. IgG is central as a drug platform, defense against microbial pathogens and prevention of inappropriate inflammation characteristic of many diseases. Yet, many questions remain. Can IgG glycoforms be programmed, or are they dynamic? Or, is the truth somewhere in between? Will glycomic engineering of antibody-based drugs increase efficacy? Can endogenous IgG glycans be manipulated directly in vivo as a therapeutic strategy, and would that have unintended consequences? The more learned about the effects of glycans on IgG, how their composition and structure are regulated and methods to manipulate them, the better armed the research community will be at finally opening the gates to the use of the glycome as the next pharmaceutical frontier.
Acknowledgements
The author would like to thank Lori SC Kreisman for critical evaluation of this manuscript, and Douglas Oswald for assistance in basic editing. Funds supporting the creation of this manuscript were provided by a NIH/NIGMS grant to BAC (R01GM115234).
Conflict of interest statement
None declared.
Abbreviations
ADCC, antibody-dependent cellular cytotoxicity; ADCP, antibody-dependent cellular phagocytosis; CDC, complement-mediated cytotoxicity; CIA, collagen-induced arthritis; CNS, central nervous system; Fc,fragment crystallizable; GPA, granulomatosis with polyangiitis; GWAS, genome-wide association study; HILIC, hydrophilic interaction liquid chromatography; ITP, murine immune thrombocytopenia; mAb, monoclonal antibody; MBP, mannose-binding lectin; MS, multiple sclerosis; OA, osteoarthritis arthritis; OST, oligosaccharyltransferase; PIT, passive immune thrombocytopenia; PMNs, polymorphonuclear cells; PSA, prostate-specific antigen; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SPF, specific pathogen-free; SPR, surface plasmon resonance; TNFα, tumor necrosis factor α.
References
- Adler Y, Lamour A, Jamin C, Menez JF, Le Corre R, Shoenfeld Y, Youinou P. 1995. Impaired binding capacity of asialyl and agalactosyl IgG to Fc gamma receptors. Clin Exp Rheumatol. 13:315–319. [PubMed] [Google Scholar]
- Ahmed AA, Giddens J, Pincetic A, Lomino JV, Ravetch JV, Wang LX, Bjorkman PJ. 2014. Structural characterization of anti-inflammatory immunoglobulin G Fc proteins. J Mol Biol. 426:3166–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert H, Collin M, Dudziak D, Ravetch JV, Nimmerjahn F. 2008. In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner. Proc Natl Acad Sci U S A. 105:15005–15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. 2011. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 475:110–U133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. 2008a. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 320:373–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. 2008b. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci U S A. 105:19571–19578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Wermeling F, Ravetch JV. 2012. Novel roles for the IgG Fc glycan. Ann N Y Acad Sci. 1253:170–180. [DOI] [PubMed] [Google Scholar]
- Aurer I, Lauc G, Dumic J, Rendic D, Matisic D, Milos M, Heffer-Lauc M, Flogel M, Labar B. 2007. Aberrant glycosylation of Igg heavy chain in multiple myeloma. Coll Antropol. 31:247–251. [PubMed] [Google Scholar]
- Axford JS, Alavi A, Bond A, Hay FC. 1994. Differential B lymphocyte galactosyltransferase activity in the MRL mouse model of rheumatoid arthritis. Autoimmunity. 17:157–163. [DOI] [PubMed] [Google Scholar]
