Abstract
BACKGROUND
Uncontrolled hypertension (HTN) is a leading modifiable stroke risk factor contributing to global stroke disparities. This study is unique in testing a transitional care model aimed at controlling HTN in black and Hispanic poststroke, home health patients, an understudied group.
METHODS
A 3-arm randomized controlled trial design compared (i) usual home care (UHC), with (ii) UHC plus a 30-day nurse practitioner transitional care program, or (iii) UHC plus nurse practitioner plus a 60-day health coach program. The trial enrolled 495 black and Hispanic, English- and Spanish- speaking adults with uncontrolled systolic blood pressure (SBP ≥ 140 mm Hg) who had experienced a first-time or recurrent stroke or transient ischemic attack. The primary outcome was change in SBP from baseline to 3 and 12 months.
RESULTS
Mean participant age was 67; 57.0% were female; 69.7% were black, non-Hispanic; and 30.3% were Hispanic. Three-month follow-up retention was 87%; 12-month retention was 81%. SBP declined 9–10 mm Hg from baseline to 12 months across all groups; the greatest decrease occurred between baseline and 3 months. The interventions demonstrated no relative advantage compared to UHC.
CONCLUSION
The significant across-the-board SBP decreases suggest that UHC nurse/patient/physician interactions were the central component of SBP reduction and that additional efforts to lower recurrent stroke risk should test incremental improvements in usual care, not resource-intensive transitional care interventions. They also suggest the potential value of pragmatic home care programs as part of a broader strategy to overcome HTN treatment barriers and improve secondary stroke prevention globally.
CLINICAL TRIALS REGISTRATION
Trial Number NCT01918891.
Keywords: blood pressure, hypertension, nurse practitioner, randomized controlled trial, stroke
Elevated systolic blood pressure (SBP ≥ 140 mm Hg) is a global health risk, affecting over 1 billion people worldwide.1,2 It is the single most important treatable stroke risk factor and a major contributor to disparities in stroke incidence and related mortality.3–6 Despite important advances in uncontrolled hypertension (HTN) treatment, blood pressure (BP) control in the United States is disproportionately low in both black and Hispanic populations.7 Moreover, the impact of elevated BP on stroke is 3 times greater for blacks than whites.8 When black and Hispanic individuals receive adequate treatment, they can achieve declines in BP comparable to those of whites.9 Evidence from randomized controlled trials suggests that multifaceted, team-based, culturally attuned interventions that address more than 1 treatment barrier at more than 2 levels (e.g., providers, patients, and system) may be the most successful in improving HTN outcomes.10,11 However, such interventions are difficult to implement and sustain. To overcome these issues, experts in HTN control have argued for rigorous evaluation of pragmatic, multilevel interventions.10,12
Here we present the primary results of a pragmatic multilevel intervention study designed to address gaps in the HTN and transitional care evidence base. The study contributes to the relatively small but growing literature on nurse-led multilevel BP interventions.13–15 Transitional care problems have already been documented in health disparities populations; this study is unique in that it seeks to rigorously test a transitional care model focused on a vulnerable poststroke black or Hispanic home care population to address disparity-related HTN treatment barriers.
CONCEPTUAL MODELS
The Chronic Care Model—encompassing community, health system, self-management support, and delivery system components16–18—guided overall intervention design. The Transitional Care Model, adapted for poststroke patients, guided intervention components, and procedures.19,20
METHODS
Overview
The study was a 3-arm randomized controlled trial comparing the effectiveness of (i) usual home care (UHC), with (ii) a nurse practitioner (NP) transitional care program, or (iii) UHC plus NP plus a 60-day health coach (UHC + NP + HC) program. Eligible patients were black or Hispanic, English- or Spanish-speaking poststroke adults with elevated SBP (target enrollment 495 patients). The trial was “pragmatic”—i.e., designed to assess the effectiveness of the interventions in “real-life” practice conditions.21,22 UHC was prescribed for all study patients, who were receiving home care for varied reasons. Detailed study methods have been published previously.23
Study setting
The setting was a large, urban home health organization. Patients are admitted through hospital, rehabilitation facility, and community referrals (primarily physicians). The study protocol was institutional review board approved.
Interventions
Usual home care.
UHC, following a physician-ordered plan of care, consists of skilled nursing and/or rehabilitative therapy; patient education and monitoring; home health aide and/or social work services as appropriate. BP is 1 of 4 vital signs assessed at each visit. UHC nurses may have multiple direct or indirect physician contacts to obtain approval or revision of initial orders, reconcile or seek medication changes, or report a patient's changing condition. Thus UHC intervenes on at least 2 levels: patient and physician.
UHC + NP transitional care arm.
