Introduction
Alopecia areata is a common dermatologic affliction that frequently affects preadolescent and adolescent children and can have a severe influence on quality of life.1 Recent advances in the pathogenesis of alopecia areata have revealed that interferon-γ and interleukin 15 are important in the mechanism of disease for alopecia areata.2,3 The use of Janus kinase (JAK) inhibitors (JAKIs) is a promising therapeutic strategy for the treatment of severe alopecia areata, with many studies demonstrating 50% or greater improvement in Severity of Alopecia Tool score in one- to two-thirds of patients treated with the JAKIs tofacitinib4,5 and ruxolitinib.6 Although responses have been investigated in adults and adolescents, few studies have examined the effect of oral JAKI (tofacitinib) in preadolescents.7,8 Here, we present a case of a preadolescent 9-year-old boy with severe alopecia areata who was successfully treated with the JAKI ruxolitinib.
Case report
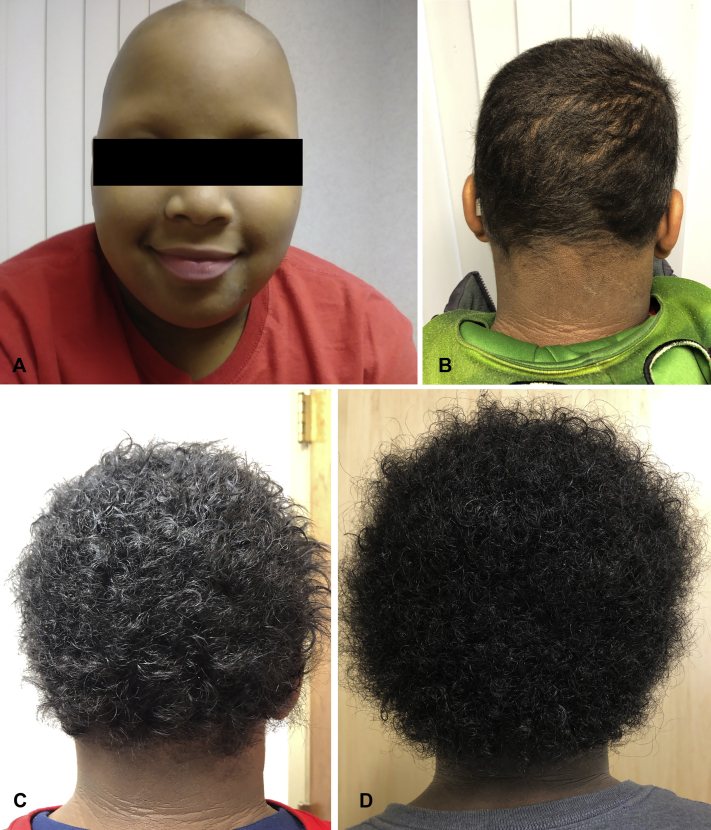
A 9-year-old obese boy first presented with a single patch of alopecia areata on his scalp that did not respond to intralesional triamcinolone. Within 2 months, he had developed several additional patches on his scalp and was treated again with intralesional triamcinolone, clobetasol solution, and oral prednisone, without response. By 8 months after his initial presentation, his disease had progressed to involve his entire scalp and eyebrows, consistent with alopecia totalis (Fig 1, A). Given lack of response to previous treatments and severe psychological distress, we initiated JAKI therapy. After denial of tofacitinib by insurance, ruxolitinib was approved and initiated with careful monitoring for hematologic effects. Screening laboratory test results for HIV, hepatitis B and C, and tuberculosis were negative before the initiation of treatment. For dosing, we followed a phase 1 study with dose escalation of ruxolitinib in patients with hematologic malignancies from aged 2 to 22 years.9 In this study, oral ruxolitinib was administered twice daily in escalating doses levels of 15, 21, 29, 39, and 50 mg/m2 per dose. Toxicities were similar across doses and included elevation of liver enzyme levels, creatinine-level elevation in the setting of acute kidney injury, anemia, lymphopenia, leukopenia, neutropenia, and thrombocytopenia.9 A discussion of potential treatment-related adverse effects with the patient and his mother ensued and included those listed earlier, as well as hypertriglyceridemia and vascular occlusion or cardiovascular stroke. After consent and baseline laboratory studies (complete blood cell count, liver function tests, serum electrolytes, renal function, and lipid panel), we opted to start at a dose of 25 mg/m2. According to the body surface area Dubois formula for 25 mg/m2 per dose in a patient with a height of 1.4 m and weight of 80 kg, the calculated dose was 40 mg ruxolitinib, and the patient began receiving ruxolitinib 20 mg twice daily. After 4 months, he had a nearly complete hair regrowth of eyebrows and scalp (Fig 1, B). The dosage was subsequently tapered to 10 mg twice daily for an additional 8 months (Fig 1C and D) without loss of efficacy. Ruxolitinib was tapered further to 10 mg once daily for 3 months, and the patient currently receives 10 mg every other day without loss of efficacy, treatment adverse effects, or laboratory-result abnormalities. Laboratory tests were performed every 3 to 4 months and included complete blood cell count, liver function tests, basic metabolic panel (including electrolyte levels and renal function), and lipid panel (including cholesterol and triglyceride levels). There were no laboratory abnormalities or changes from baseline throughout the treatment course. There were no infectious episodes during the treatment course.