- Axford JS, Mackenzie L, Lydyard PM, Hay FC, Isenberg DA, Roitt IM. 1987. Reduced B-cell galactosyltransferase activity in rheumatoid arthritis. Lancet. 2:1486–1488. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Huttner WB. 1984. Inhibition of N-glycosylation induces tyrosine sulphation of hybridoma immunoglobulin G. EMBO J. 3:2209–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba T, Harb J, Ducreux S, Koenig A, Mathias V, Rabeyrin M, Pouliquen E, Sicard A, Chartoire D, Dugast E et al. 2019. Highly Variable Sialylation Status of Donor-Specific Antibodies Does Not Impact Humoral Rejection Outcomes. Front Immunol. 10:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bas M, Terrier A, Jacque E, Dehenne A, Pochet-Beghin V, Beghin C, Dezetter AS, Dupont G, Engrand A, Beaufils B et al. 2019. Fc Sialylation Prolongs Serum Half-Life of Therapeutic Antibodies. J Immunol. 202:1582–1594. [DOI] [PubMed] [Google Scholar]
- Bond A, Cooke A, Hay FC. 1990. Glycosylation of IgG, immune complexes and IgG subclasses in the MRL-lpr/lpr mouse model of rheumatoid arthritis. Eur J Immunol. 20:2229–2233. [DOI] [PubMed] [Google Scholar]
- Bones J, Mittermayr S, O’Donoghue N, Guttman A, Rudd PM. 2010. Ultra performance liquid chromatographic profiling of serum N-glycans for fast and efficient identification of cancer associated alterations in glycosylation. Anal Chem. 82:10208–10215. [DOI] [PubMed] [Google Scholar]
- Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. 2002. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 99:754–758. [DOI] [PubMed] [Google Scholar]
- Chan KF, Shahreel W, Wan C, Teo G, Hayati N, Tay SJ, Tong WH, Yang Y, Rudd PM, Zhang P et al. 2016. Inactivation of GDP-fucose transporter gene (Slc35c1) in CHO cells by ZFNs, TALENs and CRISPR-Cas9 for production of fucose-free antibodies. Biotechnol J. 11:399–414. [DOI] [PubMed] [Google Scholar]
- Chen G, Wang Y, Qiu L, Qin X, Liu H, Wang X, Wang Y, Song G, Li F, Guo Y et al. 2012. Human IgG Fc-glycosylation profiling reveals associations with age, sex, female sex hormones and thyroid cancer. J Proteome. 75:2824–2834. [DOI] [PubMed] [Google Scholar]
- Chung AW, Crispin M, Pritchard L, Robinson H, Gorny MK, Yu X, Bailey-Kellogg C, Ackerman ME, Scanlan C, Zolla-Pazner S et al. 2014. Identification of antibody glycosylation structures that predict monoclonal antibody Fc-effector function. AIDS. 28:2523–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccimarra F, Rosen FS, Schneeberger E, Merler E. 1976. Failure of heavy chain glycosylation of IgG in some patients with common, variable agammaglobulinemia. J Clin Invest. 57:1386–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadel G, Calabro A, Sigounas G, Hascall VC, Notkins AL, Harindranath N. 1994. Human polyreactive and monoreactive antibodies: effect of glycosylation on antigen binding. Glycobiology. 4:491–496. [DOI] [PubMed] [Google Scholar]
- Einarsdottir HK, Selman MH, Kapur R, Scherjon S, Koeleman CA, Deelder AM, van der Schoot CE, Vidarsson G, Wuhrer M. 2013. Comparison of the Fc glycosylation of fetal and maternal immunoglobulin G. Glycoconj J. 30:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan A, Kohrt WM, Cui J, Deane KD, Pezer M, Yu EW, Hausmann JS, Campbell H, Kaiser UB, Rudd PM et al. 2017. Estrogens regulate glycosylation of IgG in women and men. JCI Insight. 2:e89703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espy C, Morelle W, Kavian N, Grange P, Goulvestre C, Viallon V, Chereau C, Pagnoux C, Michalski JC, Guillevin L et al. 2011. Sialylation levels of anti-proteinase 3 antibodies are associated with the activity of granulomatosis with polyangiitis (Wegener’s). Arthritis Rheum. 63:2105–2115. [DOI] [PubMed] [Google Scholar]
- Flogel M, Lauc G, Gornik I, Macek B. 1998. Fucosylation and galactosylation of IgG heavy chains differ between acute and remission phases of juvenile chronic arthritis. Clin Chem Lab Med. 36:99–102. [DOI] [PubMed] [Google Scholar]
- Gornik I, Maravic G, Dumic J, Flogel M, Lauc G. 1999. Fucosylation of IgG heavy chains is increased in rheumatoid arthritis. Clin Biochem. 32:605–608. [DOI] [PubMed] [Google Scholar]
