Abstract
Therapeutic monoclonal antibodies (mAbs) are the fastest growing group of drugs with 11 new antibodies or antibody-drug conjugates approved by the Food and Drug Administration in 2018. Many mAbs require effector function for efficacy, including antibody-dependent cell-mediated cytotoxicity triggered following contact of an immunoglobulin G (IgG)-coated particle with activating crystallizable fragment (Fc) γ receptors (FcγRs) expressed by leukocytes. Interactions between IgG1 and the FcγRs require post-translational modification of the Fc with an asparagine-linked carbohydrate (N-glycan). Though the structure of IgG1 Fc and the role of Fc N-glycan composition on disease were known for decades, the underlying mechanism of how the N-glycan affected FcγR binding was not defined until recently. This review will describe the current understanding of how N-glycosylation impacts the structure and function of the IgG1 Fc and describe new techniques that are poised to provide the next critical breakthroughs.
Keywords: antibody, Fc gamma receptor, immunoglobulin G, N-glycosylation
Introduction
Monoclonal antibodies (mAbs) are powerful drugs for cancers, autoimmune disorders and infection in addition to many other diseases. Positive drug attributes including high specificity and tolerance promote the development of mAbs for a wide variety of targets. Antibodies are complex molecules with multiple polypeptide chains and post-translational modifications; human immunoglobulin G1 (IgG1) is a 150 kDa heterotetramer with two conserved Asn-linked carbohydrate chains (N-glycans) and 16 disulfide bonds (Figure 1) (Liu and May 2012). Furthermore, proper 3D structure is critical for mAb function and an important quality attribute concerning commercial mAb production (Berkowitz et al. 2012). Thus, the development, evaluation, validation and quality control of mAbs as drugs differs substantially from small molecule therapeutics that previously represented the pharmaceutical industry’s primary products.
Figure 1.
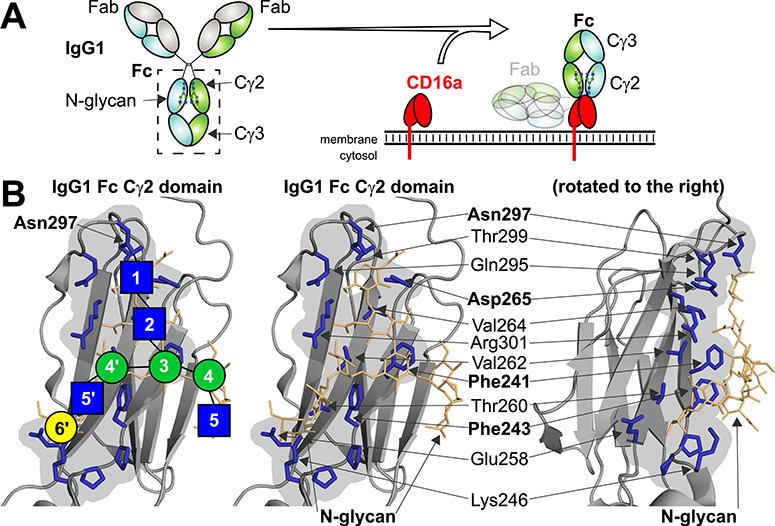
The IgG1 Fc N-glycan is essential for binding to the Fc γ receptors. (A) IgG1 Fc is a heterotetramer consisting of two Fabs and a single Fc that binds receptors. (B) The Fc N-glycan forms an interface with Cγ2 residues through noncovalent intramolecular interactions. The volume of interacting residues is shown with a gray background, and individual N-glycan and protein residues are highlighted. Blue squares represent N-acetylglucosamine residues; green circles, mannose; and yellow circles, galactose.
Complex molecules have a long history in human health that begins with undefined mixtures used as vaccines and arriving at a seminal moment in 1922 with the administration of a bovine pancreatic extract to successfully treat a young male diabetic (Bliss 2007). Each basic discovery, from defining insulin as a protein to sequencing the polypeptide chains to identifying residues that promote stability, has led to the improvement of human health through enhancing insulin formulations, production and from protein engineering (Brange et al. 1991). The development of modern insulin variants informs the future development of IgG analogs as therapeutics. MAbs are predominantly built on an IgG scaffold though atomic-level details governing critical interactions with the immune system remain undefined. Based on the recent development of mAbs and protein therapeutics in general, it is clear that basic descriptions of IgG structure/function relationships will drive development of the next generation of drugs.
Two heavy polypeptide chains and two light chains form an IgG heterotetramer (Figure 1). IgG binds to a target through specifically optimized loops contained in the antigen binding fragments (Fabs). The C-terminal halves of the heavy chains form the crystallizable fragment (Fc) that binds various surface-borne and soluble receptors. IgG Fc contains an N-glycan at Asn297 that is required for proper interactions with Fc γ receptors (FcγRs) and the therapeutic efficacy of mAbs that elicit antibody-dependent cell-mediated cytotoxicity (Nose and Wigzell 1983; Guillerey et al. 2016). Humans express as many as five activating FcγRs including the high-affinity FcγRI (CD64) and the low-affinity activating receptors FcγRIIa, FcγRIIc (expressed by ~ 20% of individuals (Ernst et al. 2002; Breunis et al. 2008)), FcγRIIIa and FcγRIIIb (CD32a, CD32c, CD16a and CD16b, respectively). Humans also express a single inhibitory receptor FcγRIIb (CD32b). IgG also binds the C1q component of complement to elicit complement-dependent cytotoxicity. Though C1q binding requires oligomerized IgG and binds to a different Fc surface than the FcγRs (Ugurlar et al. 2018), there are reports that N-glycan composition affects efficacy (Peschke et al. 2017). The neonatal Fc receptor and TRIM21 also bind IgG Fc and binding may be influenced by N-glycan composition (James et al. 2007; Jennewein et al. 2019). A few groups provided data that indicated DC-SIGN and CD23 bind sialylated IgG (reviewed in Pincetic et al. 2014), though recent results cast these conclusions into doubt (Temming et al. 2019).
N-glycan composition impacts mAb recognition, and the composition of serum IgG shows strong correlations to disease. The predominant structure found on serum IgG1 is a complex-type, core fucosylated, biantennary N-glycan (Figure 2). Modifications at the non-reducing termini can add one or two galactose residues that each may be modified with one N-acetylneuraminic acid residue. A correlation between the degree of galactose modification on IgG1 and rheumatoid arthritis provided the first indication that Fc N-glycosylation may impact antibody structure and function (Parekh et al. 1985). More recently, strong connections between the addition of a core fucose residue to autoimmune disorders among many IgG studies in serum and in vitro demonstrated that IgG N-glycan composition is a crucial factor in human health and disease (Figure 2, residue “0”) (Shields et al. 2002; Lauc et al. 2013; Chung et al. 2014; Kapur et al. 2014; Mahan et al. 2016; Sonneveld et al. 2017; Clerc et al. 2018; Doherty et al. 2018).
Figure 2.
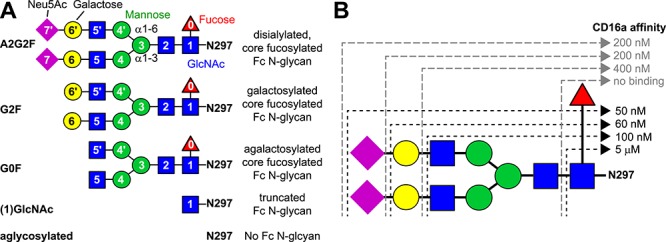
The different Fc N-glycan compositions provide Fc with variable affinity for CD16a. (A) N-glycan compositions as discussed in the text; note the abbreviated identifier to the left of the cartoon figures. (B) IgG1 Fc with each different N-glycan binds with different affinity to CD16a. These KD values were adapted directly from (Subedi and Barb 2016) but are consistent with other reports (Yamaguchi et al. 2006; Thomann et al. 2015; Dekkers et al. 2017). GlcNAc, N-acetylglucosamine; Neu5Ac, N-acetylneuraminic acid.
Surprisingly, the first atomic-resolution structure showed no direct contact between the Fc N-glycans and the FcγR (Sondermann et al. 2000). Later x-ray crystallography studies revealed small differences in IgG Fc quaternary structure, and solution nuclear magnetic resonance (NMR) spectroscopy showed subtle changes in Fc structure and FcγR binding that correlated with N-glycan composition (Krapp 2003; Yamaguchi et al. 2006). These results did not reveal how N-glycan composition impacted receptor affinity, a fact complicated by the observation that the Fc N-glycans appeared immobilized through contacts with the polypeptide (Huber et al. 1976). However, this interpretation was challenged by NMR data showing that polypeptide contacts only partially restricted motion of the N-glycan under more physiological conditions and led to the hypothesis that the N-glycan modulated receptor binding through an indirect mechanism (Barb and Prestegard 2011).
Here we will review the most recent developments toward understanding how N-glycan composition impacts IgG1 Fc structure and receptor binding. An article that thoroughly covers this entire history would prove too expansive for this format. The understanding of IgG Fc structure and function has changed dramatically in recent years, and recent studies will form the focus of this review.