The UHC + NP intervention, based on an existing evidence-based 30-day transitional care program,19,24 included eligible patients referred by hospital discharge planners or community-based physicians, and focused on reducing SBP to reduce recurrent stroke risk. The 30-day protocol recommended 3 in-home visits and 3 patient/caregiver telephone contacts. The NP was responsible for (i) conducting a comprehensive health assessment; (ii) communicating with the patient's physicians and UHC nurse; (iii) monitoring BP; (iv) ensuring appropriate medication and behavioral regimens; (v) collaborating with patients and caregivers to overcome barriers and adhere to a tailored, culturally sensitive self-management plan; and (vi) addressing patients' social support needs through referral to appropriate community resources. A bilingual NP served Spanish-speaking patients. The other primary NP was African American.
UHC + NP + HC transitional care arm.
In this arm, all patients received UHC plus the intensive 30-day NP intervention followed by 60 days of coaching/self-management support from a home health aide specially trained to be an HC. The HC protocol added 3 in-home visits and 3 telephone contacts beyond the 30-day NP intervention. Coaches were bilingual with similar racial/ethnic backgrounds as the target patient population. The HCs' main responsibilities were to (i) promote ongoing stroke prevention and risk factor awareness, (ii) support patient self-management, and (iii) facilitate patients' integration into the community after discharge.
Eligibility criteria
Patient eligibility required a first-time or recurrent stroke or transient ischemic attack at any point prior to enrollment, uncontrolled SBP at the time of enrollment, and self-identification as Hispanic and/or black based on 2 separate screening questions used to create 2 mutually exclusive categories: “Black, non-Hispanic” and “Hispanic.” Patients were excluded if they had a clinical condition that might have required specialized HTN management (e.g., end-stage renal disease), had a dementia diagnosis, or were unable to provide informed consent.
Enrollment and randomization procedures
Newly admitted home care patients were identified from electronic health records, using a variety of International Classification of Diseases (ICD) codes for poststroke care (with the vast majority 438 [ICD-9] or I69 [ICD-10] late effects of cerebrovascular disease). Stroke or transient ischemic attack history and other eligibility was confirmed through patient self-report during a telephone eligibility call. Research interviewers screened for SBP in the home, using a validated, automated oscillometric BP device (Microlife WatchBP, Golden, CO). Patients were asked not to smoke, have caffeine, or participate in physical activity prior to the interview. BP was taken in the seated position after a resting period of 5 minutes. Three BP readings were taken from both arms and averaged. The arm with the higher SBP was designated as the “dominant” arm. Patients were eligible if average SBP on their dominant arm was ≥140 mm Hg. Those who met criteria and provided informed consent were enrolled, and then completed the baseline assessment—a structured interview, largely consisting of patient self-report measures. Following the baseline interview, subjects were randomized to 1 of 3 arms. Follow-up assessments were conducted at 3 and 12 months post-baseline.
Measures
Primary outcome: change in SBP.
The primary outcome was change in SBP from baseline to 3 and 12 months. The 3-month follow-up was chosen for proximity to conclusion of the longest intervention (UHC + NP + HC); 12 months was chosen to assess medium-term effectiveness. At each of the follow-up visits, research interviewers, blinded to patient assignment group, again measured SBP 3 times using the dominant arm; the average was used for analysis.
Descriptive data.
National Institute of Neurological Disorders and Stroke Common Data Elements25 were used to collect information on multiple patient-level predisposing (e.g., demographics), enabling (e.g., insurance, informal support, health literacy), and need (e.g., stroke history, comorbidities, functional status) variables that could be potential confounders/predictors of the primary outcome. These data were used to ensure balance among patients across study arms.
Statistical procedures
Power analysis for primary outcome variable.
Power for SBP rate of change was calculated including all 3 assessment points.26 The calculation demonstrated that under intent-to-treat, a rate of reduction in SBP equivalent to 5.40 mm Hg was detectable, given the assumptions posited related to pooled variances, translating to relatively small effect sizes using Cohen's d. The proposed sample size was 165 per group.
Data analyses for primary outcome: SBP.
The main hypothesis was that those assigned to the intervention groups would, on average, exhibit greater 3- and 12-month decreases in SBP than those assigned to UHC-only. The primary analyses used mixed random effects models, and a full information maximum likelihood approach, with sensitivity analyses using generalized estimating equations regression models. The change from pre- to posttreatment values of SBP was modeled as functions of time, treatment, and the interaction of time and treatment. The general longitudinal mixed-effects model, using SAS PROC MIXED, was used to model serial correlations and group heterogeneity in residual variances if needed. The ITT analyses were designed to permit all individuals with at least 1 observation to be included. Baseline differences on key variables between completers and those lost to follow-up were examined to assess the nature of missing data. Because little missing data were observed, modeling was not required. Significance tests were 2 tailed. SBP was treated as a continuous variable and, based on graphical inspection of the distribution of the outcome, did not require prior transformation. Time was measured in months. Sensitivity analyses were conducted using an analysis of covariance mixed model approach.
Prior to analyses, baseline values of potential confounders/moderators were examined to determine if any covariates required modeling due to imbalance among study arms. In addition, to determine if interaction term analysis was warranted we examined the distribution of observed SBP means across randomized arms for 12 subgroups defined by baseline characteristics including predisposing (e.g., age, race/ethnicity (Hispanic vs. black, non-Hispanic)), enabling (e.g., education, income, insurance type), and need/illness variables (e.g., smoking status, depression).