- Gu XJ, Wang DIC. 1998. Improvement of interferon-gamma sialylation in Chinese hamster ovary cell culture by feeding of N-acetylmannosamine. Biotechnol Bioeng. 58:642–648. [PubMed] [Google Scholar]
- Guhr T, Bloem J, Derksen NI, Wuhrer M, Koenderman AH, Aalberse RC, Rispens T. 2011. Enrichment of sialylated IgG by lectin fractionation does not enhance the efficacy of immunoglobulin G in a murine model of immune thrombocytopenia. PLoS One. 6:e21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N, Liu Y, Masuda Y, Kawagoe M, Ueno Y, Kameda T, Sugiyama T. 2005. Repeated immunization induces the increase in fucose content on antigen-specific IgG N-linked oligosaccharides. Clin Biochem. 38:149–153. [DOI] [PubMed] [Google Scholar]
- Harre U, Lang SC, Pfeifle R, Rombouts Y, Fruhbeisser S, Amara K, Bang H, Lux A, Koeleman CA, Baum W et al. 2015. Glycosylation of immunoglobulin G determines osteoclast differentiation and bone loss. Nat Commun. 6:6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennet T, Chui D, Paulson JC, Marth JD. 1998. Immune regulation by the ST6Gal sialyltransferase. Proc Natl Acad Sci U S A. 95:4504–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman B, Nose M, Weigle WO. 1985. Carbohydrate chains on IgG2b: a requirement for efficient feedback immunosuppression. J Immunol. 134:4018–4023. [PubMed] [Google Scholar]
- Heyman B, Wiersma E, Nose M. 1988. Complement activation is not required for IgG-mediated suppression of the antibody response. Eur J Immunol. 18:1739–1743. [DOI] [PubMed] [Google Scholar]
- Hickman S, Kornfeld S. 1978. Effect of tunicamycin on IgM, IgA, and IgG secretion by mouse plasmacytoma cells. J Immunol. 121:990–996. [PubMed] [Google Scholar]
- Hitsumoto Y, Thompson SJ, Zhang YW, Rook GA, Elson CJ. 1992. Relationship between interleukin 6, agalactosyl IgG and pristane-induced arthritis. Autoimmunity. 11:247–254. [DOI] [PubMed] [Google Scholar]
- Jassal R, Jenkins N, Charlwood J, Camilleri P, Jefferis R, Lund J. 2001. Sialylation of human IgG-Fc carbohydrate by transfected rat alpha2,6-sialyltransferase. Biochem Biophys Res Commun. 286:243–249. [DOI] [PubMed] [Google Scholar]
- Jeddi PA, Lund T, Bodman KB, Sumar N, Lydyard PM, Pouncey L, Heath LS, Kidd VJ, Delves PJ. 1994. Reduced galactosyltransferase mRNA levels are associated with the agalactosyl IgG found in arthritis-prone MRL-lpr/lpr strain mice. Immunology. 83:484–488. [PMC free article] [PubMed] [Google Scholar]
- Jones MB, Nasirikenari M, Lugade AA, Thanavala Y, Lau JT. 2012. Anti-inflammatory IgG production requires functional P1 promoter in beta-galactoside alpha2,6-sialyltransferase 1 (ST6Gal-1) gene. J Biol Chem. 287:15365–15370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MB, Oswald DM, Joshi S, Whiteheart SW, Orlando R, Cobb BA. 2016. B-cell-independent sialylation of IgG. Proc Natl Acad Sci U S A. 113:7207–7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ST, Reddy ST, Kang TH, Borrok MJ, Sandlie I, Tucker PW, Georgiou G. 2010. Aglycosylated IgG variants expressed in bacteria that selectively bind FcgammaRI potentiate tumor cell killing by monocyte-dendritic cells. Proc Natl Acad Sci U S A. 107:604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, Nimmerjahn F, Ravetch JV. 2006. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 313:670–673. [DOI] [PubMed] [Google Scholar]
- Kanoh Y, Mashiko T, Danbara M, Takayama Y, Ohtani S, Egawa S, Baba S, Akahoshi T. 2004. Changes in serum IgG oligosaccharide chains with prostate cancer progression. Anticancer Res. 24:3135–3139. [PubMed] [Google Scholar]
- Keusch J, Levy Y, Shoenfeld Y, Youinou P. 1996. Analysis of different glycosylation states in IgG subclasses. Clin Chim Acta. 252:147–158. [DOI] [PubMed] [Google Scholar]