The structure and function of IgG1 Fc is mediated by interactions between the Fc polypeptide and N-glycan residues
IgG1 Fc N-glycan composition impacts FcγR binding
Systematic investigations into the role of Fc N-glycan composition on FcγR binding emerged in the past few years. Though it was known that FcγR binding was sensitive to glycosylation, these recent efforts probed interactions for the entire set of human FcγRs with high sensitivity and a large number of Fc glycoforms. One common feature of these studies is the unique sensitivity of CD16 affinity to Fc N-glycan composition (Thomann et al. 2015; Subedi and Barb 2016; Dekkers et al. 2017). Though CD32 likewise requires Fc N-glycosylation for binding, CD16a and CD16b show a much greater sensitivity to IgG1 Fc N-glycan composition with longer N-glycans promoting tighter interactions (Figure 2B).
The most dramatic affinity differences are due to the addition of a fucose residue to the N-linked GlcNAc residue that reduces affinity for CD16a in vitro (Shields et al. 2002; Shinkawa et al. 2003). Two studies recapitulated the negative impact of core fucosylation on CD16 affinity with a large number of Fc glycoforms, though one study using isolated Fc showed a smaller 4 to 8-fold reduction of binding (Subedi and Barb 2016) compared to another using full length antibodies with a 40-fold reduction (Dekkers et al. 2017). These differences may reflect additional sensitivity of the full-length antibody to Fc N-glycan fucosylation. These studies also showed a moderate benefit of galactosylation (~2-fold) and no consistent measurable impact resulting from sialylation. One study reported only a minimal effect of bisecting N-acetylglucosamine (linked β1–4 to the (3) mannose residue) (Dekkers et al. 2017).
Human IgG1 Fc displaying only a single N-acetylglucosamine residue attached to N297 is produced in situ by the digestion of IgG1 containing an afucosylated N-glycan by EndoS ((1)GlcNAc-Fc; Figure 2B) (Collin and Olsen 2001). Surprisingly, this Fc glycoform binds to CD16a, though with reduced affinity compared to Fc with a larger N-glycan (Subedi and Barb 2015; Okbazghi et al. 2016). Furthermore, human IgG trimmed to the single GlcNAc residue is capable of clearing B cells in a mouse xenograft model (Kao, et al. 2015). These results may be explained by the identification of the first N-glycan residue as contributing a greater degree of stability than any other residue in the glycan (Hanson et al. 2009). The structural relationship of this unique glycoform and the IgG1 Fc structure will be discussed below.
Interactions at the Fc N-glycan/polypeptide interface stabilize N-glycan motion
Following the definition of how Fc N-glycan composition affects receptor binding and the identification of N-glycan motion, multiple studies investigated N-glycan motion further to probe the relationship between N-glycan composition, motion and receptor binding affinity. An all-atom solvated molecular dynamics study demonstrated that the galactose residue attached to the (α1–6mannose) branch of a complex-type N-glycan on Fc exhibited motion (Frank et al. 2014). These simulations were initialized with the starting coordinates observed by x-ray crystallography and showed the detachment of this glycan branch from the protein surface, further supporting the earlier observation by NMR and revealing how the glycan might deform to accommodate conformational exchange (Barb and Prestegard 2011).
Studies on the motion of the Fc N-glycan with multiple glycoforms in solution show that composition impacts glycan interactions. Measurements of the galactose residues before and after sialylation using NMR spectroscopy showed that the modification slightly stabilized the (α1–6mannose) branch (Barb et al. 2012). A later study investigated the motion of the branch N-acetylglucosamine residues during N-glycan maturation starting with a hybrid Mannose5 + N-acetylglucosamine form and ending with a core-fucosylated, complex type N-glycan with N-acetylglucosamine termini (Barb 2015). These results indicated that each remodeling step increased the contact between polypeptide and N-glycan residues during the remodeling of an oligomannose precursor to a final disialylated complex-type N-glycan and are consistent with previous observations in vitro and in vivo (Butler et al. 2003; Bowden et al. 2012). This result suggests that the αmannose residues on the (α1–6mannose) branch of the oligomannose N-glycan likely form unfavorable contacts with the polypeptide and each subsequent glycosyltransferase reaction exploited different contact surfaces.
Hydrogen-deuterium exchange mass spectrometry (HDX) experiments supported the conclusion that extending the Fc N-glycan increases intramolecular interactions, revealing a reduction in deuterium uptake in the P245-T256 peptide following galactosylation (Kiyoshi et al. 2018). The galactose residue directly interacts with residues on this peptide, potentially reducing deuterium uptake by occluding solvent. The authors indicate these data represent changes in the Cγ2/Cγ3 domain orientation. A comparable HDX-MS study followed a greater number of N-glycan compositions, including aglycosylated, (1) GlcNAc, Mannose5 and a mixture of Mannose8-Mannose12 and identified less polypeptide flexibility in Fcs with longer N-glycans (More et al. 2018). Interestingly, the authors identified decreased flexibility in two key regions, the N297-containing C’E loop and the region surrounding N315 and that these two surfaces mediate aggregation. Consistent with the CD16a binding data presented above, this study also reported a substantial reduction in deuterium uptake for the (1)GlcNAc-Fc when compared to the aglycosylated Fc.
Thus far, these data indicate a relationship between N-glycan length and Fc affinity for CD16a. Furthermore, a longer N-glycan appears to stabilize glycan motion and Fc structure. An experiment to mutate phenylalanine residues at the intramolecular interface directly probed this relationship. Prior studies showed that mutating residues at the interface increased N-glycan processing during expression, potentially indicating increased motion of the N-glycans on mutated Fcs and greater exposure to glycan modifying enzymes in the Golgi (Lund et al. 1996; Yu, Baruah, et al. 2013). The observation of Kelly and coworkers that aromatic residues form the strongest interactions with carbohydrate residues through dispersive interactions led to the choice to mutate the Phe241 and Phe243 residues (Chen, Enck, et al. 2013). Mutated Fcs exhibited greater N-glycan processing, with the proteins containing two Phe to Ser mutations showing the greatest processing (Subedi, Hanson, et al. 2014). NMR analyses of mutant Fcs, enzymatically remodeled to have nearly homogeneous G2F glycans, indicated N-glycans on mutant Fcs experienced increased mobility resulting from reduced intramolecular contacts.
Interactions at the Fc N-glycan/polypeptide interface stabilize Fc polypeptide motion and receptor binding
The IgG1 Fc Phe mutants showed greater N-glycan motion with double mutants revealing greater motion than single mutants and single mutants characterized by greater motion than wild-type Fc. An additional experiment demonstrated that these double Phe mutants, enzymatically remodeled to display G2F N-glycans, also bound CD16a with less affinity that the single Phe mutants that bound with less affinity than the wild-type Fc (Subedi, Hanson, et al. 2014). Thus, weaker intramolecular interactions between N-glycan and polypeptide residues led to greater N-glycan motion and weaker receptor binding. A 2D NMR fingerprint of the wild-type and mutant Fcs using [15N]-Tyr labeling indicated that Fc structure was largely preserved in the mutants though one Tyr residue at position 300, near the site of N-glycan attachment to Asn297, showed perturbation of the local structure. A follow-up study indicated that the chemical environment surrounding Tyr300 is uniquely sensitive to the N-glycan composition and that the presence of an IgG1 Fc N-glycan largely impacts structure of the C′ strand and C’E loop, minimally affecting on other areas of the protein (shown in Figure 3) (Subedi and Barb 2015). Furthermore, the N-glycan stabilized C’E loop motions on a μs-ms timescale.
Figure 3.
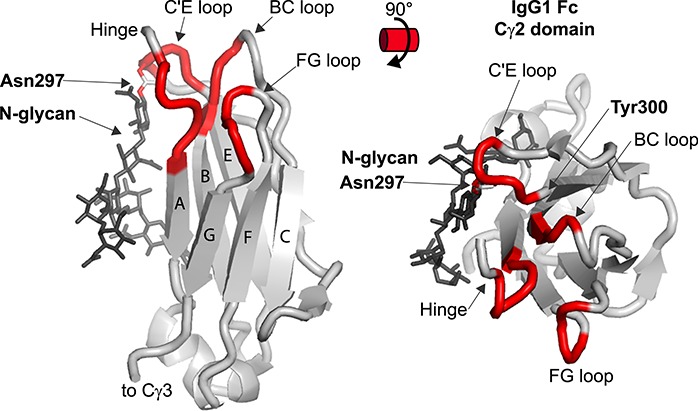
IgG1 Fc Cγ2 domain structure. Residues that are within 5 Å of CD16a are highlighted in red. These contacts are draw for residues that form contacts in either the Fc a or b chain. Strands are labeled with letters.
Extensive molecular dynamics simulations show comparable results with oligomannose-type N-glycans (Lee and Im 2017). These glycans exchange with the Fc surface and form fewer intramolecular contacts than complex type N-glycans particularly at the non-reducing termini. Notably, the presence of a glycan stabilized the C’E loop, leading to stronger receptor interactions.
Thus, the Fc N-glycan dampens motion of the C′ strand and C’E loop. This dampening is enhanced by extending the N-glycan to utilize a greater number of intramolecular interactions. Though CD16a does not directly contact the bulk of the Fc N-glycan, and certainly not the non-reducing termini, CD16a does contact the C’E loop (Sondermann, P., Huber, R., et al. 2000). These results are consistent with the HDX-MS results discussed previously indicating the presence of greater polypeptide structural heterogeneity in Fcs with less stabilizing N-glycan contacts (Kiyoshi et al. 2018; More et al. 2018). One exception to the statement that extending the N-glycan enhances contacts and CD16a binding affinity is modification of the core N-acetylglucosamine residue with fucose. This topic will be specifically addressed in Section 3.
Controversy: Does the N-glycan contribute to Fc quaternary structure?