Research involving human participants: All procedures performed in this study were in accordance with the ethical standards of the institution and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
RESULTS
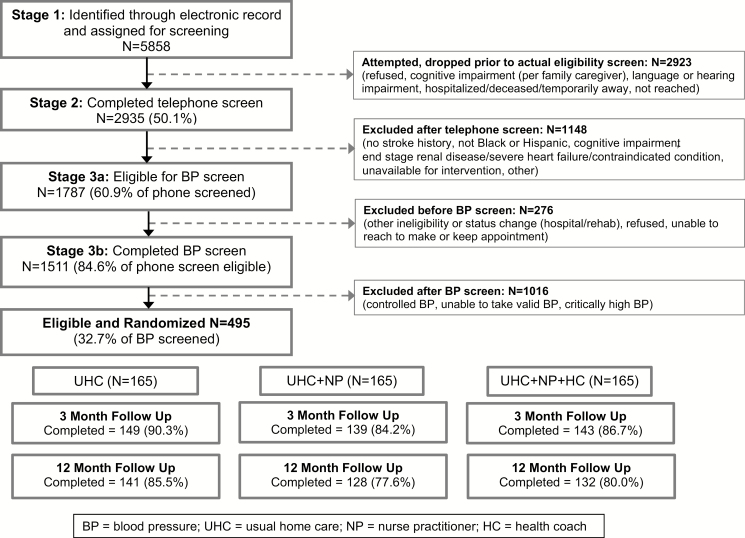
The CONSORT diagram (Figure 1) shows that of 5,858 patients identified in the electronic health record as meeting initial study criteria, 2,923 were dropped prior to the screen; of these, 22.8% could not be reached for screening, 18.6% refused screening, and 32.8% did not meet eligibility criteria. Of the 1,511 patients who participated in the BP screening, 495 (32.7%) were eligible, consented, and completed baseline interviews. Attrition was not statistically different across groups. Retention rates of 87% at the 3-month follow-up and 81% at the 12-month follow-up were achieved.
Figure 1.
CONSORT diagram. Abbreviations: BP, blood pressure; HC, health coach; NP, nurse practitioner; UHC; usual home care.
Baseline patient characteristics
Mean participant age was 66.6; 57.0% were female; 69.7% were black, non-Hispanic; and were 30.3% Hispanic (Table 1). Just over 50% were born outside of the United States. Average education was 11.3 years; 61.3% reported annual family income of less than $25,000. Over 60% reported diabetes; over 20% heart failure. Participants reported an average of 1.7 prior strokes and most were ischemic; 65% percent had their most recent stroke within 90 days prior to enrollment. Examination of characteristics across study arms showed significant differences in the baseline distribution of education (P = 0.0037) and insurance type (P = 0.018).
Table 1.
Baseline patient characteristics
| Total (N = 495) | UHC (N = 165) | NP only (N = 165) | NP + HC (N = 165) | |
|---|---|---|---|---|
| Predisposing patient characteristics | ||||
| Age (mean, SD) | 66.6 (11.2) | 66.5 (11.3) | 66.9 (11.1) | 66.4 (11.2) |
| Female (%) | 57.0 | 58.8 | 59.4 | 52.7 |
| Black, non-Hispanic (%) | 69.7 | 73.3 | 70.3 | 65.5 |
| Hispanic (%) | 30.3 | 26.7 | 29.7 | 34.5 |
| Marital status (%) | ||||
| Single/never married | 27.5 | 30.1 | 24.5 | 27.8 |
| Married/domestic partnership | 31.1 | 33.1 | 31.3 | 29.0 |
| Divorced/separated | 22.1 | 21.5 | 23.9 | 21.0 |
| Widowed | 19.3 | 15.3 | 20.2 | 22.2 |
| Country of origina: United States (%) | 49.5 | 46.7 | 54.5 | 47.3 |
| Enabling patient characteristics | ||||
| Education (mean, SD) | 11.3 (3.7) | 11.4 (3.8) | 11.3 (3.6) | 11.0 (3.9) |
| Income (%) | ||||
| $0–$9,999 annually | 27.8 | 23.6 | 24.5 | 35.4 |
| $10,000–$14,999 annually | 17.2 | 16.6 | 15.3 | 19.6 |
| $15,000–$24,999 annually | 16.3 | 19.7 | 15.3 | 13.9 |
| $25,000 and above annually | 23.6 | 26.1 | 28.2 | 16.5 |
| Unknown/refusal | 15.1 | 14.0 | 16.6 | 14.6 |
| Insurance: primary payer (%) | ||||
| Medicare | 56.0 | 49.7 | 63.0 | 55.2 |
| Medicaid | 26.7 | 28.5 | 20.0 | 31.5 |
| Private pay | 13.3 | 18.8 | 11.5 | 9.7 |
| Other | 20 | 3.0 | 5.5 | 3.6 |
| Need/illness-level characteristics | ||||
| Currently smokes (%) | 14.7 | 13.3 | 16.4 | 14.5 |
| Diabetes (%) | 62.1 | 64.2 | 62.3 | 59.9 |
| Heart attack (%) | 18.2 | 19.4 | 16.0 | 19.1 |
| Heart failure (%) | 22.5 | 23.6 | 20.9 | 22.9 |