- Kjeldsen-Kragh J, Sumar N, Bodman-Smith K, Brostoff J. 1996. Changes in glycosylation of IgG during fasting in patients with rheumatoid arthritis. Br J Rheumatol. 35:117–119. [DOI] [PubMed] [Google Scholar]
- Klasic M, Markulin D, Vojta A, Samarzija I, Birus I, Dobrinic P, Ventham NT, Trbojevic-Akmacic I, Simurina M, Stambuk J et al. 2018. Promoter methylation of the MGAT3 and BACH2 genes correlates with the composition of the immunoglobulin G glycome in inflammatory bowel disease. Clin Epigenetics. 10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodar K, Stadlmann J, Klaamas K, Sergeyev B, Kurtenkov O. 2012. Immunoglobulin G Fc N-glycan profiling in patients with gastric cancer by LC-ESI-MS: relation to tumor progression and survival. Glycoconj J. 29:57–66. [DOI] [PubMed] [Google Scholar]
- Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P. 2003. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J Mol Biol. 325:979–989. [DOI] [PubMed] [Google Scholar]
- Lastra GC, Thompson SJ, Lemonidis AS, Elson CJ. 1998. Changes in the galactose content of IgG during humoral immune responses. Autoimmunity. 28:25–30. [DOI] [PubMed] [Google Scholar]
- Lauc G, Huffman JE, Pucic M, Zgaga L, Adamczyk B, Muzinic A, Novokmet M, Polasek O, Gornik O, Kristic J et al. 2013. Loci associated with N-glycosylation of human immunoglobulin G show pleiotropy with autoimmune diseases and haematological cancers. PLoS Genet. 9:e1003225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontyev D, Katsman Y, Ma XZ, Miescher S, Kasermann F, Branch DR. 2012. Sialylation-independent mechanism involved in the amelioration of murine immune thrombocytopenia using intravenous gammaglobulin. Transfusion. 52:1799–1805. [DOI] [PubMed] [Google Scholar]
- Lin CW, Tsai MH, Li ST, Tsai TI, Chu KC, Liu YC, Lai MY, Wu CY, Tseng YC, Shivatare SS et al. 2015. A common glycan structure on immunoglobulin G for enhancement of effector functions. Proc Natl Acad Sci U S A. 112:10611–10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Chu X, Wang H, Dong J, Ge SQ, Zhao ZY, Peng HL, Sun M, Wu LJ, Song MS et al. 2018a. The changes of immunoglobulin G N-glycosylation in blood lipids and dyslipidaemia. J Transl Med. 16:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JK. 2014. The history of monoclonal antibody development—Progress, remaining challenges and future innovations. Ann Med Surg (Lond). 3:113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JN, Dolikun M, Stambuk J, Trbojevic-Akmacic I, Zhang J, Wang H, Zheng DQ, Zhang XY, Peng HL, Zhao ZY et al. 2018b. The association between subclass-specific IgG Fc N-glycosylation profiles and hypertension in the Uygur, Kazak, Kirgiz, and Tajik populations. J Hum Hypertens. 32:555–563. [DOI] [PubMed] [Google Scholar]
- Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS, Schoen MK, Tafesse F, Martin C, Leung V et al. 2016. A Functional Role for Antibodies in Tuberculosis. Cell. 167(433–443):e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J, Takahashi N, Pound JD, Goodall M, Jefferis R. 1996. Multiple interactions of IgG with its core oligosaccharide can modulate recognition by complement and human Fc gamma receptor I and influence the synthesis of its oligosaccharide chains. J Immunol. 157:4963–4969. [PubMed] [Google Scholar]
- Lund J, Takahashi N, Pound JD, Goodall M, Nakagawa H, Jefferis R. 1995. Oligosaccharide-protein interactions in IgG can modulate recognition by Fc gamma receptors. FASEB J. 9:115–119. [DOI] [PubMed] [Google Scholar]