Evidence to support the stabilization of the IgG1 Fc C′ strand and C’E loop through intramolecular contacts mediated by the N-glycan appears well established. However, recent evidence both supports and contradicts the hypothesis that the N-glycan affects Fc quaternary structure. Recent studies in this area have focused on comparing glycosylated Fc with Fc that contains either a shortened (1) GlcNAc N-glycan or completely lacks modification.
It has been assumed that deglycosylation alters Fc conformation, thereby reducing affinity for proteins that elicit effector functions. This assumption has been examined by x-ray crystallographic analysis of deglycosylated Fc. The crystal structures of aglycosylated human IgG1 Fc (produced in Escherichia coli) (Borrok et al. 2012) and enzymatically deglycosylated murine IgG1-Fc (PNGase F treatment) (Feige et al. 2009) showed a closed conformation of the two Cγ2 domains, as judged from Pro239-Pro239 Cα distances ranging from 18.9Å to 19.6 Å. Hence, an idea was proposed that the closed conformation prevents interaction with FcγRs and the N-glycan at Asn297 stabilizes the open conformation of Fc. Another model of Fc with the N-glycan truncated to a single GlcNAc residue following digestion with EndoS revealed a similarly collapsed conformation (Baruah et al. 2012); however, this glycoform binds CD16a with moderate affinity (Subedi and Barb 2015), indicating that the quaternary structures observed by crystallography fail to clearly separate Fc forms capable of binding receptor from inert forms.
In contrast to these observations, a crystal structure of fully deglycosylated human IgG1 Fc (produced in E. coli) was revealed with an open conformation with the Pro239-Pro239 Cα distance of 27.6 Å (PDB ID: 3DNK). A later study reported a crystal structure of enzymatically deglycosylated human IgG4-Fc (PNGase F treatment) that also adopted an open conformation with the Pro239-Pro239 Cα distance of 29.1 Å (Davies, Jefferis, et al. 2014). The results are inconsistent with the earlier crystallographic results. Taken together, it is likely that deglycosylated Fc adopts a set of conformations (including open and closed) and the observed structures in the crystal may be highly dependent on the crystal packing environment. Therefore, the crystal structures of deglycosylated Fc might not reflect the predominant conformation sampled in solution. Thus, other biophysical techniques are required to characterize conformation in the solution state.
One approach is small-angle x-ray scattering (SAXS), a method to analyze the conformation of macromolecules in solution. Several groups reported SAXS analysis of glycosylated and aglycosylated Fc. The radius of gyration (Rg) was obtained from the SAXS analysis, which is defined as the root mean square distance of all atoms from the common center of mass. Borrok et al. (2012) reported that the glycosylated human IgG1 Fc displayed a Rg of 27.4 Å, compared to 28.3 Å for aglycosylated Fc (E. coli expression). They suggest that in solution the absence of a glycan promotes a more open conformation. Yageta et al. (2019) performed a similar SAXS analysis of glycosylated and aglycosylated Fc (E. coli expression) that is consistent with the former result with Rg values of 26.4 Å and 28.9 Å, respectively. Owing to the availability of many Fc crystal structures, these authors analyzed the theoretical SAXS profiles based on individual crystal structures. The data indicated that both glycosylated and aglycosylated Fc adopt a “semi-closed” Cγ2 domain orientation. The conclusion is rather different from that of Borrok et al., but they reasonably assumed that the scattering from the N-glycan itself reduces the Rg of glycosylated Fc compared with the aglycosylated Fc, without any changes in the Cγ2 domain orientation. Comparable MD simulations identified smaller distances between the Cγ2 domains for Fc with truncated N-glycans (Lee and Im 2017). It is also possible that disorder of protein loops or quaternary structure changes contributed to changes in Fc tumbling.
Relative orientation of the Cγ2 and Cγ3 domains were also analyzed by stable isotope-assisted solution NMR spectroscopy (Subedi and Barb 2015). Residual dipolar couplings (RDCs) can be used to define the relative orientation of each observable amide N-H bond vector for 15N-labeled samples. RDCs from selectively 15N-labeled Fc samples (15N-Tyr and 15N-Lys labeled) were measured for both glycosylated and aglycosylated (T299A) human IgG1 Fc. RDCs reveal little difference between the predominant quaternary structures of glycosylated and aglycosylated Fc in solution.
SAXS and NMR analysis were usually analyzed assuming a single averaged conformation without consideration for a distribution of multiple sampled conformations. Recent analysis using single molecule Förster Resonance Energy transfer (FRET) is advantageous, since this technique provides a histogram of the conformations present in solution allowing conformational populations to be detected (Kelliher et al. 2014; Piraino et al. 2015). To introduce a probe into Fc, Ser258 of human IgG-Fc was mutated into Cys and the acceptor and donor fluorescent dyes were introduced to give antibody-dye conjugates with homo- and hetero-combinations of acceptor and donor dyes. A comparison of the FRET efficiency histograms obtained for glycosylated and deglycosylated human IgG indicates that the Fc region becomes more flexible and can assume a wider variety of structures upon deglycosylation with PNGase F or EndoS. This observation further supports the idea that x-ray crystallographic data of deglycosylated Fc captures one out of many Fc conformations in solution.
Then why does removing the Fc N-glycan reduce the affinity with effector molecules? Aglycosylated Fc likely samples a set of multiple conformations while glycosylated Fc experiences a relatively limited range of mobility. The more flexible nature of aglycosylated Fc may be unfavorable for the interaction with effector molecules such as the FcγRs. From a local viewpoint, the orientation of C’E loop, which harbors the Fc N-glycosylation site at Asn297, is thought to be critical for the binding to FcγRs. Solution NMR spectroscopy can provide information on macromolecular motion by measuring the relaxation parameters. By comparing the relaxation rates of glycosylated (WT) and aglycosylated (T299A) human IgG1 Fc, it was found that the C’E loop is relatively more flexible in aglycosylated Fc upon comparison to glycosylated Fc (Subedi and Barb 2015). This is consistent with the x-ray crystallographic data, in which human aglycosylated Fc structure displays high crystallographic temperature factors (B-factors) for the C’E loop, relative to the core of the protein (Borrok et al. 2012). These observations suggest that the conformation of the C’E loop is stabilized by the presence of the N-glycan and the loop conformation is essential for forming the FcγR interface.
Fc engineering targeting the C’E loop and glycan remodeling
The ultimate goal of a structural definition is to generate sufficient data to design Fcs with altered properties. A number of studies support the role of the C’E loop and N-glycan in receptor binding and Fc stability. A study by Isoda et al. (2015) tested the effect of each amino acid type substituted at position 296. This residue is a Tyr in human IgG1 Fc and located immediately adjacent to the glycosylated Asn297 residue. Tyr300 may form contacts with the core fucose residue (Matsumiya et al. 2007). With the exception of a Trp substitution, all residues reduced affinity for CD16a and CD16b. A crystal structure of the Fc Tyr296Trp variant indicated a presence of greater contacts that slightly increase affinity for CD16a.
The directed replacement of both the Gln295 and Tyr296 residues increased Fc thermal stability and decreased N-glycan processing (Chen et al. 2016). Based on an idealized structure, the Fc Gln295Phe Tyr296Ala variant revealed reduced N-glycan processing evident from the appearance of high levels of minimally-processed hybrid-type N-glycans rarely observed on IgG1 Fc. The enhanced thermal stability resulted from stabilization of the Cγ2; however, this mutation decreased binding to CD32a, CD16a and CD16b. It is evident that decreased FcγR affinity in a mAb would severely limit efficacy if FcγR-mediated effector function is desired because the high concentration of serum IgG (~10 mg/mL = 67 μM) is far above the dissociation constant for IgG1 (50–400 nM).
A mutation to the Cγ3 domain that disrupted Fc dimer formation also increased N-glycan processing (Rose et al. 2013). The Fc Tyr403Glu variant of IgG1 and IgG4 showed increased N-glycan processing similar to the Phe243 variant, indicating that Fc dimer formation contributes to restricted processing of the Fc N-glycan. These results are important for efforts to alter Fc properties by modifying N-glycan processing during protein expression.
IgG sialylation and fucosylation affects IgG Fc structure and function
Structural consequences of core fucosylation
Antibody-dependent cellular cytotoxicity is a key effector function, relying on the binding of antigen-antibody complexes to FcγRs. Most natural antibodies are highly fucosylated. It was discovered that antibodies lacking core fucosylation show a large increase in affinity for CD16a leading to an enhanced ADCC activity. This discovery stimulated the development of therapeutic antibodies with desired activity by specifically increasing or decreasing fucosylation. Many therapeutic antibodies are, however, modified with core fucose, which may indicate that FcγR-mediated activity is not desired for therapeutic purpose. One possible mechanism explaining affinity enhancement by defucosylation was revealed from x-ray crystallographic analysis, using afucosylated Fc and glycosylated CD16a. Ferrara et al. (2011) reported a crystal structure of afucosylated human IgG1 Fc in complex with a glycosylated CD16a. Afucosylated Fc was prepared by introducing the GnTIII gene into the host cell to produce bisected and afucosylated N-glycans. For the preparation of the human CD16a, three out of five N-linked glycosylation sites (38, 74 and 169) were removed by changing the Asn residues (38, 74 and 169) to Gln. Asn162 and Asn45 were kept because they are essential for the affinity toward IgG1 and for expression, respectively. Further, the CD16a was expressed in the presence of kifunensine, producing the oligomannose-type N-glycans. In the crystal structure of the afucosylated Fc-glycosylated CD16a complex, unique intermolecular carbohydrate–carbohydrate interactions appeared, connecting N-glycans of the receptor the afucosylated Fc N-glycan. In order to understand the regulatory mechanism of IgG core fucosylation, fucosylated Fc was used for comparison. In contrast to the complex with afucosylated Fc, carbohydrate–carbohydrate contact area was significantly decreased in the complex structure of fucosylated Fc with glycosylated CD16a. Core fucose linked to the Fc is oriented toward the second N-acetylglucosamine residue of the Asn162-glycan and as a result the Asn162-glycan must move and hence carbohydrate–carbohydrate contact is not properly formed.