| Heart murmur or valvular heart disease (%) | 24.3 | 26.7 | 23.2 | 23.1 |
| Diastolic blood pressure (mean, SD) | 85.8 (12.7) | 86.2 (11.9) | 85.7 (12.9) | 85.5 (13.3) |
| No. of strokes/TIAs (mean, SD) | 1.7 (1.2) | 1.7 (1.1) | 1.8 (1.5) | 1.5 (0.8) |
| Most recent stroke, type report in medical record (%) | ||||
| Ischemic | 56.4 | 61.2 | 56.4 | 51.5 |
| Hemorrhagic | 8.1 | 6.7 | 8.5 | 9.1 |
| TIA | 6.9 | 6.1 | 4.8 | 9.7 |
| Not specified/unable to obtain medical record | 28.7 | 26.1 | 30.3 | 29.7 |
| Time since most recent stroke/TIA (days, mean, SD) | 544.9 (1342.6) | 606.5 (1586.8) | 545.8 (1315.9) | 478.7 (1068.0) |
Abbreviations: HC, health coach; NP, nurse practitioner; TIA, transient ischemic attack; UHC, usual home care.
aUS territories coded as international.
Primary outcome analyses
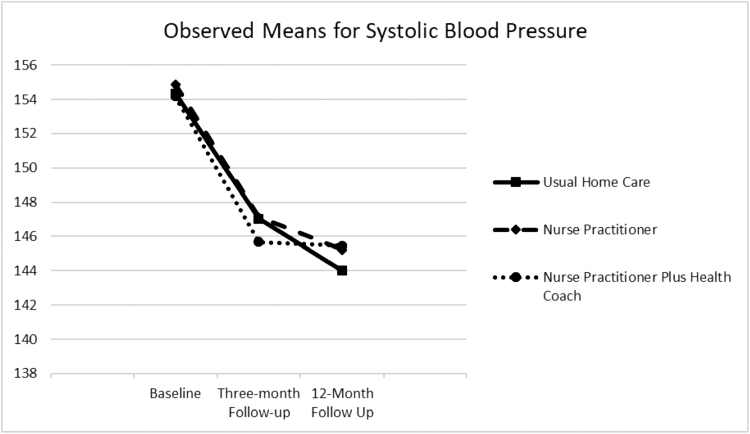
Table 2 and Figure 2 show the observed means for SBP for the total sample and each study arm at all 3 time points. Model-based means (not shown) were similar to observed means. The 3-arm means were not significantly different at each time point. Significant within-treatment groups effects were observed; each group decreased between 9 and 10 mm Hg from baseline to 12-month follow-up, with the greatest decrease occurring between baseline and 3-month follow-up. Table 3 shows the results of the longitudinal analysis comparing the groups over time. There were no significant differences in SBP reduction among the groups.
Table 2.
Observed means and SDs for systolic blood pressure over time by study arm
| Total (n = 495) | UHC (n = 165) | NP only (n = 165) | NP + HC (n = 165) | P value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | ||
| Baseline | 495 | 154.47 | (13.37) | 165 | 154.34 | (13.12) | 165 | 154.88 | (14.72) | 165 | 154.18 | (12.22) | 0.882 |
| 3-month follow-up | 427 | 146.62 | (18.85) | 147 | 147.05 | (20.31) | 138 | 147.13 | (18.10) | 142 | 145.67 | (18.09) | 0.765 |
| 12-month follow-up | 398 | 144.88 | (20.04) | 138 | 144.01 | (18.83) | 128 | 145.22 | (21.67) | 132 | 145.45 | (19.73) | 0.818 |
Abbreviations: HC, health coach; NP, nurse practitioner; UHC, usual home care.
Figure 2.
Observed means and SDs for systolic blood pressure (SBP) in mm Hg over time by randomization group (n = 495) demonstrating a significant within-group decrease in SBP from baseline to the 2 follow-up points but no differences in SBP decline between randomized groups.
Table 3.
Results of repeated measures mixed models analysis of SBP by administration and time in months (n = 495)
| Using time in months | |||
|---|---|---|---|
| Estimate | SE | P value | |
| Intercept | 151.72 | 1.1588 | <0.0001 |
| Administration (time) | −0.6171 | 0.1689 | 0.0003 |
| Group: UHC | 0.8325 | 1.6348 | 0.6108 |
| Group: NP Only | 1.1295 | 1.6417 | 0.4918 |
| Group: NP + HC | 0 | ||
| Administration (time) by group: UHC vs. NP + HC | −0.1650 | 0.2372 | 0.4869 |
| Administration (time) by group: NP vs. NP + HC | −0.0935 | 0.2400 | 0.6969 |
| Administration (time) by group: UHC vs. NP | −0.0715 | 0.2384 | 0.7644 |
Administration (baseline, first follow-up, second follow-up). Abbreviations: HC = health coach; NP, nurse practitioner; SBP, systolic blood pressure; UHC, usual home care.
