Introduction
Iododerma is a rare halogenoderma that develops after exposure to iodine-containing compounds. Three cases of iododerma in patients who had recent exposure to intravenous iodinated contrast media and confirmed elevated urine iodine levels are presented. These cases serve to illustrate that lesions can be heterogeneous, can progress rapidly, and frequently have a hemorrhagic component, all of which can cause clinical concern for disseminated infection, neutrophilic dermatosis, or vasculitis. In addition, skin biopsies in all patients demonstrated histologic features that to our knowledge have not previously been described in iododerma: a neutrophilic dermatosis with haloed structures simulating Cryptococcus, with or without vasculitis, a finding causing additional concern for disseminated infection.
Materials and methods
This study was approved by the University of Michigan institutional review board. Patients with iododerma diagnosed by the dermatology consultation team at Michigan Medicine, Ann Arbor, MI, between February and April 2019 were identified. Retrospective chart review was performed to obtain clinical and laboratory data, hospital course, and follow-up information.
Results
Case 1
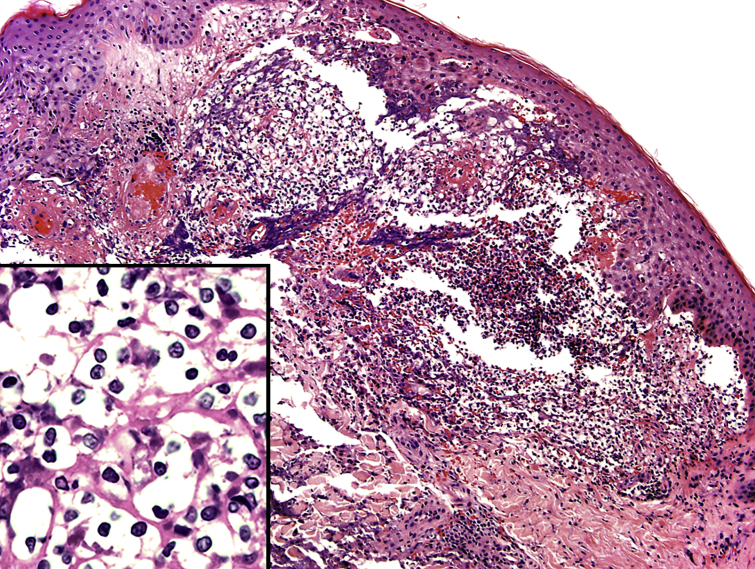
A 54-year-old man with ischemic cardiomyopathy, atrial fibrillation, diverticulitis status posthemicolectomy, hypertension, chronic kidney disease (stage 4), and type 2 diabetes mellitus presented with hemorrhagic shock from gastrointestinal tract bleeding. Computed tomography (CT) of the abdomen and pelvis with intravenous contrast (125 mL of Isovue 370; Bracco Diagnostics Inc, Princeton, NJ) and aortogram with intravenous contrast (130 mL Visipaque 320) was performed. Three days later, the patient rapidly developed multiple flesh-colored to translucent dome-shaped papules with central umbilication and hemorrhagic crust on the face, upper portion of the chest, proximal portion of the arms, and dorsal aspect of the hands (Fig 1, A). The dermatology service was consulted and skin biopsies for histopathologic evaluation and tissue cultures were performed. Skin biopsy demonstrated a neutrophilic dermatosis with haloed cryptococcal-like structures (Fig 2). Special stains for fungus were negative. The cryptococcal-like structures were positive with PU.1 immunostain, a nuclear histiocytic marker, and negative for myeloperoxidase. A neutrophilic dermatosis such as bowel-associated dermatosis arthritis syndrome or Sweet syndrome with haloed inflammatory cells was considered; however, infection remained a consideration pending tissue culture results.
Fig 1.
Cutaneous lesions in iododerma. A, Multiple flesh-colored to erythematous umbilicated papules and plaques on the face. B, Hemorrhagic papules and flaccid bullae on the dorsal aspect of the hands.
Fig 2.
Iododerma skin biopsy demonstrating an interstitial neutrophilic infiltrate in the superficial dermis. Inset highlights numerous haloed acellular structures simulating cryptococcal organisms. (Hematoxylin-eosin stain; original magnifications: ×100; inset, ×200.)