Independently, Mizushima et al. (2011) reported the crystal structure of afucosylated IgG1-Fc in complex with glycosylated CD16a. In this case, CD16a possesses a complex-type biantennary complex glycan at Asn45 and Asn162 and the other remaining N-glycosylation sites were similarly mutated to abolish N-glycosylation. Although the CD16a glycan composition differs between the two reports (high mannose vs complex), carbohydrate–carbohydrate interactions were observed. Ten sugar residues were observed with the oligomannose-type Asn162-glycan (Ferrara et al. 2011) and eight residues were detected in the complex with the complex-type Asn162-glycan (Mizushima et al. 2011).
Recently, Falconer et al. (2018) reported the crystal structure of afucoyslated IgG1 Fc in complex with CD16a with Mannose5 N-glycans, which was different from the previous reports. Among the CD16a glycoforms (Mannose5, Mannose9 and complex-type), the Mannose5 form showed the highest affinity toward afucosylated Fc. In fact, CD16a isolated from NK cells contains a substantial amount of oligomannose and hybrid-type glycans (Patel et al. 2018). In the crystal structure of the complex, in contrast with earlier reports, electron density was detected only from single N-acetylglucosamine residue attached to Asn162 of CD16a. It is unclear why the intermolecular N-glycan contacts were not observed. It is possible that intermolecular N-glycan contacts in the previous cases formed as a result of the crystal contacts. To better understand this point, the analysis of N-glycan dynamics is required.
MD simulations were performed to examine the effect of Fc afucosylation on the dynamics of the CD16a Asn162-glycan (Sakae et al. 2017). The root mean square fluctuation of Asn162-complex-type glycan was significantly higher (5.5 Å) in the fucosylated Fc system than in the afucosylated Fc system (3.7 Å). This can be interpreted as a significant disruption of proper carbohydrate-carbohydrate interactions upon Fc fucosylation. MD simulations were also performed with CD16a displaying a Mannose5 glycoform with either fucosylated or afucosylated Fc (Falconer et al. 2018). The addition of an Fc core fucose residue decreased the volume sampled by the Asn162-linked glycan from 10,800 Å3 to 9,100 Å3. Falconer et al. interpreted these results to indicate that the Fc core fucose restricts the conformational space of Asn162, introducing a fucose-dependent energetic penalty upon binding to Fc. This idea is opposed to the previously proposed mechanism that direct intermolecular glycan-glycan contacts stabilized the complex (Ferrara et al. 2011; Mizushima et al. 2011). The mechanism of affinity enhancement upon defucosylation is still controversial, but we must pay attention to the interpretation of crystal structures which rather reflect a snapshot from many possible conformations and are often affected by unwanted crystal contacts.
Structural consequences of sialylation
Another topic regarding the structure and function of the Fc N-glycan is sialylation. In general, sialic acid can be incorporated in α2–3 or α2–6 linkages to a Gal residue. Looking at the Fc part of natural IgG, sialylated glycans are only a small population and are connected only through α2–6 linkages. α2–3 Neu5Ac onto the α1–6 branch of the Fc glycan is predicted to have a big impact on the native glycan-polypeptide interface. In vivo studies have shown that intravenous immunoglobulin G (IVIG) enriched in (α2–6) sialylation of the Fc glycan increased anti-inflammatory activity by up to 10-fold when compared to nonenriched IgG (Anthony, Nimmerjahn, et al. 2008; Anthony et al. 2011). When IVIG was treated with a sialidase to remove the modification, the anti-inflammatory activity was abrogated (Kaneko et al. 2006). Furthermore, ADCC activity is enhanced by (α2–6) sialylation in the absence of core fucosylation in in vitro and in vivo assays (Li et al. 2017).
How sialylation regulates the Fc activity is still unclear, but several mechanisms were discussed and proposed. One proposed mechanism is that sialylated IgG-Fc is recognized by a specific receptor. In vivo experiments showed that the anti-inflammatory effect of Fc required expression of the murine C-type lectin receptor SIGN-R1 (Anthony, Wermeling, et al. 2008). A human orthologue for SIGN-R1 is the C-type lectin DC-SIGN, and it is also proposed to bind to sialylated Fc (Anthony, Wermeling, et al. 2008). Later, Sondermann et al. (2013) showed that α2,6-sialylated IgG binds to the IgE receptor CD23 in a cell-based ELISA assay. The model was based on a hypothesis that sialylation of IgG Fc leads to a conformational change, which triggers receptor binding. It should be noted that conflicting results have been reported (Yu, Vasiljevic, et al. 2013; Temming et al. 2019), thus further analysis will be required for the identification of bona fide receptor(s). Other candidate receptors are implicated, including DCIR (Massoud et al. 2014) and Siglecs (von Gunten and Simon 2008; Seite et al. 2010).
Attention has been paid to examine the effect of sialylation on the conformational property of Fc. Several crystal structures of sialylated Fc have been reported from several groups (Crispin et al. 2013; Ahmed et al. 2014; Chen et al. 2017). Overall, the conformations of sialylated Fc are within the range of Fc structures without sialylation. In a representative crystal structure of sialylated Fc, electron density of Neu5Ac residue is observed only on the (α1–6Mannose) branch and Neu5Ac interacted through water-mediated hydrogen bonds with residues found at the interface formed between the Cγ2 and Cγ3 domains (Chen et al. 2017). The observed interaction between Neu5Ac and residues at the Cγ2-Cγ3 interface may modulate the orientation of the Cγ2-Cγ3 domain and affect the binding affinity toward FcγRs and the neonatal Fc receptor (Chen et al. 2017). Based on the observation that certain N-glycan compositions caused increased variation in Fc crystal structures, Bjorkman and coworkers suggested that sialylation increases conformational flexibility of the Cγ2 domain which is associated with anti-inflammatory activity of the Fc (Ahmed et al. 2014). However, this hypothesis does not explain how the conformational flexibility of Fc affects receptor binding. Thus, the mechanism behind the anti-inflammatory activity of sialylated IgG is still an open question.
Studies on other IgGs
There are four human IgG antibody subclasses: IgG1, IgG2, IgG3 and IgG4, which are homologous to over 90% at the amino acid level (Vidarsson et al. 2014). The global structures of the four human IgG subclasses are thus similar. However, the subclasses have sequence variations especially in the hinge region and N-terminal Cγ2 domain (Vidarsson et al. 2014). This variation is likely linked to differing affinities for the specific FcγRs and different abilities to activate complement (Bruhns et al. 2009; Vidarsson et al. 2014). So far, most studies have been conducted for human IgG1 subclasses and a large set of human IgG1 crystal structures have been reported. In contrast, the structural information on other subclasses is rather limited.
Human IgG4 is the least abundant of the four classes of IgG in serum, but displays unique biological properties. One is heavy chain exchange, also known as Fab-arm exchange, to form a bispecific but monovalent antibody (Aalberse and Schuurman 2002; Davies et al. 2013). IgG4 binds Fcγ receptors with lower affinity than IgG3 with the exception of FcγRI (Bruhns et al. 2009) and does not activate complement (van der Zee et al. 1986); these properties make IgG4 suitable for therapeutics when effector functions are undesired (Davies and Sutton 2015). For this reason, the IgG4 subclass is collecting a lot of attention as a preferred subclass for immunotherapy, exemplified by the anti-PD1 therapeutic antibodies (pembrolizumab/Keytruda® and nivolumab/Opdivo®).
Davies, Rispens, et al. (2014) reported the high-resolution crystal structure of human IgG4 Fc, prepared from papain digestion of serum IgG4 and using recombinant Fc. The overall topology of human IgG4 Fc is very similar to human IgG1 Fc fragments. The Asn297 glycans were facing each other in the cavity formed between two Cγ2 domains. Shortly thereafter, the crystal structure of full-length human IgG4 antibody S228P (pembrolizumab) was reported at 2.3 Å resolution (Scapin et al. 2015). Looking at the Fc region, the orientation of one Cγ2 domain is different from the previously reported IgG4 Fc structure. In the structure of the full-length antibody, the orientation of one Cγ2 domain (chain B) displayed a rotation of 120o relative to the position observed in the isolated Fc, maintaining the immunoglobulin fold. Consequently, the Asn297 glycan on chain B is more solvent-exposed than chain A. This nature is verified by measuring the deglycosylation rate of IgG4 and a reference IgG1. The rate of IgG4 deglycosylation by PNGase F is faster than for IgG1, suggesting that IgG4 N-glycan is more solvent-exposed than in IgG1. However, one cannot rule out the possibility that this unusual Cγ2 conformation represents only one of many possible conformations.