Sensitivity analysis of SBP.
Results of the longitudinal analysis comparing the groups over time, including only completers, yielded results very similar to those of the main analyses. A decrease in SBP was observed over time in all 3 treatment groups, with no significant differences in SBP reduction among groups.
Analysis of potential moderators and confounding factors.
When tested for potential moderating effects, the education and insurance subgroups by randomization group interaction were not significant, indicating that these variables were not moderating the relationship of treatment group to SBP. In contrast, observed SBP means within each characteristic subgroup by randomization group evidenced significant SBP differences between the randomization groups for the currently smokes (vs. currently not smokes) subgroup (P = 0.049) and the race/ethnicity (black vs. Hispanic) subgroup (P = 0.026) (but not for any other subgroups). Because of sparse data in the currently smokes group, further analysis was not performed. (There were fewer than 30 participants in each treatment group that currently smoked.)
At baseline, black respondents evidenced higher SBP than Hispanics (155.35 vs. 152.44, respectively, P = 0.026). An interaction term was constructed for intervention group by administration by Hispanic status. The difference in slopes between the treatment groups within the Hispanic and non-Hispanic subgroups were examined using contrast estimates. The overall interaction term testing for a moderator effect for Hispanic status was significant (P = 0.0001). Although all subgroups declined over time, the black subgroup tended to have a greater reduction in SBP over time than the Hispanic subgroup (P = 0.04). The difference appeared to be in the NP group: blacks in the NP group decreased significantly more than did the Hispanics in the NP group. There was no significant difference between black and Hispanic slopes in the UHC or NP + treatment groups (P = 0.3270 and P = 0.5155, respectively). In slope analyses, the black patients in the NP group decreased 3.03 (P = 0.0396) points per wave more than Hispanics in the NP group.
DISCUSSION
This study yielded 3 main findings. First, over a 12-month period, participants experienced an average drop of nearly 10 mm Hg in SBP from baseline to final follow-up. Second, this large reduction was the same across all study arms. Third, virtually all SBP reductions occurred between baseline and the 3-month follow-up but were sustained at the 12-month follow-up.
The clinical importance of a 10 mm Hg across-the-board drop in study participants' SBP should not be underestimated. A 10 mm Hg difference in SBP has been equated to a 24% difference in stroke risk among black individuals8 and every 1 mm Hg elevation in SBP to a 1% increase in stroke mortality in the general population.27 Secondary stroke prevention randomized controlled trials and meta-analyses confirm that lowering BP significantly reduces the risk of both recurrent and first strokes.28 Because the absolute and relative risks of recurrent stroke are highest early after the first stroke,29 this study's 12-month SBP improvement may have been especially important for our population.
The results of the subgroup analyses treating Hispanic status as a moderator yielded mixed results. A relative advantage in SBP reduction among black compared to Hispanic patients in the NP group was observed. One could speculate that a reason could be less culturally sensitive delivery of the intervention in that group. However, these results were not observed in the NP + HC group, perhaps because the HC's presence somehow compensated for the NP's intervention delivery actions. A future goal could be to examine carefully the ways in which each intervention was delivered to Hispanic clients. Because the Hispanic subgroup within the NP group started with higher baseline values of SBP than any of the other groups, and because of the small subgroup sample sizes, the results may be an artifact and not robust; however, in general the results for the subgroups were similar to the overall results in that the intervention groups did not fare better than usual care.
What factors might explain the interventions' lack of relative advantage compared to UHC-only? Imperfect intervention fidelity associated with the study's pragmatic intent-to-treat design may have been 1 factor. Customary US home care practice requires (and pays for) in-home patient visits, not phone calls, and may explain NPs' and HCs' high fidelity to the interventions' visit compared to phone call protocols. Eighty-eight percent of patients in the UHC + NP group and 85% of patients in the UHC + NP + HC group received the full “dose” of prescribed NP home visits; 75% of patients in the UHC + NP + HC group received all prescribed HC visits. In contrast, just over 50% received the prescribed dose of NP and HC phone calls. Depending on patient availability and preference, NPs and HCs deviated from call schedules when they did not perceive a specific follow-up need. NP–physician outreach also was lower than expected. NPs, engaged for their training in medical doctor (MD) communication and collaboration,30 communicated with the patient's primary care provider in only about 20% of cases. Reported reasons for not communicating included the following: no designated primary care provider, UHC nurse contacted instead of MD, and NP focused on patient nonadherence instead of regimen changes requiring MD involvement. NP failure to enlist physicians as allies in HTN management may have been a missed opportunity to improve effectiveness.