- Magorivska I, Munoz LE, Janko C, Dumych T, Rech J, Schett G, Nimmerjahn F, Bilyy R, Herrmann M. 2016. Sialylation of anti-histone immunoglobulin G autoantibodies determines their capabilities to participate in the clearance of late apoptotic cells. Clin Exp Immunol. 184:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AE, Jennewein MF, Suscovich T, Dionne K, Tedesco J, Chung AW, Streeck H, Pau M, Schuitemaker H, Francis D et al. 2016. Antigen-Specific Antibody Glycosylation Is Regulated via Vaccination. PLoS Pathog. 12:e1005456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaise MG, Franchimont P, Bouillene C, Houssier C, Mahieu PR. 1987. Increased concanavalin A-binding capacity of immunoglobulin G purified from sera of patients with rheumatoid arthritis. Clin Exp Immunol. 68:543–551. [PMC free article] [PubMed] [Google Scholar]
- Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB. 1995. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat Med. 1:237–243. [DOI] [PubMed] [Google Scholar]
- McCulloch J, Zhang YW, Dawson M, Harkiss GD, Peterhans E, Vogt HR, Lydyard PM, Rook GA. 1995. Glycosylation of IgG during potentially arthritogenic lentiviral infections. Rheumatol Int. 14:243–248. [DOI] [PubMed] [Google Scholar]
- Mehta AS, Long RE, Comunale MA, Wang M, Rodemich L, Krakover J, Philip R, Marrero JA, Dwek RA, Block TM. 2008. Increased levels of galactose-deficient anti-Gal immunoglobulin G in the sera of hepatitis C virus-infected individuals with fibrosis and cirrhosis. J Virol. 82:1259–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura Y, Church S, Ghirlando R, Ashton PR, Dong S, Goodall M, Lund J, Jefferis R. 2000. The influence of glycosylation on the thermal stability and effector function expression of human IgG1-Fc: properties of a series of truncated glycoforms. Mol Immunol. 37:697–706. [DOI] [PubMed] [Google Scholar]
- Mimura Y, Sondermann P, Ghirlando R, Lund J, Young SP, Goodall M, Jefferis R. 2001. Role of oligosaccharide residues of IgG1-Fc in Fc gamma RIIb binding. J Biol Chem. 276:45539–45547. [DOI] [PubMed] [Google Scholar]
- Mizuochi T, Hamako J, Nose M, Titani K. 1990. Structural changes in the oligosaccharide chains of IgG in autoimmune MRL/Mp-lpr/lpr mice. J Immunol. 145:1794–1798. [PubMed] [Google Scholar]
- Mizushima T, Yagi H, Takemoto E, Shibata-Koyama M, Isoda Y, Iida S, Masuda K, Satoh M, Kato K. 2011. Structural basis for improved efficacy of therapeutic antibodies on defucosylation of their Fc glycans. Genes Cells. 16:1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JS, Wu X, Kulhavy R, Tomana M, Novak J, Moldoveanu Z, Brown R, Goepfert PA, Mestecky J. 2005. Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. AIDS. 19:381–389. [DOI] [PubMed] [Google Scholar]
- Nandakumar KS, Collin M, Olsen A, Nimmerjahn F, Blom AM, Ravetch JV, Holmdahl R. 2007. Endoglycosidase treatment abrogates IgG arthritogenicity: importance of IgG glycosylation in arthritis. Eur J Immunol. 37:2973–2982. [DOI] [PubMed] [Google Scholar]
- Nose M, Wigzell H. 1983. Biological significance of carbohydrate chains on monoclonal antibodies. Proc Natl Acad Sci U S A. 80:6632–6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmi Y, Ise W, Harazono A, Takakura D, Fukuyama H, Baba Y, Narazaki M, Shoda H, Takahashi N, Ohkawa Y et al. 2016. Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis. Nat Commun. 7:11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki A, Shoji-Hosaka E, Nakamura K, Wakitani M, Uchida K, Kakita S, Tsumoto K, Kumagai I, Shitara K. 2004. Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcgammaRIIIa. J Mol Biol. 336:1239–1249. [DOI] [PubMed] [Google Scholar]
- Pagan JD, Kitaoka M, Anthony RM. 2018. Engineered Sialylation of Pathogenic Antibodies In Vivo Attenuates Autoimmune Disease. Cell. 172(564–577):e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh R, Roitt I, Isenberg D, Dwek R, Rademacher T. 1988a. Age-related galactosylation of the N-linked oligosaccharides of human serum IgG. J Exp Med. 167:1731–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T, Matsuta K et al. 1985. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 316:452–457. [DOI] [PubMed] [Google Scholar]