IgG3 is the third most abundant human IgG subclass. It contains a long hinge region which is thought to provide additional flexibility toward antigen binding (Vidarsson, G., Dekkers, G., et al. 2014). A high-resolution (1.8 Å) crystal structure utilized human IgG3 Fc expressed with a modified yeast strain that added homogeneous Mannose5 N-glycans (Shah et al. 2017). Out of the five sugar residues, the first three sugar residues corresponding to Mannose(β1–4)N-acetylglucosamine(β1–4)N-acetylglucosamine revealed electron density. The terminal mannose residues were not observed. The protein-carbohydrate interactions for the three visible sugar residues were identical to those previously reported for human IgG1 Fc structures (Deisenhofer 1981, Nagae and Yamaguchi 2012).
Human IgG2 is known to form structural isomers that originate from alternative disulfide bond formation of between the cysteines in the hinge region (Wypych et al. 2008). It is suggested that the activity of each human IgG2 isomer will be different (Dillon et al. 2008), and hence this point needs to be analyzed in detail. So far two IgG2 Fc crystal structures were reported, one is isolated after papain digestion and the other is from a recombinant construct (Teplyakov et al. 2013). The human IgG2 Fc structures are very similar to human IgG1 Fc structures. It is likely that sequence differences in the lower high region between IgG1 and IgG2 account for differences in FcγR binding affinity.
Structural studies on non-human IgG are limited. Recently, a comprehensive study was performed for all IgG subclasses, IgG1, IgG2, IgG3 and IgG4-Fc of an Old-World monkey, Rhesus macaque (Macaca mulatta, Mm) (Tolbert et al. 2019). Crystal structures of the Fc from MmIgG1–4 were solved with resolutions from 2.8 Å to 3.5 Å. The overall topologies of the MmIgG1–4 Fc resemble that of human IgG1 Fc. Solution NMR spectroscopy was employed to analyze the dynamics of the Fc-glycan at Asn297, with the aid of metabolic 13C-labeling (Tolbert et al. 2019). N-glycan dynamics were evaluated from the linewidth of anomeric 1H signals from Asn297-linked N-acetylglucosamine residues. The linewidth is in the following order: human IgG1-Fc (125 Hz) > Mm IgG1-Fc (102 Hz) > Mm IgG2-Fc (83 Hz) > Mm IgG4-Fc (75 Hz) > Mm IgG3 (66 Hz). This indicates that among Mm IgGs, the Fc N-glycan dynamics is the most restricted in Mm IgG1. This data is supported by the observation that the N-glycan of Mm IgG1-Fc is more stabilized through glycan-polypeptide interaction than Mm IgG2–4. Interestingly, Mm IgG1 experienced the most glycan processing as compared to other Mm IgGs, suggesting that the Mm IgG1 N-glycan is more accessible to glycosyltransferases (galactosyltransferase, sialyltransferase etc.) and this may be correlated with the slowest dynamics of Mm IgG1 Fc glycan.
Mice express different IgG subclasses that vary by strain and include IgG1, IgG2a, IgG2b, IgG2c and IgG3. Subclass nomenclature has arisen independently for each species and there is no general relationship between the subclasses from different species. A recent study indicates that mouse FcγRIV is the homolog of human FcγRIIIa (Dekkers et al. 2018). However, much less is known about mouse IgG2 or mouse FcγRIV. Crystal structures of mouse IgG2c Fc have been reported recently (Falconer and Barb 2018) and compared with that of mouse IgG2b Fc. These two Fcs showed a high degree of similarity with differences in loop residues including Tyr296. Solution NMR analysis of the Asn297-linked N-acetylglucosamine residue shows differences between mouse IgG2b, mouse IgG2c and human IgG1, in terms of anomeric 1H chemical shift, and that correlates to receptor binding affinity. Even in highly conserved molecules, structural and functional differences are present which should be further investigated to fully establish structure/function relationships across important model species.
Noteworthy technical achievements
Recent technical advances have provided a wealth of new tools to probe Fc structure and motion. Progress is focused in two primary areas. Developments in IgG1 Fc expression, purification and glycoengineering are substantial; this topic is directly related to pharmaceutical development and too broad to appropriately cover here (see Loos and Steinkellner 2012; Subedi et al. 2015; Mimura et al. 2018). The second major group of advances results from improvements to analytical techniques or new analyses of IgG. These reports have not themselves elucidated new aspects of the IgG Fc structure/function relationship; however, these techniques warrant a separate section highlighting significant progress in the past few years that may be a foundation for future advances.
Solution NMR studies designed to probe protein motion require assignment of the backbone nuclei. This allows the extraction of atomic-level detail regarding protein structure and motion. Kato and coworkers reported a major advance with the backbone resonance assignment of the glycosylated IgG1 Fc fragment (Yagi et al. 2015). Ordinarily, Fc would be a challenging NMR target due to the size of the molecule alone (~52 kDa). However, the challenges are magnified because appropriately glycosylated IgG1 Fc cannot be expressed in prokaryotic hosts and mammalian hosts are often used instead. This requirement introduces two additional limitations. First, proteins expressed in mammalian hosts, like the CHO cells used in this study, cannot be deuterated because high deuterium content of the medium is toxic to the host. Second, labeling expressed protein with stable 13C and 15N isotopes is much more expensive in a mammalian host compared to a prokaryotic host. The authors reported the nearly complete assignment of IgG1 Fc (99% of HN, N and Cα). This assignment provided a crucial starting point for at least two studies described above (Subedi et al. 2014; Subedi and Barb 2015).
IgG1 Fc binds multiple protein ligands through many different interfaces (Hanson and Barb 2015). The Fc-binding portion of one ligand, protein A, was developed to simultaneously bind IgG1 Fc and lanthanide ions with high affinity (Barb and Subedi 2016). Paramagnetic lanthanide ions provide a means to study distance and orientation up to 50 Å from the ion using solution NMR spectroscopy, but some ions also provide luminescence for microscopy and fluorescence-type solution measurements, unpaired electrons for EPR, and electron dense nuclei for electron and x-ray diffraction (Su and Otting 2010; Koehler and Meiler 2011). This specific construct revealed minimal motion of the paramagnetic ion relative to IgG1 Fc, expanding the utility of the tag for probing glycan and protein motion and conformation (Barb and Subedi 2016).
An additional advance included the development of a suite of NMR experiments to probe commercial antibodies and other therapeutic proteins. One challenge facing the pharmaceutical industry is appropriately validating the composition and structure of each product batch. Unlike small molecule drugs, three-dimensional conformation is an essential property of therapeutic proteins and is challenging to probe with traditional techniques used by the manufacturers. A simple mass analysis is insufficient. Developing techniques with suitable sensitivity, resolution and throughput represents a major challenge. Marino and coworkers developed multiple methods that provide sufficient resolution and are capable of characterizing pharmaceutical products using natural abundance of 13C and 15N. One method utilizes the unique property of methyl groups that provide superior detection sensitivity and resolution (Arbogast et al. 2015). Their developments reduced experimental time to 30 min and were sensitive enough to show resolvable differences in antibodies with different N-glycan composition. The next experiments correlated backbone amide protons to the nitrogen nuclei, an approach that is inherently less sensitive than detecting methyl correlations but provides a fingerprint of the protein that is likely more influenced by subtle conformational changes including changes in hydrogen bonding (Arbogast et al. 2016). These experiments required more instrument time of ~17 h for a single analysis, but provided a greater magnitude of changes, detecting large differences in peak positions following deglycosylation and applying a rigorous statistical analysis to the spectra (Arbogast et al. 2017). Thus, NMR is capable of detecting large conformational differences and small structural differences resulting from processing (Brinson et al. 2019). A related study by a group at Pfizer identified spectral features from 1H-13C methyl groups that correlated with aggregation (Majumder et al. 2018). Aggregation is another significant problem in pharmaceutical production, and is specific to individual mAb clones. This report indicates that surprisingly melting temperature did not correlate with aggregation behavior in a pilot study using three mAbs, but spectral features measured by NMR did. This result may represent a significant advance toward characterizing therapeutic proteins, though future studies with a larger number of proteins will determine if spectral features are reliable probes of aggregation behavior.
Though the common application of mass spectrometry provides mass information and generally does not probe molecular shape, advances in ion mobility mass spectrometry are providing new insight into protein structure and function. Tian and Ruotolo (2018) reported the unfolding of different antibody glycoforms in the gas phase. Though they observed greater sensitivity using the isolated Fc fragment, this team differentiated antibodies that differed only in glycoform.
Future considerations
Fundamental properties of IgG emerged in the past few years that define how Fc composition impacts structure and receptor binding. This may appear surprising due to the importance and intense scrutiny focused on this molecule since the description of IgG in 1939 and high-resolution models of the Fab and Fc in 1973 and 1976, respectively (Tiselius and Kabat 1939; Poljak et al. 1973; Huber et al. 1976). Recent advances were largely driven by developments in physical techniques and Fc preparation methods, allowing researchers to break free from the bounds imposed by the crystal lattice. It is then worthwhile to consider which remaining undefined aspects may lead to better therapies once defined. Here we identify three primary areas for future research.
1. Future studies of unglycosylated antibodies may provide insight into which conformations are disfavored by intramolecular N-glycan interactions. A quantitative analysis of conformation states sampled by the FcγR-binding loops of aglycosylated Fc that defines the populations of each state will guide Fc engineering to limit the exposure of residues that promote aggregation and potentially enhance FcγR binding affinity. Similarly, it is likely that the Fc N-glycan completely dissociates from the protein surface and becomes accessible to glycan modifying enzymes. The structure of this state, and the definition of the conformational rearrangements that promote sampling this state, will impact Fc engineering to modify N-glycan composition during expression.