In the following 2 days, hemorrhagic bullae developed on the dorsal aspect of the hands (Fig 1, B) and buttocks. Violaceous retiform plaques were noted on the elbows. Because of this progression and concern for vasculitis, a repeated skin biopsy was performed, confirming vasculitis of small- and medium-sized vessels, in addition to a neutrophilic dermatosis with similar cryptococcal-like structures.
There was also interval development of pulmonary nodules identified on chest CT, concerning for disseminated fungal infection. Results for serum cryptococcal antigen, HIV testing, blood cultures, and tissue cultures were negative. The patient was treated with intravenous methylprednisolone and empirical intravenous amphotericin.
A urine iodine sample collected 3 days after the initial eruption was 185,174 μg/L (normal 26-705 μg/L). Shortly thereafter, the patient had recurrent gastrointestinal bleeding and died secondary to hemorrhagic shock.
Case 2
A 56-year-old woman with a medical history of alcoholic cirrhosis complicated by esophageal varices and hepatic encephalopathy, hypertension, chronic kidney disease (stage 4), drug-induced lupus erythematosus secondary to hydralazine, and antineutrophilic cytoplasmic antibody small-vessel vasculitis was admitted for altered mental status. Evaluation included CT of the chest, abdomen, and pelvis with intravenous contrast (125 mL Isovue 300). Two days later, she developed erythematous to violaceous papules with crusting and central umbilication on the face, chest, and dorsal aspect of the hands. During the next few days, the eruption became vesiculobullous and hemorrhagic. The dermatology service was consulted. Two skin biopsies were obtained and both demonstrated a dermal neutrophilic infiltrate with cryptococcal-like acellular bodies. These bodies were positive with PU.1 and negative for myeloperoxidase. Vasculitis was present in 1 of the biopsies. Results for special stains for microorganisms were negative.
The patient concurrently developed pulmonary nodular and masslike airspace opacities on chest CT, interpreted as suspicious for invasive aspergillosis. Infectious evaluation results were negative, including blood cultures for bacteria and fungus, tissue cultures, HIV, urine fungal antigens, lumbar puncture, and bronchoalveolar lavage. A 24-hour urine iodine level collected 3 days after the initial eruption was greater than 488,960 μg/24 hours (normal 75-851 μg/24 hours).
The patient was treated with triamcinolone 0.1% ointment and a prednisone taper with near-complete clearing of lesions. Repeated 24-hour urine iodine level 8 days after the initial eruption showed that the level had normalized to 632 μg/24 hours.
Case 3
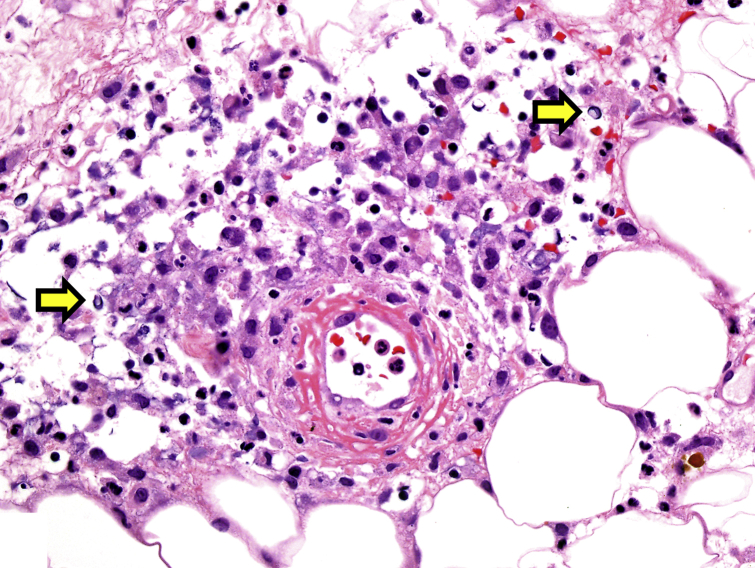
A 61-year-old man with a history of heart failure, atrial fibrillation, chronic obstructive pulmonary disease, hypertension, and chronic kidney disease (stage 5) on hemodialysis presented with septic shock, cardiac arrest, and a rash. Eight days before presentation, he underwent CT of the abdomen and pelvis with intravenous contrast (100 mL Isovue 370) for evaluation of abdominal pain. On admission, evaluation included CT angiography of the chest with intravenous contrast (70 mL Isovue 370) and CT of the abdomen and pelvis with intravenous contrast (125 mL Isovue 300). Skin examination showed intact vesicles on the forearms and lower extremities, flaccid ruptured bullae on the dorsal aspect of the hands, and hemorrhagic crusted erythematous papules on the forehead (Fig 3) and trunk. Skin biopsy of the right arm revealed a dermal neutrophilic infiltrate with haloed structures mimicking Cryptococcus and vasculitis of small- and medium-sized vessels (Fig 4). Results for special stains for fungus were negative. The haloed structures were positive with PU.1 and negative for myeloperoxidase. Results for tissue and blood cultures, HIV, and transthoracic echocardiogram were negative.