- Parekh RB, Roitt IM, Isenberg DA, Dwek RA, Ansell BM, Rademacher TW. 1988b. Galactosylation of IgG associated oligosaccharides: reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet. 1:966–969. [DOI] [PubMed] [Google Scholar]
- Park DI, Stambuk J, Razdorov G, Pucic-Bakovic M, Martins-de-Souza D, Lauc G, Turck CW. 2018. Blood plasma/IgG N-glycome biosignatures associated with major depressive disorder symptom severity and the antidepressant response. Sci Rep. 8:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavic T, Dilber D, Kifer D, Selak N, Keser T, Ljubicic D, Vukic Dugac A, Lauc G, Rumora L, Gornik O. 2018. N-glycosylation patterns of plasma proteins and immunoglobulin G in chronic obstructive pulmonary disease. J Transl Med. 16:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse RN, Kawabe T, Bolland S, Guinamard R, Kurosaki T, Ravetch JV. 1999. SHIP recruitment attenuates Fc gamma RIIB-induced B cell apoptosis. Immunity. 10:753–760. [DOI] [PubMed] [Google Scholar]
- Peipp M, Lammerts van Bueren JJ, Schneider-Merck T, Bleeker WW, Dechant M, Beyer T, Repp R, van Berkel PH, Vink T, van de Winkel JG et al. 2008. Antibody fucosylation differentially impacts cytotoxicity mediated by NK and PMN effector cells. Blood. 112:2390–2399. [DOI] [PubMed] [Google Scholar]
- Pekelharing JM, Hepp E, Kamerling JP, Gerwig GJ, Leijnse B. 1988. Alterations in carbohydrate composition of serum IgG from patients with rheumatoid arthritis and from pregnant women. Ann Rheum Dis. 47:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppard JV, Hobbs SM, Jackson LE. 1989. Role of carbohydrate in binding of IgG to the Fc receptor of neonatal rat enterocytes. Mol Immunol. 26:495–500. [DOI] [PubMed] [Google Scholar]
- Pereira NA, Chan KF, Lin PC, Song Z. 2018. The “less-is-more” in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. MAbs. 10:693–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucic M, Knezevic A, Vidic J, Adamczyk B, Novokmet M, Polasek O, Gornik O, Supraha-Goreta S, Wormald MR, Redzic I et al. 2011. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol Cell Proteomics. 10(M111):010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast I, Keller CW, Maurer MA, Giddens JP, Tackenberg B, Wang LX, Munz C, Nimmerjahn F, Dalakas MC, Lunemann JD. 2015. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J Clin Invest. 125:4160–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radaev S, Sun PD. 2001. Recognition of IgG by Fcgamma receptor. The role of Fc glycosylation and the binding of peptide inhibitors. J Biol Chem. 276:16478–16483. [DOI] [PubMed] [Google Scholar]
- Rademacher TW, Williams P, Dwek RA. 1994. Agalactosyl glycoforms of IgG autoantibodies are pathogenic. Proc Natl Acad Sci U S A. 91:6123–6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GA, Steele J, Brealey R, Whyte A, Isenberg D, Sumar N, Nelson JL, Bodman KB, Young A, Roitt IM et al. 1991. Changes in IgG glycoform levels are associated with remission of arthritis during pregnancy. J Autoimmun. 4:779–794. [DOI] [PubMed] [Google Scholar]
- Rothman RJ, Perussia B, Herlyn D, Warren L. 1989. Antibody-dependent cytotoxicity mediated by natural killer cells is enhanced by castanospermine-induced alterations of IgG glycosylation. Mol Immunol. 26:1113–1123. [DOI] [PubMed] [Google Scholar]
- Schwaber J, Rosen FS. 1984. Lymphoid cell lines from patients with “non-secretory” agammaglobulinemia produce glycosylated heavy chains which are reduced in molecular weight. J Mol Cell Immunol. 1:279–291. [PubMed] [Google Scholar]
- Scott SD. 1998. Rituximab: a new therapeutic monoclonal antibody for non-Hodgkin’s lymphoma. Cancer Pract. 6:195–197. [DOI] [PubMed] [Google Scholar]