2. IgG1 is now a well-defined molecule, though some questions still remain. Much less is known about IgG2,3,4, IgD, IgM and IgE. Each of these share an N-glycan at a position homologous to N297 of IgG1 Fc and display an aromatic residue similar to F243 (Subedi, G.P., Hanson, Q.M., et al. 2014). IgE likewise shares a comparable receptor binding mode though the role of N-glycosylation in receptor interactions is less well defined thatn IgG (Shade et al. 2019). It is currently not known how N-glycosylation impacts structure and function for these less-studied antibodies. Furthermore, little is known about antibodies from other species which may provide complementary evolutionarily-selected solutions to stability and receptor binding. Unique among the five human antibody classes, IgA is much different, lacking homologous glycosylation, the key aromatic residue, and IgA displays a different receptor binding mode (Herr et al. 2003).
3. Differences between the behavior of IgG1 and isolated Fc and how Fc modulates interactions between two antibodies may provide insight into improving Fc designs. The 10-fold greater impact of IgG1 Fc fucosylation when compared to isolated Fc in measurements of CD16a binding indicates that the presence of Fab domains may impact Fc activity. Detailed studies to define these interactions will lead to a more complete definition of antibody function. Hints to the presence of intra- and inter-antibody interactions exist, including the observation that IgG1 forms hexamers on surfaces (Saphire et al. 2001; Ugurlar et al. 2018). N-glycans are poised to modulate these interactions as well, though sensitive techniques must be developed to investigate these phenomena further.
It is likely studies of antibody structure and function will continue for decades into the future. The results of these studies stand to improve existing therapies and create engineered mAb backbones for a wide range of future clinical applications, if investigators are sufficiently bold and inventive to identify key features and residues that may be altered for improved drug properties.
Funding
This work was supported by the National Institutes of Health (grant number R01 GM115489) to A.W.B. and by Japan Society for the Promotion of Science Kakenhi (grant numbers JP16H04758 and JP19H03362) to Y.Y.
References
- Aalberse R, Schuurman J. 2002. IgG4 breaking the rules. Immunology. 105:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed AA, Giddens J, Pincetic A, Lomino JV, Ravetch JV, Wang LX, Bjorkman PJ. 2014. Structural characterization of anti-inflammatory immunoglobulin G Fc proteins. J Mol Biol. 426:3166–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. 2011. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 475:110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. 2008. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 320:373–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. 2008. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci U S A. 105:19571–19578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbogast LW, Brinson RG, Formolo T, Hoopes JT, Marino JP. 2016. 2D (1)H(N), (15) N correlated NMR methods at natural abundance for obtaining structural maps and statistical comparability of monoclonal antibodies. Pharm Res. 33:462–475. [DOI] [PubMed] [Google Scholar]
- Arbogast LW, Brinson RG, Marino JP. 2015. Mapping monoclonal antibody structure by 2D 13C NMR at natural abundance. Anal Chem. 87:3556–3561. [DOI] [PubMed] [Google Scholar]
- Arbogast LW, Delaglio F, Schiel JE, Marino JP. 2017. Multivariate analysis of two-dimensional (1) H, (13) C methyl NMR spectra of monoclonal antibody therapeutics to facilitate assessment of higher order structure. Anal Chem. 89:11839–11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barb AW. 2015. Intramolecular N-glycan/polypeptide interactions observed at multiple N-glycan remodeling steps through [(13)C,(15)N]-N-acetylglucosamine labeling of immunoglobulin G1. Biochemistry. 54:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barb AW, Meng L, Gao Z, Johnson RW, Moremen KW, Prestegard JH. 2012. NMR characterization of immunoglobulin G Fc glycan motion on enzymatic sialylation. Biochemistry. 51:4618–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barb AW, Prestegard JH. 2011. NMR analysis demonstrates immunoglobulin G N-glycans are accessible and dynamic. Nat Chem Biol. 7:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barb AW, Subedi GP. 2016. An encodable lanthanide binding tag with reduced size and flexibility for measuring residual dipolar couplings and pseudocontact shifts in large proteins. J Biomol NMR. 64:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruah K, Bowden TA, Krishna BA, Dwek RA, Crispin M, Scanlan CN. 2012. Selective deactivation of serum IgG: A general strategy for the enhancement of monoclonal antibody receptor interactions. J Mol Biol. 420:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz SA, Engen JR, Mazzeo JR, Jones GB. 2012. Analytical tools for characterizing biopharmaceuticals and the implications for biosimilars. Nat Rev Drug Discov. 11:527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss M. 2007. The Discovery of Insulin. 25th anniversary ed. Chicago: University of Chicago Press. [Google Scholar]
- Borrok MJ, Jung ST, Kang TH, Monzingo AF, Georgiou G. 2012. Revisiting the role of glycosylation in the structure of human IgG Fc. ACS Chem Biol. 7:1596–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden TA, Baruah K, Coles CH, Harvey DJ, Yu X, Song BD, Stuart DI, Aricescu AR, Scanlan CN, Jones EY et al. 2012. Chemical and structural analysis of an antibody folding intermediate trapped during glycan biosynthesis. J Am Chem Soc. 134:17554–17563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brange J, Dodson G, Xiao B. 1991. Designing insulin for diabetes therapy by protein engineering. Current Opinion in Structural Biology. 1:934–940. [Google Scholar]
- Breunis WB, van Mirre E, Bruin M, Geissler J, de Boer M, Peters M, Roos D, de Haas M, Koene HR, Kuijpers TW. 2008. Copy number variation of the activating FCGR2C gene predisposes to idiopathic thrombocytopenic purpura. Blood. 111:1029–1038. [DOI] [PubMed] [Google Scholar]
- Brinson RG, Marino JP, Delaglio F, Arbogast LW, Evans RM, Kearsley A, Gingras G, Ghasriani H, Aubin Y, Pierens GK et al. 2019. Enabling adoption of 2D-NMR for the higher order structure assessment of monoclonal antibody therapeutics. MAbs. 11:94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daeron M. 2009. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 113:3716–3725. [DOI] [PubMed] [Google Scholar]
- Butler M, Quelhas D, Critchley AJ, Carchon H, Hebestreit HF, Hibbert RG, Vilarinho L, Teles E, Matthijs G, Schollen E et al. 2003. Detailed glycan analysis of serum glycoproteins of patients with congenital disorders of glycosylation indicates the specific defective glycan processing step and provides an insight into pathogenesis. Glycobiology. 13:601–622. [DOI] [PubMed] [Google Scholar]
- Chen CL, Hsu JC, Lin CW, Wang CH, Tsai MH, Wu CY, Wong CH, Ma C. 2017. Crystal structure of a homogeneous IgG-fc glycoform with the N-glycan designed to maximize the antibody dependent cellular cytotoxicity. ACS Chem Biol. 12:1335–1345. [DOI] [PubMed] [Google Scholar]
- Chen W, Enck S, Price JL, Powers DL, Powers ET, Wong CH, Dyson HJ, Kelly JW. 2013. Structural and energetic basis of carbohydrate-aromatic packing interactions in proteins. J Am Chem Soc. 135:9877–9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Kong L, Connelly S, Dendle JM, Liu Y, Wilson IA, Powers ET, Kelly JW. 2016. Stabilizing the CH2 domain of an antibody by engineering in an enhanced aromatic sequon. ACS Chem Biol. 11:1852–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AW, Crispin M, Pritchard L, Robinson H, Gorny MK, Yu X, Bailey-Kellogg C, Ackerman ME, Scanlan C, Zolla-Pazner S et al. 2014. Identification of antibody glycosylation structures that predict monoclonal antibody Fc-effector function. AIDS. 28:2523–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc F, Novokmet M, Dotz V, Reiding KR, de Haan N, Kammeijer GSM, Dalebout H, Bladergroen MR, Vukovic F, Rapp E et al. 2018. Plasma N-glycan signatures are associated with features of inflammatory bowel diseases. Gastroenterology. 155:829–843. [DOI] [PubMed] [Google Scholar]
- Collin M, Olsen A. 2001. EndoS, a novel secreted protein from streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 20:3046–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispin M, Yu X, Bowden TA. 2013. Crystal structure of sialylated IgG fc: Implications for the mechanism of intravenous immunoglobulin therapy. Proc Natl Acad Sci U S A. 110:E3544–E3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AM, Jefferis R, Sutton BJ. 2014. Crystal structure of deglycosylated human IgG4-fc. Mol Immunol. 62:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AM, Rispens T, den Bleker TH, McDonnell JM, Gould HJ, Aalberse RC, Sutton BJ. 2013. Crystal structure of the human IgG4 C(H)3 dimer reveals the role of Arg409 in the mechanism of fab-arm exchange. Mol Immunol. 54:1–7. [DOI] [PubMed] [Google Scholar]