Published pragmatic studies also have found BP improvements in usual care groups comparable to those in intervention groups.31 These studies share several commonalities with ours. First, usual care participants—all blacks or Hispanics with uncontrolled HTN—were high risk, chronically ill patients. Second, they were not receiving minimal or no services as is common in many community-based BP studies, but were receiving many services, potentially including HTN management. Thus, the interventions may have had an unusually high benefit threshold to exceed. Third, clinicians throughout the host organizations often were aware of ongoing HTN research, resulting possibly in heightened BP management efforts in usual care. Fourth, most study protocols used obtrusive assessment instruments, including BP measurements, for all study arms, which may have heightened usual care patients' BP awareness and self-management efforts.
Secular events and trends also may have affected outcomes. Shortly before our study started the Eighth Joint National Committee (JNC 8) issued new BP guidelines,32 which raised the SBP target for individuals aged 60 years or older to <150 mm Hg rather than <140 mm Hg. These guidelines have been challenged, and were never endorsed by the American Heart Association or the American College of Cardiology, but they received much publicity, possibly influencing clinicians' decisions. Clinical inertia in other studies has been attributed to disagreements about appropriate BP targets.33,34
Virtually all of the SBP reduction observed across the study arms occurred by the 3-month follow-up, the period most proximate to UHC nurse involvement. The lack of an additive effect of NPs and HCs suggests that their contribution to BP management was negligible and that SBP reductions were more likely attributable to the interactions between UHC nurses, their patients, and physicians during the course of routine home health care. It also suggests that we may have underestimated UHC as an effective multilevel agent for addressing uncontrolled HTN. Whether the transitional care interventions produced comparative improvements in other secondary patient outcomes (e.g., weight or self-efficacy) remains to be explored
Strengths and limitations
This study's pragmatic design, intended to maximize sustainability in the home care setting, was both a strength and a limitation, introducing some unmeasurable but unavoidable bias that may have mitigated the interventions' demonstrable effect. However, we worked to constrain bias (e.g., through patient randomization, use of independent “blinded” research interviewers, and use of detailed intervention protocols). Further, despite unavoidable bias, our findings likely indicate more accurately than a “purer” trial the challenges transitional care programs face in the home care environment and their difficulties in achieving comparative advantage over UHC. Conversely, the study findings also suggest that the strengths of UHC in chronic care management may be underrated.
An additional strength/limitation of this study was that it enrolled only US black and Hispanic individuals, unlike most transitional care studies, which have enrolled mostly white populations.19,20 The advantage was that our resources could be devoted to developing culturally tailored interventions for 2 populations with well-documented health disparities. The disadvantage was that our findings are silent on possible advantages for a non-Hispanic white, nonurban, non-US population. Another limitation/advantage was that the study was conducted in a single organization. That limitation was offset by the fact that the host organization has one of the largest black and Hispanic home care populations in the United States, making study recruitment effective and efficient. Nevertheless, additional research on interventions for poststroke patients in other geographical and organizational settings would enhance the generalizability of our findings.
Conclusion
Many common barriers to successful HTN management exist internationally at the system, provider, and individual level.5,35 Home- and community-based nurse and coaching interventions to overcome treatment barriers are far more common in the United States than elsewhere. However, European, Asian and African countries increasingly recognize home- and community-based services as an important vehicle for improving access to and effectiveness of primary and chronic care in both urban and rural communities.36,37 Thus, the implications of this study potentially extend far beyond its urban US setting. This is the second large pragmatic trial to demonstrate that UHC produced significant, clinically important reductions in the SBP of older hypertensive patients and that interventions augmenting UHC yielded no comparative advantage.15 UHC in this study reduced average SBP from 154 to 144 mm Hg—a 10.33 mm Hg reduction that was, nevertheless, still above the target of <140/90 mm Hg recommended by current poststroke guidelines.38,39 These findings suggest that additional efforts to lower recurrent stroke risk should test incremental improvements in UHC rather than more resource-intensive add-on transitional care interventions. They also point to the potential importance of pragmatic home- and community-based nursing programs as a broader strategy to overcome HTN treatment barriers and improve secondary prevention in the United States and abroad.
DISCLOSURE
The authors declared no conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number U54NS081765. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank all our study participants who gave their time to help us evaluate this initiative.