- Selman MH, de Jong SE, Soonawala D, Kroon FP, Adegnika AA, Deelder AM, Hokke CH, Yazdanbakhsh M, Wuhrer M. 2012. Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination. Mol Cell Proteomics. 11(M111):014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman MH, Niks EH, Titulaer MJ, Verschuuren JJ, Wuhrer M, Deelder AM. 2011. IgG fc N-glycosylation changes in Lambert-Eaton myasthenic syndrome and myasthenia gravis. J Proteome Res. 10:143–152. [DOI] [PubMed] [Google Scholar]
- Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. 2002. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 277:26733–26740. [DOI] [PubMed] [Google Scholar]
- Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, Xie D, Lai J, Stadlen A, Li B et al. 2001. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 276:6591–6604. [DOI] [PubMed] [Google Scholar]
- Shikata K, Yasuda T, Takeuchi F, Konishi T, Nakata M, Mizuochi T. 1998. Structural changes in the oligosaccharide moiety of human IgG with aging. Glycoconj J. 15:683–689. [DOI] [PubMed] [Google Scholar]
- Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M et al. 2003. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 278:3466–3473. [DOI] [PubMed] [Google Scholar]
- Stadlmann J, Weber A, Pabst M, Anderle H, Kunert R, Ehrlich HJ, Peter Schwarz H, Altmann F. 2009. A close look at human IgG sialylation and subclass distribution after lectin fractionation. Proteomics. 9:4143–4153. [DOI] [PubMed] [Google Scholar]
- Stanley P, Chaney W. 1985. Control of carbohydrate processing: the lec1A CHO mutation results in partial loss of N-acetylglucosaminyltransferase I activity. Mol Cell Biol. 5:1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo M, Yamaguchi Y, Spath PJ, Matsumoto-Morita K, Ong BK, Shahrizaila N, Yuki N. 2014. Different IVIG glycoforms affect in vitro inhibition of anti-ganglioside antibody-mediated complement deposition. PLoS One. 9:e107772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton BJ, Phillips DC. 1983. The three-dimensional structure of the carbohydrate within the Fc fragment of immunoglobulin G. Biochem Soc Trans. 11:130–132. [PubMed] [Google Scholar]
- Tao MH, Morrison SL. 1989. Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J Immunol. 143:2595–2601. [PubMed] [Google Scholar]
- Temming AR, Dekkers G, van de Bovenkamp FS, Plomp HR, Bentlage AEH, Szittner Z, Derksen NIL, Wuhrer M, Rispens T, Vidarsson G. 2019. Human DC-SIGN and CD23 do not interact with human IgG. Sci Rep. 9:9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomann M, Schlothauer T, Dashivets T, Malik S, Avenal C, Bulau P, Ruger P, Reusch D. 2015. In vitro glycoengineering of IgG1 and its effect on Fc receptor binding and ADCC activity. PLoS One. 10:e0134949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SJ, Hitsumoto Y, Zhang YW, Rook GA, Elson CJ. 1992. Agalactosyl IgG in pristane-induced arthritis. Pregnancy affects the incidence and severity of arthritis and the glycosylation status of IgG. Clin Exp Immunol. 89:434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomana M, Schrohenloher RE, Koopman WJ, Alarcon GS, Paul WA. 1988. Abnormal glycosylation of serum IgG from patients with chronic inflammatory diseases. Arthritis Rheum. 31:333–338. [DOI] [PubMed] [Google Scholar]
- Tradtrantip L, Ratelade J, Zhang H, Verkman AS. 2013. Enzymatic deglycosylation converts pathogenic neuromyelitis optica anti-aquaporin-4 immunoglobulin G into therapeutic antibody. Ann Neurol. 73:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Geijn FE, Wuhrer M, Selman MH, Willemsen SP, de Man YA, Deelder AM, Hazes JM, Dolhain RJ. 2009. Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res Ther. 11:R193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl A, Kasela S, Carnero-Montoro E, van Iterson M, Stambuk J, Sharma S, van den Akker E, Klaric L, Benedetti E, Razdorov G et al. 2018. IgG glycosylation and DNA methylation are interconnected with smoking. Biochim Biophys Acta Gen Subj. 1862:637–648. [DOI] [PubMed] [Google Scholar]