- Davies AM, Rispens T, Ooijevaar-de Heer P, Gould HJ, Jefferis R, Aalberse RC, Sutton BJ. 2014. Structural determinants of unique properties of human IgG4-fc. J Mol Biol. 426:630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AM, Sutton BJ. 2015. Human IgG4: A structural perspective. Immunol Rev. 268:139–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J. 1981. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 20:2361–2370. [PubMed] [Google Scholar]
- Dekkers G, Bentlage AEH, Plomp R, Visser R, Koeleman CAM, Beentjes A, Mok JY, van Esch WJE, Wuhrer M, Rispens T et al. 2018. Conserved FcgammaR- glycan discriminates between fucosylated and afucosylated IgG in humans and mice. Mol Immunol. 94:54–60. [DOI] [PubMed] [Google Scholar]
- Dekkers G, Treffers L, Plomp R, Bentlage AEH, de Boer M, Koeleman CAM, Lissenberg-Thunnissen SN, Visser R, Brouwer M, Mok JY et al. 2017. Decoding the human immunoglobulin G-glycan repertoire reveals a spectrum of Fc-receptor- and complement-mediated-effector activities. Front Immunol. 8:877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon TM, Ricci MS, Vezina C, Flynn GC, Liu YD, Rehder DS, Plant M, Henkle B, Li Y, Deechongkit S et al. 2008. Structural and functional characterization of disulfide isoforms of the human IgG2 subclass. J Biol Chem. 283:16206–16215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty M, Theodoratou E, Walsh I, Adamczyk B, Stockmann H, Agakov F, Timofeeva M, Trbojevic-Akmacic I, Vuckovic F, Duffy F et al. 2018. Plasma N-glycans in colorectal cancer risk. Sci Rep. 8:8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst LK, Metes D, Herberman RB, Morel PA. 2002. Allelic polymorphisms in the FcgammaRIIC gene can influence its function on normal human natural killer cells. J Mol Med (Berl). 80:248–257. [DOI] [PubMed] [Google Scholar]
- Falconer DJ, Barb AW. 2018. Mouse IgG2c fc loop residues promote greater receptor-binding affinity than mouse IgG2b or human IgG1. PLoS One. 13: e0192123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer DJ, Subedi GP, Marcella AM, Barb AW. 2018. Antibody fucosylation lowers the FcgammaRIIIa/CD16a affinity by limiting the conformations sampled by the N162-glycan. ACS Chem Biol. 13:2179–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige MJ, Nath S, Catharino SR, Weinfurtner D, Steinbacher S, Buchner J. 2009. Structure of the murine unglycosylated IgG1 Fc fragment. J Mol Biol. 391:599–608. [DOI] [PubMed] [Google Scholar]
- Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M et al. 2011. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 108:12669–12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Walker RC, Lanzilotta WN, Prestegard JH, Barb AW. 2014. Immunoglobulin G1 fc domain motions: Implications for Fc engineering. J Mol Biol. 426:1799–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillerey C, Huntington ND, Smyth MJ. 2016. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. 17:1025–1036. [DOI] [PubMed] [Google Scholar]
- Hanson QM, Barb AW. 2015. A perspective on the structure and receptor binding properties of immunoglobulin G Fc. Biochemistry. 54:2931–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SR, Culyba EK, Hsu TL, Wong CH, Kelly JW, Powers ET. 2009. The core trisaccharide of an N-linked glycoprotein intrinsically accelerates folding and enhances stability. Proc Natl Acad Sci U S A. 106:3131–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr AB, Ballister ER, Bjorkman PJ. 2003. Insights into IgA-mediated immune responses from the crystal structures of human FcalphaRI and its complex with IgA1-Fc. Nature. 423:614–620. [DOI] [PubMed] [Google Scholar]
- Huber R, Deisenhofer J, Colman PM, Matsushima M, Palm W. 1976. Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature. 264:415–420. [DOI] [PubMed] [Google Scholar]
- Isoda Y, Yagi H, Satoh T, Shibata-Koyama M, Masuda K, Satoh M, Kato K, Iida S. 2015. Importance of the side chain at position 296 of antibody fc in interactions with FcgammaRIIIa and other Fcgamma receptors. PLoS One. 10:e0140120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LC, Keeble AH, Khan Z, Rhodes DA, Trowsdale J. 2007. Structural basis for PRYSPRY-mediated tripartite motif (TRIM) protein function. Proc Natl Acad Sci U S A. 104:6200–6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennewein MF, Goldfarb I, Dolatshahi S, Cosgrove C, Noelette FJ, Krykbaeva M, Das J, Sarkar A, Gorman MJ, Fischinger S et al. 2019. Fc glycan-mediated regulation of placental antibody transfer. Cell. 178202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, Nimmerjahn F, Ravetch JV. 2006. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 313:670–673. [DOI] [PubMed] [Google Scholar]
- Kao D, Danzer H, Collin M, Gross A, Eichler J, Stambuk J, Lauc G, Lux A, Nimmerjahn F. 2015. A monosaccharide residue is sufficient to maintain mouse and human IgG subclass activity and directs IgG effector functions to cellular Fc receptors. Cell Rep. 13:2376–2385. [DOI] [PubMed] [Google Scholar]
- Kapur R, Kustiawan I, Vestrheim A, Koeleman CA, Visser R, Einarsdottir HK, Porcelijn L, Jackson D, Kumpel B, Deelder AM et al. 2014. A prominent lack of IgG1-Fc fucosylation of platelet alloantibodies in pregnancy. Blood. 123:471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher MT, Jacks RD, Piraino MS, Southern CA. 2014. The effect of sugar removal on the structure of the Fc region of an IgG antibody as observed with single molecule Forster resonance energy transfer. Mol Immunol. 60:103–108. [DOI] [PubMed] [Google Scholar]
- Kiyoshi M, Caaveiro JMM, Tada M, Tamura H, Tanaka T, Terao Y, Morante K, Harazono A, Hashii N, Shibata H et al. 2018. Assessing the heterogeneity of the Fc-glycan of a therapeutic antibody using an engineered FcgammaReceptor IIIa-immobilized column. Sci Rep. 8:3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler J, Meiler J. 2011. Expanding the utility of NMR restraints with paramagnetic compounds: Background and practical aspects. Prog Nucl Magn Reson Spectrosc. 59:360–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P. 2003. Structural analysis of human IgG-fc glycoforms reveals a correlation between glycosylation and structural integrity. J Mol Biol. 325:979–989. [DOI] [PubMed] [Google Scholar]
- Lauc G, Huffman JE, Pucic M, Zgaga L, Adamczyk B, Muzinic A, Novokmet M, Polasek O, Gornik O, Kristic J et al. 2013. Loci associated with N-glycosylation of human immunoglobulin G show pleiotropy with autoimmune diseases and haematological cancers. PLoS Genet. 9:e1003225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Im W. 2017. Effects of N-glycan composition on structure and dynamics of IgG1 Fc and their implications for antibody engineering. Sci Rep. 7:12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, DiLillo DJ, Bournazos S, Giddens JP, Ravetch JV, Wang LX. 2017. Modulating IgG effector function by Fc glycan engineering. Proc Natl Acad Sci U S A. 114:3485–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, May K. 2012. Disulfide bond structures of IgG molecules: Structural variations, chemical modifications and possible impacts to stability and biological function. MAbs. 4:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos A, Steinkellner H. 2012. IgG-Fc glycoengineering in non-mammalian expression hosts. Arch Biochem Biophys. 526:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J, Takahashi N, Pound JD, Goodall M, Jefferis R. 1996. Multiple interactions of IgG with its core oligosaccharide can modulate recognition by complement and human Fc gamma receptor I and influence the synthesis of its oligosaccharide chains. J Immunol. 157:4963–4969. [PubMed] [Google Scholar]
- Mahan AE, Jennewein MF, Suscovich T, Dionne K, Tedesco J, Chung AW, Streeck H, Pau M, Schuitemaker H, Francis D et al. 2016. Antigen-specific antibody glycosylation is regulated via vaccination. PLoS Pathog. 12:e1005456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S, Jones MT, Kimmel M, Alphonse IA. 2018. Probing conformational diversity of Fc domains in aggregation-prone monoclonal antibodies. Pharm Res. 35:220. [DOI] [PubMed] [Google Scholar]
- Massoud AH, Yona M, Xue D, Chouiali F, Alturaihi H, Ablona A, Mourad W, Piccirillo CA, Mazer BD. 2014. Dendritic cell immunoreceptor: A novel receptor for intravenous immunoglobulin mediates induction of regulatory T cells. J Allergy Clin Immunol. 133:853–863.. [DOI] [PubMed] [Google Scholar]
- Matsumiya S, Yamaguchi Y, Saito J, Nagano M, Sasakawa H, Otaki S, Satoh M, Shitara K, Kato K. 2007. Structural comparison of fucosylated and nonfucosylated Fc fragments of human immunoglobulin G1. J Mol Biol. 368:767–779. [DOI] [PubMed] [Google Scholar]