REFERENCES
- 1. Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Hassen Abate K, Akinyemiju TF, Ali R, Alvis-Guzman N, Azzopardi P, Banerjee A, Bärnighausen T, Basu A, Bekele T, Bennett DA, Biadgilign S, Catalá-López F, Feigin VL, Fernandes JC, Fischer F, Gebru AA, Gona P, Gupta R, Hankey GJ, Jonas JB, Judd SE, Khang YH, Khosravi A, Kim YJ, Kimokoti RW, Kokubo Y, Kolte D, Lopez A, Lotufo PA, Malekzadeh R, Melaku YA, Mensah GA, Misganaw A, Mokdad AH, Moran AE, Nawaz H, Neal B, Ngalesoni FN, Ohkubo T, Pourmalek F, Rafay A, Rai RK, Rojas-Rueda D, Sampson UK, Santos IS, Sawhney M, Schutte AE, Sepanlou SG, Shifa GT, Shiue I, Tedla BA, Thrift AG, Tonelli M, Truelsen T, Tsilimparis N, Ukwaja KN, Uthman OA, Vasankari T, Venketasubramanian N, Vlassov VV, Vos T, Westerman R, Yan LL, Yano Y, Yonemoto N, Zaki ME, Murray CJ. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA 2017; 317:165–182. [DOI] [PubMed] [Google Scholar]
- 2. WHO. Fact Sheet. Hypertension Key Facts. https://www.who.int/news-room/fact-sheets/detail/hypertension. Accessed 7 January 2019.
- 3. Graham G. Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev 2015; 11:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pool LR, Ning H, Lloyd-Jones DM, Allen NB. Trends in racial/ethnic disparities in cardiovascular health among US adults from 1999–2012. J Am Heart Assoc 2017; 6:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 Countries. Circulation 2016; 134:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mittal BV, Singh AK. Hypertension in the developing world: challenges and opportunities. Am J Kidney Dis 2010; 55:590–598. [DOI] [PubMed] [Google Scholar]
- 7. Mensah GA, Galis ZS, Fine LJ, Garcia ME, Levy DF, Gibbons GH. Building on a legacy of hypertension research: charting our future together. Hypertension 2017; 69:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Howard G, Lackland DT, Kleindorfer DO, Kissela BM, Moy CS, Judd SE, Safford MM, Cushman M, Glasser SP, Howard VJ. Racial differences in the impact of elevated systolic blood pressure on stroke risk. JAMA Intern Med 2013; 173:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Margolis KL, Piller LB, Ford CE, Henriquez MA, Cushman WC, Einhorn PT, Colon PJ Sr, Vidt DG, Christian R, Wong ND, Wright JT Jr, Goff DC Jr; Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group . Blood pressure control in Hispanics in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Hypertension 2007; 50:854–861. [DOI] [PubMed] [Google Scholar]
- 10. Whelton PK, Einhorn PT, Muntner P, Appel LJ, Cushman WC, Diez Roux AV, Ferdinand KC, Rahman M, Taylor HA, Ard J, Arnett DK, Carter BL, Davis BR, Freedman BI, Cooper LA, Cooper R, Desvigne-Nickens P, Gavini N, Go AS, Hyman DJ, Kimmel PL, Margolis KL, Miller ER III, Mills KT, Mensah GA, Navar AM, Ogedegbe G, Rakotz MK, Thomas G, Tobin JN, Wright JT, Yoon SS, Cutler JA; National Heart, Lung, and Blood Institute Working Group on Research Needs to Improve Hypertension Treatment and Control in African Americans . Research needs to improve hypertension treatment and control in African Americans. Hypertension 2016; 68:1066–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bartolome RE, Chen A, Handler J, Platt ST, Gould B. Population care management and team-based approach to reduce racial disparities among African Americans/blacks with hypertension. Perm J 2016; 20:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mueller M, Purnell TS, Mensah GA, Cooper LA. Reducing racial and ethnic disparities in hypertension prevention and control: what will it take to translate research into practice and policy? Am J Hypertens 2015; 28:699–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clark CE, Smith LF, Taylor RS, Campbell JL. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010; 341:c3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cooper LA, Marsteller JA, Noronha GJ, Flynn SJ, Carson KA, Boonyasai RT, Anderson CA, Aboumatar HJ, Roter DL, Dietz KB, Miller ER III, Prokopowicz GP, Dalcin AT, Charleston JB, Simmons M, Huizinga MM. A multi-level system quality improvement intervention to reduce racial disparities in hypertension care and control: study protocol. Implement Sci 2013; 8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feldman PH, McDonald MV, Barrón Y, Gerber LM, Peng TR. Home-based interventions for black patients with uncontrolled hypertension: a cluster randomized controlled trial. J Comp Eff Res 2016; 5:155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q 1996; 74:511–544. [PubMed] [Google Scholar]
- 17. Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001; 20:64–78. [DOI] [PubMed] [Google Scholar]
- 18. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA 2002; 288:1775–1779. [DOI] [PubMed] [Google Scholar]
- 19. Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc 2004; 52:675–684. [DOI] [PubMed] [Google Scholar]