- Walker MR, Lund J, Thompson KM, Jefferis R. 1989. Aglycosylation of human IgG1 and IgG3 monoclonal antibodies can eliminate recognition by human cells expressing Fc gamma RI and/or Fc gamma RII receptors. Biochem J. 259:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Li X, Wang X, Liu D, Zhang X, Cao W, Zheng Y, Guo Z, Li D, Xing W et al. 2019. Next-Generation (Glycomic) Biomarkers for Cardiometabolic Health: A Community-Based Study of Immunoglobulin G N-Glycans in a Chinese Han Population. OMICS. [DOI] [PubMed] [Google Scholar]
- Wang J, Balog CI, Stavenhagen K, Koeleman CA, Scherer HU, Selman MH, Deelder AM, Huizinga TW, Toes RE, Wuhrer M. 2011. Fc-glycosylation of IgG1 is modulated by B-cell stimuli. Mol Cell Proteomics. 10(M110):004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Maamary J, Tan GS, Bournazos S, Davis CW, Krammer F, Schlesinger SJ, Palese P, Ahmed R, Ravetch JV. 2015. Anti-HA Glycoforms Drive B Cell Affinity Selection and Determine Influenza Vaccine Efficacy. Cell. 162:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn N, Schwab I, Ortiz D, Bhatnagar N, Lansing JC, Medeiros A, Tyler S, Mekala D, Cochran E, Sarvaiya H et al. 2015. Controlled tetra-Fc sialylation of IVIg results in a drug candidate with consistent enhanced anti-inflammatory activity. Proc Natl Acad Sci U S A. 112:E1297–E1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng WK, Levy R. 2003. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 21:3940–3947. [DOI] [PubMed] [Google Scholar]
- Williams PJ, Arkwright PD, Rudd P, Scragg IG, Edge CJ, Wormald MR, Rademacher TW. 1995. Short communication: selective placental transport of maternal IgG to the fetus. Placenta. 16:749–756. [DOI] [PubMed] [Google Scholar]
- Wright A, Morrison SL. 1994. Effect of altered CH2-associated carbohydrate structure on the functional properties and in vivo fate of chimeric mouse-human immunoglobulin G1. J Exp Med. 180:1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuhrer M, Selman MH, McDonnell LA, Kumpfel T, Derfuss T, Khademi M, Olsson T, Hohlfeld R, Meinl E, Krumbholz M. 2015. Pro-inflammatory pattern of IgG1 Fc glycosylation in multiple sclerosis cerebrospinal fluid. J Neuroinflammation. 12:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Ito K, Furukawa J, Nakata J, Alvarez M, Verbeek JS, Shinohara Y, Izui S. 2013. Galactosylation of IgG1 modulates FcgammaRIIB-mediated inhibition of murine autoimmune hemolytic anemia. J Autoimmun. 47:104–110. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Nishimura M, Nagano M, Yagi H, Sasakawa H, Uchida K, Shitara K, Kato K. 2006. Glycoform-dependent conformational alteration of the Fc region of human immunoglobulin G1 as revealed by NMR spectroscopy. Biochim Biophys Acta. 1760:693–700. [DOI] [PubMed] [Google Scholar]
- Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, Kusunoki M, Iida S, Nakano R, Wakitani M, Niwa R, Sakurada M, Uchida K et al. 2004. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng. 87:614–622. [DOI] [PubMed] [Google Scholar]
- Yang WH, Aziz PV, Heithoff DM, Mahan MJ, Smith JW, Marth JD. 2015. An intrinsic mechanism of secreted protein aging and turnover. Proc Natl Acad Sci U S A. 112:13657–13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Vasiljevic S, Mitchell DA, Crispin M, Scanlan CN. 2013. Dissecting the molecular mechanism of IVIg therapy: the interaction between serum IgG and DC-SIGN is independent of antibody glycoform or Fc domain. J Mol Biol. 425:1253–1258. [DOI] [PubMed] [Google Scholar]
- Zhao ZY, Liu D, Cao WJ, Sun M, Song MS, Wang W, Wang YX. 2018. Association between IgG N-glycans and nonalcoholic fatty liver disease in Han Chinese. Biomed Environ Sci. 31:454–458. [DOI] [PubMed] [Google Scholar]