- Mimura Y, Katoh T, Saldova R, O'Flaherty R, Izumi T, Mimura-Kimura Y, Utsunomiya T, Mizukami Y, Yamamoto K, Matsumoto T et al. 2018. Glycosylation engineering of therapeutic IgG antibodies: Challenges for the safety, functionality and efficacy. Protein Cell. 9:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima T, Yagi H, Takemoto E, Shibata-Koyama M, Isoda Y, Iida S, Masuda K, Satoh M, Kato K. 2011. Structural basis for improved efficacy of therapeutic antibodies on defucosylation of their fc glycans. Genes Cells. 16:1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More AS, Toth RT, Okbazghi SZ, Middaugh CR, Joshi SB, Tolbert TJ, Volkin DB, Weis DD. 2018. Impact of glycosylation on the local backbone flexibility of well-defined IgG1-Fc glycoforms using hydrogen exchange-mass spectrometry. J Pharm Sci. 107:2315–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagae M, Yamaguchi Y. 2012. Function and 3D structure of the N-glycans on glycoproteins. Int J Mol Sci. 13:8398–8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose M, Wigzell H. 1983. Biological significance of carbohydrate chains on monoclonal antibodies. Proc Natl Acad Sci U S A. 80:6632–6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okbazghi SZ, More AS, White DR, Duan S, Shah IS, Joshi SB, Middaugh CR, Volkin DB, Tolbert TJ. 2016. Production, characterization, and biological evaluation of well-defined IgG1 Fc glycoforms as a model system for biosimilarity analysis. J Pharm Sci. 105:559–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T, Matsuta K et al. 1985. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 316:452–457. [DOI] [PubMed] [Google Scholar]
- Patel KR, Roberts JT, Subedi GP, Barb AW. 2018. Restricted processing of CD16a/Fc gamma receptor IIIa N-glycans from primary human NK cells impacts structure and function. J Biol Chem. 293:3477–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschke B, Keller CW, Weber P, Quast I, Lunemann JD. 2017. Fc-Galactosylation of human immunoglobulin gamma Isotypes improves C1q binding and enhances complement-dependent cytotoxicity. Front Immunol. 8:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincetic A, Bournazos S, DiLillo DJ, Maamary J, Wang TT, Dahan R, Fiebiger BM, Ravetch JV. 2014. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol. 15:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piraino MS, Kelliher MT, Aburas J, Southern CA. 2015. Single molecule Forster resonance energy transfer studies of the effect of EndoS deglycosylation on the structure of IgG. Immunol Lett. 167:29–33. [DOI] [PubMed] [Google Scholar]
- Poljak RJ, Amzel LM, Avey HP, Chen BL, Phizackerley RP, Saul F. 1973. Three-dimensional structure of the Fab' fragment of a human immunoglobulin at 2,8-A resolution. Proc Natl Acad Sci U S A. 70:3305–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RJ, van Berkel PH, van den Bremer ET, Labrijn AF, Vink T, Schuurman J, Heck AJ, Parren PW. 2013. Mutation of Y407 in the CH3 domain dramatically alters glycosylation and structure of human IgG. MAbs. 5:219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakae Y, Satoh T, Yagi H, Yanaka S, Yamaguchi T, Isoda Y, Iida S, Okamoto Y, Kato K. 2017. Conformational effects of N-glycan core fucosylation of immunoglobulin G fc region on its interaction with Fcgamma receptor IIIa. Sci Rep. 7:13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saphire EO, Parren PW, Pantophlet R, Zwick MB, Morris GM, Rudd PM, Dwek RA, Stanfield RL, Burton DR, Wilson IA. 2001. Crystal structure of a neutralizing human IGG against HIV-1: A template for vaccine design. Science. 293:1155–1159. [DOI] [PubMed] [Google Scholar]
- Scapin G, Yang X, Prosise WW, McCoy M, Reichert P, Johnston JM, Kashi RS, Strickland C. 2015. Structure of full-length human anti-PD1 therapeutic IgG4 antibody pembrolizumab. Nat Struct Mol Biol. 22:953–958. [DOI] [PubMed] [Google Scholar]
- Seite JF, Cornec D, Renaudineau Y, Youinou P, Mageed RA, Hillion S. 2010. IVIg modulates BCR signaling through CD22 and promotes apoptosis in mature human B lymphocytes. Blood. 116:1698–1704. [DOI] [PubMed] [Google Scholar]
- Shade KT, Conroy ME, Anthony RM. 2019. IgE glycosylation in health and disease. Curr Top Microbiol Immunol. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah IS, Lovell S, Mehzabeen N, Battaile KP, Tolbert TJ. 2017. Structural characterization of the Man5 glycoform of human IgG3 fc. Mol Immunol. 92:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. 2002. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 277:26733–26740. [DOI] [PubMed] [Google Scholar]
- Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M et al. 2003. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 278:3466–3473. [DOI] [PubMed] [Google Scholar]
- Sondermann P, Huber R, Oosthuizen V, Jacob U. 2000. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature. 406:267–273. [DOI] [PubMed] [Google Scholar]
- Sondermann P, Pincetic A, Maamary J, Lammens K, Ravetch JV. 2013. General mechanism for modulating immunoglobulin effector function. Proc Natl Acad Sci U S A. 110:9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneveld ME, de Haas M, Koeleman C, de Haan N, Zeerleder SS, Ligthart PC, Wuhrer M, van der Schoot CE, Vidarsson G. 2017. Patients with IgG1-anti-red blood cell autoantibodies show aberrant Fc-glycosylation. Sci Rep. 7:8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su XC, Otting G. 2010. Paramagnetic labelling of proteins and oligonucleotides for NMR. J Biomol NMR. 46:101–112. [DOI] [PubMed] [Google Scholar]
- Subedi GP, Barb AW. 2015. The structural role of antibody N-glycosylation in receptor interactions. Structure. 23:1573–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subedi GP, Barb AW. 2016. The immunoglobulin G1 N-glycan composition affects binding to each low affinity Fc gamma receptor. MAbs. 8:1512–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subedi GP, Hanson QM, Barb AW. 2014. Restricted motion of the conserved immunoglobulin G1 N-glycan is essential for efficient FcgammaRIIIa binding. Structure. 22:1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subedi GP, Johnson RW, Moniz HA, Moremen KW, Barb A. 2015. High yield expression of recombinant human proteins with the transient transfection of HEK293 cells in suspension. J Vis Exp. 106: e53568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temming AR, Dekkers G, van de FS, Plomp HR, Bentlage AEH, Szittner Z, Derksen NIL, Wuhrer M, Rispens T, Vidarsson G. 2019. Human DC-SIGN and CD23 do not interact with human IgG. Sci Rep. 9:9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teplyakov A, Zhao Y, Malia TJ, Obmolova G, Gilliland GL. 2013. IgG2 Fc structure and the dynamic features of the IgG CH2-CH3 interface. Mol Immunol. 56:131–139. [DOI] [PubMed] [Google Scholar]
- Thomann M, Schlothauer T, Dashivets T, Malik S, Avenal C, Bulau P, Ruger P, Reusch D. 2015. In vitro glycoengineering of IgG1 and its effect on Fc receptor binding and ADCC activity. PLoS One. 10:e0134949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Ruotolo B. 2018. Collision induced unfolding detects subtle differences in intact antibody glycoforms and associated fragments. Int J Mass Spectrom. 425:1–9. [Google Scholar]
- Tiselius A, Kabat EA. 1939. An electrophoretic study of immune sera and purified antibody preparations. J Exp Med. 69:119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert WD, Subedi GP, Gohain N, Lewis GK, Patel KR, Barb AW, Pazgier M. 2019. From rhesus macaque to human: Structural evolutionary pathways for immunoglobulin G subclasses. MAbs. 11:709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurlar D, Howes SC, de Kreuk BJ, Koning RI, de Jong RN, Beurskens FJ, Schuurman J, Koster AJ, Sharp TH, Parren P et al. 2018. Structures of C1-IgG1 provide insights into how danger pattern recognition activates complement. Science. 359:794–797. [DOI] [PubMed] [Google Scholar]
- van der Zee JS, van Swieten P, Aalberse RC. 1986. Inhibition of complement activation by IgG4 antibodies. Clin Exp Immunol. 64:415–422. [PMC free article] [PubMed] [Google Scholar]
- Vidarsson G, Dekkers G, Rispens T. 2014. IgG subclasses and allotypes: From structure to effector functions. Front Immunol. 5:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gunten S, Simon HU. 2008. Natural anti-Siglec autoantibodies mediate potential immunoregulatory mechanisms: Implications for the clinical use of intravenous immunoglobulins (IVIg). Autoimmun Rev. 7:453–456. [DOI] [PubMed] [Google Scholar]
- Wypych J, Li M, Guo A, Zhang Z, Martinez T, Allen MJ, Fodor S, Kelner DN, Flynn GC, Liu YD et al. 2008. Human IgG2 antibodies display disulfide-mediated structural isoforms. J Biol Chem. 283:16194–16205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yageta S, Imamura H, Shibuya R, Honda S. 2019. CH2 domain orientation of human immunoglobulin G in solution: Structural comparison of glycosylated and aglycosylated Fc regions using small-angle X-ray scattering. MAbs. 11:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H, Zhang Y, Yagi-Utsumi M, Yamaguchi T, Iida S, Yamaguchi Y, Kato K. 2015. Backbone (1) H, (13) C, and (15) N resonance assignments of the Fc fragment of human immunoglobulin G glycoprotein. Biomol NMR Assign. 9:257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Nishimura M, Nagano M, Yagi H, Sasakawa H, Uchida K, Shitara K, Kato K. 2006. Glycoform-dependent conformational alteration of the Fc region of human immunoglobulin G1 as revealed by NMR spectroscopy. Biochim Biophys Acta. 1760:693–700. [DOI] [PubMed] [Google Scholar]
- Yu X, Baruah K, Harvey DJ, Vasiljevic S, Alonzi DS, Song BD, Higgins MK, Bowden TA, Scanlan CN, Crispin M. 2013. Engineering hydrophobic protein-carbohydrate interactions to fine-tune monoclonal antibodies. J Am Chem Soc. 135:9723–9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Vasiljevic S, Mitchell DA, Crispin M, Scanlan CN. 2013. Dissecting the molecular mechanism of IVIg therapy: The interaction between serum IgG and DC-SIGN is independent of antibody glycoform or fc domain. J Mol Biol. 425:1253–1258. [DOI] [PubMed] [Google Scholar]