Fig 1.
Response to treatment. Patient at baseline (A) and 4 months after beginning receipt of ruxolitinib 20 mg twice daily (B), followed by ruxolitinib 10 mg twice daily for 3 months (7 months' total treatment) (C) and 8 months (12 months' total treatment) (D).
Discussion
Treatment for severe alopecia areata is rapidly evolving. JAKIs work by blocking the JAK/signal transducer and activator of transcription pathway to halt CD8+ T-cell–mediated attack of hair follicles.5 Tofacitinib, ruxolitinib, baricitinib, and upadacitinib are Food and Drug Administration–approved JAKIs, but none are approved for alopecia areata. Tofacitinib, ruxolitinib, and baricitinib have shown efficacy in adults with alopecia areata,5,6,10 whereas 2 studies with oral tofacitinib showed benefit for preadolescent children with alopecia totalis or alopecia universalis.7,8 Further work for the use of JAKIs for alopecia areata is under way with adult patients. However, there is a paucity of literature when it comes to the use of JAKIs for alopecia areata in pediatric patients. This is a vulnerable population for whom treatment of alopecia areata, alopecia totalis, or alopecia universalis with JAKIs results in increased quality of life. We present a 9-year-old boy who demonstrated hair regrowth after the initiation of ruxolitinib.
Alopecia areata is a chronic autoimmune disease that will likely require long-term treatment. Initiation of JAKIs can effectively treat alopecia areata, but usually recurs with discontinuation of therapy. There are no clear guidelines for tapering JAKIs in any population, let alone preadolescent children. Here, we were able to taper ruxolitinib to 10 mg once every other day without loss of efficacy.
Tofacitinib, ruxolitinib, and baricitinib are first-generation nonselective inhibitors that target multiple JAKs. Next-generation JAKIs that are more selective for a single JAK are currently under investigation for other dermatologic and rheumatologic diseases. In accordance with pathogenesis and biomarker studies,3 interferon-γ and interleukin 15 appear to be critical for alopecia areata development, and therefore selective targeting of JAK1 (eg, interferon-γ, interleukin 15) or JAK3 (interleukin 15) may be beneficial.
Current clinical trials with JAKIs will help elucidate their efficacy and safety for alopecia areata. However, few clinical trials include children, and to our knowledge no current clinical trials in alopecia areata with JAKIs include preadolescent children. Further work investigating the efficacy and response of pediatric patients to JAKIs in alopecia areata is needed. Additionally, systematic evaluation of the use and tapering algorithms of current and future JAKIs in pediatric patients is warranted.
Acknowledgments
We are grateful to Brett A. King, MD, PhD for critical evaluation of the manuscript.
Footnotes
Funding sources: Dr Vesely is supported by a Dermatology Foundation Career Development Award, Melanoma Research Alliance, and National Center for Advancing Translational Sciences (KL2 TR001862).
Conflicts of interest: Dr Vesely's spouse is an employee of Regeneron Pharmaceuticals. Dr Peterson has no conflicts of interest to declare.
References
- 1.Liu L.Y., King B.A., Craiglow B.G. Alopecia areata is associated with impaired health-related quality of life: a survey of affected adults and children and their families. J Am Acad Dermatol. 2018;79(3):556–558.e1. doi: 10.1016/j.jaad.2018.01.048. [DOI] [PubMed] [Google Scholar]
- 2.Xing L., Dai Z., Jabbari A. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20(9):1043–1049. doi: 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pratt C.H., King L.E., Jr., Messenger A.G., Christiano A.M., Sundberg J.P. Alopecia areata. Nat Rev Dis Primers. 2017;3:17011. doi: 10.1038/nrdp.2017.11. 1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L.Y., Craiglow B.G., Dai F., King B.A. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76(1):22–28. doi: 10.1016/j.jaad.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy Crispin M., Ko J.M., Craiglow B.G. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1(15):e89776. doi: 10.1172/jci.insight.89776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackay-Wiggan J., Jabbari A., Nguyen N. Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata. JCI Insight. 2016;1(15):e89790. doi: 10.1172/jci.insight.89790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craiglow B.G., King B.A. Tofacitinib for the treatment of alopecia areata in preadolescent children. J Am Acad Dermatol. 2019;80(2):568–570. doi: 10.1016/j.jaad.2018.08.041. [DOI] [PubMed] [Google Scholar]
- 8.Dai Y.X., Chen C.C. Tofacitinib therapy for children with severe alopecia areata. J Am Acad Dermatol. 2019;80(4):1164–1166. doi: 10.1016/j.jaad.2018.12.041. [DOI] [PubMed] [Google Scholar]
- 9.Loh M.L., Tasian S.K., Rabin K.R. A phase 1 dosing study of ruxolitinib in children with relapsed or refractory solid tumors, leukemias, or myeloproliferative neoplasms: a Children's Oncology Group phase 1 consortium study (ADVL1011) Pediatr Blood Cancer. 2015;62(10):1717–1724. doi: 10.1002/pbc.25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jabbari A., Dai Z., Xing L. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EBioMedicine. 2015;2(4):351–355. doi: 10.1016/j.ebiom.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]