Fig 3.

Cutaneous lesions in iododerma. A, Hemorrhagic vesiculopapules on the forehead. B, Flaccid bullae and plaques with necrotic eschar on the dorsal aspect of the hand and fingers.
Fig 4.
Iododerma skin biopsy highlighting the dermal neutrophilic infiltrate with acellular haloed structures resembling cryptococcal organisms (arrows) and fibrinoid necrosis of a vessel wall consistent with vasculitis. (Hematoxylin-eosin stain; original magnification: ×400.)
A 24-hour urine iodine level collected 2 days after presentation was greater than 17,920 μg/24 hours (normal 75-851 μg/24 hours). The patient's hospital course was complicated by multidrug-resistant pseudomonal pneumonia resulting in respiratory failure. He died shortly thereafter.
Discussion
Iododerma is a rare cutaneous eruption caused by exposure to iodine-containing compounds. In addition to iododerma after iodinated contrast media exposure, it may occur after potassium iodide ingestion,1 amiodarone use,2 topical povidone-iodine application,3 and radioactive iodine 131 administration.4 Iododerma can present a diagnostic challenge both clinically and histologically. Although an acneiform pustular eruption or vegetative plaque is the more common presentation,5 hemorrhagic plaques,6 bullae,6 and vasculitis7 are less well-recognized manifestations.
These cases serve to highlight several critical points. Iododerma can progress rapidly and be polymorphous in its clinical presentation. All cases had a neutrophilic dermatosis with prominent cryptococcal-like acellular bodies, causing even greater initial concern for infection. Ko et al8 were the first to describe acellular bodies surrounded by capsule-like vacuolated spaces mimicking Cryptococcus in the setting of neutrophilic dermatosis. Since then, there have been additional rare reports of cryptococcoid Sweet syndrome,9,10 as well as cryptococcoid-like changes with vasculitis.11 None of these reports mentioned iodine levels. In our cases, the presumed nuclear fragments within the haloed spaces stained with PU.1, suggesting that the structures were likely degenerating histiocytes.
In addition, all of our patients also had biopsy-confirmed vasculitis. One of our patients had a known history of antineutrophilic cytoplasmic antibody-associated vasculitis; however, this was quiescent before admission. It seems reasonable that the vasculitis noted in our cases was secondary to iodine toxicity. Although the exact pathogenesis of vasculitis in iododerma remains unclear, it has been theorized that iodide increases recruitment of polymorphonuclear leukocytes, induces apoptosis, and produces circulating immune complexes, which can result in vasculitis.7
Two of our patients developed lung nodules, adding to the clinical concern for disseminated fungal infection. Although pulmonary findings are underrecognized, there are reports describing them in iododerma.1,7 Iodine toxicity can cause a multitude of systemic findings, including gastrointestinal bleeding, dysrhythmia, laryngeal edema, and increased bronchial secretions leading to nodular pulmonary infiltrates.12 Thus, in the setting of iodine toxicity, it is important to remember that there may be systemic manifestations, in addition to cutaneous lesions. The clinical picture can be complex as patients become progressively more toxic appearing with involvement of multiple organ systems. Thus, an accurate and expedient diagnosis is paramount.
The diagnosis of iododerma is largely one of exclusion. Elevated blood or urine iodine levels support the diagnosis. Renal insufficiency appears to be a risk factor because of decreased renal clearance and accumulation of iodine.6,7 All of our patients had severe renal insufficiency, likely predisposing them to the condition. With normal renal function, the elimination half-life of iodinated contrast agents is 90 to 120 minutes.13 All of our patients had elevated urine iodine levels several days after contrast administration, indicating delayed elimination. Two of our patients were receiving hydralazine. Iododerma after intravenous iodinated contrast in a patient receiving hydralazine has been previously reported.14 In addition, a patient with hydralazine-induced autoimmune syndrome, chronic kidney disease, vasculitis, and cryptococcoid-like Sweet syndrome has been recently described15; however, there was no mention of iodine levels. In patients with impaired renal function, iodinated contrast media and the concurrent use of hydralazine may confer an increased risk of iodine toxicity.