- 20. Naylor MD, Aiken LH, Kurtzman ET, Olds DM, Hirschman KB. The care span: the importance of transitional care in achieving health reform. Health Aff (Millwood) 2011; 30:746–754. [DOI] [PubMed] [Google Scholar]
- 21. Patsopoulos NA. A pragmatic view on pragmatic trials. Dialogues Clin Neurosci 2011; 13:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ford I, Norrie J. Pragmatic trials. N Engl J Med 2016; 375:454–463. [DOI] [PubMed] [Google Scholar]
- 23. Feldman PH, McDonald MV, Trachtenberg MA, Schoenthaler A, Coyne N, Teresi J. Center for stroke disparities solutions community- based care transition interventions: study protocol of a randomized controlled trial. Trials 2015; 16:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parry C, Coleman EA, Smith JD, Frank J, Kramer AM. The care transitions intervention: a patient-centered approach to ensuring effective transfers between sites of geriatric care. Home Health Care Serv Q 2003; 22:1–17. [DOI] [PubMed] [Google Scholar]
- 25. Grinnon ST, Miller K, Marler JR, Lu Y, Stout A, Odenkirchen J, Kunitz S. National Institute of Neurological Disorders and Stroke Common Data Element Project—approach and methods. Clin Trials 2012; 9:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Diggle PJ Liang KY, Zeger SL.. Analysis of Longitudinal Data. Oxford University Press: New York, 1994. [Google Scholar]
- 27. Palmer AJ, Bulpitt CJ, Fletcher AE, Beevers DG, Coles EC, Ledingham JG, O'Riordan PW, Petrie JC, Rajagopalan BE, Webster J. Relation between blood pressure and stroke mortality. Hypertension 1992; 20:601–605. [DOI] [PubMed] [Google Scholar]
- 28. Hong KS. Blood pressure management for stroke prevention and in acute stroke. J Stroke 2017; 19:152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Edwards JD, Kapral MK, Fang J, Swartz RH. Long-term morbidity and mortality in patients without early complications after stroke or transient ischemic attack. CMAJ 2017; 189:E954–E961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hain D, Fleck LM. Barriers to NP practice that impact healthcare redesign. Online J Issues Nurs 2014; 19:2. [PubMed] [Google Scholar]
- 31. Pavlik VN, Chan W, Hyman DJ, Feldman P, Ogedegbe G, Schwartz JE, McDonald M, Einhorn P, Tobin JN. Designing and evaluating health systems level hypertension control interventions for African-Americans: lessons from a pooled analysis of three cluster randomized trials. Curr Hypertens Rev 2015; 11:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. [DOI] [PubMed] [Google Scholar]
- 33. Kerry SM, Markus HS, Khong TK, Cloud GC, Tulloch J, Coster D, Ibison J, Oakeshott P. Home blood pressure monitoring with nurse-led telephone support among patients with hypertension and a history of stroke: a community-based randomized controlled trial. CMAJ 2013; 185:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agarwal R, Bills JE, Hecht TJ, Light RP. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension 2011; 57:29–38. [DOI] [PubMed] [Google Scholar]
- 35. Morgenstern LB, Kissela BM. Stroke disparities: large global problem that must be addressed. Stroke 2015; 46:3560–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bolarinwa OA, Juni MH, Nor Afiah MZ, Salmiah MS, Akande TM. Mid-term impact of home-based follow-up care on health-related quality of life of hypertensive patients at a teaching hospital in Ilorin, Nigeria. Niger J Clin Pract 2019; 22:69–78. [DOI] [PubMed] [Google Scholar]
- 37. Bernocchi P, Scalvini S, Bertacchini F, Rivadossi F, Muiesan ML. Home based telemedicine intervention for patients with uncontrolled hypertension–a real life non-randomized study. BMC Med Inform Decis Mak 2014; 14:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nerenberg KA, Zarnke KB, Leung AA, Dasgupta K, Butalia S, McBrien K, Harris KC, Nakhla M, Cloutier L, Gelfer M, Lamarre-Cliche M, Milot A, Bolli P, Tremblay G, McLean D, Padwal RS, Tran KC, Grover S, Rabkin SW, Moe GW, Howlett JG, Lindsay P, Hill MD, Sharma M, Field T, Wein TH, Shoamanesh A, Dresser GK, Hamet P, Herman RJ, Burgess E, Gryn SE, Grégoire JC, Lewanczuk R, Poirier L, Campbell TS, Feldman RD, Lavoie KL, Tsuyuki RT, Honos G, Prebtani APH, Kline G, Schiffrin EL, Don-Wauchope A, Tobe SW, Gilbert RE, Leiter LA, Jones C, Woo V, Hegele RA, Selby P, Pipe A, McFarlane PA, Oh P, Gupta M, Bacon SL, Kaczorowski J, Trudeau L, Campbell NRC, Hiremath S, Roerecke M, Arcand J, Ruzicka M, Prasad GVR, Vallée M, Edwards C, Sivapalan P, Penner SB, Fournier A, Benoit G, Feber J, Dionne J, Magee LA, Logan AG, Côté AM, Rey E, Firoz T, Kuyper LM, Gabor JY, Townsend RR, Rabi DM, Daskalopoulou SS; Hypertension Canada . Hypertension Canada's 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol 2018; 34:506–525. [DOI] [PubMed] [Google Scholar]
- 39. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45:2160–2236. [DOI] [PubMed] [Google Scholar]