Dermatology is poised to be in the forefront to recognize iododerma and iodine toxicity with its varied presentation. Lesions may evolve rapidly, causing clinical concern for infection, neutrophilic dermatosis, or vasculitis. Systemic manifestations, including pulmonary infiltrates, may develop. Cryptococcoid neutrophilic dermatosis with or without vasculitis should be recognized as part of the histologic spectrum of iododerma.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Pranteda G., Grimaldi M., Salzetta M.L. Vegetating iododerma and pulmonary eosinophilic infiltration. A simple co-occurrence? Acta Derm Venereol. 2004;84:480–481. doi: 10.1080/000155584480481. [DOI] [PubMed] [Google Scholar]
- 2.Ricci C., Krasovec M., Frank E. Amiodarone induced iododerma treated by cyclosporine. Ann Dermatol Venereol. 1997;124:260–263. [PubMed] [Google Scholar]
- 3.Aliagaoglu C., Turan H., Uslu E. Iododerma following topical povidone-iodine application. Cutan Ocul Toxicol. 2013;32:339–340. doi: 10.3109/15569527.2013.780181. [DOI] [PubMed] [Google Scholar]
- 4.Paul A.K., Al-Nahhas A., Ansari S.M., Islam N. Skin eruptions following treatment with iodine-131 for hyperthyroidism: a rare and un-reported early/intermediate side effect. Nucl Med Rev Cent East Eur. 2005;8:125–127. [PubMed] [Google Scholar]
- 5.Obrien T.J. Iodic eruptions. Australas J Dermatol. 1987;28:119–122. doi: 10.1111/j.1440-0960.1987.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 6.Young A., Grossman M. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377–1379. doi: 10.1111/bjd.12852. [DOI] [PubMed] [Google Scholar]
- 7.Vaillant L., Pengloan J., Blanchier D. Iododerma and acute respiratory distress with leucocytoclastic vasculitis following the intravenous injection of contrast medium. Clin Exp Dermatol. 1990;15:232–233. doi: 10.1111/j.1365-2230.1990.tb02079.x. [DOI] [PubMed] [Google Scholar]
- 8.Ko J.S., Fernandez A.P., Anderson K.A. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38–45. doi: 10.1111/cup.12019. [DOI] [PubMed] [Google Scholar]
- 9.Byekova Y.A., Shedd A.D., Schiro J.A. An additional case of neutrophilic dermatosis histopathologically mimicking Cryptococcus in a patient with Sweet’s syndrome. J Cutan Pathol. 2014;41:972–974. doi: 10.1111/cup.12407. [DOI] [PubMed] [Google Scholar]
- 10.Wilson J., Gleghorn K., Kelly B. Cryptococcoid Sweet's syndrome: two reports of Sweet's syndrome mimicking cutaneous cryptococcosis. J Cutan Pathol. 2017;44:413–419. doi: 10.1111/cup.12921. [DOI] [PubMed] [Google Scholar]
- 11.Fresco A., Wang J., Krausz A. Cryptococcus-like changes in the setting of vasculitis. J Cutan Pathol. 2019;46:143–147. doi: 10.1111/cup.13380. [DOI] [PubMed] [Google Scholar]
- 12.Namasivayam S., Kalra M.K., Torres W.E., Small W.C. Adverse reactions to intravenous iodinated contrast media: an update. Curr Probl Diagn Radiol. 2006;35:164–169. doi: 10.1067/j.cpradiol.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Pasternak J.J., Williamson E.E. Clinical pharmacology, uses, and adverse reactions: a primer for the non-radiologist. Mayo Clin Proc. 2012;87:390–402. doi: 10.1016/j.mayocp.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang M.W., Miner J.E., Moiin A., Hashimoto K. Iododerma after computed tomographic scan with intravenous radiopaque contrast media. J Am Acad Dermatol. 1997;36:1014–1016. doi: 10.1016/s0190-9622(97)80291-5. [DOI] [PubMed] [Google Scholar]
- 15.Skaljic M., Agarwa A., Smith R.J. A hydralazine-induced triumvirate: lupus, cutaneous vasculitis, and cryptococcoid Sweet syndrome. JAAD Case Rep. 2019;5:1006–1009. doi: 10.1016/j.jdcr.2019.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]